Innovative Chemical Process for Recycling Thermosets Cured with Recyclamines® by Converting Bio-Epoxy Composites in Reusable Thermoplastic—An LCA Study
Abstract
:1. Introduction
2. Description of the Recycling Process
2.1. Process Details and Assumptions
2.1.1. Dissolving Phase
- Heating the solution to 70 °C
- -
- Initial temperature of the acetic acid solution and composites is 25 °C.
- -
- Heat capacity of water: 4.2 KJ/Kg °C.
- -
- Heat capacity of Acetic acid: 2.0 KJ/Kg °C.
- -
- Heat capacity of composite: 1 KJ/Kg °C.
- -
- Energy to heat the vessel to 70 °C plus the wasted energy assumed to be 10% of required energy to heat the solution.
- Keeping the solution temperature at 70 °C
2.1.2. Thermoplastic Precipitation Phase (PPT)
2.1.3. Washing Phase
2.1.4. Drying Phase
- -
- Vacuum: 1 HP
- -
- Tumbler: 1.5 HP
- -
- Heater: 800 W
2.2. Reclaimed Materials
2.2.1. Carbon Fibres
2.2.2. Epoxy Thermoplastic Properties
3. LCA Method Description
3.1. Goal and Scope and Inventory Analysis
3.2. Life Cycle Impact Assessment (LCIA)
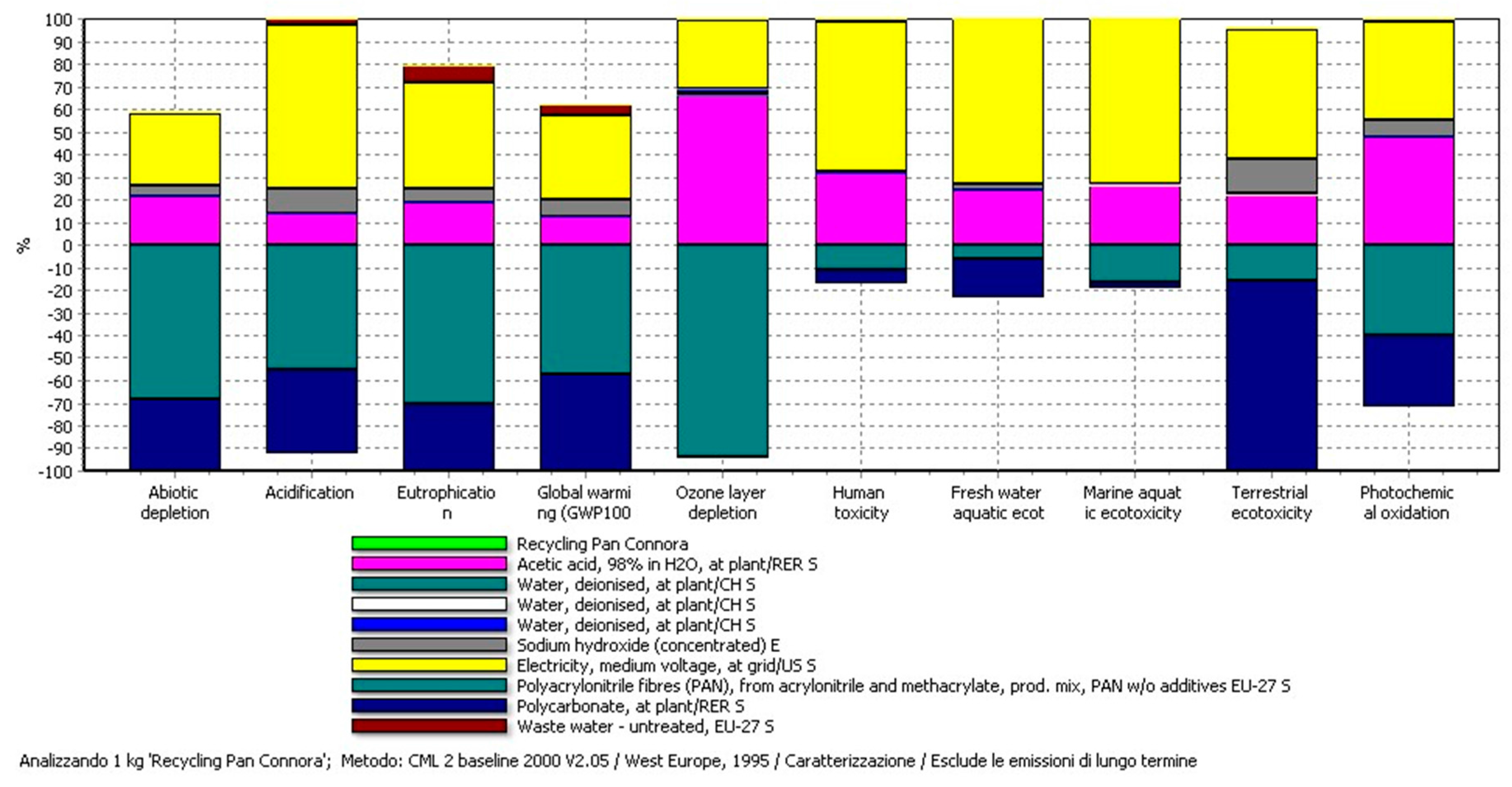
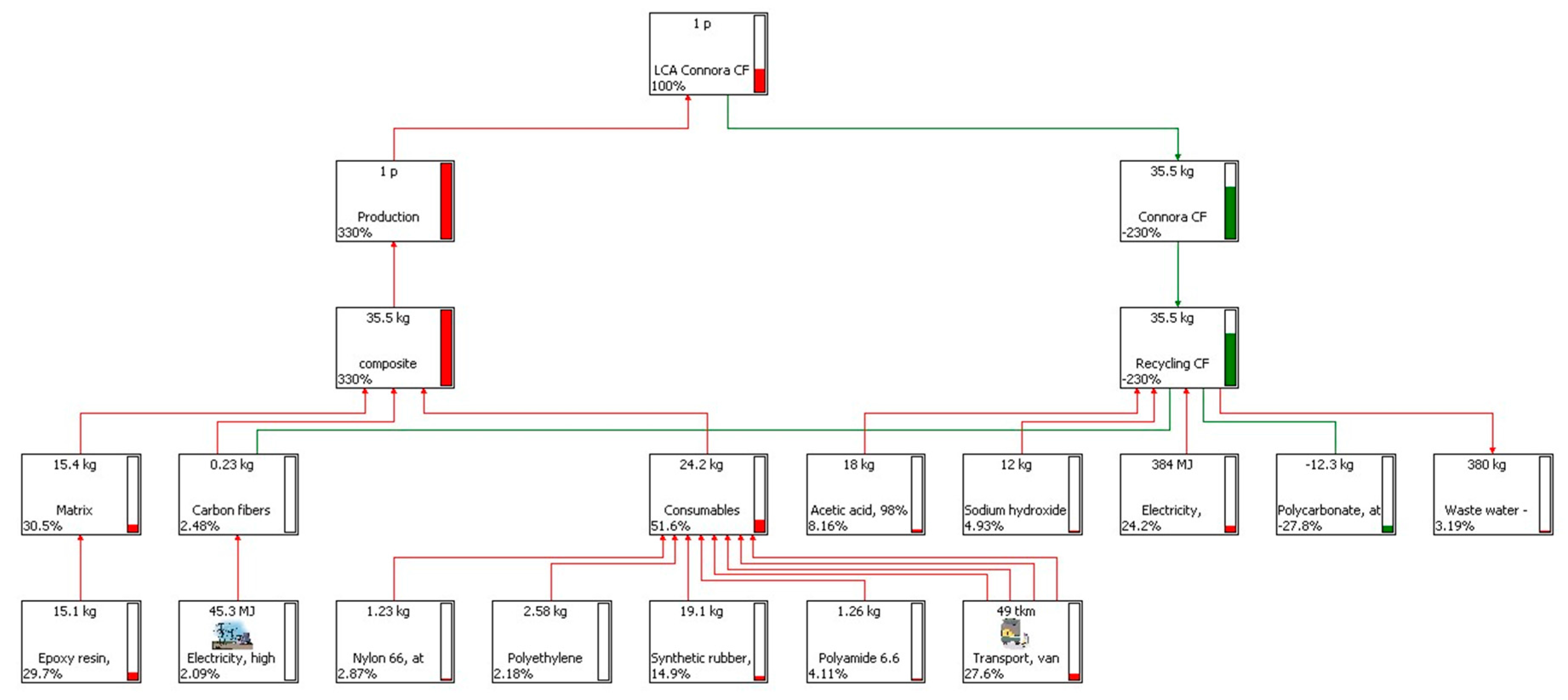
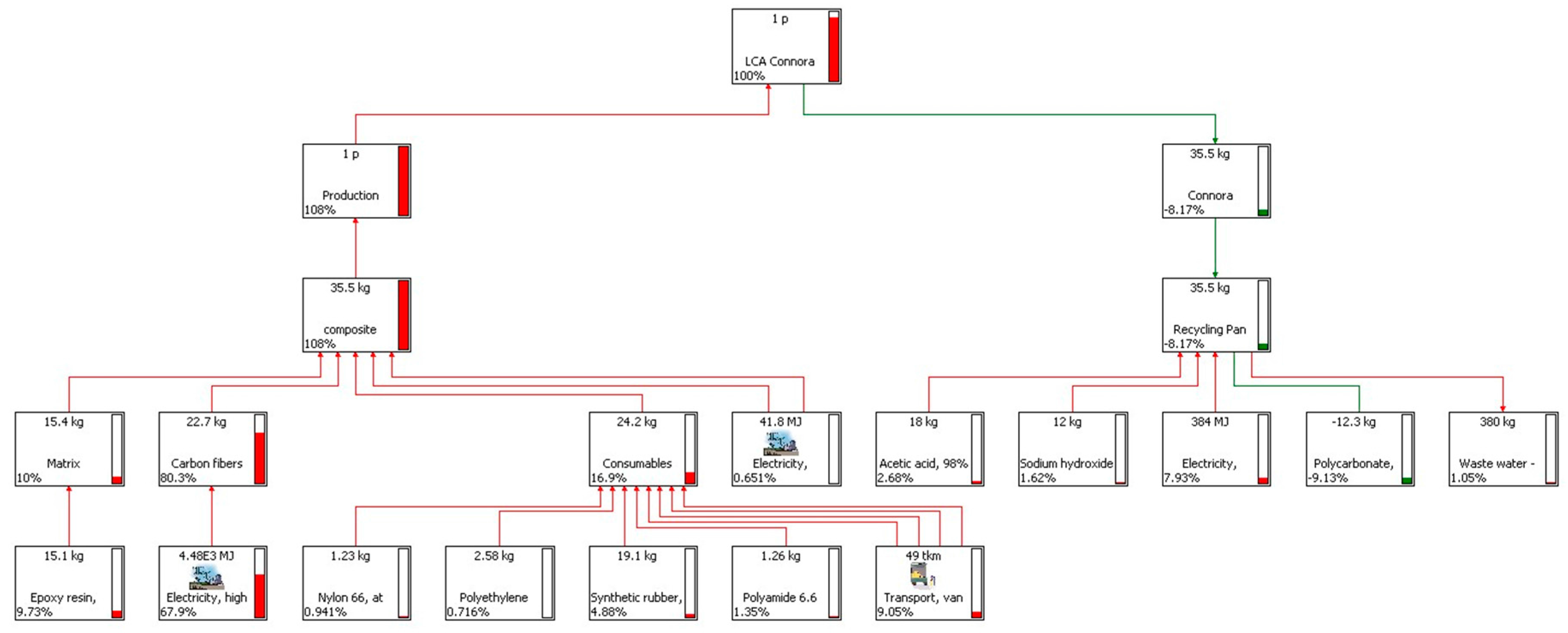
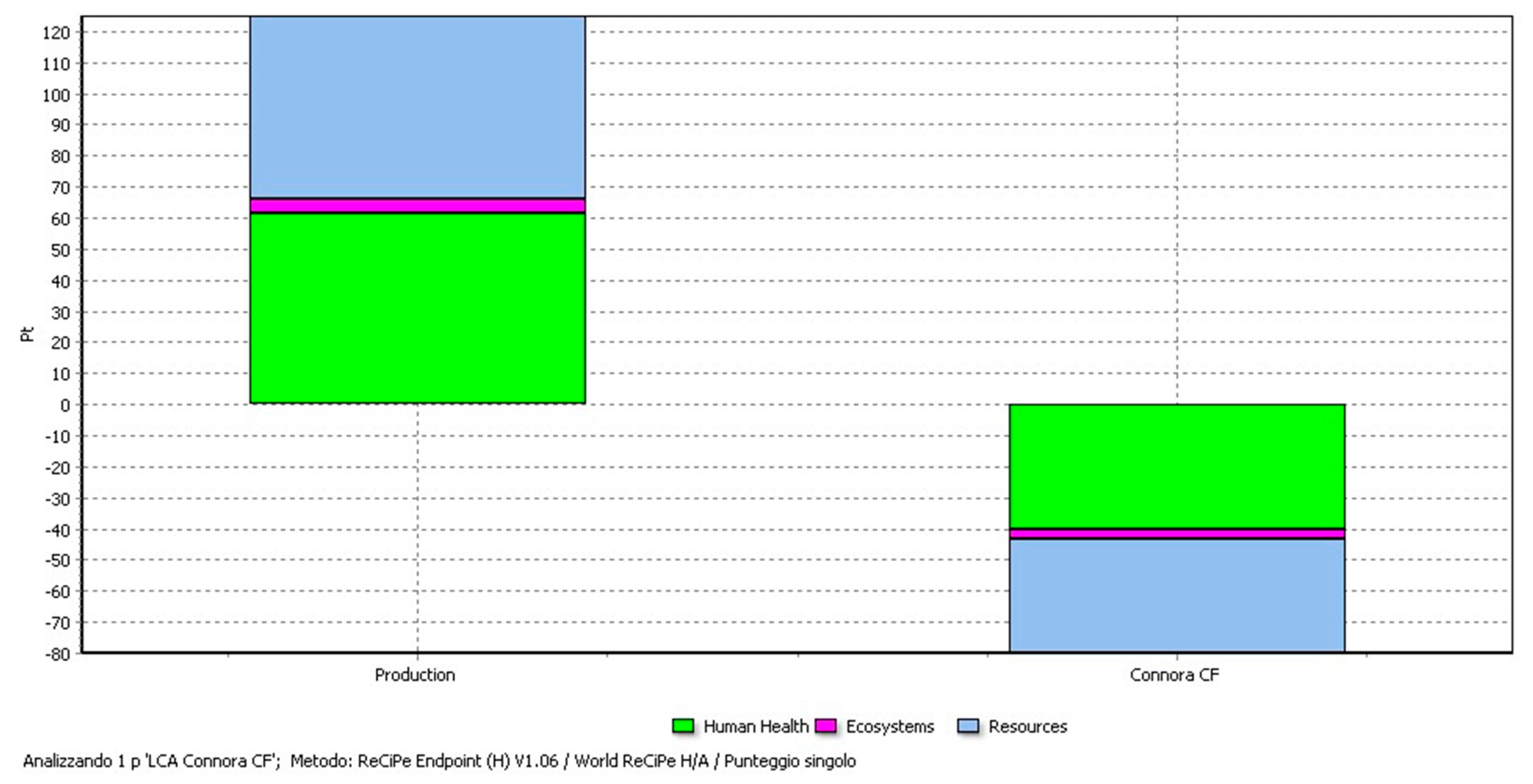
4. LCA Results and Discussion
4.1. Results of the Recycling Process
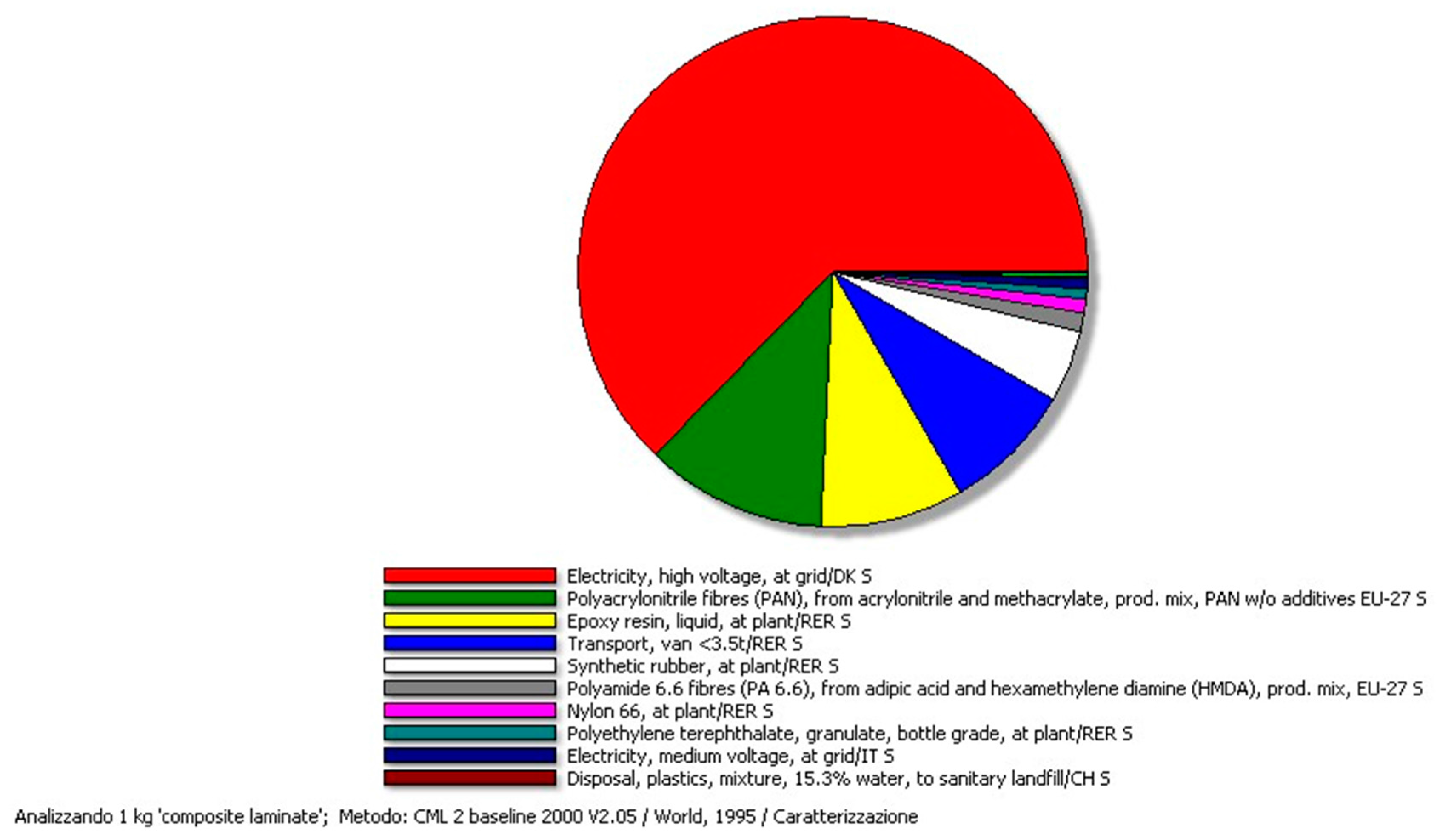
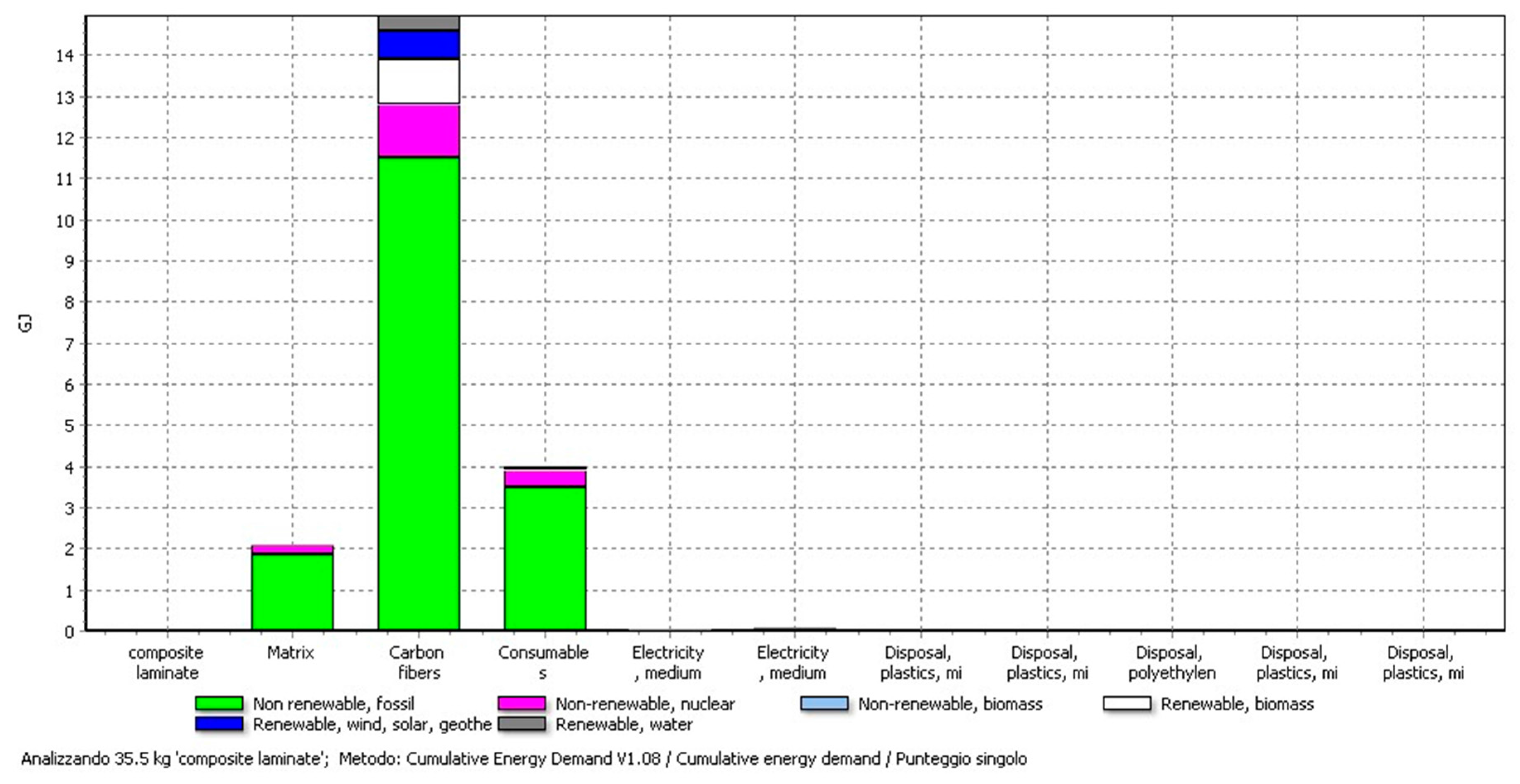
4.2. Results of the CF-Panel Production
4.3. LCA Results of the Production and the Recycling Phases
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dutta, A.; Tekalur, S.A. Synthetic staggered architecture composites. Mater. Des. 2013, 46, 802–808. [Google Scholar] [CrossRef]
- Dutta, A.; Tekalur, S.A. Crack tortuousity in the nacreous layer–Topological dependence and biomimetic design guideline. Int. J. Solids Struct. 2014, 51, 325–335. [Google Scholar] [CrossRef]
- Shibata, K. FRP Recycling Technologies, Network. Polymer 2007, 28, 247–255. [Google Scholar]
- The Japan Society of Epoxy Resin Technology. Epoxy Resin Composite Materials Recycling Technology. In Review of Epoxy Resin; Epoxy Resin Technology Association: Tokyo, Japan, 2009; pp. 195–201. [Google Scholar]
- Fukuda, H. Recycling of Composite Materials. In Review of Composite Materials and Technologies; Ben, G., Suemasu, H., Eds.; Industrial Technology Service Center: Winnipeg, MB, Canada, 2011; pp. 829–837. [Google Scholar]
- Pinero-Hernanz, R.; Dodds, C.; Hyde, J.; García-Serna, J.; Poliakoff, M.; Lester, E.; José Cocero, M.; Kingma, S.; Pickerin, S.; Wong, K.H. Chemical Recycling of Carbon Fibre Reinforced Composites in Nearcritical and Supercritical Water. Compos. Part A Appl. Sci. Manuf. 2008, 39, 454–461. [Google Scholar] [CrossRef]
- Goto, M. Chemical Recycling of Plastics Using Sub- and Supercritical Fluids. J. Supercrit. Fluids 2009, 47, 500–507. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, Z.; Feng, L. Chemical Recycling of Carbon Fibers Reinforced Epoxy Resin Composites in Oxygen in Supercritical Water. Mater. Des. 2010, 31, 999–1002. [Google Scholar] [CrossRef]
- Benedetti, M.; Cafiero, L.; De Angelis, D.; Tuffi, R.; Ciprioti, S.V. Pyrolysis of WEEE plastics using catalysts produced from fly ash of coal gasification. Front. Environ. Sci. Eng. 2017, 11. [Google Scholar] [CrossRef]
- Katsuji Shibata, Mitsutoshi Nakagawa, Hitachi Chemical Technical Report No.56. Available online: http://www.hitachi-chem.co.jp/english/report/056/56.pdf (accessed on 26 February 2018).
- Front Page of the Morning Edition; Nihon Keizai Shimbun: Tokyo, Japan, 2008.
- Santo, I. Environmental Load Performance of Carbon Fiber and Recycling. JSCE Annu. Meet. 2008, 10–11. [Google Scholar]
- Okajima, I.; Sako, T. Recycling of Carbon Fiber- Glass Reinforced Plastics. Ind. Mater. 2008, 56, 70–72. [Google Scholar]
- NEDO, Material Engineering, Faculty of Engineering, Shizuoka University. 2008. Available online: http://www.nedo.go.jp/english/index.html (accessed on 26 February 2018).
- Kuwabara, J.; Sasaki, M.; Goto, M. Recover of Materials Through Subcritical Fluid Processing of Fiber- Reinforced Plastic. Chem. Eng. 2008, 53, 501–504. [Google Scholar]
- Goto, M. Recycling Fiber-Reinforced Plastic using Supercritical and Subcritical Fluids. Fiber J. 2009, 65, 62–66. [Google Scholar]
- White, J.E.; Silvis, H.C.; Winkler, M.S.; Glass, T.W.; Kirkpatrick, D.E. Poly(hydroxyaminoethers): A New Family of Epoxy-Based Thermoplastics. Adv. Mater. 2000, 12, 1791–1800. [Google Scholar] [CrossRef]
- White, J.E.; Brennan, D.J.; Silvis, H.C.; Mang, M.N. Specialty Monomers and Polymers. In Epoxy-Based Thermoplastics: New Polymers with Unusual Property Profiles; American Chemical Society: Washington, DC, USA, 2000; Chapter 10; pp. 132–146. [Google Scholar]
- Cicala, G.; Pergolizzi, E.; Piscopo, F.; Carbone, D.; Recca, G. Hybrid composites manufactured by resin infusion with a fully recyclable bioepoxy resin. Compos. Part B Eng. 2018, 132, 69–76. [Google Scholar] [CrossRef]
- Cicala, G.; Mannino, S.; La Rosa, A.D.; Banatao, D.R.; Pastine, S.J.; Kosinski, S.T.; Scarpa, F. Hybrid Biobased Recyclable Epoxy Composites for Mass Production. Polym. Compos. 2017. [Google Scholar] [CrossRef]
- Bovea, M.D.; Vidal, R. Increasing product value by integrating environmental impacts, costs and customer valuation. J. Resour. Conserv. Recycl. 2004, 41, 133–145. [Google Scholar] [CrossRef]
- SimaPro 8.01, Pre Product Ecology Consultants, Amersfoort, NL, USA. Available online: www.pre.nl/simapro/ (accessed on 22 February 2018).
- Ecoinvent v3. Available online: https://www.ecoinvent.org/database/database.html (accessed on 26 February 2018).
- International Organization for Standardization. ISO 14040—Environmental Management—Life Cycle Assessment—Principles and Framework; ISO 14000 International Standards Compendium: Geneva, Switzerland, 2006. [Google Scholar]
- International Organization for Standardization. ISO 14044—Environmental Management—Life Cycle Assessment—Requirements and Guidelines; ISO 14000 International Standards Compendium: Geneva, Switzerland, 2006. [Google Scholar]
- La Rosa, A.D.; Banatao, D.R.; Pastine, S.J.; Latteri, A.; Cicala, G. Recycling treatment of carbon fibers/epoxy composites: Materials recovery and characterization and environmental impacts through LCA. Compos. B Eng. 2016, 104, 17–25. [Google Scholar] [CrossRef]
- Tony Roberts. The Carbon Fibre Industry Worldwide 2011–2020. Available online: http://www.carbonfiber-report.com/ (accessed on 26 February 2018).



| Materials (Kg) | |||
|---|---|---|---|
| Phase | Water | Acetic Acid | NaOH |
| Dissolving | 54.44 | 18.15 | 0.00 |
| PPT | 68.27 | 0.00 | 11.98 |
| Washing | 255.60 | 0.00 | 0.00 |
| Drying | 0.00 | 0.00 | 0.00 |
| Total | 378.31 | 18.15 | 11.98 |
| Equipment and Phase | Power (W) | Time (h) | Energy (KJ) | |
|---|---|---|---|---|
| Dissolving | 3 | 27,296.6 | ||
| Thermoplastic recovery | ||||
| PPT | Mixer | negligible | negligible | |
| Washing | Centrifuge | 2238 | 1.97 | 15,889.8 |
| Drying | Vacuum | 746 | 35.5 | 95,338.8 |
| Tumbler | 1119 | 35.5 | 143,008.2 | |
| Heater | 800 | 35.5 | 102,240.0 | |
| Total energy consumption | ||||
| 383,773.4 | ||||
| Glass transition temperature (Tg) | 40–60 °C |
| Melting temperature (Tm) | 120–140 °C |
| Tensile modulus | 2.4 GPa |
| Tensile strength | 57 MPa |
| Elongation | 45% |
| Shore D hardness | 80 |
| Input | Quantity |
| Dissolution | |
| Acetic acid | 18 kg |
| NaOH | 12 kg |
| Water | 55 kg |
| Thermoplastic precipitation (PPT) and washing | |
| Water (PPT) | 70 kg |
| Water (washing) | 225 kg |
| Total electricity consumption | 383,773 kJ |
| Output | |
| CF recovered | 22.49 kg |
| CF waste | 0.23 |
| Thermoplastic recovered | 12.33 kg |
| Thermoplastic waste | 0.45 |
| Waste water production | 380 kg |
| Input | Quantity |
|---|---|
| Composite | 35.5 kg |
| Materials | 18 kg |
| Matrix (epoxy + Recyclamine®) | 12.78 kg |
| Carbon fibres | 22.72 kg |
| Process (VRTM) | |
| Electricity for vacuum pump | 4.46 kWh |
| Electricity for oven | 7.14 kWh |
| Consumables and scrap: | |
| Vacuum bag (Nylon 6,6) | 1.228 kg |
| Breather (PET) | 2.579 kg |
| Tacky tape (synthetic rubber) | 19.11 kg |
| Aspiration tubes (PA 6,6) | 1.256 kg |
| Total electricity consumption | 383,773 kJ |
| Transport | |
| Matrix | 10,500 km (USA) |
| Carbon fibres | 2224 km (Germany) |
| Vacuum bag (Nylon 6,6) | 1354 km (Milan, Italy) |
| Breather (PET) | 1354 km (Milan, Italy) |
| Tacky tape (synthetic rubber) | 2224 km (Germany) |
| Aspiration tubes (PA 6,6) | 1271 km (Varese, Italy) |
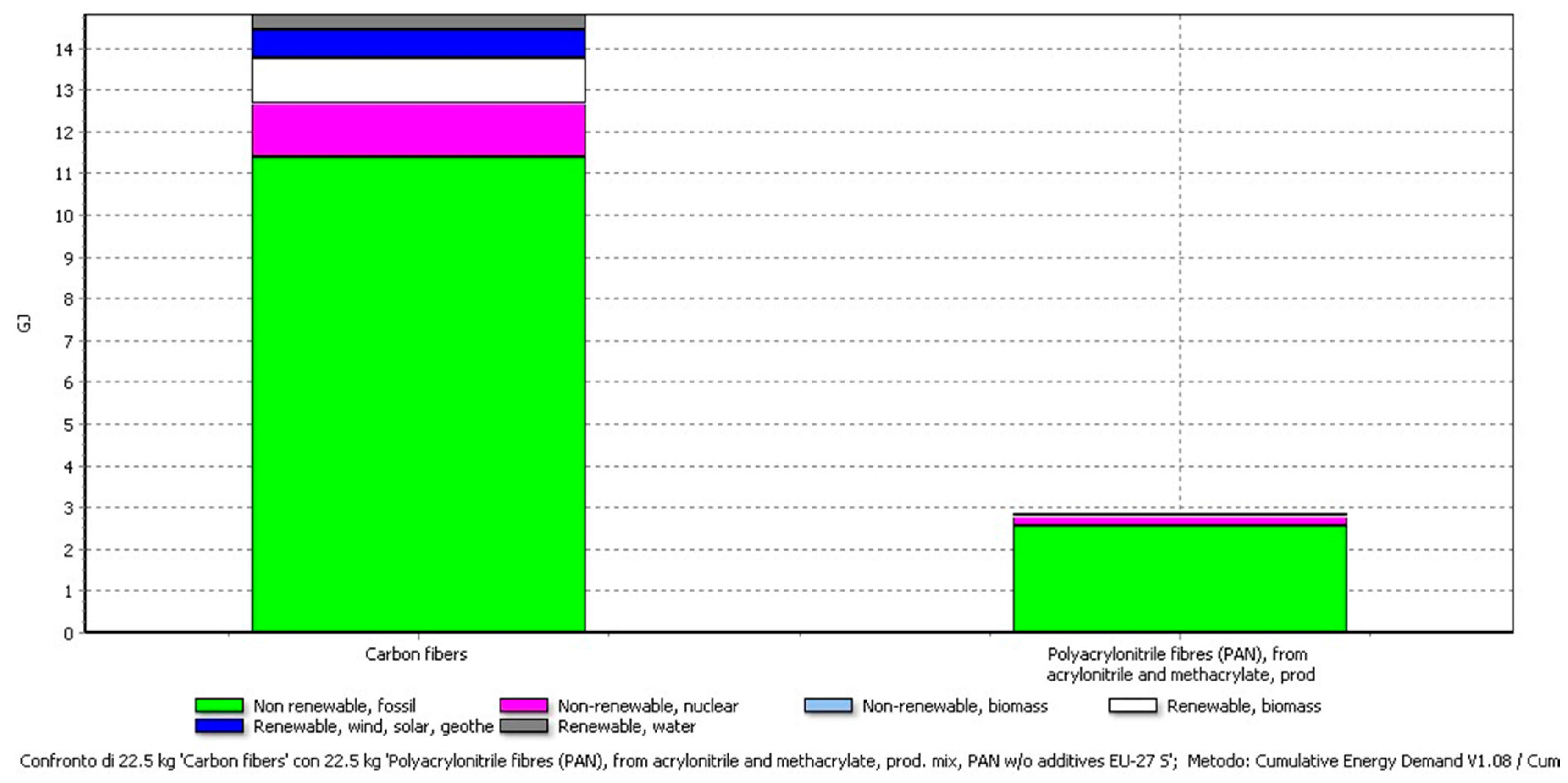
| Recovered Item | Amount | Cumulative Energy Demand (CED) |
|---|---|---|
| Carbon fibres | 22.49 kg | 14.8 GJ |
| PAN | 22.49 kg | 2.8 GJ |
| Impact Category | Unit | Pan | CF |
|---|---|---|---|
| Abiotic depletion | Kg Sb eq | −0.8 | −6.3 |
| Acidification | Kg SO2 eq | 0.1 | −1.7 |
| Eutrophication | Kg PO43− eq | 0.1 | −0.6 |
| Global warming (GWP100) | Kg CO2 eq | −84.5 | −779.0 |
| Ozone layer depletion (ODP) | Kg CFC-11 eq | 0.0 | 0.0 |
| Human toxicity | Kg 1,4-DB eq | −49.4 | −89.7 |
| Fresh water aquatic ecotoxicity | Kg 1,4-DB eq | −36.6 | −82.5 |
| Marine aquatic ecotoxicity | Kg 1,4-DB eq | −82,524.2 | −190,801.6 |
| Terrestrial ecotoxicity | Kg 1,4-DB eq | 0.0 | −0.7 |
| Photochemical oxidation | Kg C2H4 eq | 0.0 | −0.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
La Rosa, A.D.; Blanco, I.; Banatao, D.R.; Pastine, S.J.; Björklund, A.; Cicala, G. Innovative Chemical Process for Recycling Thermosets Cured with Recyclamines® by Converting Bio-Epoxy Composites in Reusable Thermoplastic—An LCA Study. Materials 2018, 11, 353. https://doi.org/10.3390/ma11030353
La Rosa AD, Blanco I, Banatao DR, Pastine SJ, Björklund A, Cicala G. Innovative Chemical Process for Recycling Thermosets Cured with Recyclamines® by Converting Bio-Epoxy Composites in Reusable Thermoplastic—An LCA Study. Materials. 2018; 11(3):353. https://doi.org/10.3390/ma11030353
Chicago/Turabian StyleLa Rosa, Angela D., Ignazio Blanco, Diosdado R. Banatao, Stefan J. Pastine, Anna Björklund, and Gianluca Cicala. 2018. "Innovative Chemical Process for Recycling Thermosets Cured with Recyclamines® by Converting Bio-Epoxy Composites in Reusable Thermoplastic—An LCA Study" Materials 11, no. 3: 353. https://doi.org/10.3390/ma11030353
APA StyleLa Rosa, A. D., Blanco, I., Banatao, D. R., Pastine, S. J., Björklund, A., & Cicala, G. (2018). Innovative Chemical Process for Recycling Thermosets Cured with Recyclamines® by Converting Bio-Epoxy Composites in Reusable Thermoplastic—An LCA Study. Materials, 11(3), 353. https://doi.org/10.3390/ma11030353







