Glassy Carbon Electrocorticography Electrodes on Ultra-Thin and Finger-Like Polyimide Substrate: Performance Evaluation Based on Different Electrode Diameters
Abstract
1. Introduction
2. Materials and Methods
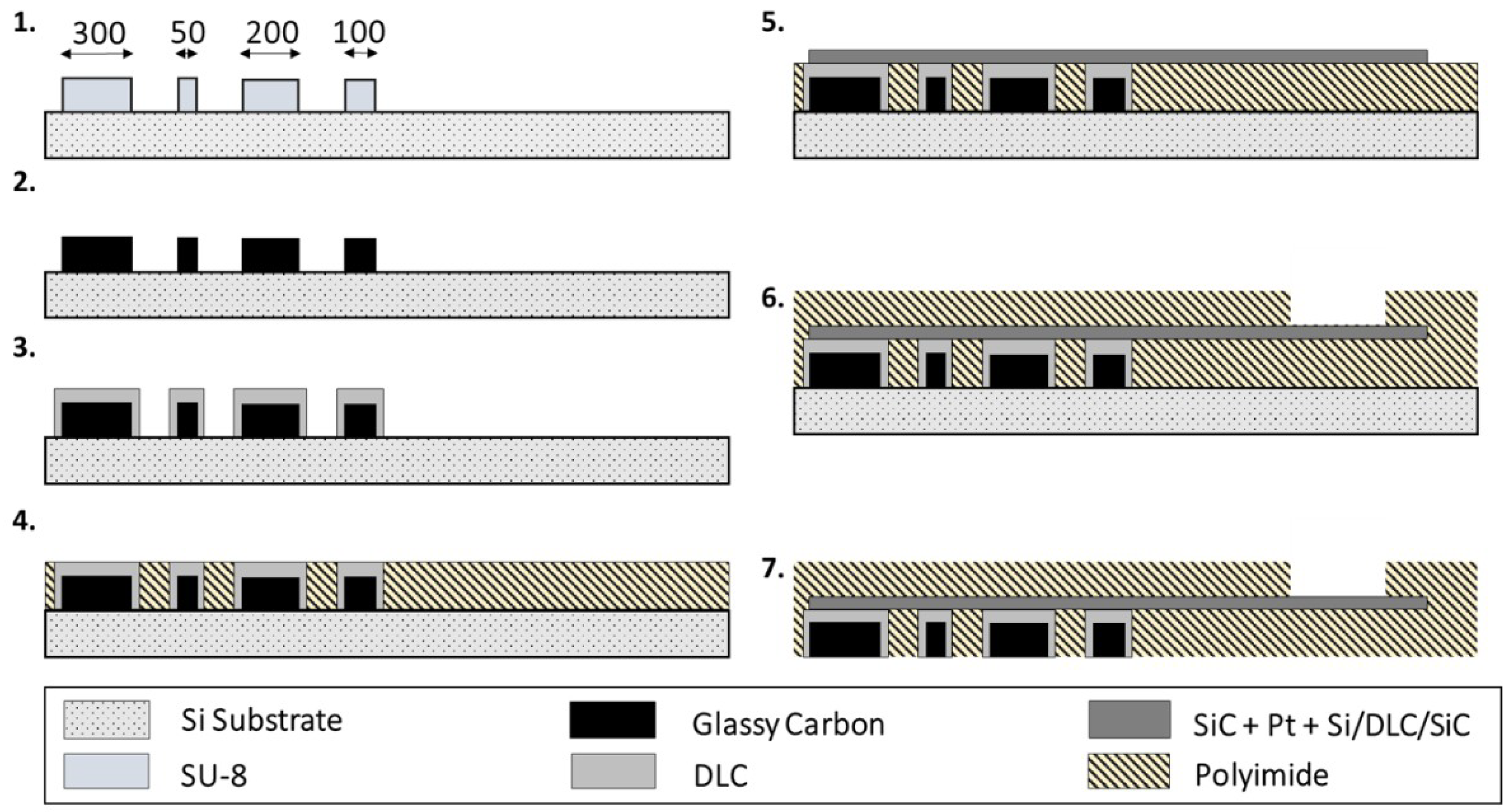
2.1. Fabrication of the ECoG Arrays and PEDOT:PSS Deposition
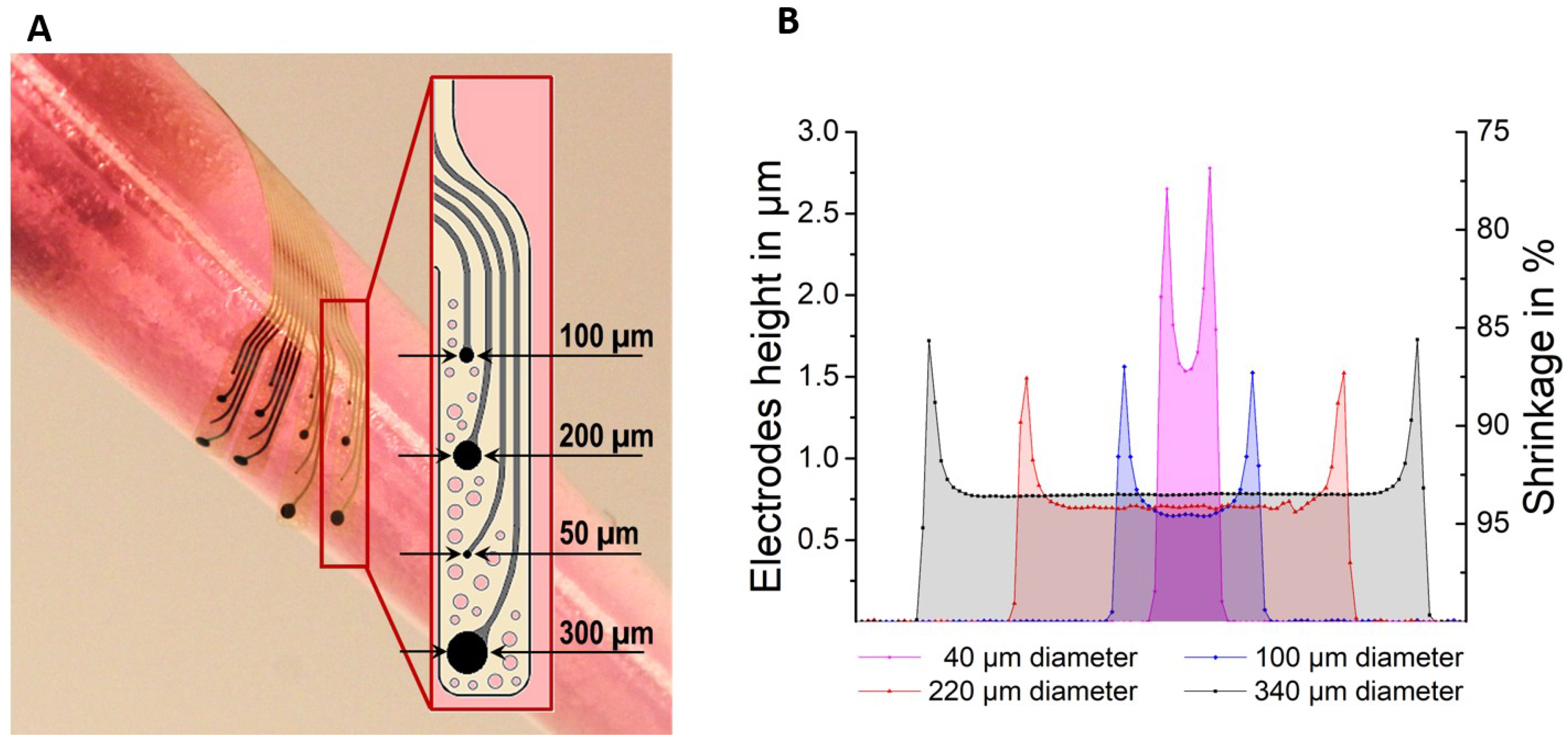
2.2. Shrinkage Measurements
2.3. Electrochemical Characterization
2.4. Biphasic Pulse Stimulation
2.5. X-Ray Photoelectron Spectroscopy (XPS)
2.6. Animals and Surgical Procedure
2.7. Somatosensory Evoked Potentials Recording
2.8. In Vivo Data Analysis
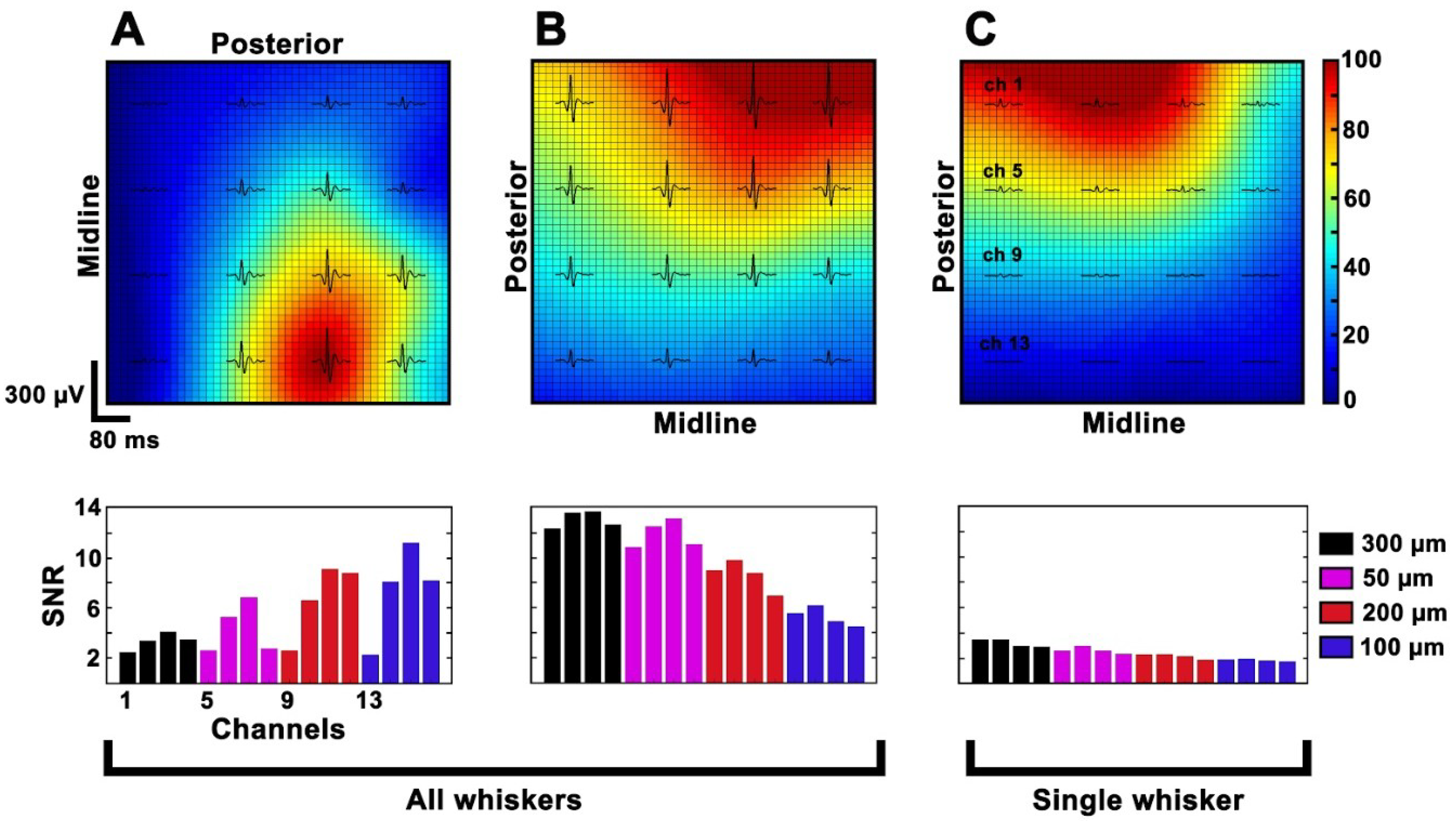
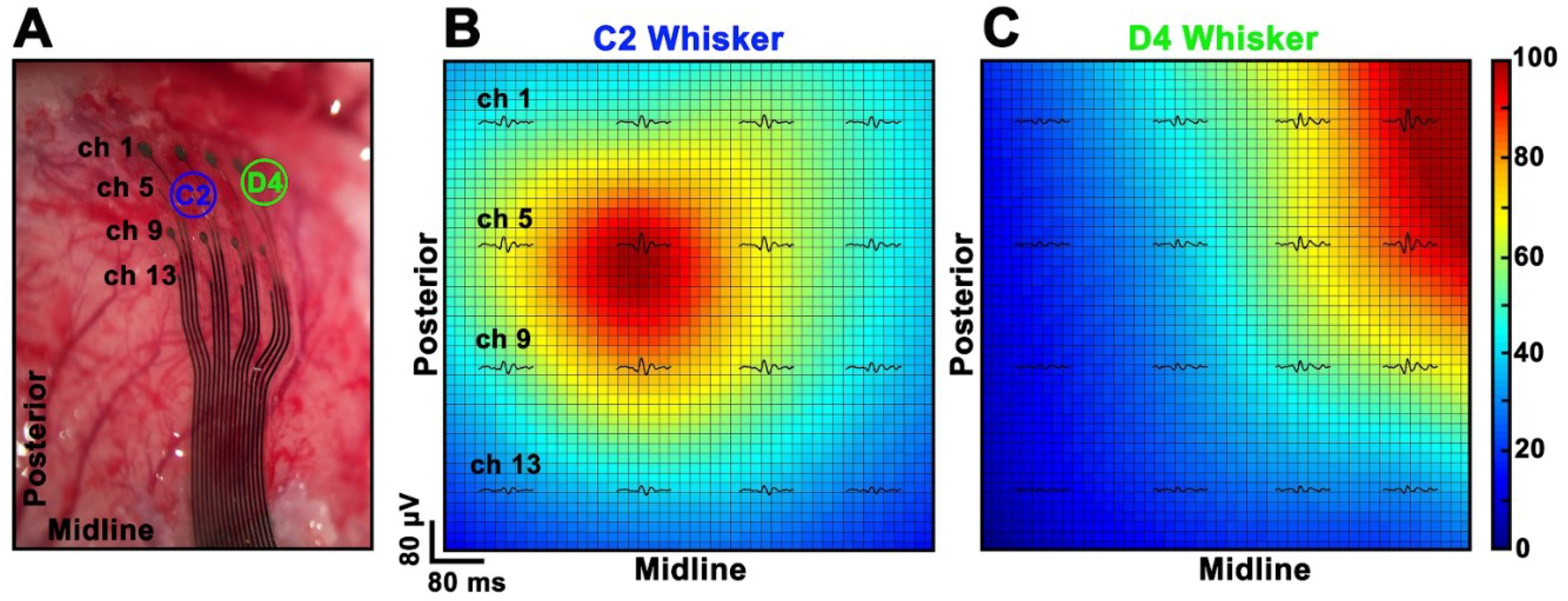
2.8.1. Estimation of HFCs’ Spatial Distribution
2.8.2. Signal-to-Noise Ratio
3. Results
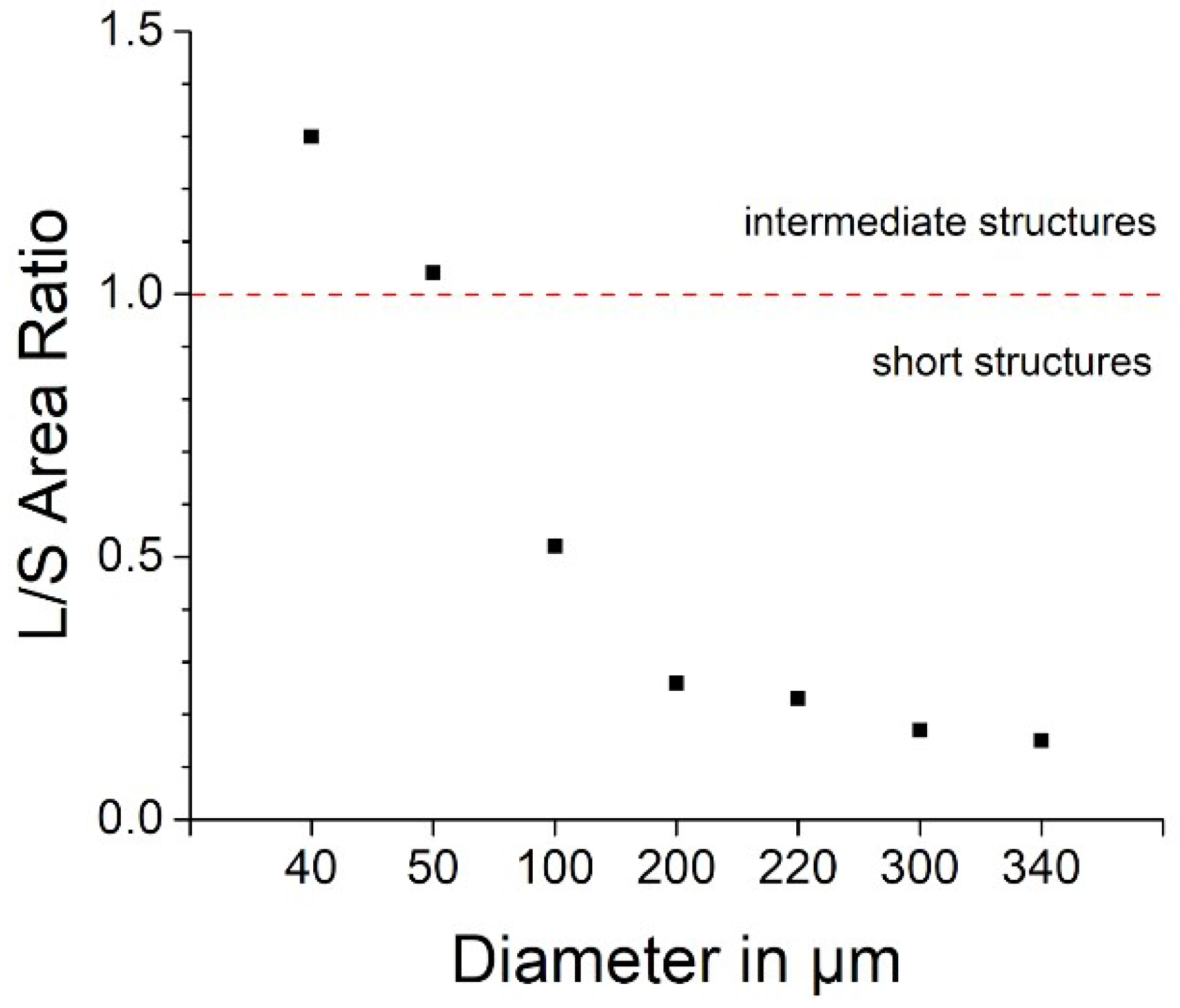
3.1. Fabrication of Devices and Pyrolysis-Related Shrinkage of the GC Microelectrodes
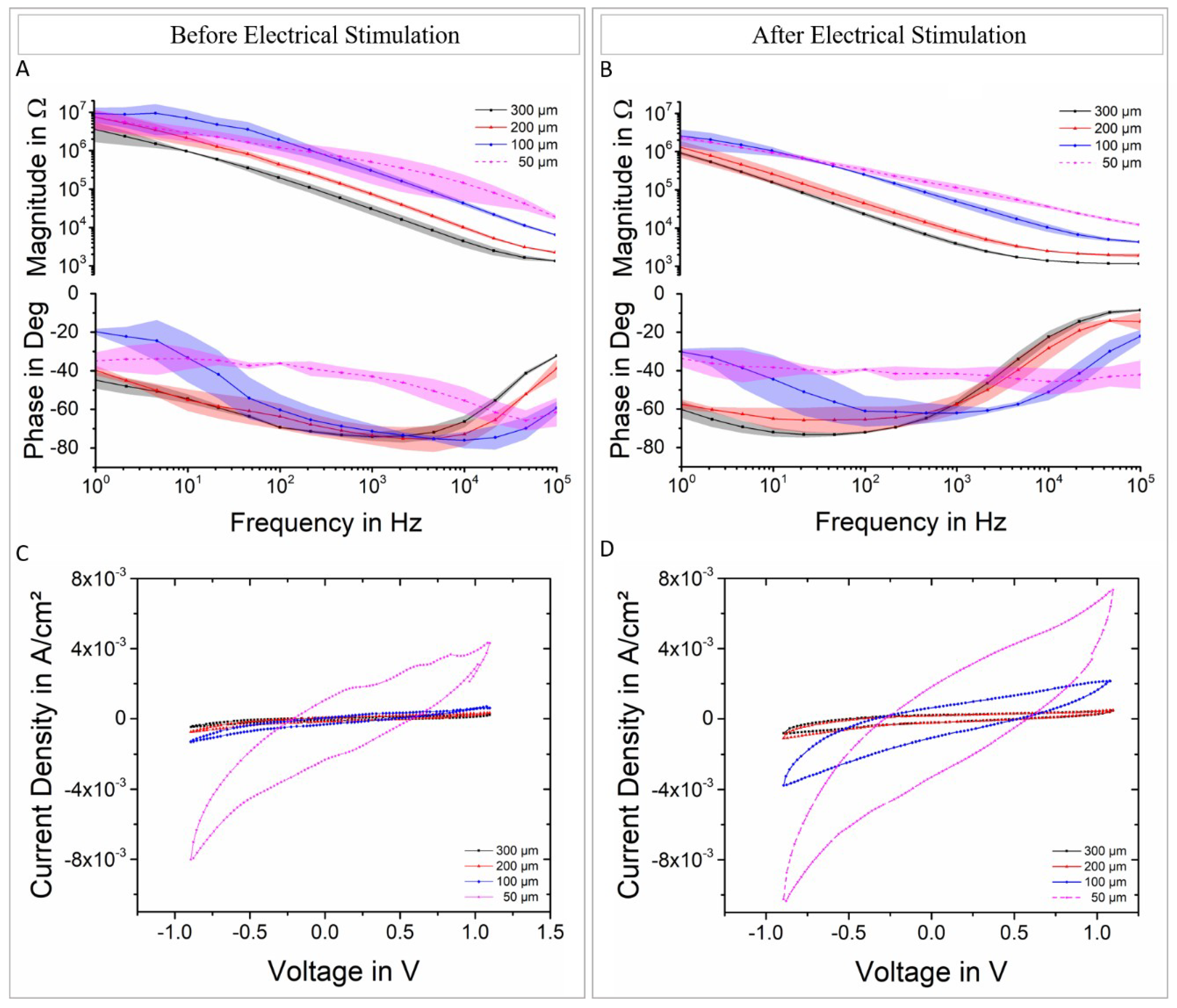
3.2. Electrochemical Characterization of the Devices (EIS, CV)
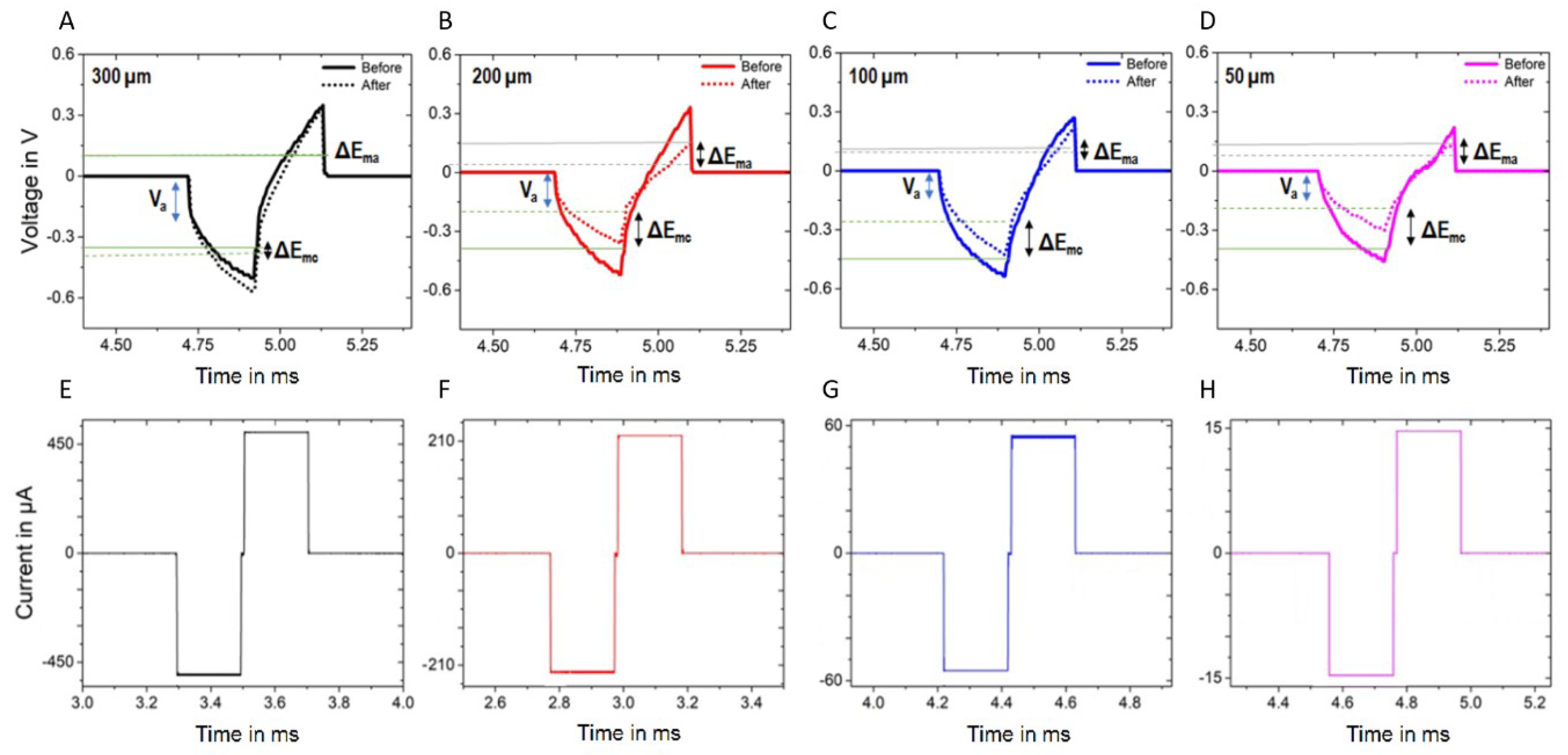
3.3. Electrical Stimulation and Voltage Transient
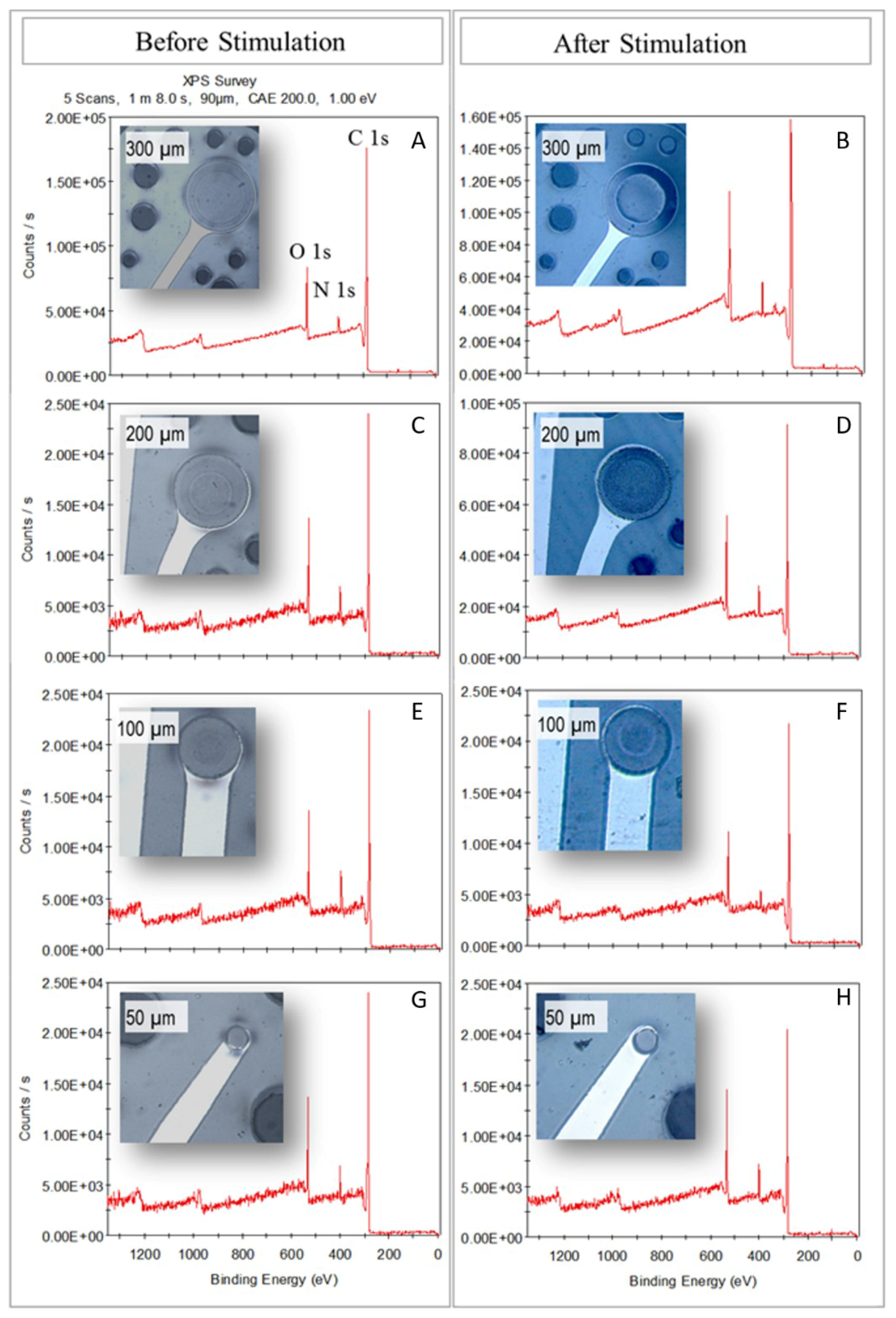
3.4. X-Ray Photoelectron Spectroscopy (XPS)
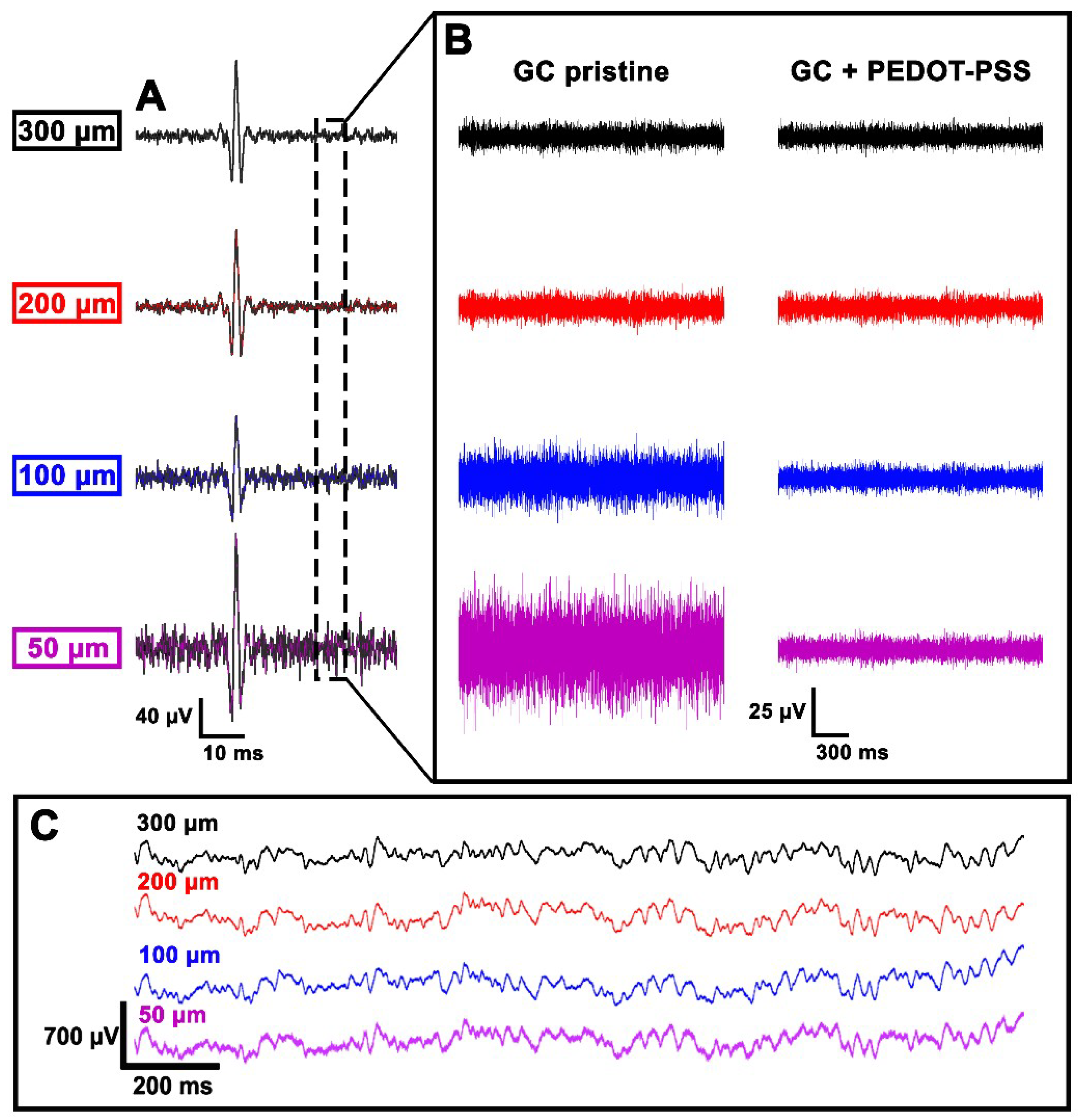
3.5. In Vivo Experiments
4. Discussion
5. Conclusion
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Mushahwar, V.K.; Collins, D.F.; Prochazka, A. Spinal cord microstimulation generates functional limb movements in chronically implanted cats. Exp. Neurol. 2000, 163, 422–429. [Google Scholar] [CrossRef]
- Hassler, C.; Boretius, T.; Stieglitz, T. Polymers for neural implants. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 18–33. [Google Scholar] [CrossRef]
- Schendel, A.A.; Nonte, M.W.; Vokoun, C.; Richner, T.J.; Brodnick, S.K.; Atry, F.; Frye, S.; Bostrom, P.; Pashaie, R.; Thongpang, S.; et al. The effect of micro-ECoG substrate footprint on the meningeal tissue response. J. Neural. Eng. 2014, 11, 046011. [Google Scholar] [CrossRef]
- Vomero, M.; Dryg, I.; Maxfield, T.; Shain, W.; Perlmutter, S.; Kassegne, S. In-vivo characterization of glassy carbon µ-electrodes and histological analysis of brain tissue after chronic implants. ECS Trans. 2016, 72, 91–96. [Google Scholar] [CrossRef]
- Henle, C.; Raab, M.; Cordeiro, J.G.; Doostkam, S.; Schulze-Bonhage, A.; Stieglitz, T.; Rickert, J. First long term in vivo study on subdurally implanted micro-ECoG electrodes, manufactured with a novel laser technology. Biomed. Microdevices 2011, 13, 59–68. [Google Scholar] [CrossRef]
- Kim, Y.T.; Bridge, M.J.; Tresco, P.A. The influence of the foreign body response evoked by fibroblast transplantation on soluble factor diffusion in surrounding brain tissue. J. Controll. Release 2007, 118, 340–347. [Google Scholar] [CrossRef]
- Seymour, J.P.; Kipke, D.R. Neural probe design for reduced tissue encapsulation in CNS. Biomaterials 2007, 28, 3594–3607. [Google Scholar] [CrossRef]
- Castagnola, E.; Maiolo, L.; Maggiolini, E.; Minotti, A.; Marrani, M.; Maita, F.; Pecora, A.; Angotzi, G.N.; Ansaldo, A.; Boffini, M.; et al. PEDOT-CNT-coated low-impedance, ultra-flexible, and brain-conformable micro-ECoG arrays. IEEE Trans. Neural Syst. Rehabil. Eng. 2015, 23, 342–350. [Google Scholar] [CrossRef]
- Khodagholy, D.; Gelinas, J.N.; Thesen, T.; Doyle, W.; Devinsky, O.; Malliaras, G.G.; Buzsáki, G. NeuroGrid: recording action potentials from the surface of the brain. Nat. Neurosci. 2015, 18, 310. [Google Scholar] [CrossRef]
- Minev, I.R.; Musienko, P.; Hirsch, A.; Barraud, Q.; Wenger, N.; Moraud, E.M.; Gandar, J.; Capogrosso, M.; Milekovic, T.; Asboth, L.; et al. Electronic dura mater for long-term multimodal neural interfaces. Science 2015, 347, 159–163. [Google Scholar] [CrossRef]
- Kassegne, S.; Vomero, M.; Gavuglio, R.; Hirabayashi, M.; Özyilmaz, E.; Nguyen, S.; Rodriguez, J.; Özyilmaz, E.; van Niekerk, P.; Khosla, A. Electrical impedance, electrochemistry, mechanical stiffness, and hardness tunability in glassy carbon MEMS μECoG electrodes. Microelectron. Eng. 2015, 133, 36–44. [Google Scholar] [CrossRef]
- Vomero, M.; Van Niekerk, P.; Nguyen, V.; Gong, N.; Hirabayashi, M.; Cinopri, A.; Logan, K.; Moghadasi, A.; Varma, P.; Kassegne, S. A novel pattern transfer technique for mounting glassy carbon microelectrodes on polymeric flexible substrates. J. Micromech. Microeng. 2016, 26, 025018. [Google Scholar] [CrossRef]
- Goshi, N.; Castagnola, E.; Vomero, M.; Gueli, C.; Cea, C.; Zucchini, E.; Bjanes, D.; Maggiolini, E.; Moritz, C.; Kassegne, S.; et al. Glassy carbon MEMS for novel origami-styled 3D integrated intracortical and epicortical neural probes. J. Micromech. Microeng. 2018, 28, 0960–1317. [Google Scholar] [CrossRef]
- Vomero, M.; Castagnola, E.; Ciarpella, F.; Maggiolini, E.; Goshi, N.; Zucchini, E.; Carli, S.; Fadiga, L.; Kassegne, S.; Ricci, D. Highly stable glassy carbon interfaces for long-term neural stimulation and low-noise recording of brain activity. Sci. Rep. 2017, 7, 40332. [Google Scholar] [CrossRef]
- Vomero, M.; Castagnola, E.; Ordonez, J.S.; Carli, S.; Zucchini, E.; Maggiolini, E.; Gueli, C.; Goshi, N.; Ciarpella, F.; Cea, C.; et al. Incorporation of silicon carbide and diamond-like carbon as adhesion promoters improves in vitro and in vivo stability of thin-film glassy carbon electrocorticography arrays. Adv. Biosyst. 2018, 2, 1700081. [Google Scholar] [CrossRef]
- Vomero, M.; Castagnola, E.; Ordonez, J.S.; Carli, S.; Zucchini, E.; Maggiolini, E.; Gueli, C.; Goshi, N.; Fadiga, L.; Ricci, D.; et al. Improved long-term stability of thin-film glassy carbon electrodes through the use of silicon carbide and amorphous carbon. In Proceedings of the 8th International IEEE/EMBS Conference on Neural Engineering (NER), Shanghai, China, 25–28 May 2017; pp. 288–291. [Google Scholar]
- Shigemitsu, T.; Matsumoto, G.; Tsukahara, S. Electrical properties of glassy-carbon electrodes. Med. Biol. Eng. Comput. 1979, 17, 465–470. [Google Scholar] [CrossRef]
- Van der Linden, W.E.; Dieker, J.W. Glassy carbon as electrode material in electro-analytical chemistry. Anal. Chim. Acta 1980, 119, 1–24. [Google Scholar] [CrossRef]
- Nimbalkar, S.; Castagnola, E.; Balasubramani, A.; Scarpellini, A.; Samejima, S.; Khorasani, A.; Boissenin, A.; Thongpang, S.; Moritz, C.; Kassegne, S. Ultra-capacitive carbon neural probe allows simultaneous long-term electrical stimulations and high-resolution neurotransmitter detection. Sci. Rep. 2018, 8, 6958. [Google Scholar] [CrossRef]
- Natu, R.; Islam, M.; Gilmore, J.; Martinez-Duarte, R. Shrinkage of SU-8 microstructures during carbonization. J. Anal. Appl. Pyrolysis 2018, 131, 17–27. [Google Scholar] [CrossRef]
- Singh, A.; Jayaram, J.; Madou, M.; Akbar, S. Pyrolysis of negative photoresists to fabricate carbon structures for microelectromechanical systems and electrochemical applications. J. Electrochem. Soc. 2002, 149, E78–E83. [Google Scholar] [CrossRef]
- Park, B.Y.; Taherabadi, L.; Wang, C.; Zoval, J.; Madou, M.J. Electrical properties and shrinkage of carbonized photoresist films and the implications for carbon microelectromechanical systems devices in conductive media. J. Electrochem. Soc. 2005, 152, J136–J143. [Google Scholar] [CrossRef]
- Ranganathan, S.; Mccreery, R.; Majji, S.M.; Madou, M. Photoresist-derived carbon for microelectromechanical systems and electrochemical applications. J. Electrochem. Soc. 2000, 147, 277–282. [Google Scholar] [CrossRef]
- Martinez-Duarte, R. SU-8 photolithography as a toolbox for carbon MEMS. Micromachines 2014, 5, 766–782. [Google Scholar] [CrossRef]
- Parry, K.L.; Shard, A.G.; Short, R.D.; White, R.G.; Whittle, J.D.; Wright, A. ARXPS characterisation of plasma polymerised surface chemical gradients. Surf. Interface Anal. 2006, 38, 1497–1504. [Google Scholar] [CrossRef]
- Scofield, J.H. Hartree-Slater subshell photoionization cross-sections at 1254 and 1487 eV. J. Electron. Spectrosc. Relat. Phenom. 1976, 8, 129–137. [Google Scholar] [CrossRef]
- Tanuma, S.; Powell, C.J.; Penn, D.R. Calculations of electron inelastic mean free paths. V. Data for 14 organic compounds over the 50–2000 eV range. Surf. Interface Anal. 1994, 21, 165–176. [Google Scholar] [CrossRef]
- TDT System 3 Manual. Available online: https://www.tdt.com/files/manuals/TDTSys3_Manual.pdf (accessed on 26 October 2018).
- Vomero, M.; Porto Cruz, M.F.; Zucchini, E.; Shabanian, A.; Delfino, E.; Carli, S.; Fadiga, L.; Ricci, D.; Stieglitz, T. Achieving ultra-conformability with polyimide-based ECoG arrays. In Proceedings of the 40th International Engineering in Medicine and Biology Conference (EMBC), Honolulu, Hawaii, 17–21 July 2018. [Google Scholar]
- Nagaoka, T.; Yoshino, T. Surface properties of electrochemically pretreated glassy carbon. Anal. Chem. 1986, 58, 1037–1042. [Google Scholar] [CrossRef]
- Cogan, S.F. Neural stimulation and recording electrodes. Annu. Rev. Biomed. Eng. 2008, 10, 275–309. [Google Scholar] [CrossRef]
- Petersen, C.C.H. The functional organization of the barrel cortex. Neuron 2007, 56, 339–355. [Google Scholar] [CrossRef]
- Diamond, M.E.; Von Heimendahl, M.; Knutsen, P.M.; Kleinfeld, D.; Ahissar, E. ‘Where’ and ‘what’ in the whisker sensorimotor system. Nat. Rev. Neurosci. 2008, 9, 601–612. [Google Scholar] [CrossRef]
- Bockhorst, T.; Pieper, F.; Engler, G.; Stieglitz, T.; Galindo-Leon, E.; Engel, A.K. Synchrony surfacing: Epicortical recording of correlated action potentials. Eur. J. Neurosci. 2018, 48, 3583–3596. [Google Scholar] [CrossRef]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vomero, M.; Zucchini, E.; Delfino, E.; Gueli, C.; Mondragon, N.C.; Carli, S.; Fadiga, L.; Stieglitz, T. Glassy Carbon Electrocorticography Electrodes on Ultra-Thin and Finger-Like Polyimide Substrate: Performance Evaluation Based on Different Electrode Diameters. Materials 2018, 11, 2486. https://doi.org/10.3390/ma11122486
Vomero M, Zucchini E, Delfino E, Gueli C, Mondragon NC, Carli S, Fadiga L, Stieglitz T. Glassy Carbon Electrocorticography Electrodes on Ultra-Thin and Finger-Like Polyimide Substrate: Performance Evaluation Based on Different Electrode Diameters. Materials. 2018; 11(12):2486. https://doi.org/10.3390/ma11122486
Chicago/Turabian StyleVomero, Maria, Elena Zucchini, Emanuela Delfino, Calogero Gueli, Norma Carolina Mondragon, Stefano Carli, Luciano Fadiga, and Thomas Stieglitz. 2018. "Glassy Carbon Electrocorticography Electrodes on Ultra-Thin and Finger-Like Polyimide Substrate: Performance Evaluation Based on Different Electrode Diameters" Materials 11, no. 12: 2486. https://doi.org/10.3390/ma11122486
APA StyleVomero, M., Zucchini, E., Delfino, E., Gueli, C., Mondragon, N. C., Carli, S., Fadiga, L., & Stieglitz, T. (2018). Glassy Carbon Electrocorticography Electrodes on Ultra-Thin and Finger-Like Polyimide Substrate: Performance Evaluation Based on Different Electrode Diameters. Materials, 11(12), 2486. https://doi.org/10.3390/ma11122486






