Green Fabrication of Supported Platinum Nanoparticles by Supercritical CO2 Deposition
Abstract
:1. Introduction
2. Materials and Methods
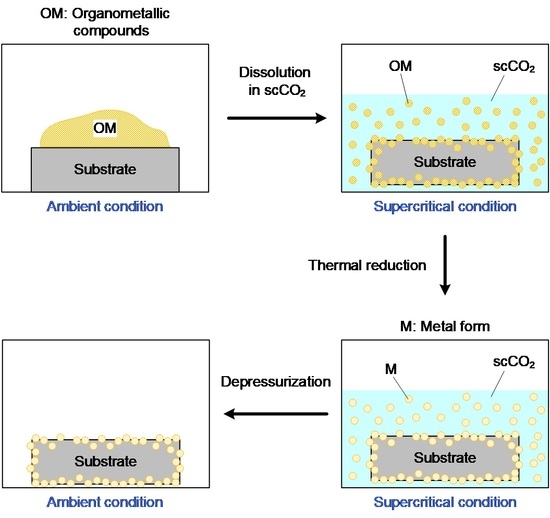
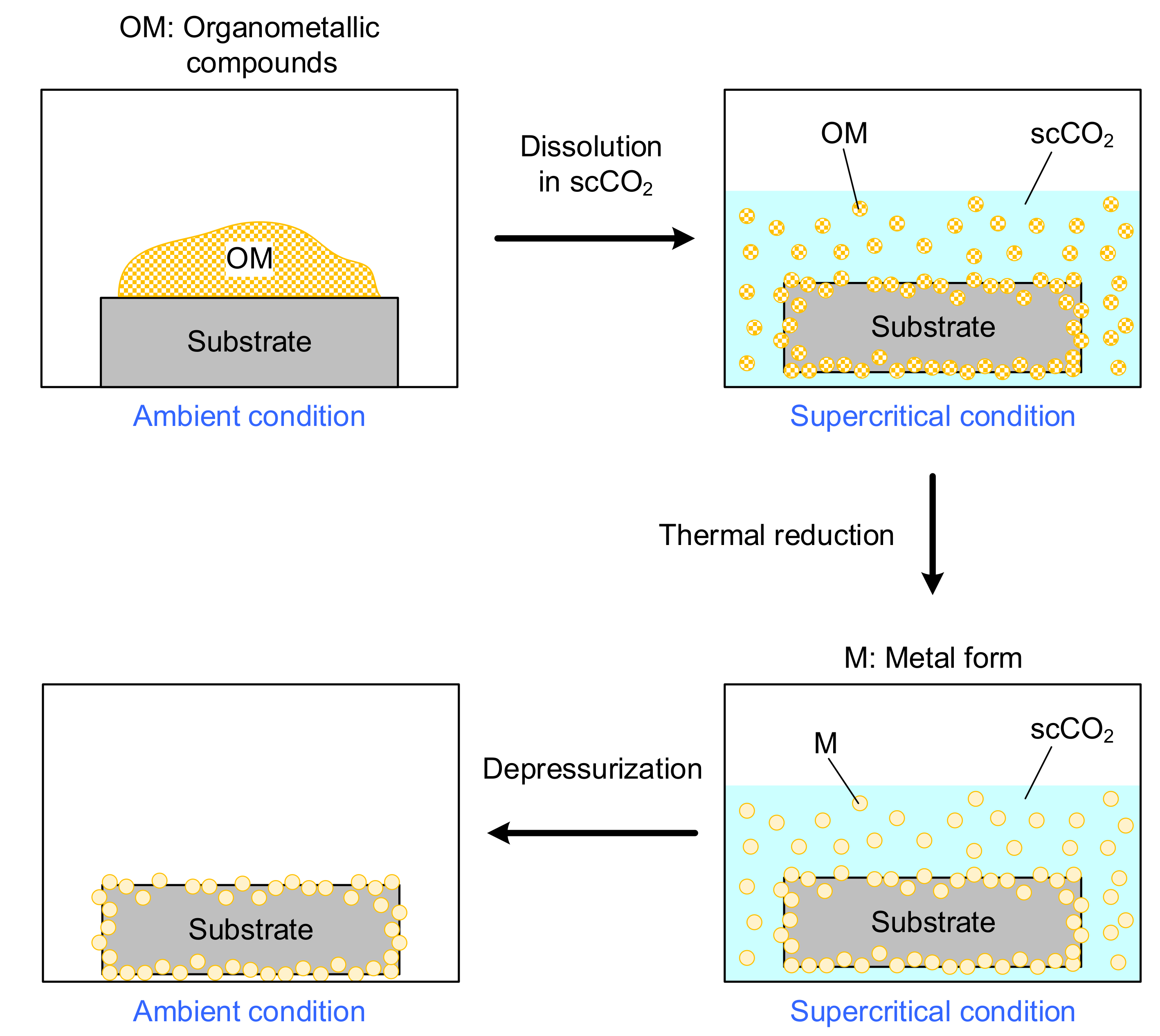
2.1. Preparation of Pt Nanoparticles via Supercritical CO2 Deposition (SCD)
2.2. Full 24 Factorial Design
2.3. Characterization
3. Results and Discussion
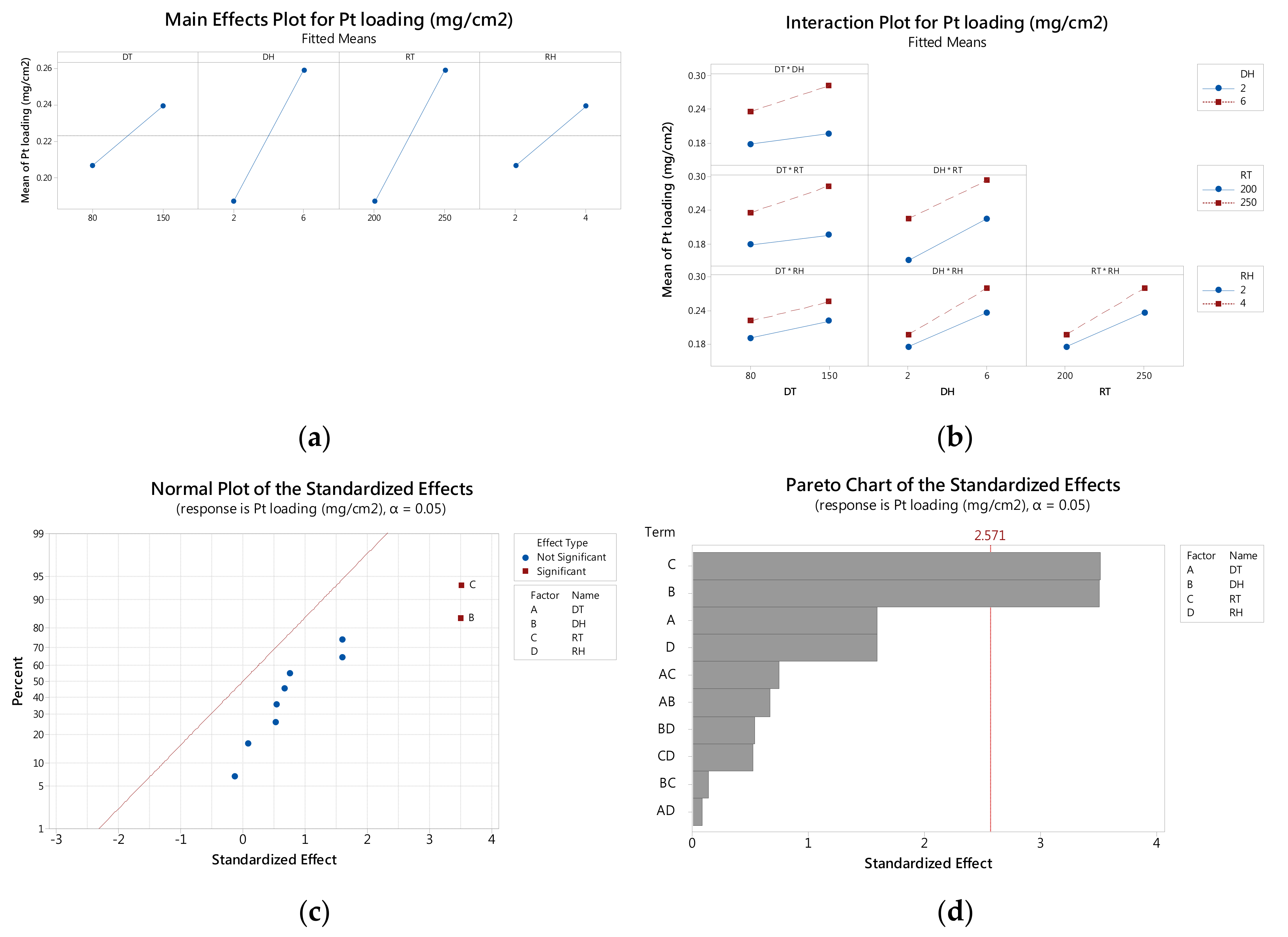
3.1. Analysis of 24 Factorial Design
3.2. Main and Interaction Effects
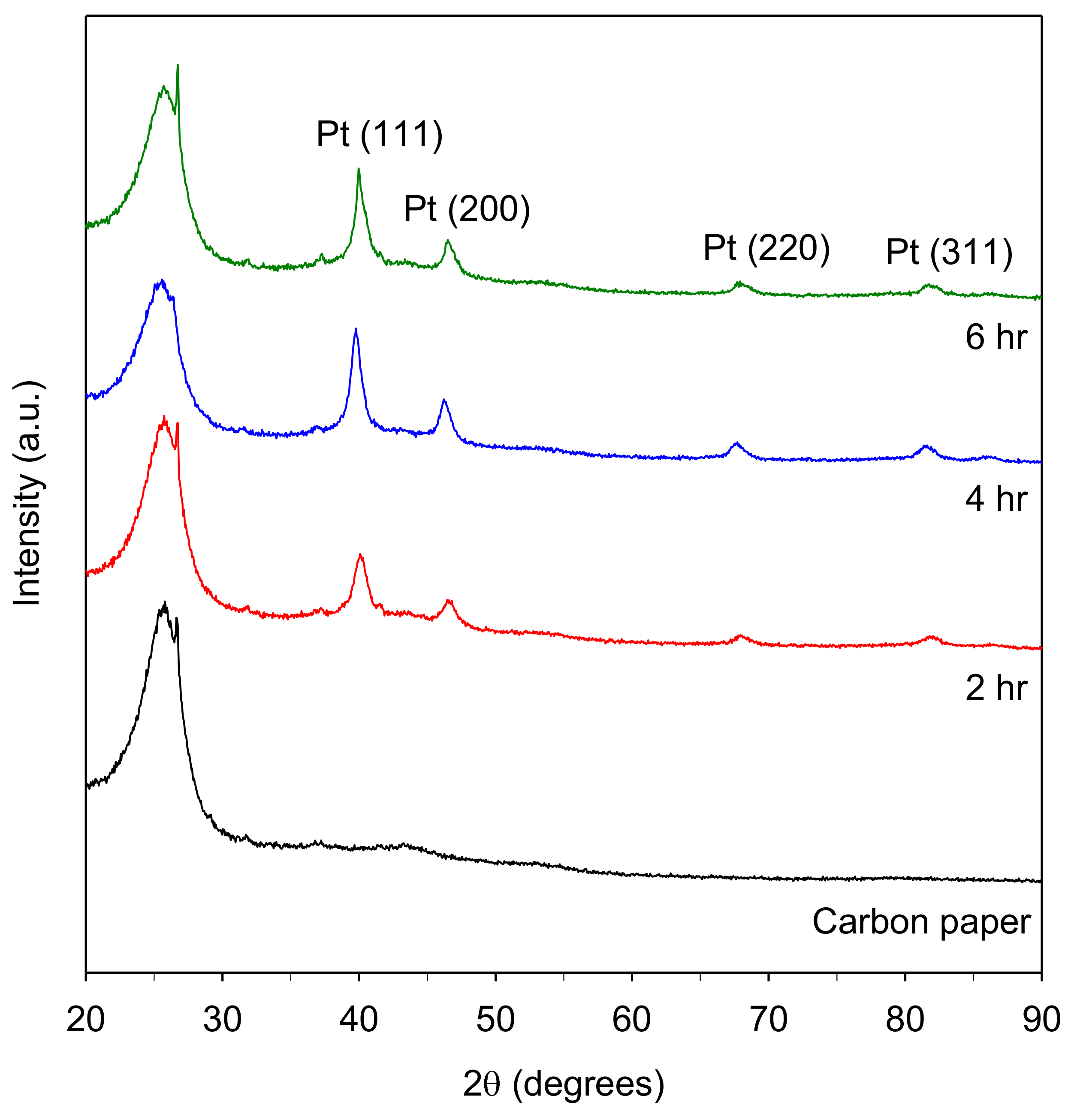
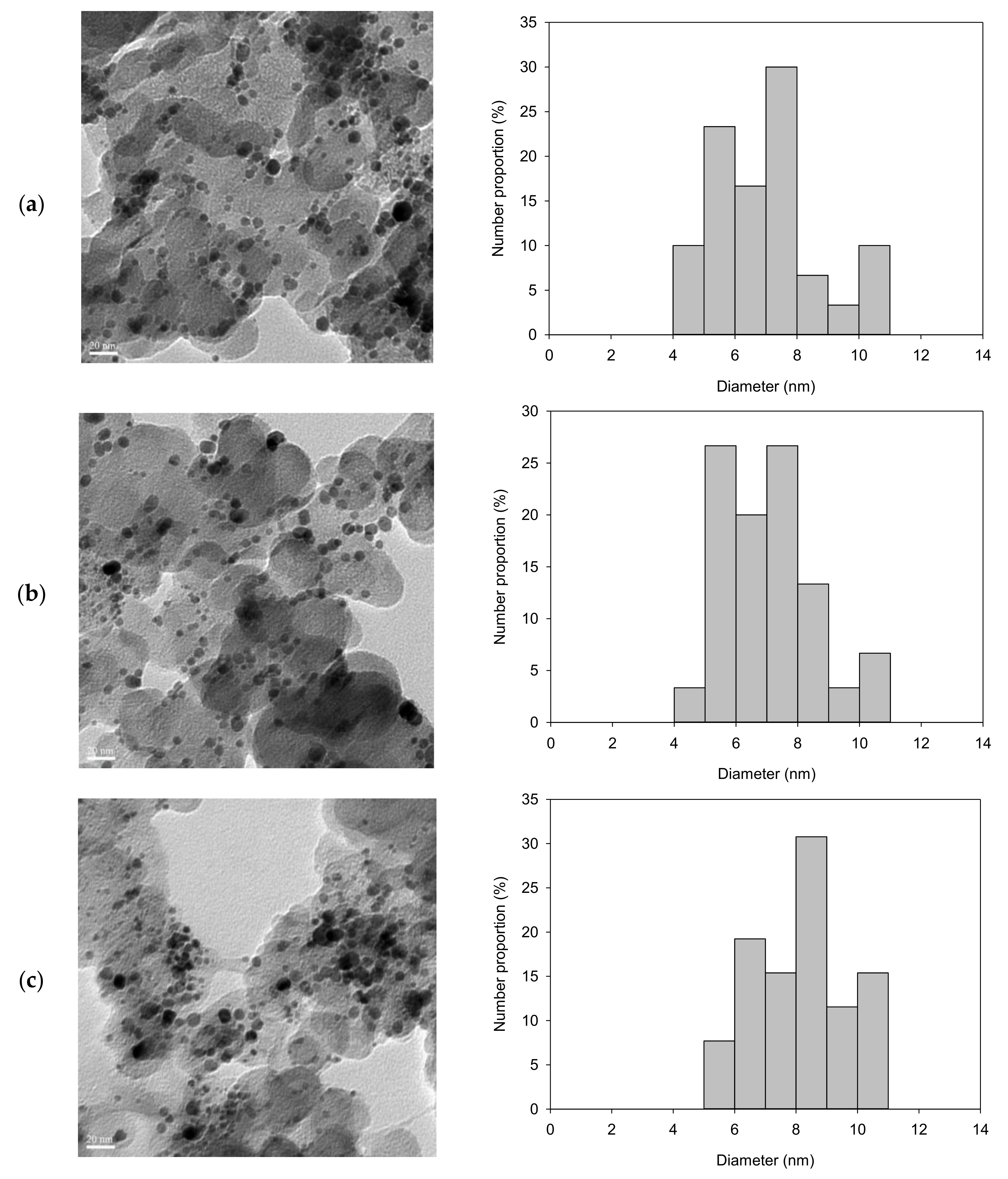
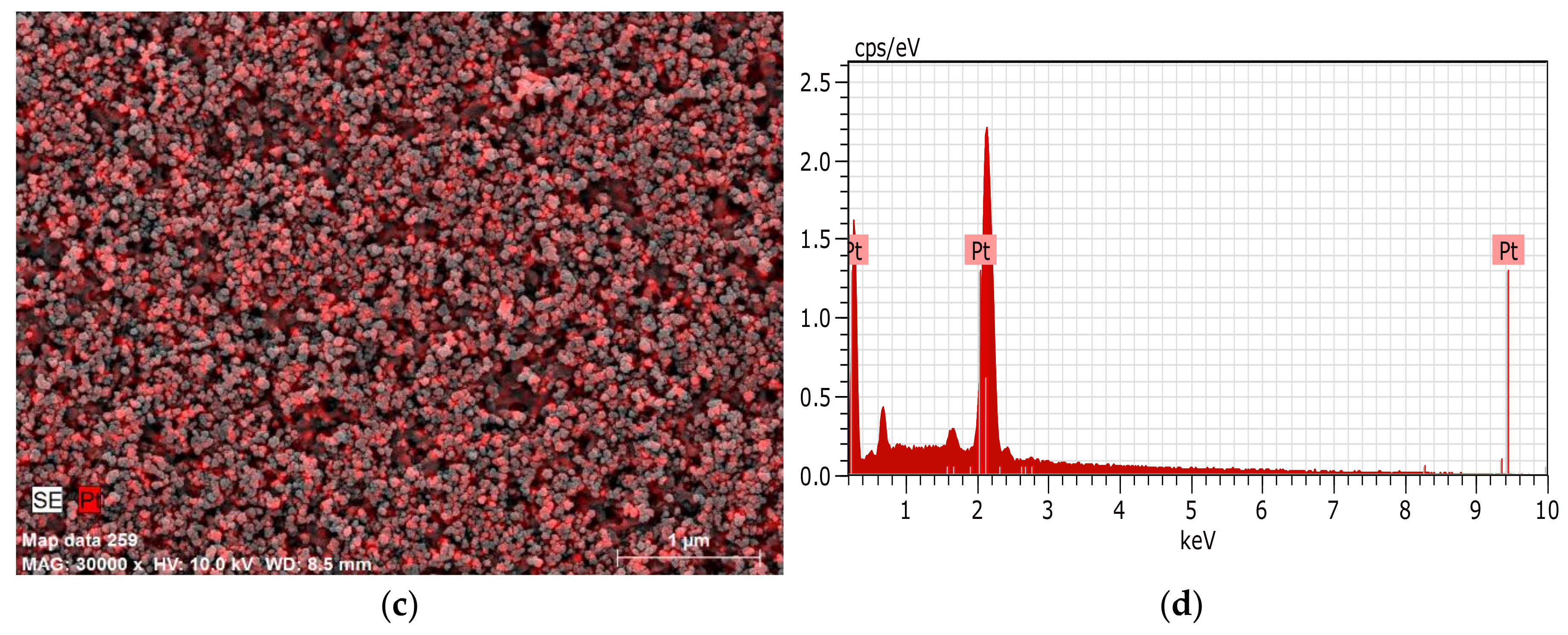
3.3. Characterization of Pt/Carbon Paper Composites
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Türk, M.; Erkey, C. Synthesis of supported nanoparticles in supercritical fluids by supercritical fluid reactive deposition: Current state, further perspectives and needs. J. Supercrit. Fluids 2018, 134, 176–183. [Google Scholar] [CrossRef]
- Sánchez-Miguel, E.; Tenorio, M.J.; Morère, J.; Cabañas, A. Green preparation of PtRu and PtCu/SBA-15 catalysts using supercritical CO2. J. CO2 Util. 2017, 22, 382–391. [Google Scholar] [CrossRef]
- Bozbag, S.E.; Erkey, C. Supercritical deposition: Current status and perspectives for the preparation of supported metal nanostructures. J. Supercrit. Fluids 2015, 96, 298–312. [Google Scholar] [CrossRef]
- Huang, K.L.; Lai, Y.C.; Tsai, C.H. Effects of sputtering parameters on the performance of electrodes fabricated for proton exchange membrane fuel cells. J. Power Sources 2006, 156, 224–231. [Google Scholar] [CrossRef]
- Sui, S.; Wang, X.; Zhou, X.; Su, Y.; Riffat, S.; Liu, C. A comprehensive review of Pt electr Catalysts for the oxygen reduction reaction: Nanostructure, activity, mechanism and carbon support in PEM fuel cells. J. Mater. Chem. A 2017, 5, 1808–1825. [Google Scholar] [CrossRef]
- Lai, Y.C.; Huang, K.L.; Tsai, C.H.; Lee, W.J.; Chen, Y.L. Sputtered Pt loadings of membrane electrode assemblies in proton exchange membrane fuel cells. Int. J. Energy Res. 2012, 36, 918–927. [Google Scholar] [CrossRef]
- Firtina, I.; Güner, S.; Albostan, A. Preparation and characterization of membrane electrode assembly (MEA) for PEMFC. Int. J. Energy Res. 2010, 35, 146–152. [Google Scholar] [CrossRef]
- Yu, W.; Porosoff, M.D.; Chen, J.G. Review of Pt-based bimetallic catalysis: from model surfaces to supported catalysts. Chem. Rev. 2012, 112, 5780–5817. [Google Scholar] [CrossRef] [PubMed]
- Sápi, A.; Varga, A.; Samu, G.F.; Dobó, D.G.; Juhász, K.L.; Takács, B.; Varga, E.; Kukovecz, Á.; Kónya, Z.; Janáky, C. Photoelectr chemistry by design: Tailoring the nanoscale structure of Pt/NiO composites leads to enhanced photoelectr chemical hydrogen evolution performance. J. Phys. Chem. C 2017, 121, 12148–12158. [Google Scholar] [CrossRef] [PubMed]
- Sápi, A.; Halasi, G.; Kiss, J.; Dobó, D.G.; Juhász, K.L.; Kolcsár, V.J.; Ferencz, Z.; Vári, G.; Matolin, V.; Erdőhelyi, A.; et al. In Situ DRIFTS and NAP-XPS Exploration of the Complexity of CO2 Hydrogenation over Size-Controlled Pt Nanoparticles Supported on Mesoporous NiO. J. Phys. Chem. C 2018, 122, 5553–5565. [Google Scholar] [CrossRef]
- Saquing, C.D.; Kang, D.; Aindow, M.; Erkey, C. Investigation of the supercritical deposition of platinum nanoparticles into carbon aerogel. Microporous Mesoporous Mater. 2005, 80, 11–23. [Google Scholar] [CrossRef]
- Aspromonte, S.; Sastre, A.; Boix, A.; Cero, M.J.C.; Alonso, E. Optimization and modelling of the supercritical CO2 deposition of CoxOy nanoparticles in MCM41. J. Supercrit. Fluids 2016, 110, 47–55. [Google Scholar] [CrossRef]
- Oztuna, F.E.S.; Barim, S.B.; Bozbag, S.E.; Yu, H.B.; Aindow, M.; Unal, U.; Erkey, C. Graphene aerogel supported pt electro Catalysts for oxygen reduction reaction by supercritical deposition. Electr Chim. Acta 2017, 250, 174–184. [Google Scholar] [CrossRef]
- Türk, M.; Crone, M.; Upper, G. Effect of gas pressure on the phase behaviour of organometallic compounds. J. Supercrit. Fluids 2011, 58, 1–6. [Google Scholar] [CrossRef]
- Chen, A.Z.; Wang, G.Y.; Wang, S.B.; Feng, J.G.; Liu, Y.G.; Kang, Y.Q. Preparation of poly-(methyl vinyl ether-co-maleic anhydride) nanoparticles by solution-enhanced dispersion by supercritical CO2. Materials 2012, 5, 1841–1852. [Google Scholar] [CrossRef]
- Montgomery, D.C. Design and Analysis of Experiments, 3rd ed.; Wiley: New York, NY, USA, 1991. [Google Scholar]
- Berger, P.D.; Maurer, R.E. Experimental Design, 1st ed.; Easter Press Pvt. Ltd: Bangalore, India, 2002. [Google Scholar]
- Sterne, J.A.C.; Smith, G.D. Sifting the evidence—What’s wrong with significance tests? Phys. Therapy 2001, 81, 1464–1469. [Google Scholar] [CrossRef] [PubMed]
- Farooq, S.; Saeed, A.; Sharif, M.; Hussain, J.; Mabood, F.; Iftekhar, M. Pr Cess optimization studies of crystal violet dye adsorption onto novel, mixed metal Ni0.5Co0.5Fe2O4 ferrospinel nanoparticles using factorial design. J. Water Pr Cess Eng. 2017, 16, 132–141. [Google Scholar] [CrossRef]
- Camacho, L.M.; Fox, J.A.; Ajedegba, J.O. Optimization of electrodialysis metathesis (EDM) desalination using factorial design methodology. Desalination 2017, 403, 136–143. [Google Scholar] [CrossRef]
- Bozbag, S.E.; Yasar, N.S.; Zhang, L.C.; Aindow, M.; Erkey, C. Absorption of Pt(cod)me2 onto organic aerogels from supercritical solutions for the synthesis of supported platinum nanoparticles. J. Supercrit. Fluids 2011, 56, 105–113. [Google Scholar] [CrossRef]
- Barım, Ş.B.; Bayrakçeken, A.; Bozbağ, S.E.; Zhang, L.; Kızılel, R.; Aindow, M.; Erkey, C. Control of average particle size of carbon aerogel supported platinum nanoparticles by supercritical deposition. Microporous Mesoporous Mater. 2017, 245, 94–103. [Google Scholar] [CrossRef]



| Factors | Levels | |
|---|---|---|
| Low(−1) | High(+1) | |
| Deposition Temperature (DT) | 80 °C | 150 °C |
| Deposition Time (DH) | 2 h | 6 h |
| Reduction Temperature (RT) | 200 °C | 250 °C |
| Reduction Time (RH) | 2 h | 4 h |
| Run | Factor Combination | Factors | Pt Loading (mg/cm2) | |||
|---|---|---|---|---|---|---|
| Deposition Temperature (A) | Deposition Time (B) | Reduction Temperature (C) | Reduction Time (D) | |||
| 1 | 1 | −1 | −1 | −1 | −1 | 0.122 |
| 2 | A | +1 | −1 | −1 | −1 | 0.147 |
| 3 | B | −1 | +1 | −1 | −1 | 0.183 |
| 4 | AB | +1 | +1 | −1 | −1 | 0.253 |
| 5 | C | −1 | −1 | +1 | −1 | 0.196 |
| 6 | AC | +1 | −1 | +1 | −1 | 0.242 |
| 7 | BC | −1 | +1 | +1 | −1 | 0.265 |
| 8 | ABC | +1 | +1 | +1 | −1 | 0.248 |
| 9 | D | −1 | −1 | −1 | +1 | 0.189 |
| 10 | AD | +1 | −1 | −1 | +1 | 0.142 |
| 11 | BD | −1 | +1 | −1 | +1 | 0.220 |
| 12 | ABD | +1 | +1 | −1 | +1 | 0.242 |
| Source | Degree of Freedom (DF) | Adjust Sum of Squares (SS) | Adjust Mean Squares (MS) | F-Value | P-Value |
|---|---|---|---|---|---|
| DT | 1 | 0.00424 | 0.00424 | 2.53 | 0.173 |
| DH | 1 | 0.020606 | 0.020606 | 12.29 | 0.017 |
| RT | 1 | 0.020696 | 0.020696 | 12.35 | 0.017 |
| RH | 1 | 0.004234 | 0.004234 | 2.53 | 0.173 |
| DT*DH | 1 | 0.000752 | 0.000752 | 0.45 | 0.533 |
| DT*RT | 1 | 0.000931 | 0.000931 | 0.56 | 0.490 |
| DT*RH | 1 | 0.000011 | 0.000011 | 0.01 | 0.939 |
| DH*RT | 1 | 0.000032 | 0.000032 | 0.02 | 0.896 |
| DH*RH | 1 | 0.000486 | 0.000486 | 0.29 | 0.613 |
| RT*RH | 1 | 0.000446 | 0.000446 | 0.27 | 0.628 |
| Error | 5 | 0.008381 | 0.001676 | ||
| Total | 15 | 0.060814 | |||
| S = 0.0409422; R2 = 86.22% | |||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-L.; Tsai, C.-H.; Chen, M.-Y.; Lai, Y.-C. Green Fabrication of Supported Platinum Nanoparticles by Supercritical CO2 Deposition. Materials 2018, 11, 2587. https://doi.org/10.3390/ma11122587
Chen Y-L, Tsai C-H, Chen M-Y, Lai Y-C. Green Fabrication of Supported Platinum Nanoparticles by Supercritical CO2 Deposition. Materials. 2018; 11(12):2587. https://doi.org/10.3390/ma11122587
Chicago/Turabian StyleChen, Ying-Liang, Cheng-Hsien Tsai, Mei-Yin Chen, and Yi-Chieh Lai. 2018. "Green Fabrication of Supported Platinum Nanoparticles by Supercritical CO2 Deposition" Materials 11, no. 12: 2587. https://doi.org/10.3390/ma11122587
APA StyleChen, Y.-L., Tsai, C.-H., Chen, M.-Y., & Lai, Y.-C. (2018). Green Fabrication of Supported Platinum Nanoparticles by Supercritical CO2 Deposition. Materials, 11(12), 2587. https://doi.org/10.3390/ma11122587