Copper-Based Volumetric Filler Dedicated for Ag Paste for Depositing the Front Electrodes by Printing on Solar Si Cells
Abstract
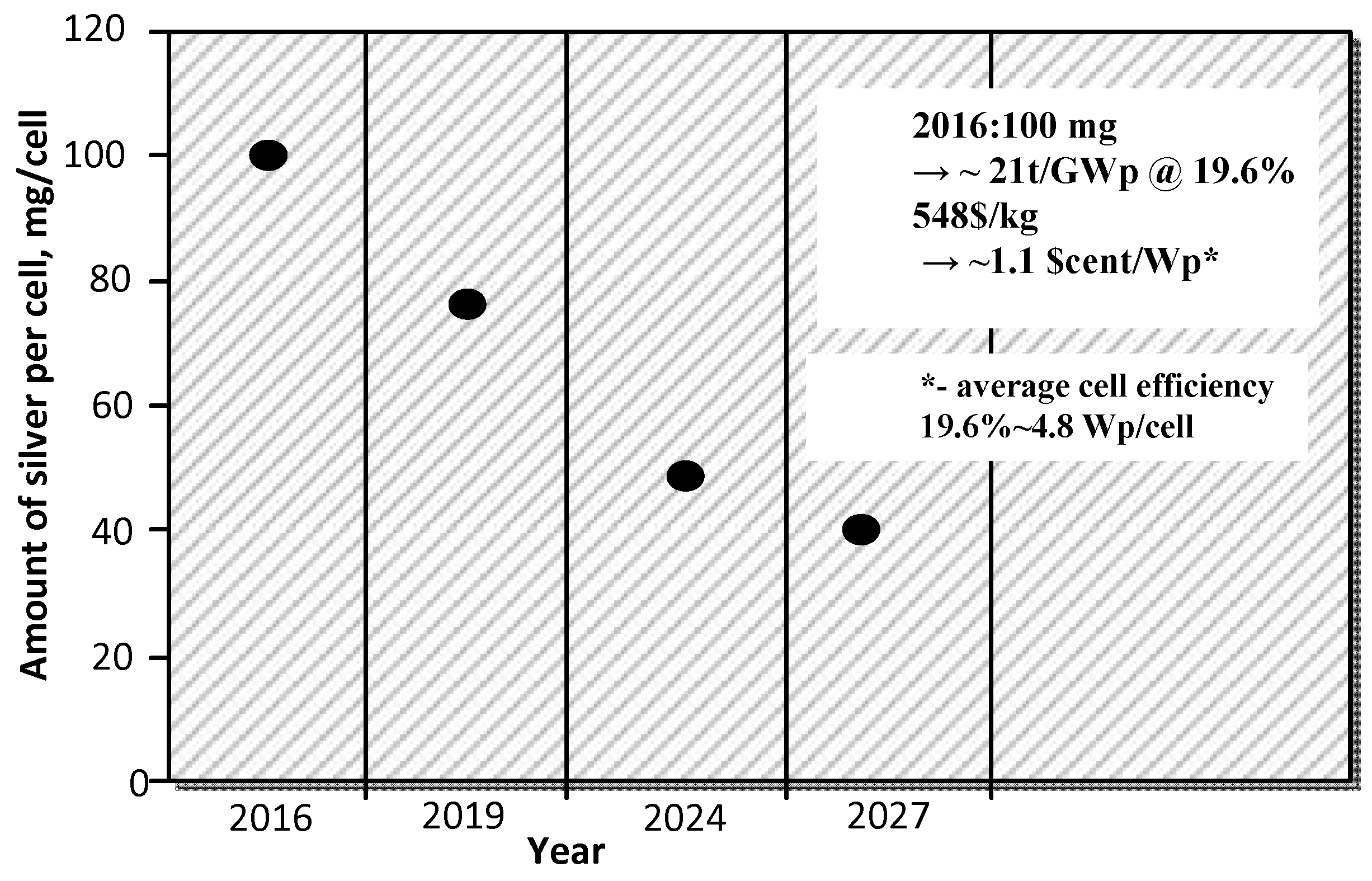
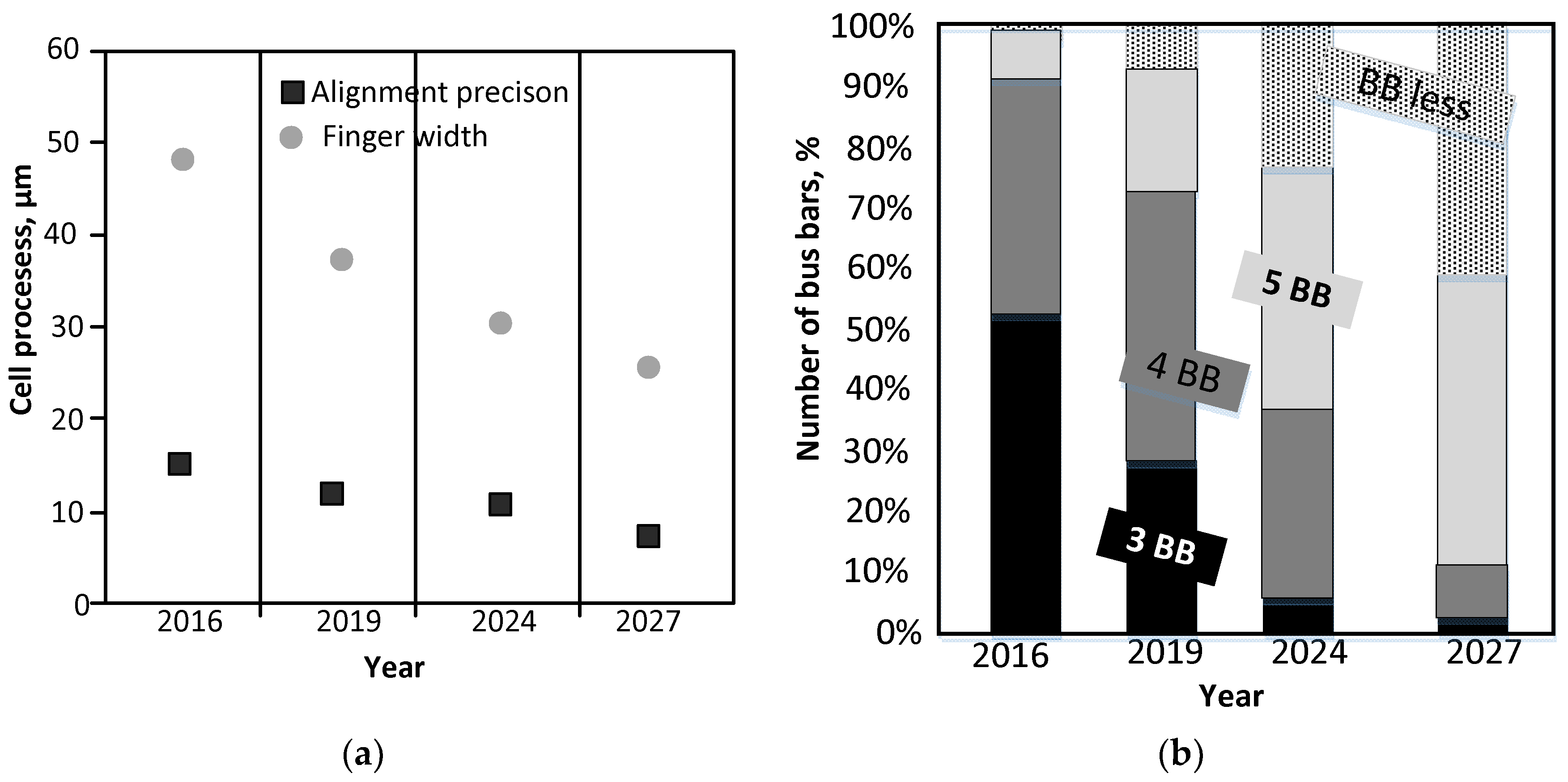
:1. Introduction
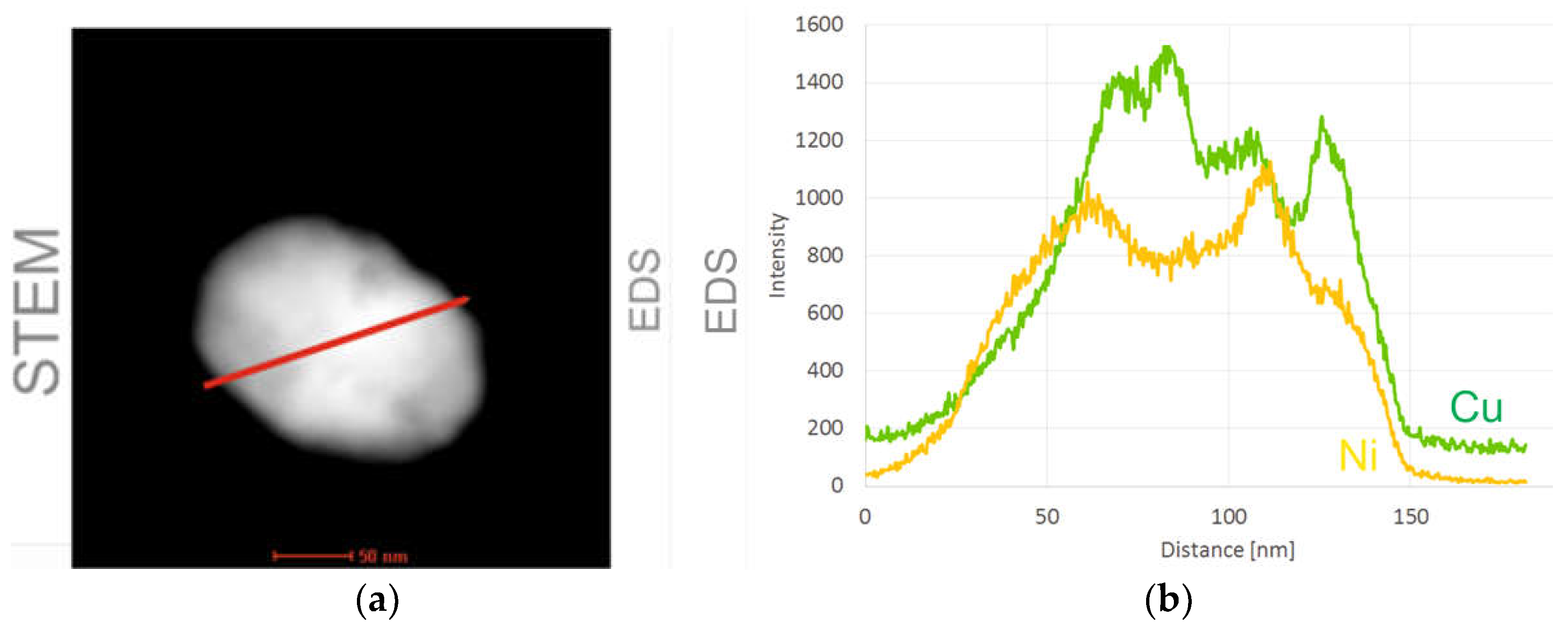
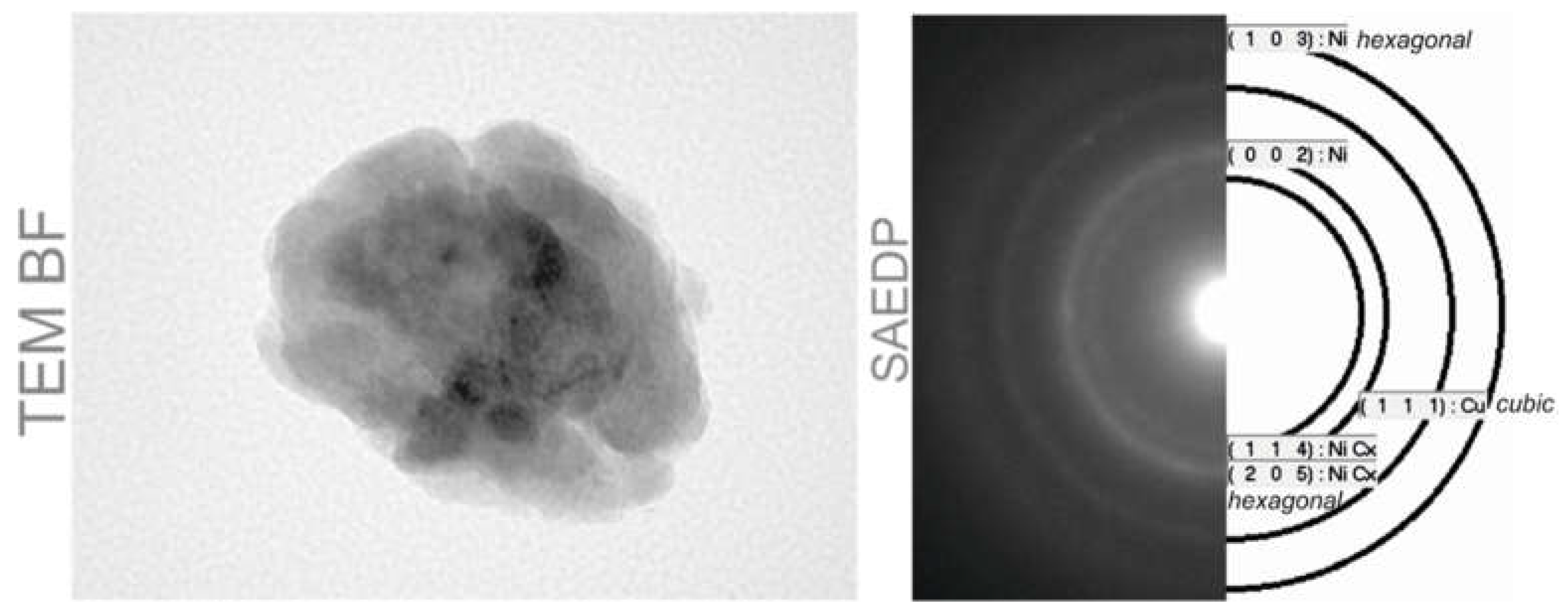
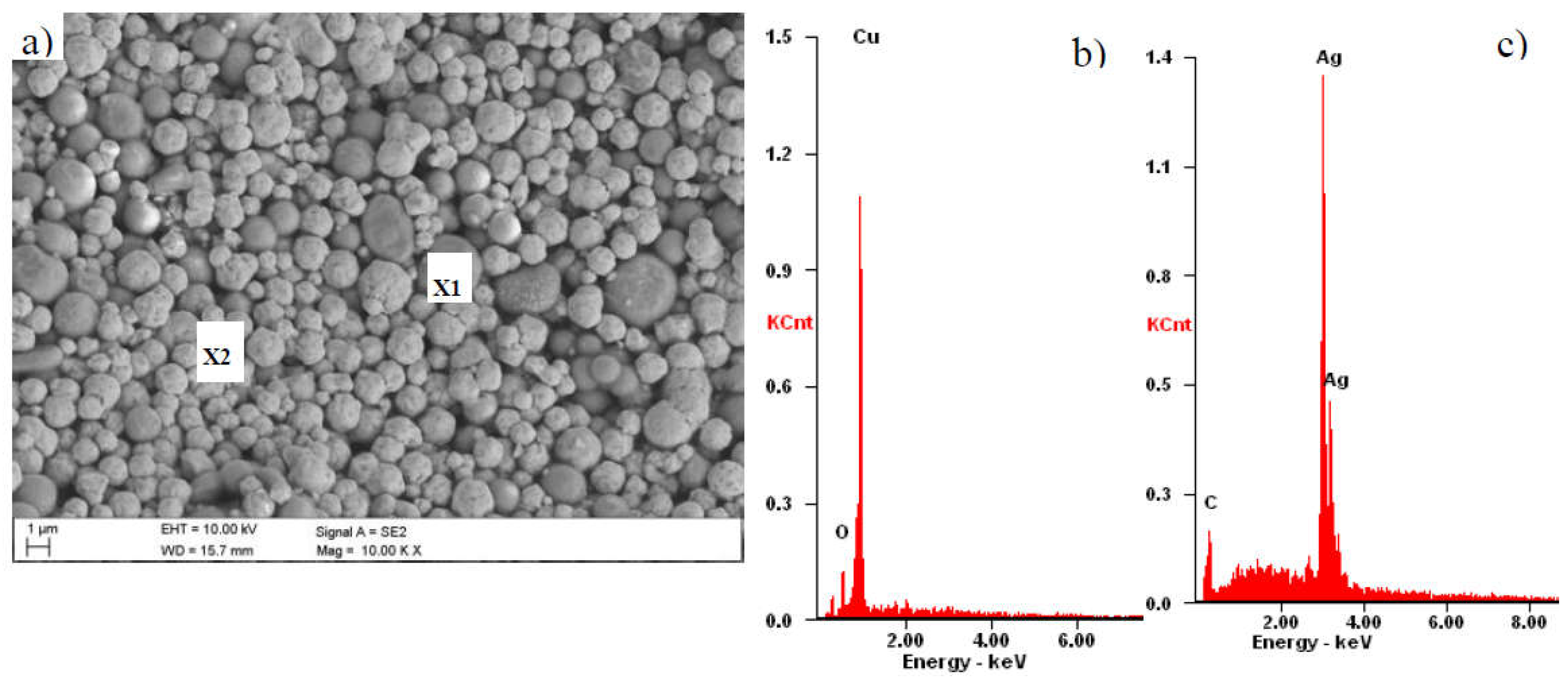
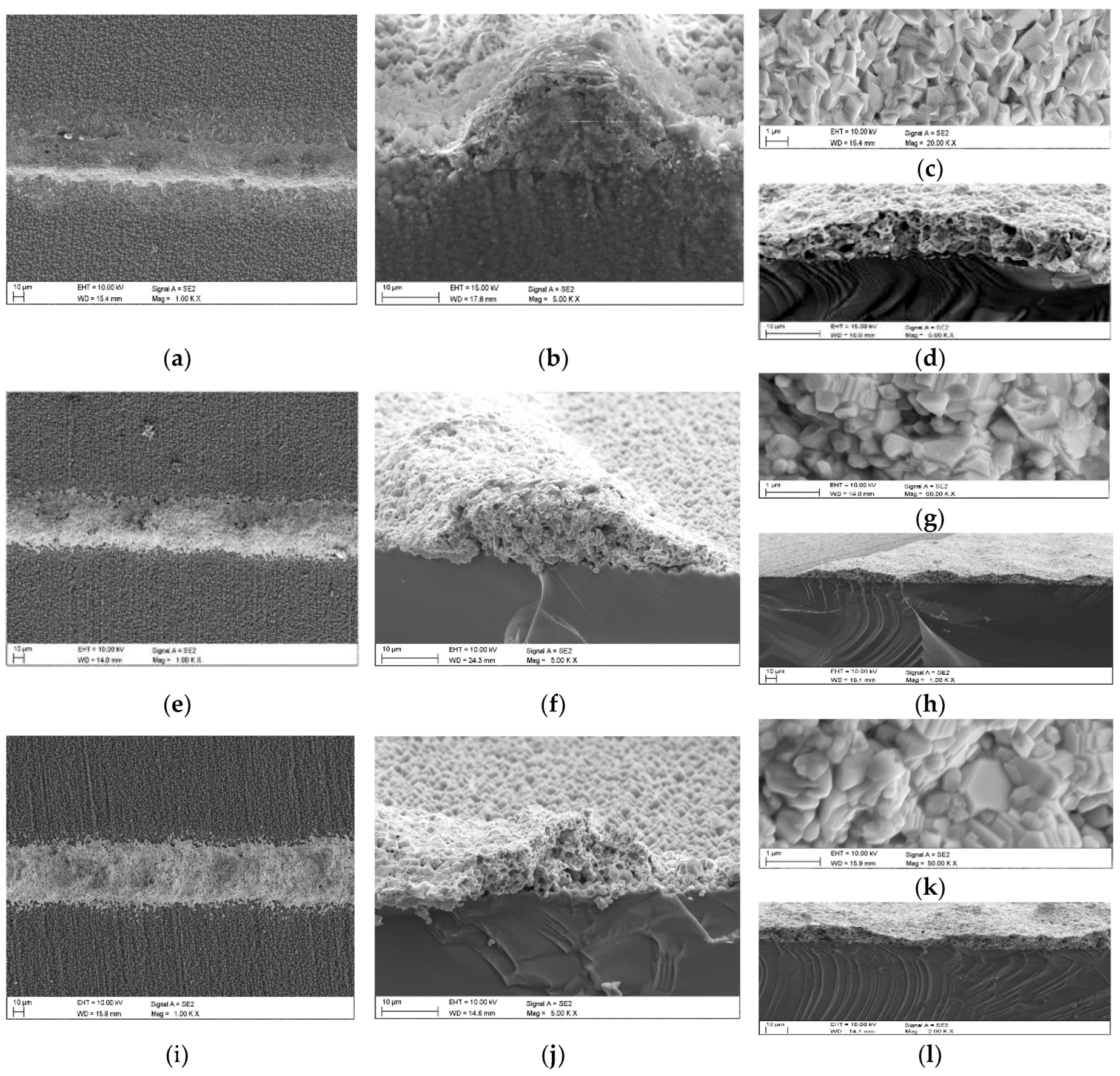
2. Fabrication Process of the CuXX Component and Paste
3. Material and Experiment
- p-type mono Cz-Si material,
- front side featured a POCl3 based n+ emitter and an 80 nm SiNx thick coating,
- rear side featured laser patterned AlOx/SiNx dielectric and a local area Al-BSF including Ag soldering pads (only PERC concept),
- quantity of 110 cells was prepared: 10 reference cells (standard front Ag PV20A paste), 50 cells for metallization with NPCuXX paste A (including 25 cells for firing optimization), 50 cells for metallization with NPCuXX paste B (including 25 cells for firing optimization).
4. Silicon Solar Cells Parameters
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Goodrich, A.; Hacke, P.; Wang, Q.; Sopori, B.; Margolis, R.; James, T.L.; Woodhouse, M. A wafer-based monocrystalline silicon photovoltaics road map: Utilizing known technology improvement opportunities for further reductions in manufacturing costs. Sol. Energy Mater. Sol. Cells 2013, 114, 110–135. [Google Scholar] [CrossRef]
- Green, M.A.; Hishikawa, Y.; Dunlop, E.D.; Levi, D.H.; Hohl-Ebinger, J.; Ho-Baillie, A.W.Y. Solar cell efficiency tables (version 51). Prog. Photovolt. Res. Appl. 2018, 26, 3–12. [Google Scholar] [CrossRef]
- Musztyfaga-Staszuk, M.; Dobrzanski, L.A.; Rusz, S.; Staszuk, M. Application examples for the different measurement modes of electrical properties of the solar cells. Arch. Metall. Mater. 2014, 59, 247–252. [Google Scholar] [CrossRef]
- Rudolph, D.; Olibet, S.; Hoornstra, J.; Weeber, A.; Cabrera, E.; Carr, A.; Koppes, M.; Kopecek, R. Replacement of silver in silicon solar cell metallization pastes containing a highly reactive glass frit: Is it possible? Energy Procedia 2013, 43, 44–53. [Google Scholar] [CrossRef]
- Metz, A.; Fischer, M.; Trube, J. International technology roadmap for photovoltaics (ITRPV) 8th edition: Crystalline silicon technology-current status and outlook. In Proceedings of the PV Manufacturing in Europe Conference, Brussels, Belgium, 18–19 May 2017. [Google Scholar]
- Available online: https://zloto.bullionvault.pl/wykres-cen-zlota.do (accessed on 20 September 2018).
- Ralph, E.L. Recent advancements in low cost solar cell processing. In Proceedings of the 11th Photovoltaic Specialists Conference, Scottsdale, AZ, USA, 6–8 May 1975. [Google Scholar]
- Berwind, J. PV manufacturing materials: Technological and process-related options for cost reduction. Photovolt. Int. 2012, 15, 36–49. [Google Scholar]
- Schuler, S.; Luck, I. Cell metallization by screen printing: Cost, limits and alternatives. Photovolt. Int. 2014, 23. [Google Scholar]
- Available online: pvinsight.com (accessed on 20 September 2018).
- Available online: https://pv-system.pl/blog/do-2025r-energia-sloneczna-bedzie-tansza-niz-z-wegla-lub-gazu/ (accessed on 22 September 2018).
- Socha, R.; Panek, P.; Putynkowaski, P.; Mordarski, G.; Balawander, P.; Musztyfag-Staszuk, M. A Method for Manufacturing Modified Electrically-Conductive Copper Particles and Modified Electrically-Conductive Copper Particles. Patent Application No. 18190412.9-1105, 13 November 2018. [Google Scholar]
- Caballero, L.J. Contact Definition in industrial silicon solar cells. Sol. Energy 2006, 375–398. [Google Scholar]
- Bhosale1, D.; Wagh, M.; Shinde, N. Review on front contact metallization paste using silver nano particles for performance improvement in polycrystalline silicon solar cell. Int. J. Sci. Eng. Appl. Sci. 2015, 1, 58–62. [Google Scholar]
- Dobrzański, L.A.; Musztyfaga, M. Effect of the front electrode metallisation process on electrical parameters of a silicon solar cell. J. Achiev. Mater. Manuf. Eng. 2011, 48, 115–144. [Google Scholar]
- Hong, K.K. Mechanism for the formation of Ag crystallites in the Ag thick-film contacts of crystalline Si solar cells. Sol. Energy Mater. Sol. Cells 2009, 93, 898–904. [Google Scholar] [CrossRef]
- Lin, C.H.; Tsai, S.Y.; Hsu, S.P. Investigation of Ag-bulk/glassy-phase/Si heterostructures of printed Ag contacts on crystalline Si solar cells. Sol. Energy Mater. Sol. Cells 2008, 92, 1011–1015. [Google Scholar] [CrossRef]
- Zhou, J.; Xu, N.; Yang, H.; Zhang, Q. Effect of Ag powder and glass frit in Ag paste on front contact of silicon solar cells. Procedia Eng. 2014, 94, 1–5. [Google Scholar] [CrossRef]
- Hoenig, R. Impact of screen printing silver paste components on the space charge region recombination losses of industrial silicon solar cells. Sol. Energy Mater. Sol. Cells 2012, 106, 7–10. [Google Scholar] [CrossRef]
- Panek, P.; Socha, R.; Putynkowski, G.; Slaoui, A. The new copper composite of pastes for Si solar cells front electrode application. Energy Procedia 2016, 92, 962–970. [Google Scholar] [CrossRef]
- Musztyfaga-Staszuk, M. SLS: One of the Modern Technologies of Laser Surface Treatment. Int. J. Thermophys. 2017, 38, 130. [Google Scholar] [CrossRef]
- Chiu, Y.S.; Cheng, C.L.; Whang, T.J.; Chen, C.C. Development of screen-printed texture-barrier paste for single-side texturization of interdigitated back-contact silicon solar cell applications. Materials 2013, 6, 4565–4573. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Hsu, S.P.; Hsu, W.C. Silicon solar cells: Structural properties of Ag-contacts/Si-substrate. In Solar Cells—Silicon Wafer-Based Technologies; IntechOpen: London, UK, 2011. [Google Scholar]
- Shin, D.Y.; Seo, J.Y.; Tak, H.; Byun, D. Bimodally dispersed silver paste for the metallization of a crystalline silicon solar cell using electrohydrodynamic jet printing. Sol. Energy Mater. Sol. Cells 2015, 136, 148–156. [Google Scholar] [CrossRef]
- Musztyfaga-Staszuk, M.; Woźny, K.; Putynkowski, G.; Zięba, P.; Panek, P.; Marynowski, P. The properties of the developed technology for production of copper component and paste used in the production process of electrical contacts of silicon cells. In Proceedings of the 26th International Conference on Metallurgy and Materials, Brno, Czech Republic, 24–26 May 2017; pp. 1983–1989. [Google Scholar]
- Musztyfaga-Staszuk, M.; Major, Ł.; Putynkowski, G.; Sypień, A.; Gawlińska, K.; Panek, P.; Zięba, P. New kind of Cu based paste for Si solar cells front contact formation. Mater. Sci. Pol. 2018. [Google Scholar] [CrossRef]


| Components | wt.% | Literature |
|---|---|---|
| Silver powder | 70–85 | [14,15,16] |
| Glass frits (composition); PbO—86%, B2O3—11%, SiO2—3% PbO, SiO2, B2O3, Al2O3, CaO, ZnO; PbO, ZnO, B2O3, Bi2O3; PbO, SiO2, ZnO, Al2O3, B2O5; SiO2 | 0–5 3 - - - 2 | [16,17,18,19,20,21] |
| Cellulosic resin: ethyl cellulose ethoce | 3–15 | [22] |
| Solvent: solvent with small molecular weight solvent | 3–15 - - | [14,23] |
| Additives (rheological modifiers and surfactants): ethyl cellulose+ terpineol; ethyl cellulose * | 0–2 16 - | [14,24] |
| Technical Parameter | Value and Size of the Parameter |
|---|---|
| The content of CuXX particles | >50% by weight |
| Addition: silver paste | yes |
| Application technique | screen printing, pattern |
| The force of adhesion of the soldered connection strip to a 2 mm wide path | >1 N |
| Possible path height and width | 20–25 μm, 40–80 μm |
| It is possible to create a cell with specific serial resistance | <1 Ω·cm2 |
| Resistivity | <4 mΩ·cm |
| Viscosity of the paste | 260–420 Pa·s |
| The possibility of getting a contact to the type emitter | 50–100 Ω/square |
| Additives | glazes, oxides, organic thinners, other * |
| Guarantee period | 6 months |
| Storage temperature | 5–25 °C |
| Deposition and print temperature | 15–25 °C |
| Thixotropy of the paste | the paste should be mixed in the rolling process at speeds below 30 rpm and during 12–48 h with temperature 5–35 °C |
| Co-firing temperature | over 700 °C |
| Cell Number | Isc (A) | Voc (V) | Im (A) | Vm (V) | Pm (W) | FF (%) | Eff (%) | Rsh (Ω) | Rs (Ω) | |
|---|---|---|---|---|---|---|---|---|---|---|
| PV20A paste | ||||||||||
| REF solar cells | REF-1 | 9.360 | 0.641 | 8.745 | 0.545 | 4.767 | 79.384 | 19.537 | 29.16 | 0.0025 |
| REF-2 | 9.397 | 0.643 | 8.786 | 0.547 | 4.808 | 79.541 | 19.705 | 50.40 | 0.0025 | |
| REF-3 | 9.406 | 0.643 | 8.776 | 0.548 | 4.809 | 79.469 | 19.712 | 35.46 | 0.0025 | |
| NPCuXX-paste A | ||||||||||
| Solar cells from A | A-1 | 9.233 | 0.632 | 8.554 | 0.530 | 4.534 | 77.690 | 18.582 | 30.54 | 0.0037 |
| A-2 | 9.256 | 0.631 | 8.636 | 0.527 | 4.557 | 77.918 | 18.679 | 34.91 | 0.0038 | |
| A-3 | 9.278 | 0.632 | 8.648 | 0.524 | 4.534 | 77.267 | 18.585 | 42.83 | 0.0043 | |
| A-4 | 9.256 | 0.632 | 8.551 | 0.531 | 4.543 | 77.557 | 18.621 | 34.39 | 0.0039 | |
| Cell Number on the ECN List | Isc (A) | Voc (V) | Im (A) | Vm (V) | Pm (W) | FF (%) | Eff (%) | Rsh (Ω) | Rs (Ω) | |
|---|---|---|---|---|---|---|---|---|---|---|
| PV20A paste | ||||||||||
| REF solar cells | REF-4 (940 °C) | 9.579 | 0.655 | 8.953 | 0.548 | 4.912 | 78.266 | 20.132 | 102.47 | 0.0037 |
| REF-5 (930 °C) | 9.560 | 0.658 | 8.898 | 0.552 | 4.917 | 78.151 | 20.153 | 129.68 | 0.0038 | |
| NPCuXX-A paste | ||||||||||
| Solar cells from A | A-5 (930 °C) | 9.484 | 0.645 | 8.813 | 0.539 | 4.756 | 77.697 | 19.491 | 36.04 | 0.0039 |
| A-6 (930 °C) | 9.474 | 0.645 | 8.807 | 0.538 | 4.744 | 77.517 | 19.443 | 68.59 | 0.0042 | |
| Investigated Paste | Cell Number | J01 (A/cm2) | J02 (A/cm2) | τ (μs) |
|---|---|---|---|---|
| Solar cell Al-BSF type | ||||
| PV20A | REF-1 | 4.62 × 10−13 | 1.91 × 10−9 | 101.94 |
| REF-2 | 6.18 × 10−13 | 4.84 × 10−9 | 69.72 | |
| REF-3 | 5.99 × 10−13 | 4.56 × 10−9 | 73.01 | |
| NPCuXX-A | A-1 | 5.82 × 10−13 | 3.86 × 10−9 | 76.88 |
| A-2 | 8.46 × 10−13 | 6.61 × 10−9 | 51.76 | |
| A-3 | 5.73 × 10−13 | 4.93 × 10−9 | 75.33 | |
| A-4 | 8.43 × 10−13 | 3.50 × 10−9 | 55.22 | |
| Solar cell PERC type | ||||
| PV20A | REF-4 | 8.19 × 10−13 | 5.17 × 10−9 | 55.29 |
| REF-5 | 7.40 × 10−13 | 2.20 × 10−9 | 66.40 | |
| NPCuXX-A | A-5 | 3.58 × 10−13 | 2.95 × 10−9 | 126.99 |
| A-6 | 5.47 × 10−13 | 2.73 × 10−9 | 84.19 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musztyfaga-Staszuk, M.; Putynkowski, G.; Socha, R.; Stodolny, M.; Panek, P. Copper-Based Volumetric Filler Dedicated for Ag Paste for Depositing the Front Electrodes by Printing on Solar Si Cells. Materials 2018, 11, 2493. https://doi.org/10.3390/ma11122493
Musztyfaga-Staszuk M, Putynkowski G, Socha R, Stodolny M, Panek P. Copper-Based Volumetric Filler Dedicated for Ag Paste for Depositing the Front Electrodes by Printing on Solar Si Cells. Materials. 2018; 11(12):2493. https://doi.org/10.3390/ma11122493
Chicago/Turabian StyleMusztyfaga-Staszuk, Małgorzata, Grzegorz Putynkowski, Robert Socha, Maciej Stodolny, and Piotr Panek. 2018. "Copper-Based Volumetric Filler Dedicated for Ag Paste for Depositing the Front Electrodes by Printing on Solar Si Cells" Materials 11, no. 12: 2493. https://doi.org/10.3390/ma11122493
APA StyleMusztyfaga-Staszuk, M., Putynkowski, G., Socha, R., Stodolny, M., & Panek, P. (2018). Copper-Based Volumetric Filler Dedicated for Ag Paste for Depositing the Front Electrodes by Printing on Solar Si Cells. Materials, 11(12), 2493. https://doi.org/10.3390/ma11122493







