Application of Silver Nanostructures Synthesized by Cold Atmospheric Pressure Plasma for Inactivation of Bacterial Phytopathogens from the Genera Dickeya and Pectobacterium
Abstract
1. Introduction
2. Results
2.1. Characterization of the PEC-AgNPs and SDS-AgNPs
2.1.1. Optical Properties of the Ag Nanostructures
2.1.2. Morphology of the Ag Nanostructures
2.1.3. Surface Functionalization of the Ag Nanostructures
2.2. Concentration of the Ag Nanostructures
2.3. Antibacterial Properties of the Ag Nanostructures against Dickeya spp. and Pectobacterium spp.
3. Discussion
4. Materials and Methods
4.1. Reagents and Solutions
4.2. Synthesis of PEC-AgNPs and SDS-AgNPs in the dc-APGD-Based Reaction-Discharge System
4.3. Characterization of the PEC-AgNPs and SDS-AgNPs
4.4. Purification of PEC-AgNPs and SDS-AgNPs from Ag(I) Ions
4.5. Determination of the Production Efficiency of the Purified PEC-AgNPs and SDS-AgNPs
4.6. Bacterial Strains and Their Growth Conditions
4.7. Assessment of the Antibacterial Properties of PEC-AgNPs and SDS-AgNPs against Dickeya spp. and Pectobacterium spp.
5. Patents
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AgNPs | silver nanoparticles |
| ATR FT-IR | attenuated total reflection-Fourier transformation infrared spectroscopy |
| CAPP | cold atmospheric pressure plasma |
| dc-APGD | direct-current atmospheric pressure glow discharge |
| Dsol | Dickeya solani |
| EDX | energy-dispersive X-ray spectroscopy |
| FAAS | flame atomic absorption spectrometry |
| fcc | face cubic centered |
| FLA | flowing liquid anode |
| IFB UG&MUG | Intercollegiate Faculty of Biotechnology of the University of Gdansk and Medical University of Gdansk |
| LSPR | localized surface plasmon resonance |
| MBC | minimal bactericidal concentration |
| MIC | minimal inhibitory concentration |
| NPs | nanoparticles |
| Pba | Pectobacterium atrosepticum |
| Pcbr | Pectobacterium carotovorum subsp. brasiliense |
| Pcc | Pectobacterium carotovorum subsp. carotovorum |
| PEC | Pectins |
| Ppa | Pectobacterium parmentieri |
| ROS | reactive oxygen species |
| RNS | reactive nitrogen species |
| SADP | selected area diffraction pattern |
| SAED | selected area electron diffraction |
| SDS | sodium dodecyl sulfate |
| TEM | transmission electron microscopy |
| TSA | tryptone soya agar |
| TSB | tryptone soya broth |
References
- Khan, M.R.; Rizvi, T.F. Nanotechnology: Scope and application in plant disease management. Plant Pathol. J. 2014, 13, 214–231. [Google Scholar] [CrossRef]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of silver nanoparticles: Chemical, physical and biological methods. Res. Pharm. Sci. 2014, 9, 385–406. [Google Scholar] [PubMed]
- Dzimitrowicz, A.; Berent, S.; Motyka, A.; Jamroz, P.; Kurcbach, K.; Sledz, W.; Pohl, P. Comparison of the characteristics of gold nanoparticles synthesized using aqueous plant extracts and natural plant essential oils of Eucalyptus globulus and Rosmarinus officinalis. Arab. J. Chem. 2016. [Google Scholar] [CrossRef]
- Dzimitrowicz, A.; Jamroz, P.; diCenzo, G.C.; Sergiel, I.; Kozlecki, T.; Pohl, P. Preparation and characterization of gold nanoparticles prepared with aqueous extracts of Lamiaceae plants and the effect of follow-up treatment with atmospheric pressure glow microdischarge. Arab. J. Chem. 2016. [Google Scholar] [CrossRef]
- Tochikubo, F.; Shirai, N.; Uchida, S. Liquid-phase reactions induced by atmospheric pressure glow discharge with liquid electrode. J. Phys. Conf. Ser. 2014, 565, 12010. [Google Scholar] [CrossRef]
- Tochikubo, F.; Shimokawa, Y.; Shirai, N.; Uchida, S. Chemical reactions in liquid induced by atmospheric-pressure dc glow discharge in contact with liquid. Jpn. J. Appl. Phys. 2014, 53, 126201. [Google Scholar] [CrossRef]
- Mariotti, D.; Patel, J.; Švrček, V.; Maguire, P. Plasma-liquid interactions at atmospheric pressure for nanomaterials synthesis and surface engineering. Plasma Process. Polym. 2012, 9, 1074–1085. [Google Scholar] [CrossRef]
- De Vos, C.; Baneton, J.; Witzke, M.; Dille, J.; Godet, S.; Gordon, M.J.; Sankaran, R.M.; Reniers, F. A comparative study of the reduction of silver and gold salts in water by a cathodic microplasma electrode. J. Phys. D Appl. Phys. 2017, 50, 105206. [Google Scholar] [CrossRef]
- Dzimitrowicz, A.; Jamroz, P.; Nyk, M.; Pohl, P. Application of direct current atmospheric pressure glow microdischarge generated in contact with a flowing liquid solution for synthesis of Au-Ag core-shell nanoparticles. Materials 2016, 9, 268. [Google Scholar] [CrossRef] [PubMed]
- Dzimitrowicz, A.; Lesniewicz, T.; Greda, K.; Jamroz, P.; Nyk, M.; Pohl, P. Production of gold nanoparticles using atmospheric pressure glow microdischarge generated in contact with a flowing liquid cathode—A design of experiments study. RSC Adv. 2015, 5, 90534–90541. [Google Scholar] [CrossRef]
- Dzimitrowicz, A.; Jamroz, P.; Pogoda, D.; Nyk, M.; Pohl, P. Direct current atmospheric pressure glow discharge generated between a pin-type solid cathode and a flowing liquid anode as a new tool for silver nanoparticles production. Plasma Process. Polym. 2017, 14, 1600251. [Google Scholar] [CrossRef]
- Mijnendonckx, K.; Leys, N.; Mahillon, J.; Silver, S.; Van Houdt, R. Antimicrobial silver: Uses, toxicity and potential for resistance. BioMetals 2013, 26, 609–621. [Google Scholar] [CrossRef] [PubMed]
- Ghormade, V.; Deshpande, M.V.; Paknikar, K.M. Perspectives for nano-biotechnology enabled protection and nutrition of plants. Biotechnol. Adv. 2011, 29, 792–803. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, J.; Genin, S.; Magori, S.; Citovsky, V.; Sriariyanum, M.; Ronald, P.; Dow, M.; Verdier, V.; Beer, S.V.; Machado, M.A.; et al. Top 10 plant pathogenic bacteria in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 614–629. [Google Scholar] [CrossRef] [PubMed]
- Perombelon, M.C.M.; Kelman, A. Ecology of the soft rot Erwinias. Annu. Rev. Phytopathol. 1980, 18, 361–387. [Google Scholar] [CrossRef]
- Hugouvieux-Cotte-Pattat, N.; Condemine, G.; Nasser, W.; Reverchon, S. Regulation of pectinolysis in Erwinia chrysanthemi. Annu. Rev. Microbiol. 1996, 50, 213–257. [Google Scholar] [CrossRef] [PubMed]
- Toth, I.K.; van der Wolf, J.M.; Saddler, G.; Lojkowska, E.; Hélias, V.; Pirhonen, M.; Tsror Lahkim, L.; Elphinstone, J.G. Dickeya species: An emerging problem for potato production in Europe. Plant Pathol. 2011, 60, 385–399. [Google Scholar] [CrossRef]
- Ma, B.; Hibbing, M.E.; Kim, H.-S.; Reedy, R.M.; Yedidia, I.; Breuer, J.; Breuer, J.; Glasner, J.D.; Perna, N.T.; Kelman, A.; et al. Host range and molecular phylogenies of the soft rot enterobacterial genera Pectobacterium and Dickeya. Phytopathology 2007, 97, 1150–1163. [Google Scholar] [CrossRef] [PubMed]
- Czajkowski, R.; Pérombelon, M.C.M.; van Veen, J.A.; van der Wolf, J.M. Control of blackleg and tuber soft rot of potato caused by Pectobacterium and Dickeya species: A review. Plant Pathol. 2011, 60, 999–1013. [Google Scholar] [CrossRef]
- Motyka, A.; Zoledowska, S.; Sledz, W.; Lojkowska, E. Molecular methods as tools to control plant diseases caused by Dickeya and Pectobacterium spp: A minireview. New Biotechnol. 2017, 39, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Dzimitrowicz, A.; Greda, K.; Lesniewicz, T.; Jamroz, P.; Nyk, M.; Pohl, P. Size-controlled synthesis of gold nanoparticles by a novel atmospheric pressure glow discharge system with a metallic pin electrode and a flowing liquid electrode. RSC Adv. 2016, 6, 80773–80783. [Google Scholar] [CrossRef]
- Kora, A.; Beedu, S.; Jayaraman, A. Size-controlled green synthesis of silver nanoparticles mediated by gum ghatti (Anogeissus latifolia) and its biological activity. Org. Med. Chem. Lett. 2012, 2, 17. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Hussain, J.I.; Hashmi, A.A.; AL-Thabaiti, S.A. Preparation and characterization of silver nanoparticles using aniline. Arab. J. Chem. 2017, 10, S1506–S1511. [Google Scholar] [CrossRef]
- Shi, L.; Gunasekaran, S. Preparation of Pectin-ZnO Nanocomposite. Nanoscale Res. Lett. 2008, 3, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.-G.; Zhu, L.-M.; Branford-White, C.J.; Yang, J.-H.; Wang, X.; Li, Y.; Qian, W. Solid dispersions in the form of electrospun core-sheath nanofibers. Int. J. Nanomed. 2011, 6, 3271–3280. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.K.; Agarwal, A.; Gopal, R.; Swarnkar, R.K.; Kotnala, R.K. Dumbbell shaped nickel nanocrystals synthesized by a laser induced fragmentation method. J. Mater. Chem. 2011, 21, 11074–11079. [Google Scholar] [CrossRef]
- Viana, R.B.; da Silva, A.B.F.; Pimentel, A.S. Infrared spectroscopy of anionic, cationic, and zwitterionic surfactants. Adv. Phys. Chem. 2012, 2012, 1–14. [Google Scholar] [CrossRef]
- Morales-Avila, E.; Ferro-Flores, G.; Ocampo-García, B.E.; López-Téllez, G.; López-Ortega, J.; Rogel-Ayala, D.G.; Sánchez-Padilla, D. Antibacterial efficacy of gold and silver nanoparticles functionalized with the ubiquicidin (29–41) antimicrobial peptide. J. Nanomater. 2017, 2017, 5831959. [Google Scholar] [CrossRef]
- Mishra, S.; Singh, H.B. Biosynthesized silver nanoparticles as a nanoweapon against phytopathogens: Exploring their scope and potential in agriculture. Appl. Microbiol. Biotechnol. 2015, 99, 1097–1107. [Google Scholar] [CrossRef] [PubMed]
- Vigneshwaran, N.; Nachane, R.P.; Balasubramanya, R.H.; Varadarajan, P.V. A novel one-pot “green” synthesis of stable silver nanoparticles using soluble starch. Carbohydr. Res. 2006, 341, 2012–2018. [Google Scholar] [CrossRef] [PubMed]
- Niska, K.; Knap, N.; Kędzia, A.; Jaskiewicz, M.; Kamysz, W.; Inkielewicz-Stepniak, I. Capping agent-dependent toxicity and antimicrobial activity of silver nanoparticles: An in vitro study. Concerns about potential application in dental practice. Int. J. Med. Sci. 2016, 13, 772–782. [Google Scholar] [CrossRef] [PubMed]
- Hugouvieux-Cotte-Pattat, N.; Blot, N.; Reverchon, S. Identification of TogMNAB, an ABC transporter which mediates the uptake of pectic oligomers in Erwinia chrysanthemi 3937. Mol. Microbiol. 2008, 41, 1113–1123. [Google Scholar] [CrossRef]
- Pomogailo, A.D.; Kestelman, V.N. Metallopolymer Nanocomposites; Springer: Berlin, Germany, 2005. [Google Scholar]
- López-Miranda, A.; López-Valdivieso, A.; Viramontes-Gamboa, G. Silver nanoparticles synthesis in aqueous solutions using sulfite as reducing agent and sodium dodecyl sulfate as stabilizer. J. Nanopart. Res. 2012, 14, 1101. [Google Scholar] [CrossRef]
- Ocsoy, I.; Paret, M.L.; Ocsoy, M.A.; Kunwar, S.; Chen, T.; You, M.; Tan, W. Nanotechnology in plant disease management: DNA-directed silver nanoparticles on graphene oxide as an antibacterial against Xanthomonas perforans. ACS Nano 2013, 7, 8972–8980. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, N.; Rajkamal, P.; Cholan, S.; Kannadasan, N.; Sathishkumar, K.; Viruthagiri, G.; Sundaramanickam, A. Biosynthesis of silver nanoparticles from the marine seaweed Sargassum wightii and their antibacterial activity against some human pathogens. Appl. Nanosci. 2014, 4, 881–888. [Google Scholar] [CrossRef]
- Sathishkumar, G.; Gobinath, C.; Karpagam, K.; Hemamalini, V.; Premkumar, K.; Sivaramakrishnan, S. Phyto-synthesis of silver nanoscale particles using Morinda citrifolia L. and its inhibitory activity against human pathogens. Colloids Surf. B Biointerfaces 2012, 95, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Johari, S.A.; Kalbassi, M.R.; Soltani, M.; Yu, I.J. Study of fungicidal properties of colloidal silver nanoparticles (AgNPs) on trout egg pathogen, Saprolegnia sp. Int. J. Aquat. Biol. 2015, 3, 191–198. [Google Scholar]
- Swain, P.; Nayak, S.K.; Sasmal, A.; Behera, T.; Barik, S.K.; Swain, S.K.; Mishra, S.S.; Sen, A.K.; Das, J.K.; Jayasankar, P. Antimicrobial activity of metal based nanoparticles against microbes associated with diseases in aquaculture. World J. Microbiol. Biotechnol. 2014, 30, 2491–2502. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.K.; Kim, B.H.; Jung, G. Antifungal activity of silver ions and nanoparticles on phytopathogenic fungi. Plant Dis. 2009, 93, 1037–1043. [Google Scholar] [CrossRef]
- Lamsal, K.; Kim, S.W.; Jung, J.H.; Kim, Y.S.; Kim, K.S.; Lee, Y.S. Application of silver nanoparticles for the control of Colletotrichum species in vitro and pepper anthracnose disease in field. Mycobiology 2011, 39, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, V.; Velusamy, P. Extracellular biosynthesis of silver nanoparticles using Bacillus sp. GP-23 and evaluation of their antifungal activity towards Fusarium oxysporum. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 106, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Krishnaraj, C.; Ramachandran, R.; Mohan, K.; Kalaichelvan, P.T. Optimization for rapid synthesis of silver nanoparticles and its effect on phytopathogenic fungi. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 93, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-J.; Kim, S.-H.; Kim, H.-J.; Choi, S.-H. A new composition of nanosized silica-silver for control of various plant diseases. Plant Pathol. J. 2006, 22, 295–302. [Google Scholar] [CrossRef]
- Chua, H.; Kim, H.J.; Kim, J.S.; Kim, M.S.; Byung-Dae, Y.; Hae-Jun, P.; Kim, C.Y. A nanosized Ag–silica hybrid complex prepared by γ-irradiation activates the defense response in Arabidopsis. Radiat. Phys. Chem. 2012, 81, 180–184. [Google Scholar] [CrossRef]
- Chahardooli, M.; Khodadadi, E.; Khodadadi, E. Green synthesis of silver nanoparticles using oak leaf and fruit extracts (Quercus) and its antibacterial activity against plant pathogenic bacteria. Int. J. Biosci. 2014, 4, 97–103. [Google Scholar]
- Zoledowska, S.; Motyka, A.; Zukowska, D.; Sledz, W.; Lojkowska, E. Population structure and biodiversity of Pectobacterium parmentieri isolated from potato fields in temperate climate. Plant Dis. 2018, 102, 154–164. [Google Scholar] [CrossRef]
- Benoit, R.; Wilkinson, K.J.; Sauvé, S. Partitioning of silver and chemical speciation of free Ag in soils amended with nanoparticles. Chem. Cent. J. 2013, 7, 75. [Google Scholar] [CrossRef] [PubMed]
- DiCenzo, G.C.; Zamani, M.; Milunovic, B.; Finan, T.M. Genomic resources for identification of the minimal N2-fixing symbiotic genome. Environ. Microbiol. 2016, 18, 2534–2547. [Google Scholar] [CrossRef] [PubMed]
- Zamani, M.; Finan, T.M.; Cowie, A.; diCenzo, G.C. Proline auxotrophy in Sinorhizobium meliloti results in a plant-specific symbiotic phenotype. Microbiology 2015, 161, 2341–2351. [Google Scholar] [CrossRef]
- Choi, O.; Hu, Z. Size dependent and reactive oxygen species related nanosilver toxicity to nitrifying bacteria. Environ. Sci. Technol. 2008, 42, 4583–4588. [Google Scholar] [CrossRef] [PubMed]
- Sledz, W.; Motyka, A.; Zoledowska, S.; Paczek, A.; Los, E.; Rischka, J. Influence of exogenously supplemented caffeine on cell division, germination, and growth of economically important plants. In The Question of Caffeine; InTech: London, UK, 2017. [Google Scholar]
- Lee, W.-M.; Kwak, J., II; An, Y.-J. Effect of silver nanoparticles in crop plants Phaseolus radiatus and Sorghum bicolor: Media effect on phytotoxicity. Chemosphere 2012, 86, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Salama, H. Effects of silver nanoparticles in some crop plants, common bean (Phaseolus vulgaris L.) and corn (Zea mays L.). Int. Res. J. Biotechnol. 2012, 3, 190–197. [Google Scholar]
- Vannini, C.; Domingo, G.; Onelli, E.; Prinsi, B.; Marsoni, M.; Espen, L.; Bracale, M. Morphological and proteomic responses of Eruca sativa exposed to silver nanoparticles or silver nitrate. PLoS ONE 2013, 8, e68752. [Google Scholar] [CrossRef] [PubMed]
- Mazzaglia, A.; Fortunati, E.; Kenny, J.M.; Torre, L.; Balestra, G.M. Nanomaterials in Plant Protection. In Nanoscience and Nanotechnology for Agriculture and Food; Axelos, M.A.V., van De Voorde, M., Eds.; Wiley: Hoboken, NJ, USA, 2017; pp. 115–134. [Google Scholar]
- Kah, M.; Beulke, S.; Tiede, K.; Hofmann, T. Nanopesticides: State of knowledge, environmental fate, and exposure modeling. Crit. Rev. Environ. Sci. Technol. 2013, 43, 1823–1867. [Google Scholar] [CrossRef]
- Loeschner, K.; Hadrup, N.; Qvortrup, K.; Larsen, A.; Gao, X.; Vogel, U.; Mortensen, A.; Lam, H.; Larsen, E.H. Distribution of silver in rats following 28 days of repeated oral exposure to silver nanoparticles or silver acetate. Part. Fibre Toxicol. 2011, 8, 18. [Google Scholar] [CrossRef] [PubMed]
- Slawiak, M.; Lojkowska, E.; van der Wolf, J.M. First report of bacterial soft rot on potato caused by Dickeya sp. (syn. Erwinia chrysanthemi) in Poland. Plant Pathol. 2009, 58, 794. [Google Scholar] [CrossRef]
- Duarte, V.; De Boer, S.H.; Ward, L.J.; Oliveira, A.M.R. Characterization of atypical Erwinia carotovora strains causing blackleg of potato in Brazil. J. Appl. Microbiol. 2004, 96, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Nykyri, J.; Niemi, O.; Koskinen, P.; Nokso-Koivisto, J.; Pasanen, M.; Broberg, M.; Plyusnin, I.; Törönen, P.; Holm, L.; Pirhonen, M.; et al. Revised phylogeny and novel horizontally acquired virulence determinants of the model soft rot phytopathogen Pectobacterium wasabiae SCC3193. PLoS Pathog. 2012, 8, e1003013. [Google Scholar] [CrossRef] [PubMed]
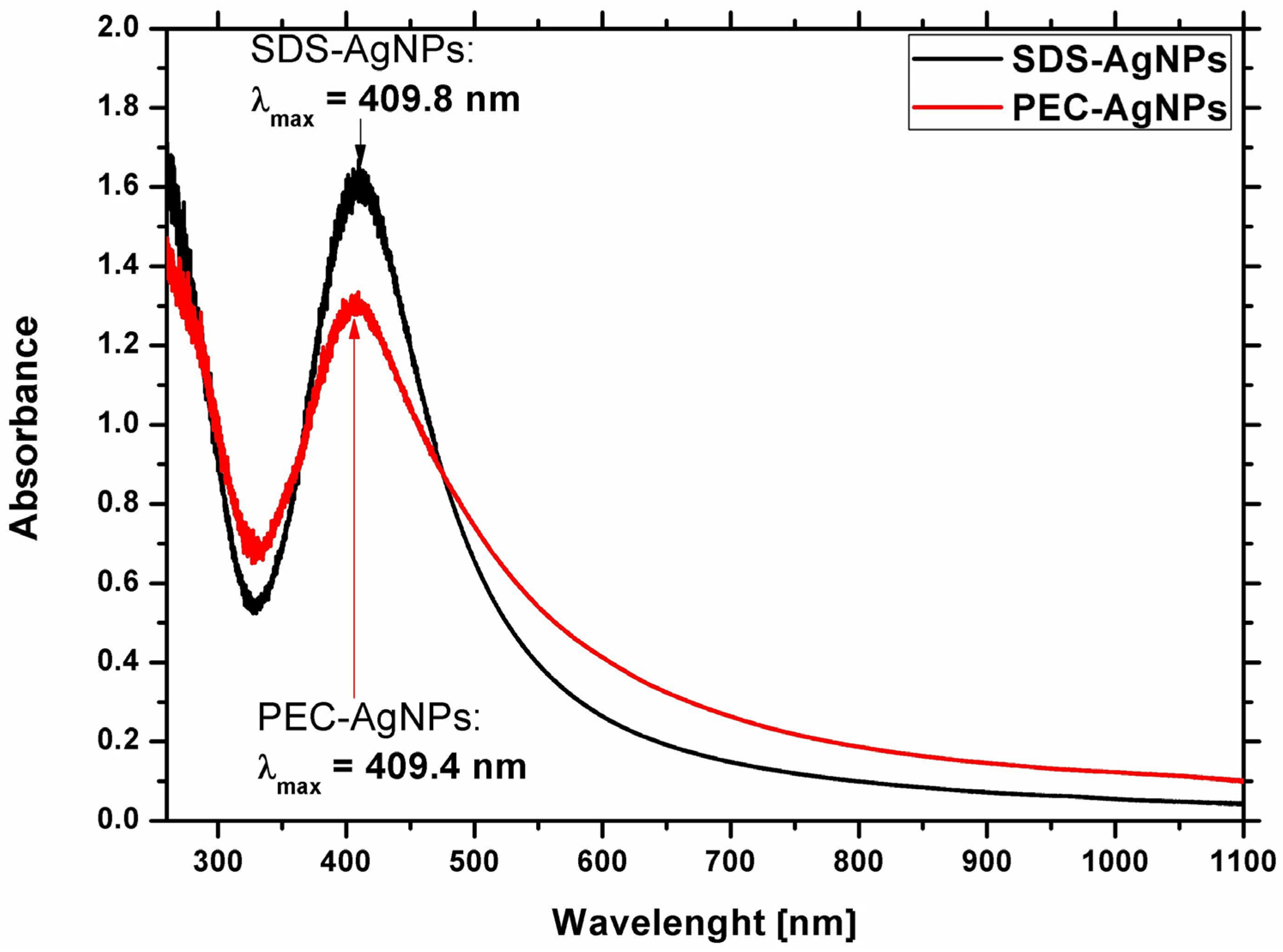


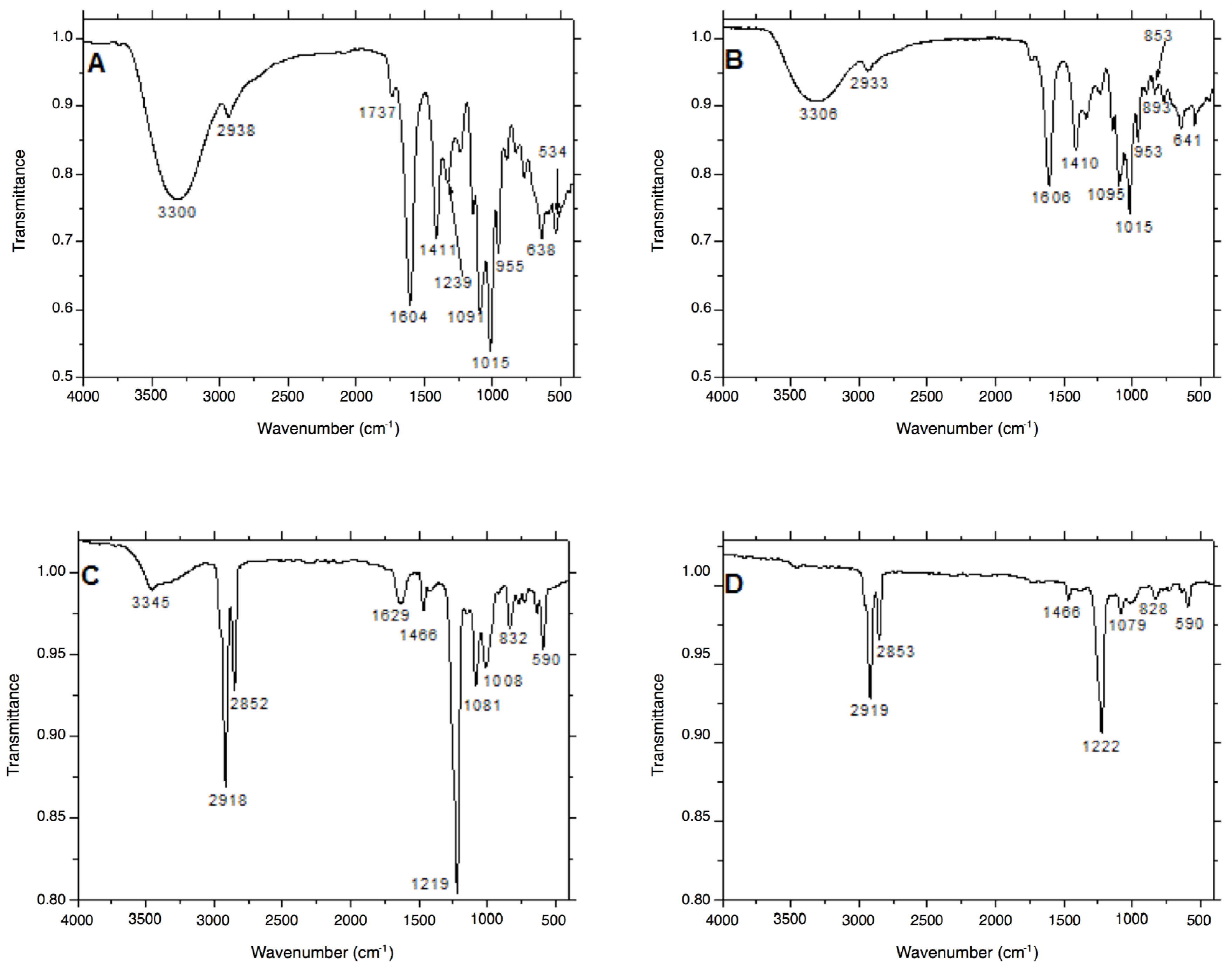
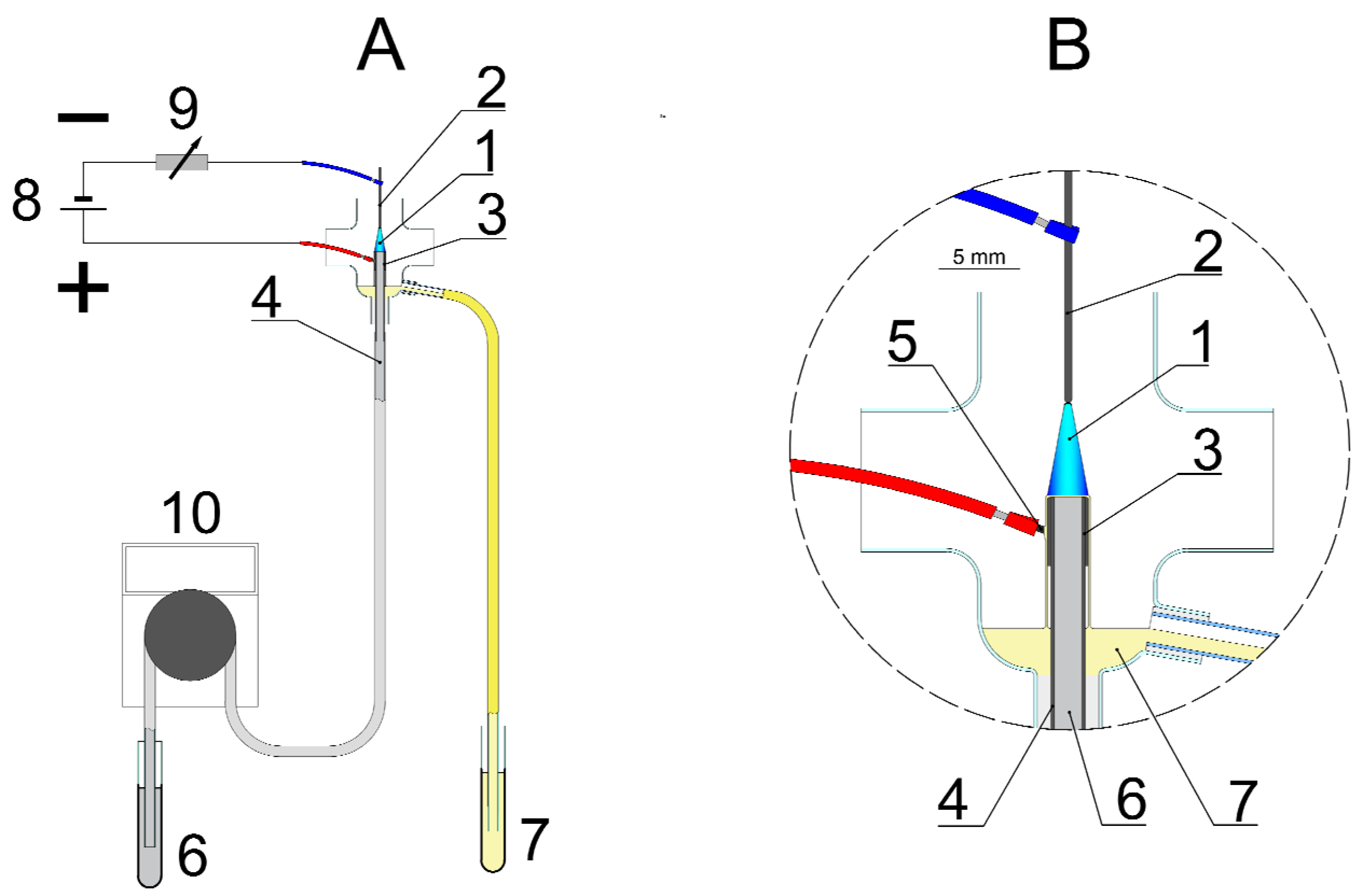
| Strain | PEC-AgNPs | SDS-AgNPs | ||
|---|---|---|---|---|
| MIC (mg∙L−1) | MBC (mg∙L−1) | MIC (mg∙L−1) | MBC (mg∙L−1) | |
| Dsol IFB0099 | 5.5 | 5.5 | 3.0 | 3.0 |
| Pba IFB5103 | 5.5 | 5.5 | 0.75 | 0.75 |
| Pcbr IFB5390 | 5.5 | 5.5 | 3.0 | 3.0 |
| Pcc IFB5118 | 5.5 | 5.5 | 3.0 | 3.0 |
| Ppa IFB5308 | 5.5 | 5.5 | 3.0 | 3.0 |
| Species | No. IFB UG&MUG Collection | Nos. in Other Collections | Host Plant | Country of Isolation | Reference |
|---|---|---|---|---|---|
| Dickeya solani (Dsol) | IFB0099 | IPO 2276, LMG 28824 | Solanum tuberosum | Poland, 2005 | Slawiak et al. (2009) [59] |
| Pectobacterium atrosepticum (Pba) | IFB5103 | SCRI 1086 | Solanum tuberosum | Canada, 1985 | SCRI collection b |
| Pectobacterium carotovorum subsp. brasiliense (Pcbr) | IFB5390 | LMG21371 | Solanum tuberosum | Brasil, 2002 | Duarte et al. (2004) [60] |
| Pectobacterium carotovorum subsp. carotovorum (Pcc) | IFB5118 | SCRI 136 | Solanum tuberosum | USA, NA a | SCRI collection b |
| Pectobacterium parmentieri (Ppa) | IFB5308 | SCC3193 | Solanum tuberosum | Finland, 1980s | Nykyri et al. (2012) [61] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dzimitrowicz, A.; Motyka, A.; Jamroz, P.; Lojkowska, E.; Babinska, W.; Terefinko, D.; Pohl, P.; Sledz, W. Application of Silver Nanostructures Synthesized by Cold Atmospheric Pressure Plasma for Inactivation of Bacterial Phytopathogens from the Genera Dickeya and Pectobacterium. Materials 2018, 11, 331. https://doi.org/10.3390/ma11030331
Dzimitrowicz A, Motyka A, Jamroz P, Lojkowska E, Babinska W, Terefinko D, Pohl P, Sledz W. Application of Silver Nanostructures Synthesized by Cold Atmospheric Pressure Plasma for Inactivation of Bacterial Phytopathogens from the Genera Dickeya and Pectobacterium. Materials. 2018; 11(3):331. https://doi.org/10.3390/ma11030331
Chicago/Turabian StyleDzimitrowicz, Anna, Agata Motyka, Piotr Jamroz, Ewa Lojkowska, Weronika Babinska, Dominik Terefinko, Pawel Pohl, and Wojciech Sledz. 2018. "Application of Silver Nanostructures Synthesized by Cold Atmospheric Pressure Plasma for Inactivation of Bacterial Phytopathogens from the Genera Dickeya and Pectobacterium" Materials 11, no. 3: 331. https://doi.org/10.3390/ma11030331
APA StyleDzimitrowicz, A., Motyka, A., Jamroz, P., Lojkowska, E., Babinska, W., Terefinko, D., Pohl, P., & Sledz, W. (2018). Application of Silver Nanostructures Synthesized by Cold Atmospheric Pressure Plasma for Inactivation of Bacterial Phytopathogens from the Genera Dickeya and Pectobacterium. Materials, 11(3), 331. https://doi.org/10.3390/ma11030331









