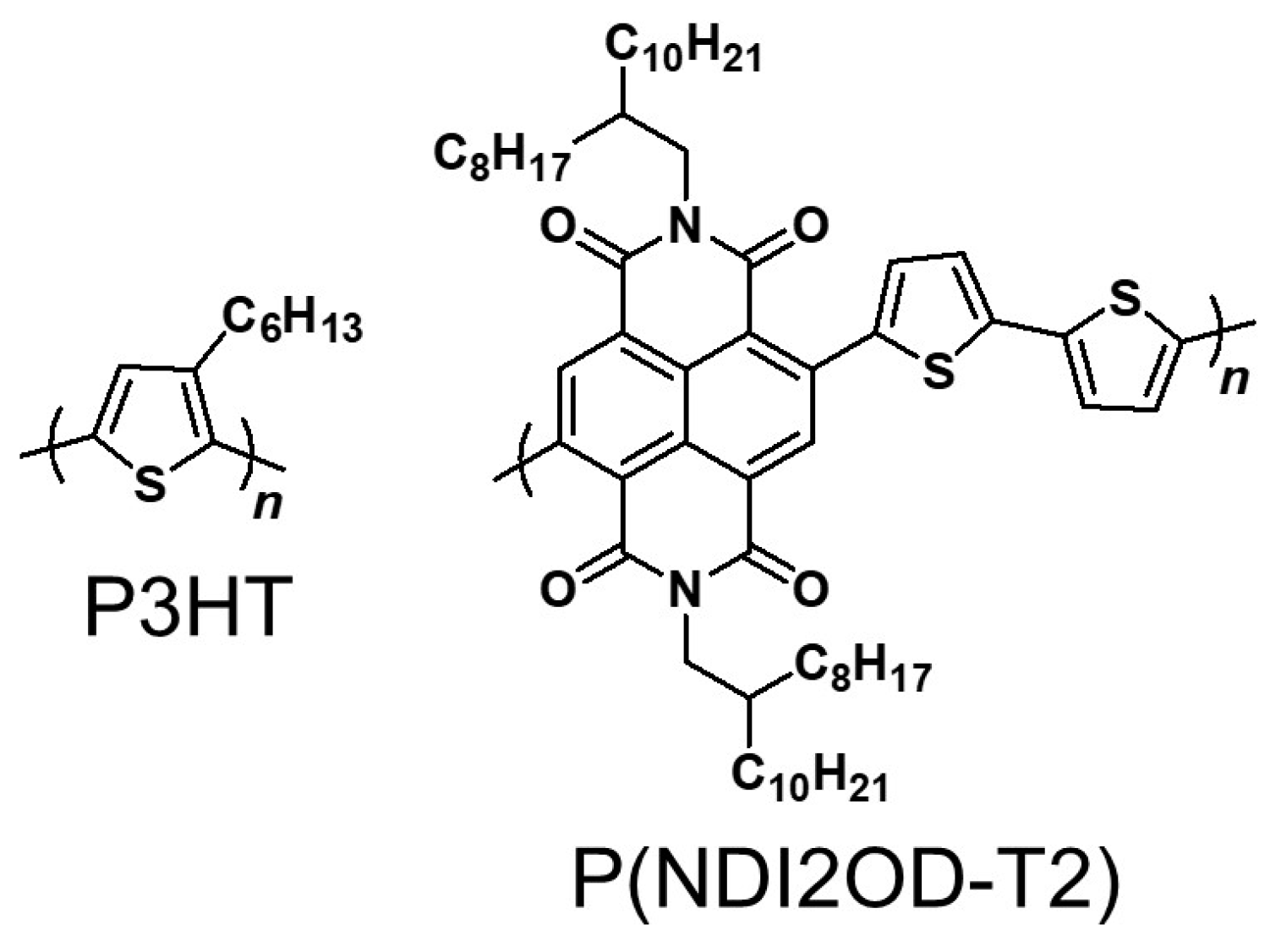
Fabrication of Completely Polymer-Based Solar Cells with p- and n-Type Semiconducting Block Copolymers with Electrically Inert Polystyrene
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. P3HT-b-PSt
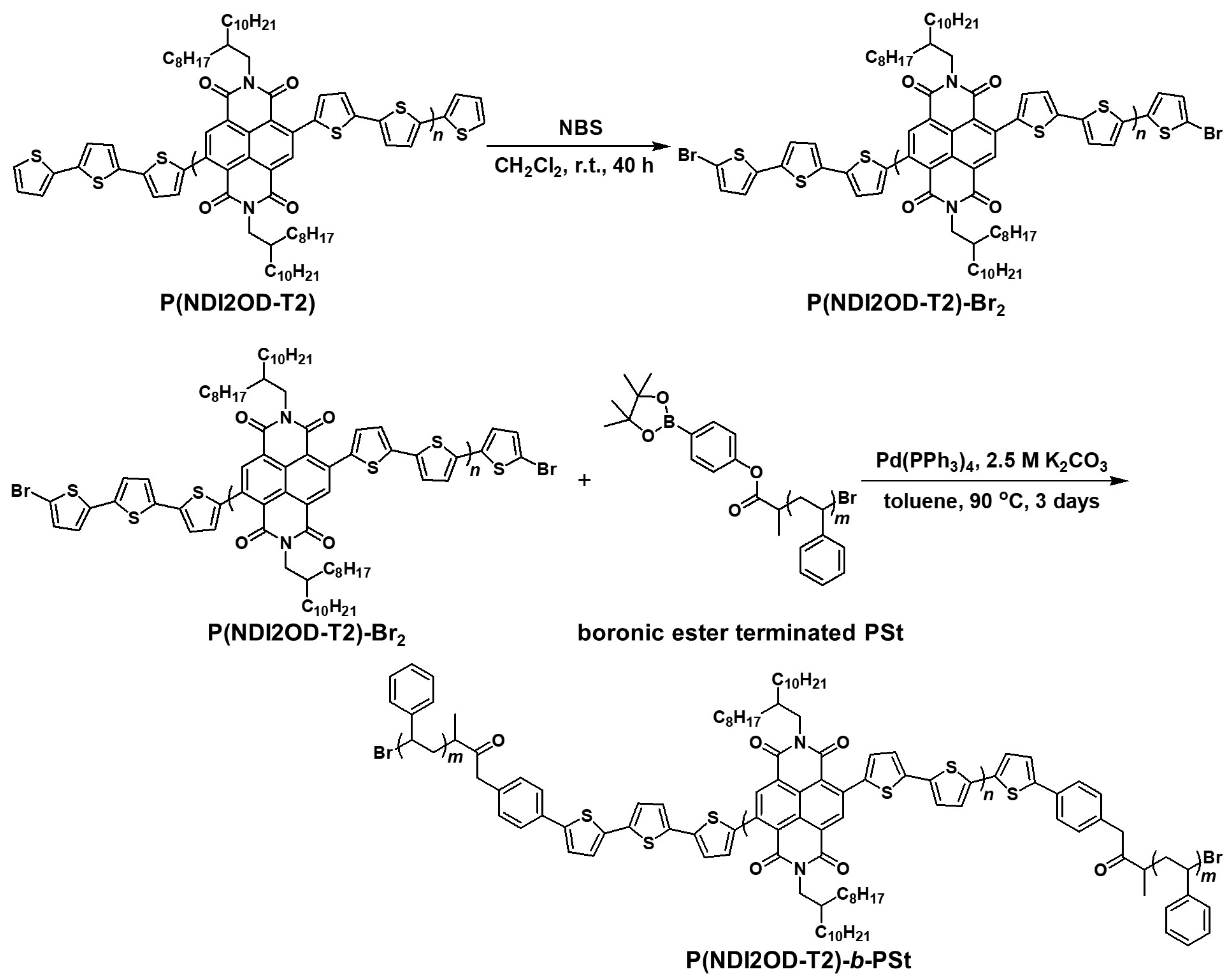
2.1.2. P(NDI2OD-T2)-Br2
2.1.3. P(NDI2OD-T2)-b-PSt
2.2. Characterizations
2.3. Device Fabrication of Space Charge Limited Current (SCLC) Measurements
2.4. Fabrication of OPV (J-V) Device and Measurements
3. Results and Discussion
3.1. Synthesis of P(NDI2OD-T2)-b-PSt
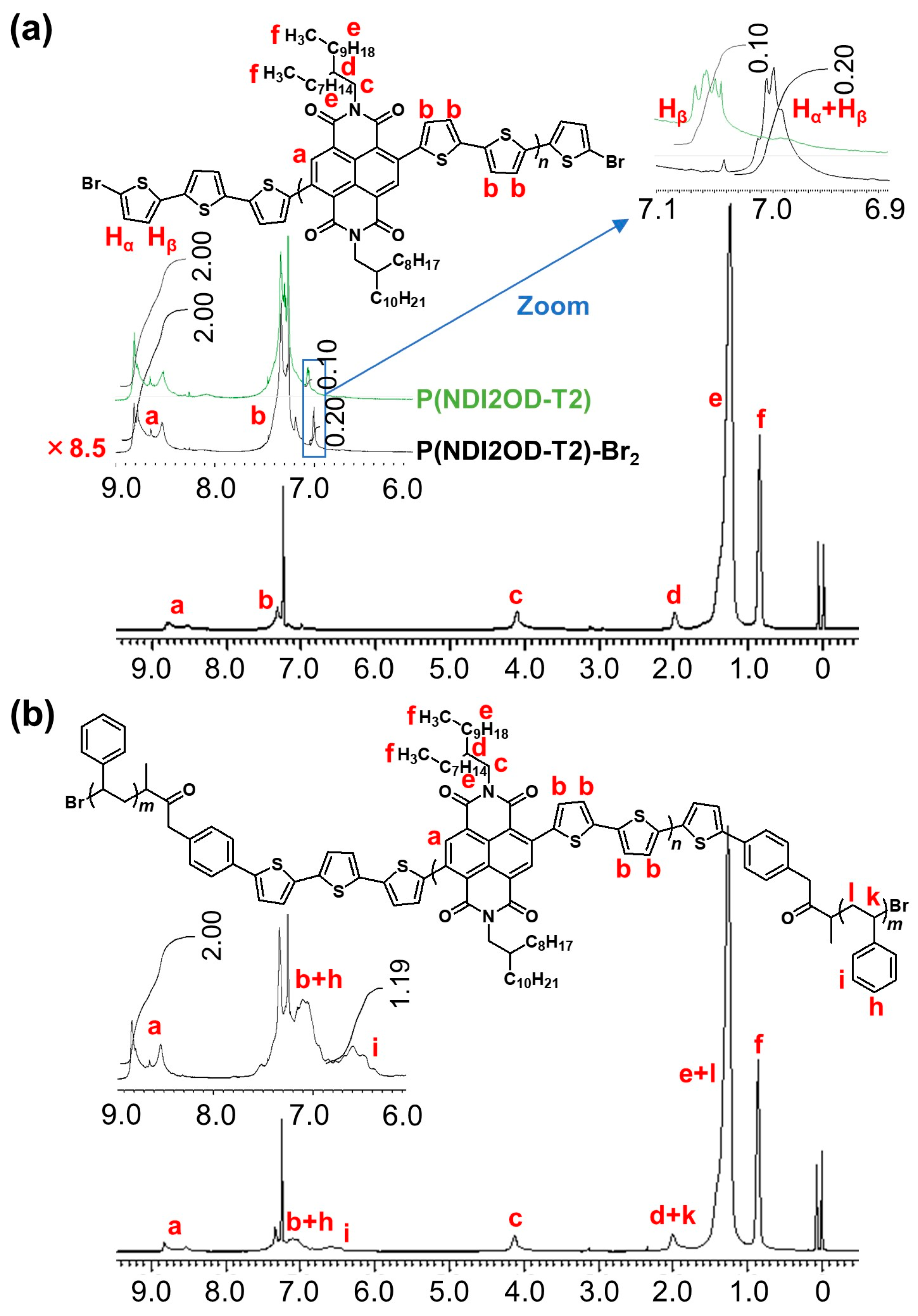
3.2. Characteristics of P(NDI2OD-T2)-b-PSt
3.4. UV/vis Absorption in Film State
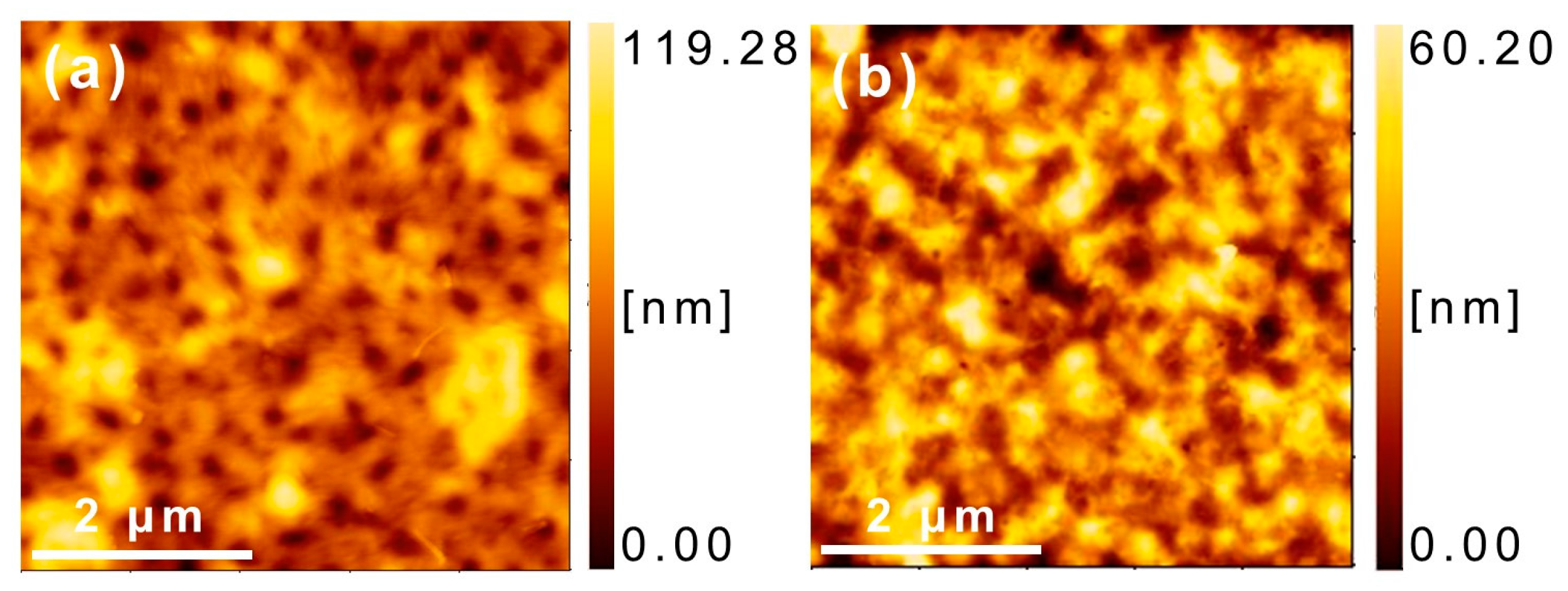
3.5. Morphology
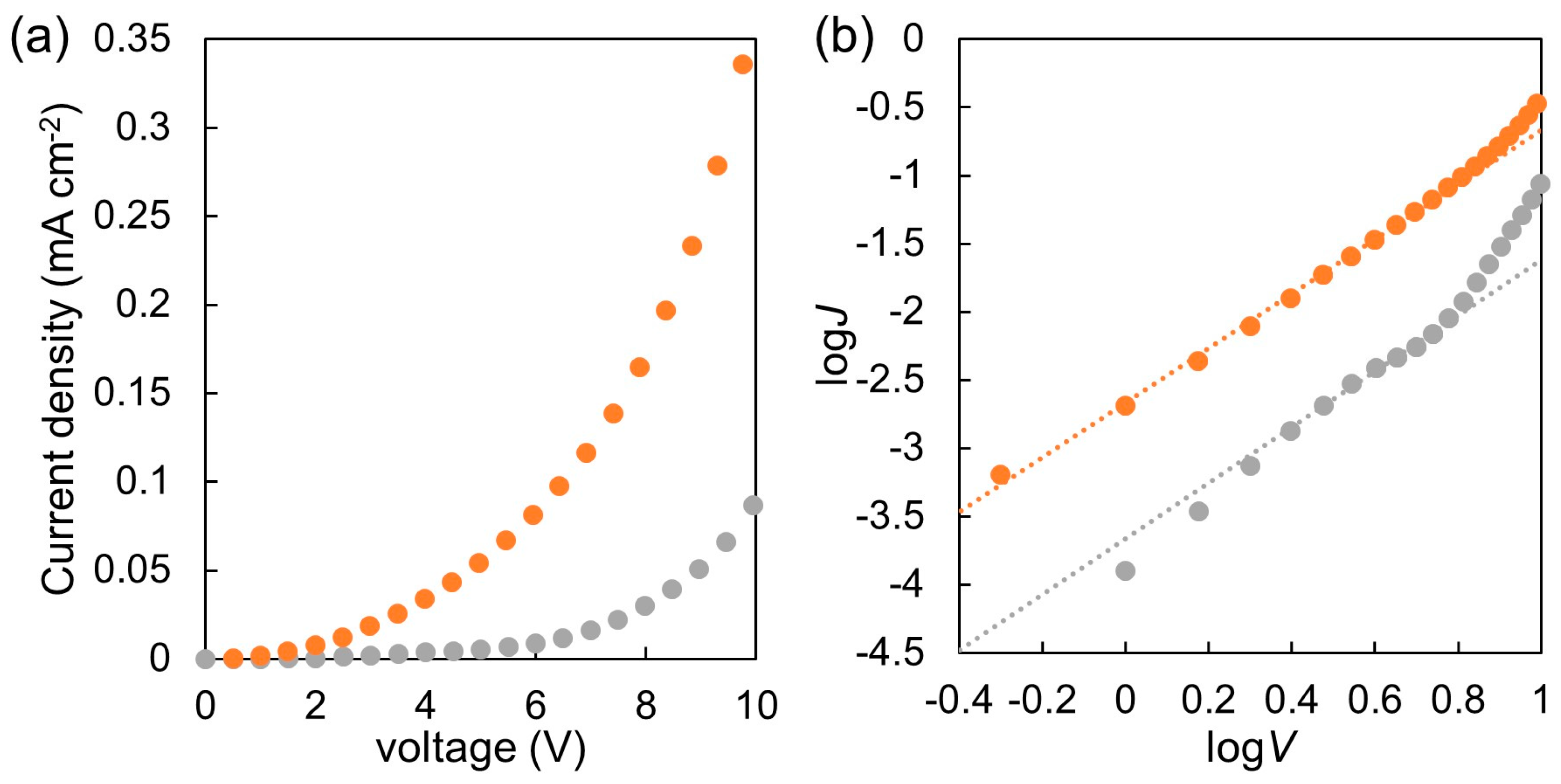
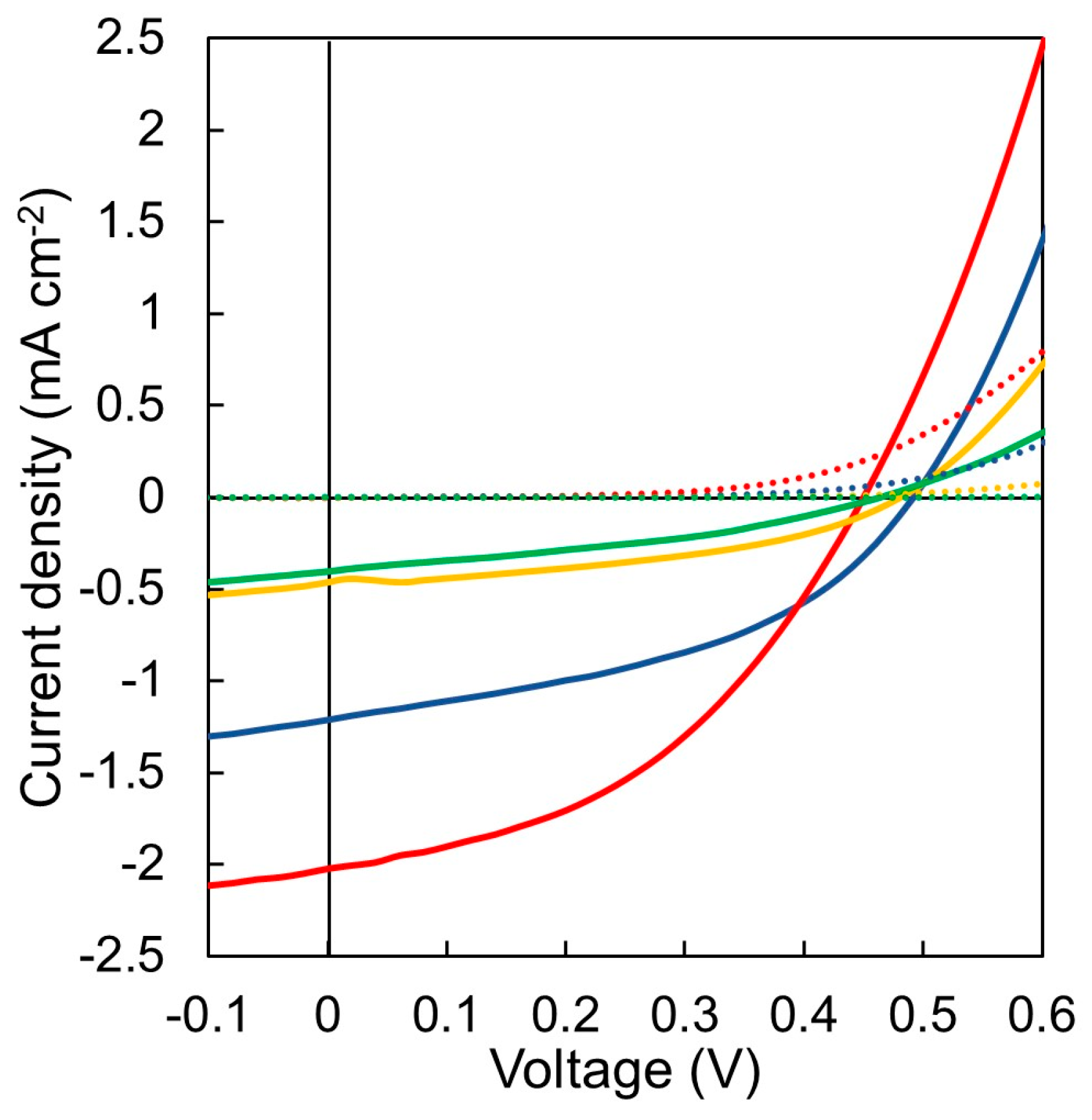
3.6. Photovoltaic Device Evaluation
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hains, A.W.; Liang, Z.; Woodhouse, M.A.; Gregg, B.A. Molecular semiconductors in organic photovoltaic cells. Chem. Rev. 2010, 110, 6689–6735. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Yu, L. Development of semiconducting polymers for solar energy harvesting. Polym. Rev. 2010, 50, 454–473. [Google Scholar] [CrossRef]
- Dou, L.; You, J.; Hong, Z.; Xu, Z.; Li, G.; Street, R.A.; Yang, Y.A. Decade of organic/polymeric photovoltaic research. Adv. Mater. 2013, 25, 6642–6671. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Shrotriya, V.; Huang, J.; Yao, Y.; Moriarty, T.; Emery, K.; Yang, Y. High-efficiency solution processable polymer photovoltaic cells by self-organization of polymer blends. Nat. Mater. 2005, 4, 864–868. [Google Scholar] [CrossRef]
- Dennler, G.; Scharber, M.C.; Brabec, C.J. Polymer-fullerene bulk-heterojunction solar cells. Adv. Mater. 2009, 21, 1323–1338. [Google Scholar] [CrossRef]
- Lin, Y.; Zhan, X. Non-fullerene acceptors for organic photovoltaics: An emerging horizon. Mater. Horiz. 2014, 1, 470–488. [Google Scholar] [CrossRef]
- Sonar, P.; Lim, J.P.F.; Chan, K.L. Organic non-fullerene acceptors for organic photovoltaics. Energy Environ. Sci. 2011, 4, 1558–1574. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, Z.-G.; Xue, L.; Min, J.; Zhang, J.; Wei, Z.; Li, Y. All-Polymer solar cells based on absorption-complementary polymer donor and acceptor with high power conversion efficiency of 8.27%. Adv. Mater. 2016, 28, 1884–1890. [Google Scholar] [CrossRef] [PubMed]
- Facchetti, A. Polymer donor–polymer acceptor (all-polymer) solar cells. Mater. Today 2013, 16, 123–132. [Google Scholar] [CrossRef]
- Moore, J.R.; Albert-Seifried, S.; Rao, A.; Massip, S.; Watts, B.; Morgan, D.J.; Friend, R.H.; McNeill, C.R.; Sirringhaus, H. Polymer blend solar cells based on a high-mobility naphthalenediimide-based polymer acceptor: Device physics, photophysics and morphology. Adv. Energy Mater. 2011, 1, 230–240. [Google Scholar] [CrossRef]
- Mori, D.; Benten, H.; Ohkita, H.; Ito, S.; Miyake, K. Polymer/polymer blend solar cells improved by using high-molecular-weight fluorene-based copolymer as electron acceptor. ACS Appl. Mater. Interfaces 2012, 4, 3325–3329. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.; Dolfen, D.; Frisch, J.; Roland, S.; Steyrleuthner, R.; Stiller, B.; Chen, Z.; Scherf, U.; Koch, N.; Facchetti, A.; et al. Influence of aggregation on the performance of all-polymer solar cells containing low-bandgap naphthalenediimide copolymers. Adv. Energy Mater. 2012, 2, 369–380. [Google Scholar] [CrossRef]
- Mori, D.; Benten, H.; Kosaka, J.; Ohkita, H.; Ito, S.; Miyake, K. Polymer/polymer blend solar cells with 2.0% efficiency developed by thermal purification of nanoscale-phase-separated morphology. ACS Appl. Mater. Interfaces 2011, 3, 2924–2927. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Xiong, Y.; Li, S.; Ghasemi, M.; Balar, N.; Turner, J.; Gadisa, A.; Hou, J.; O’Connor, B.T.; Ade, H. Precise manipulation of multilength scale morphology and its influence on eco-friendly printed all-polymer solar cells. Adv. Funct. Mater. 2017, 27, 1702016. [Google Scholar] [CrossRef]
- Ye, L.; Xiong, Y.; Yao, H.; Gadisa, A.; Zhang, H.; Li, S.; Ghasemi, M.; Balar, N.; Hunt, A.; O’Connor, B.T.; et al. High performance organic solar cells processed by blade coating in air from a benign food additive solution. Chem. Mater. 2016, 28, 7451–7458. [Google Scholar] [CrossRef]
- Yan, H.; Chen, Z.; Zheng, Y.; Newman, C.; Quinn, J.R.; Dotz, F.; Kastler, M.; Facchetti, A. A high-mobility electron-transporting polymer for printed transistors. Nature 2009, 457, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.M.; Hong, Z.; Li, G.; Yang, Y. Recent progress in polymer solar cells: Manipulation of polymer: Fullerene morphology and the formation of efficient inverted polymer solar cells. Adv. Mater. 2009, 21, 1434–1449. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, H.S.; Lee, M.S.; Jo, W.H. Morphologies of binary AB/AC diblock copolymer blends. Macromol. Chem. Phys. 2002, 203, 2188–2195. [Google Scholar] [CrossRef]
- Tomita, E.; Kim, K.; Minegishi, K.; Nakamura, A.; Kanehashi, S.; Ogino, K. Enhancement of out-of-plane hole mobility in poly(3-hexylthiophene)-b-poly(styrene) Film. Chem. Commun. 2018. under review. [Google Scholar]
- Iovu, M.C.; Sheina, E.E.; Gil, R.R.; McCullough, R.D. Experimental evidence for the quasi-“living” nature of the grignard metathesis method for the synthesis of regioregular poly(3-alkylthiophenes). Macromolecules 2005, 38, 8649–8656. [Google Scholar] [CrossRef]
- Maeda, Y.; Shimoi, Y.; Ogino, K. Fabrication of microporous films utilizing amphiphilic block copolymers and their use as templates in poly(aniline) preparation. Polym. Bull. 2005, 53, 315–321. [Google Scholar] [CrossRef]
- Gu, Z.; Tan, Y.; Tsuchiya, K.; Shimomura, T.; Ogino, K. Synthesis and characterization of poly(3-hexylthiophene)-b-polystyrene for photovoltaic application. Polymers 2011, 3, 558–570. [Google Scholar] [CrossRef]
- Chen, Z.; Zheng, Y.; Yan, H.; Facchetti, A. Naphthalenedicarboximide- vs. perylenedicarboximide-based copolymers. Synthesis and semiconducting properties in bottom-gate n-channel organic transistors. J. Am. Chem. Soc. 2009, 131, 8–9. [Google Scholar] [CrossRef] [PubMed]
- Chaignon, F.; Falkenström, M.; Karlsson, S.; Blart, E.; Odobel, F.; Hammarstöm, L. Very large acceleration of the photoinduced electron transfer in a Ru(bpy)3-naphthalene bisimide dyad bridged on the naphthyl core. Chem. Comm. 2007, 1, 64–66. [Google Scholar] [CrossRef] [PubMed]
- Steckler, T.T.; Henriksson, P.; Mollinger, S.; Lundin, A.; Salleo, A.; Andersson, M.R. Very low band gap thiadiazoloquinoxaline donor-acceptor polymers as multi-tool conjugated polymers. J. Am. Chem. Soc. 2014, 136, 1190–1193. [Google Scholar] [CrossRef] [PubMed]
- Amemori, S.; Kokado, K.; Sada, K. Polymer phase-transition behavior driven by a charge-transfer interaction. Angew. Chem. Int. Ed. 2013, 52, 4174–4178. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zhao, Y.; Zhong, C.; Gao, S.; Yu, G.; Liu, Y.; Qin, J. Effect of polymer chain conformation on field-effect transistor performance: Synthesis and properties of two arylene imide based D–A copolymers. J. Matter. Chem. 2012, 22, 14639–14644. [Google Scholar] [CrossRef]
- Ogino, K.; Sato, H.; Tsuchiya, K.; Suzuki, H.; Moriguchi, S. Synthesis of monodisperse macroreticular styrene-divinylbenzene gel particles by a single-step swelling and polymerization method. J. Chromatogr. A 1995, 699, 59–66. [Google Scholar] [CrossRef]
- Albers, W.M.; Canters, G.W.; Redijk, J. Preparation of extended di(4-pyridyl)thiophene oligomers. Tetrahedron 1995, 51, 3895–3904. [Google Scholar] [CrossRef]
- Li, Z.; Lin, J.D.A.; Phan, H.; Sharenko, A.; Proctor, C.M.; Zalar, P.; Chen, Z.; Facchetti, A.; Nguyen, T.Q. Competitive absorption and inefficient exciton harvesting: Lessons learned from bulk heterojunction organic photovoltaics utilizing the polymer acceptor P(NDI2OD-T2). Adv. Funct. Mater. 2014, 24, 6989–6998. [Google Scholar] [CrossRef]
- Singh, C.R.; Gupta, G.; Lohwasser, R.; Engmann, S.; Balko, J.; Thelakkat, M.; Thurn-Albrecht, T.; Hoppe, H. Correlation of charge transport with structural order in highly ordered melt-crystallized poly(3-hexylthiophene) thin films. J. Polym. Sci. Part B Polym. Phys. 2013, 51, 943–951. [Google Scholar] [CrossRef]
- Zhang, B.; Liang, J.; Hu, L.; Peng, F.; Chen, G.; Yang, W. Triphenylamine-based broad band-gap polymers for bulk-heterojunction polymer solar cells. J. Mater. Sci. 2015, 50, 5609–5619. [Google Scholar] [CrossRef]
- Lim, H.; Chao, C.-Y.; Su, W.-F. Modulating crystallinity of poly(3-hexylthiophene) via microphase separation of poly(3-hexylthiophene)–polyisoprene block copolymers. Macromolecules 2015, 48, 3269–3281. [Google Scholar] [CrossRef]
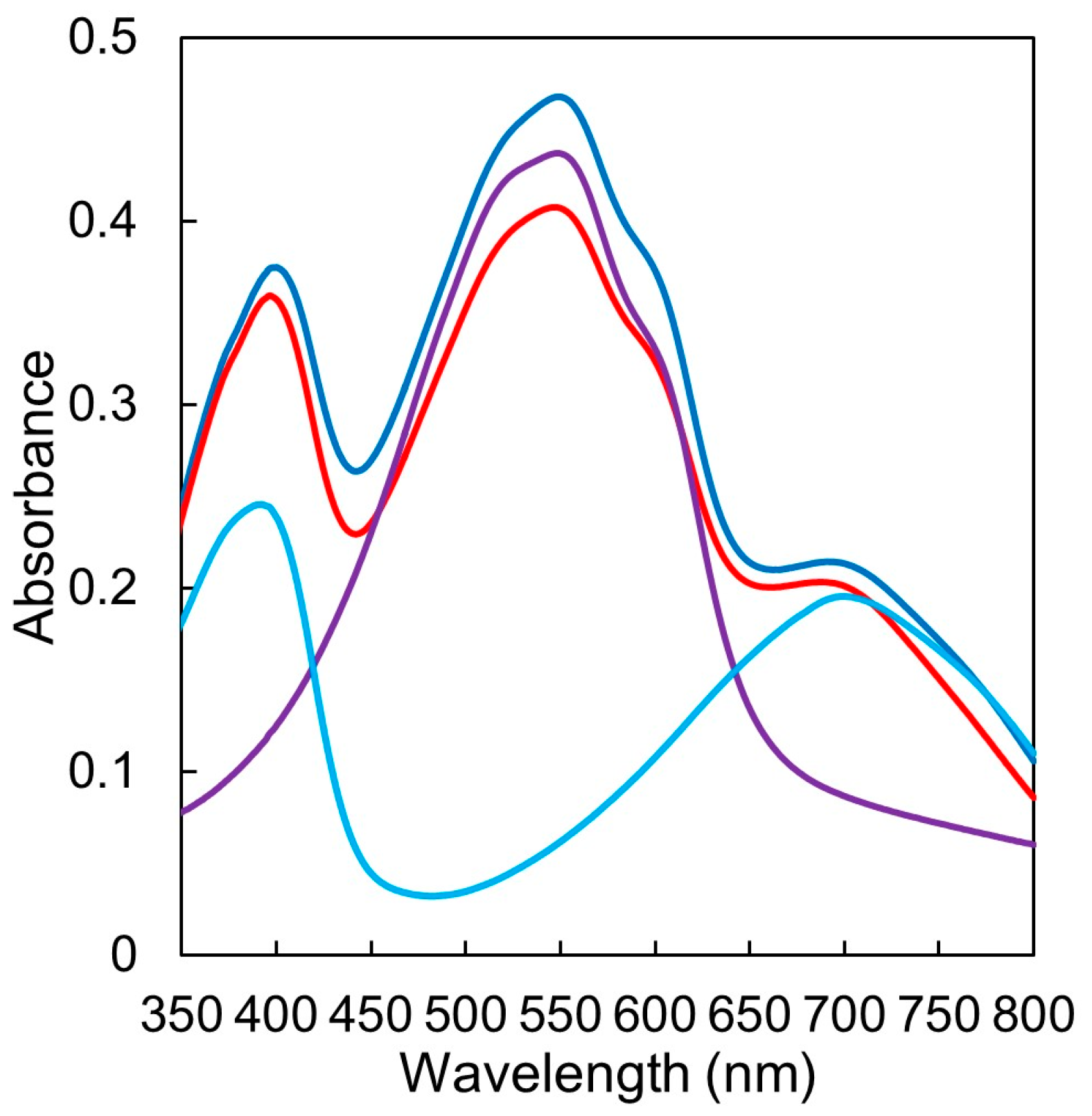
| Polymer | λmax (nm) 1 | LUMO (eV) 2 | SCLC Electron Mobility (cm2 V−1 s−1) 3 |
|---|---|---|---|
| P(NDI2OD-T2) | 369, 383, 610 | −4.0 | 4.5 × 10−6 |
| P(NDI2OD-T2)-b-PSt | 369, 384, 618 | −4.1 | 3.6 × 10−5 |
| Polymers | VOC 1 (V) | JSC 1 (mA cm−2) | FF 1 | PCE (%) | |
|---|---|---|---|---|---|
| Best | Average | ||||
| P3HT/P(NDI2OD-T2) | 0.50 ± 0.004 | 1.16 ± 0.05 | 0.42 ± 0.02 | 0.26 | 0.24 ± 0.02 |
| P3HT-l/P(NDI2OD-T2)-l | 0.53 ± 0.008 | 1.07 ± 0.06 | 0.35 ± 0.02 | 0.21 | 0.20 ± 0.02 |
| P3HT-b-PSt/P(NDI2OD-T2)-b-PSt | 0.45 ± 0.005 | 1.73 ± 0.20 | 0.41 ± 0.02 | 0.39 | 0.32 ± 0.07 |
| P3HT-l-b-PSt/P(NDI2OD-T2)-l-b-PSt | 0.55 ± 0.005 | 0.78 ± 0.06 | 0.31 ± 0.04 | 0.16 | 0.13 ± 0.03 |
| P3HT-b-PSt/P(NDI2OD-T2) | 0.47 ± 0.007 | 0.32 ± 0.13 | 0.36 ± 0.05 | 0.10 | 0.06 ± 0.04 |
| P3HT/P(NDI2OD-T2)-b-PSt | 0.46 ± 0.006 | 0.25 ± 0.12 | 0.35 ± 0.02 | 0.07 | 0.04 ± 0.03 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomita, E.; Kanehashi, S.; Ogino, K. Fabrication of Completely Polymer-Based Solar Cells with p- and n-Type Semiconducting Block Copolymers with Electrically Inert Polystyrene. Materials 2018, 11, 343. https://doi.org/10.3390/ma11030343
Tomita E, Kanehashi S, Ogino K. Fabrication of Completely Polymer-Based Solar Cells with p- and n-Type Semiconducting Block Copolymers with Electrically Inert Polystyrene. Materials. 2018; 11(3):343. https://doi.org/10.3390/ma11030343
Chicago/Turabian StyleTomita, Eri, Shinji Kanehashi, and Kenji Ogino. 2018. "Fabrication of Completely Polymer-Based Solar Cells with p- and n-Type Semiconducting Block Copolymers with Electrically Inert Polystyrene" Materials 11, no. 3: 343. https://doi.org/10.3390/ma11030343
APA StyleTomita, E., Kanehashi, S., & Ogino, K. (2018). Fabrication of Completely Polymer-Based Solar Cells with p- and n-Type Semiconducting Block Copolymers with Electrically Inert Polystyrene. Materials, 11(3), 343. https://doi.org/10.3390/ma11030343






