Thermal Decomposition and Thermal Reaction Process of PTFE/Al/MnO2 Fluorinated Thermite
Abstract
1. Introduction
2. Experimental Section
2.1. Sample Preparation
2.2. Experimental Process
3. Results and Discussion
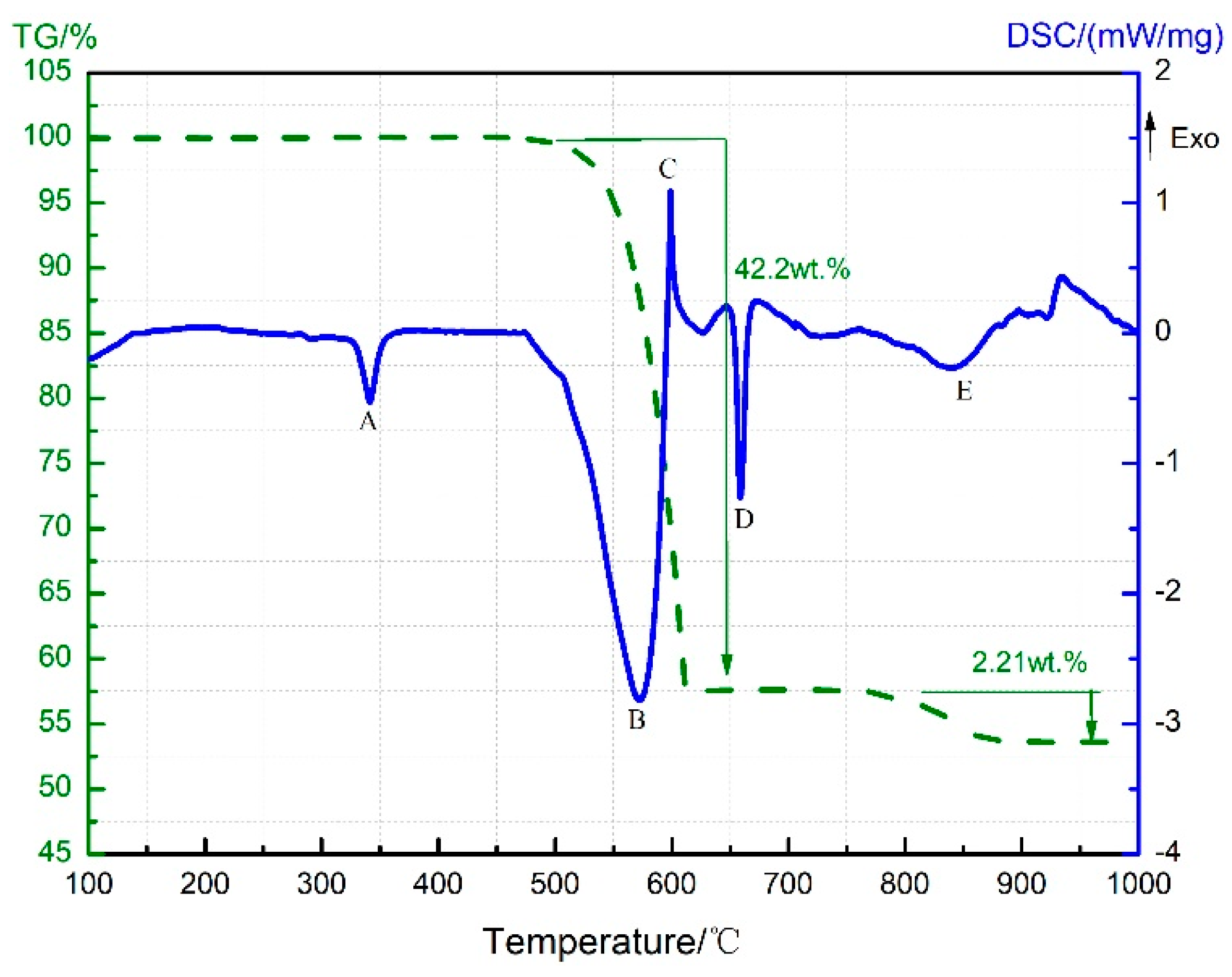
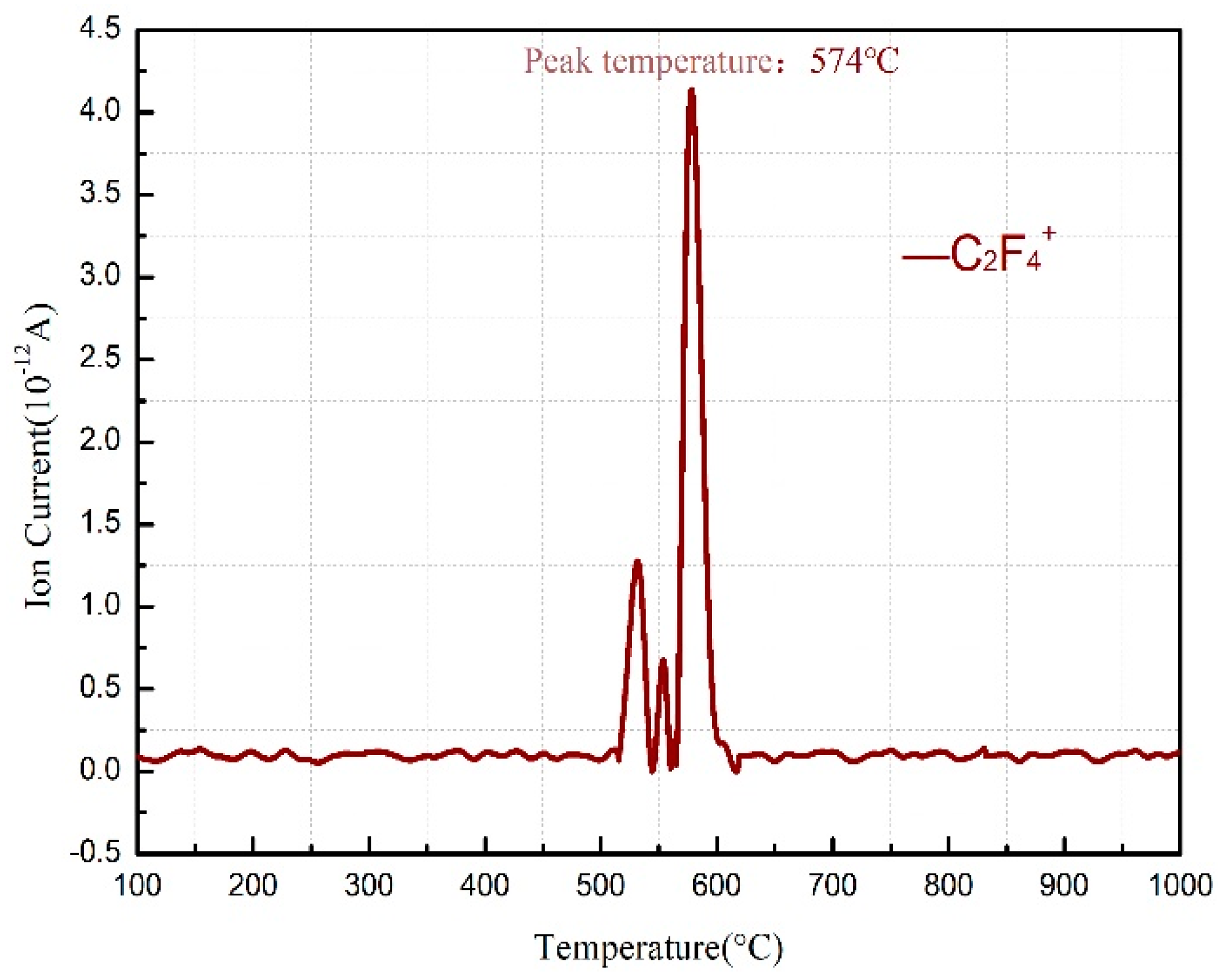
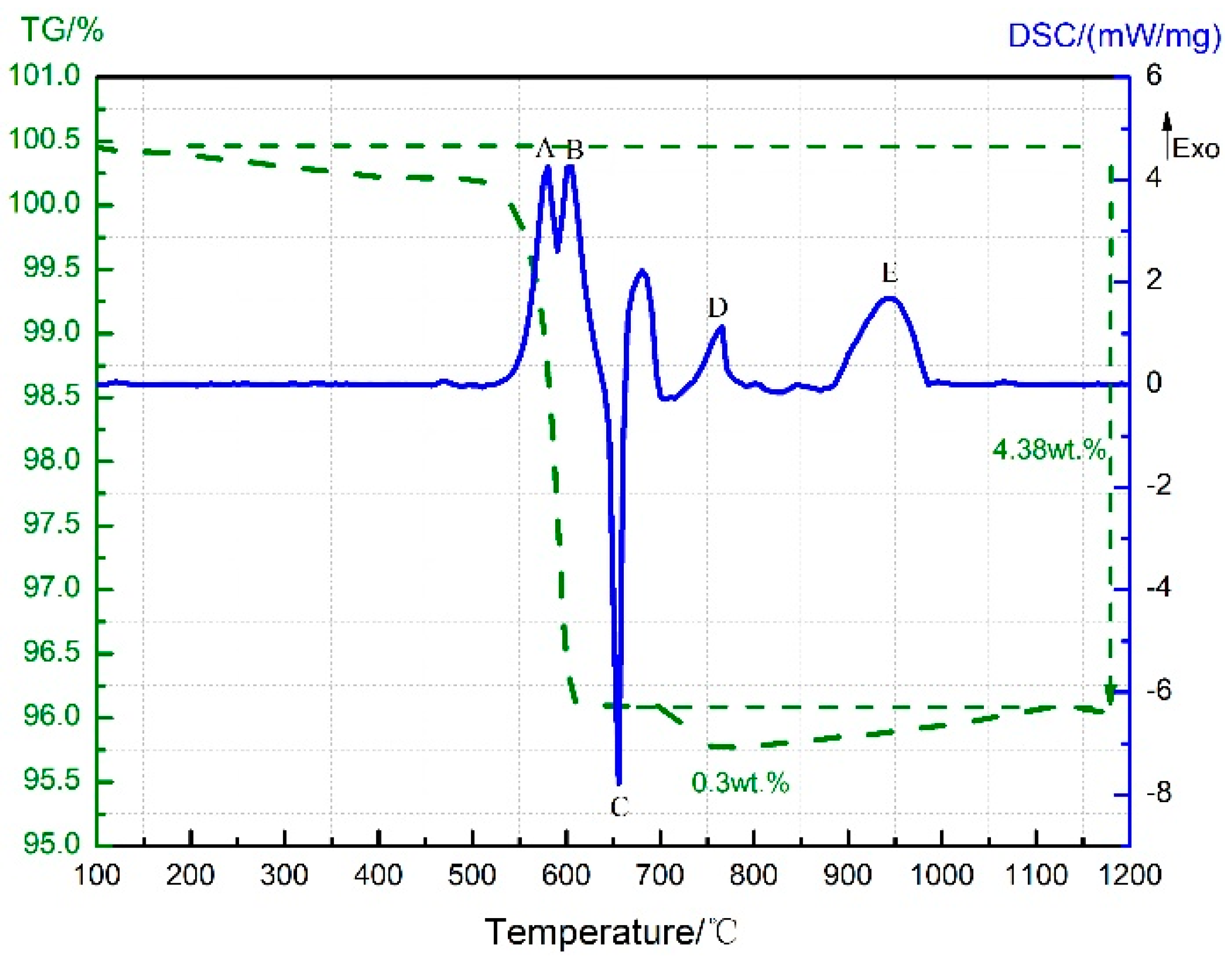
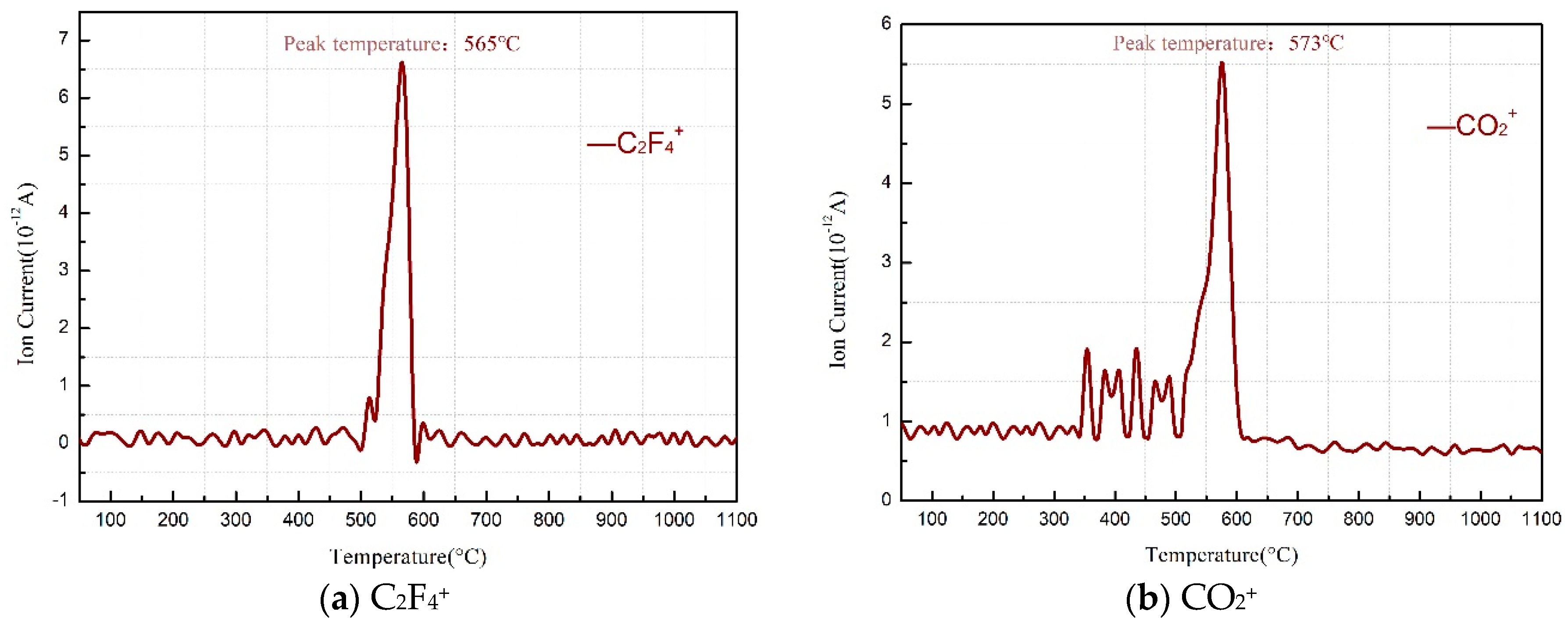
3.1. Thermal Decomposition and Thermal Reaction Processes of PTFE/Al
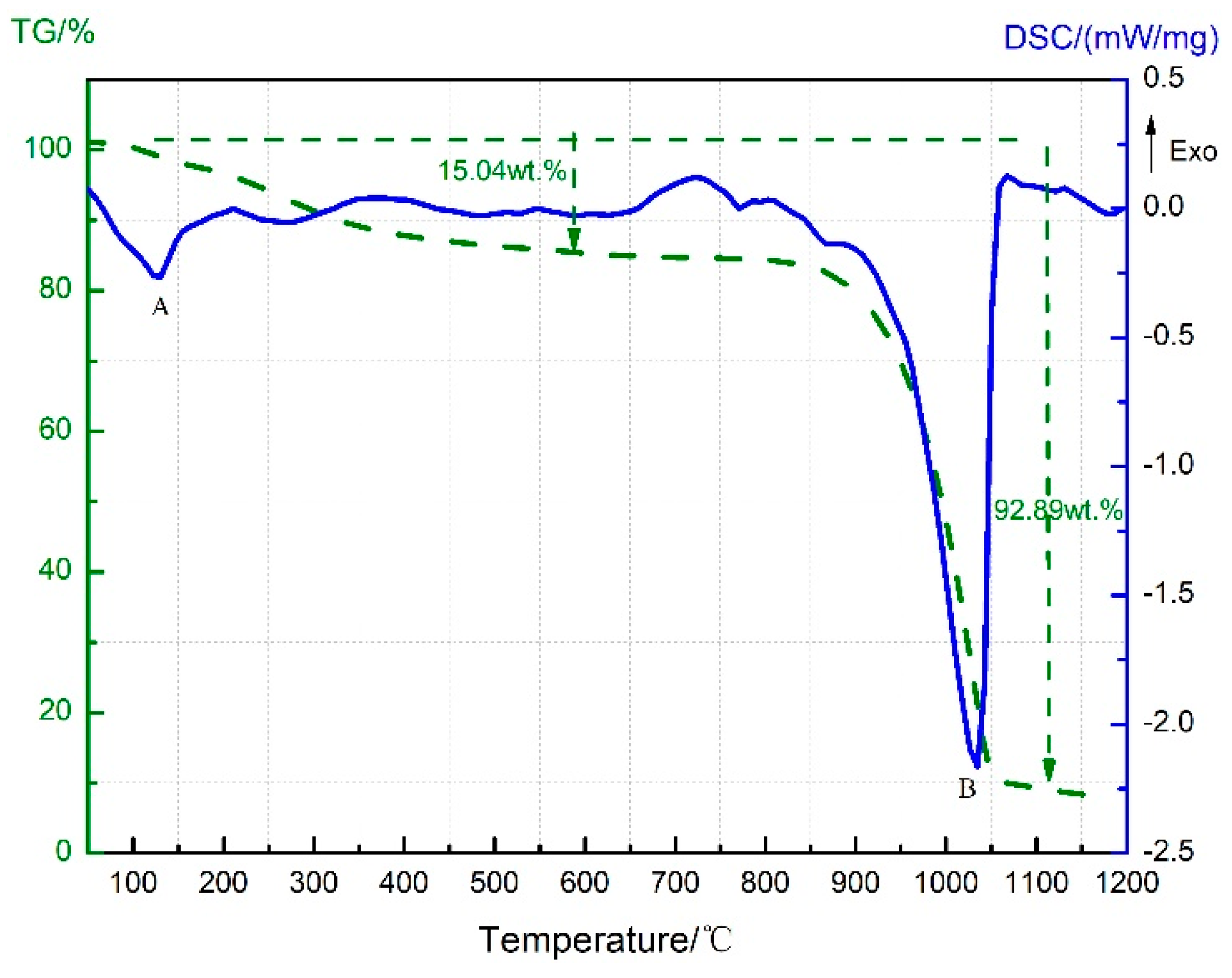
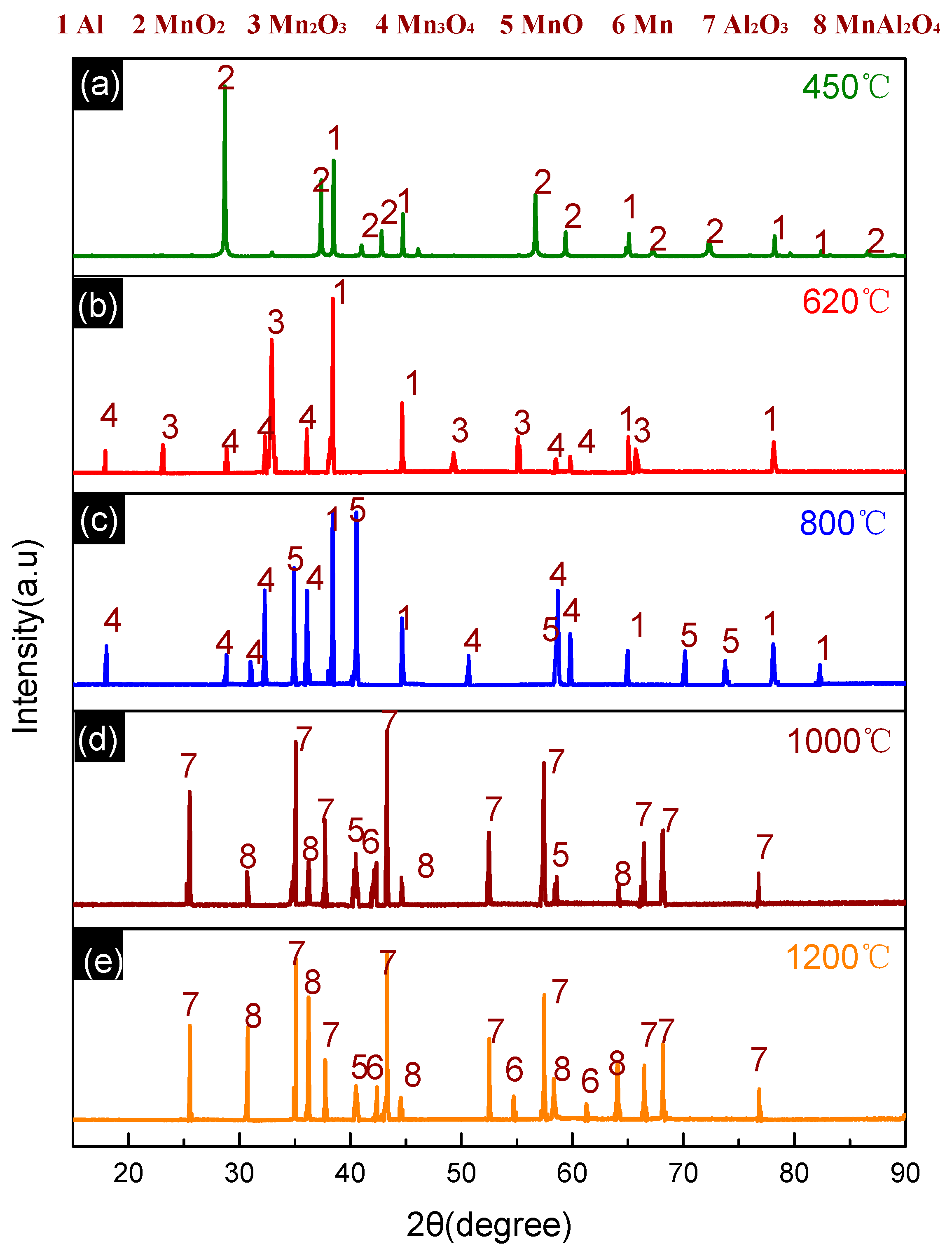
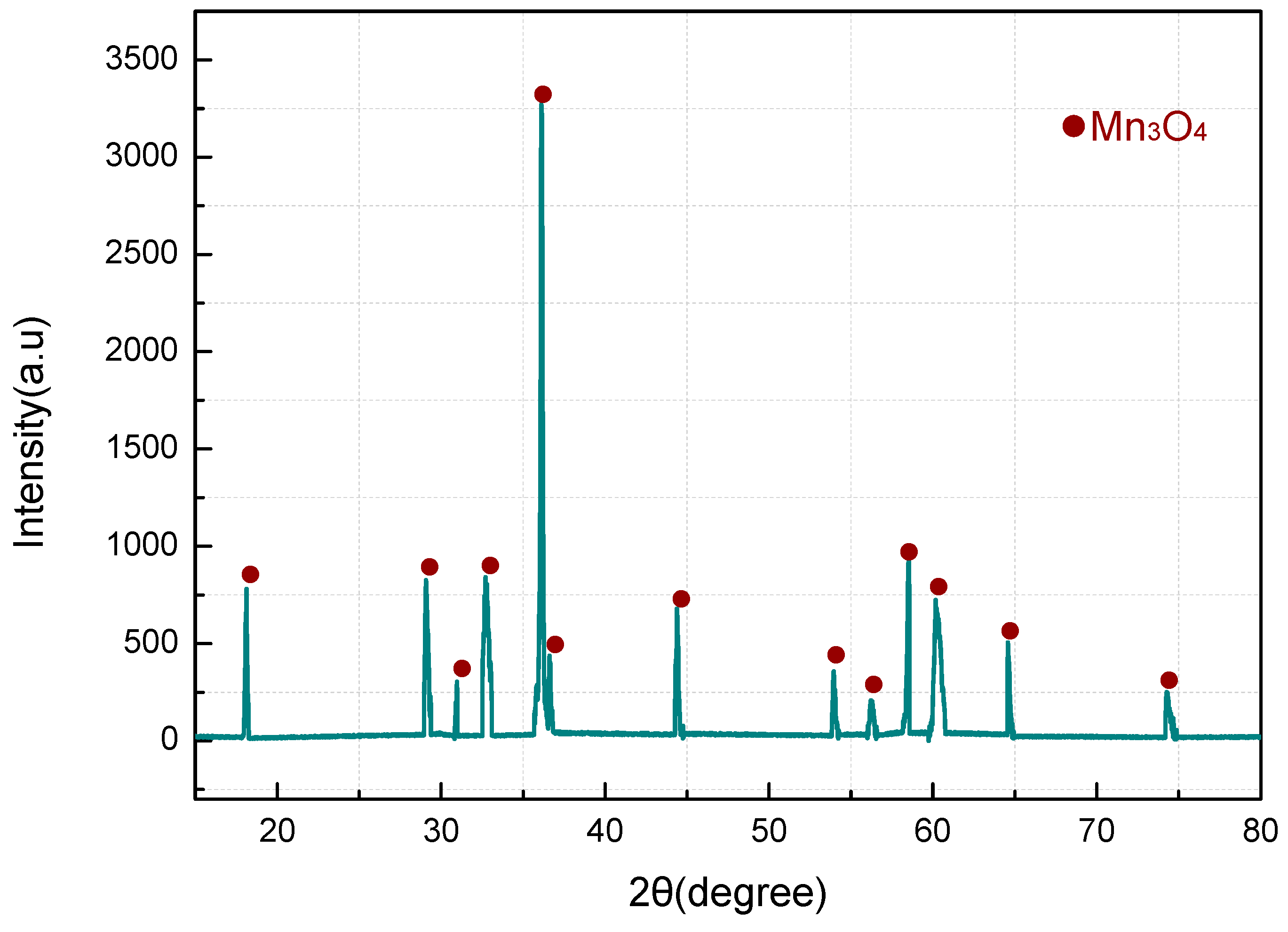
3.2. Thermal Decomposition and Thermal Reaction Process of Al/MnO2
3.3. Thermal Decomposition and Thermal Reaction Process of PTFE/MnO2
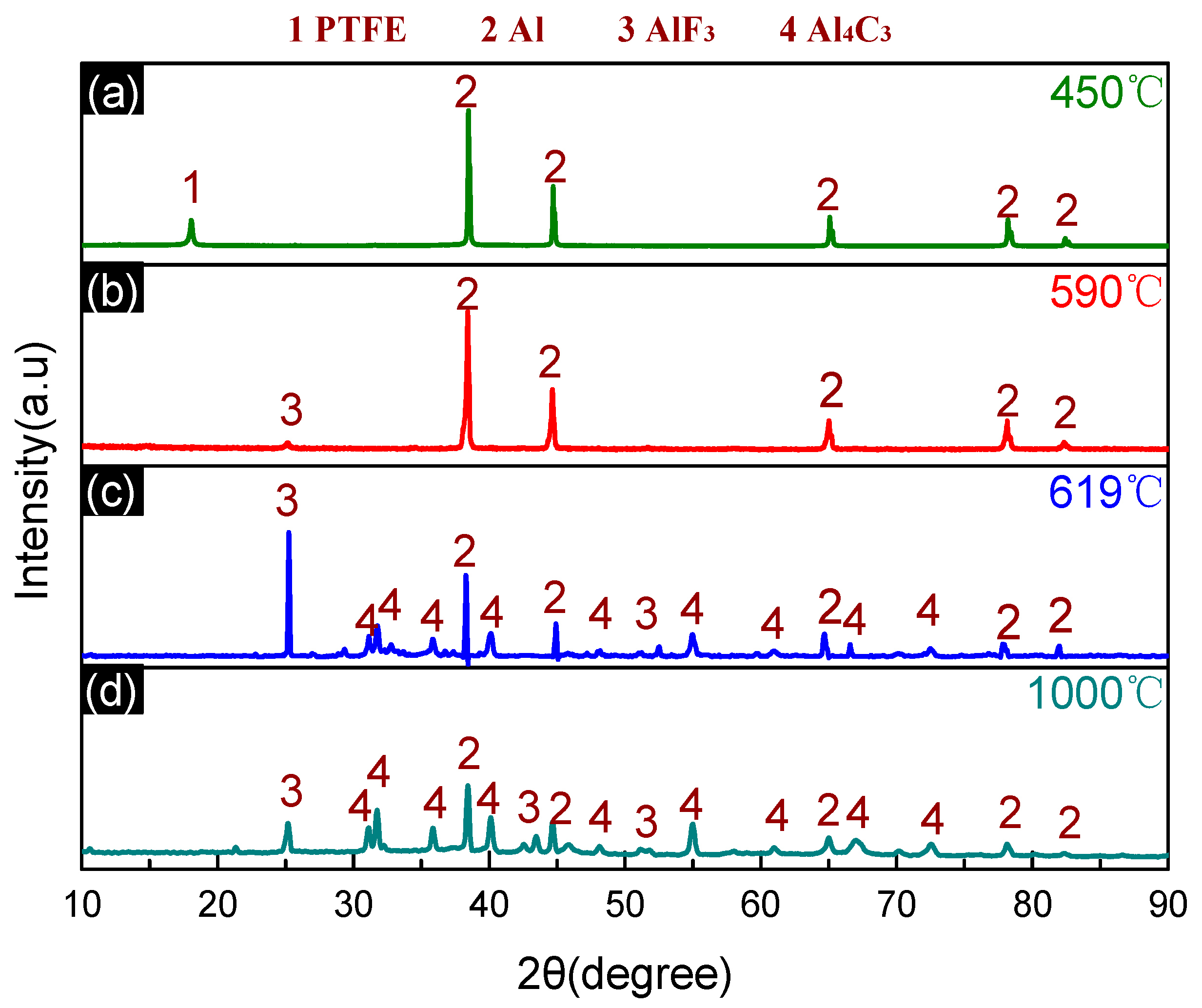
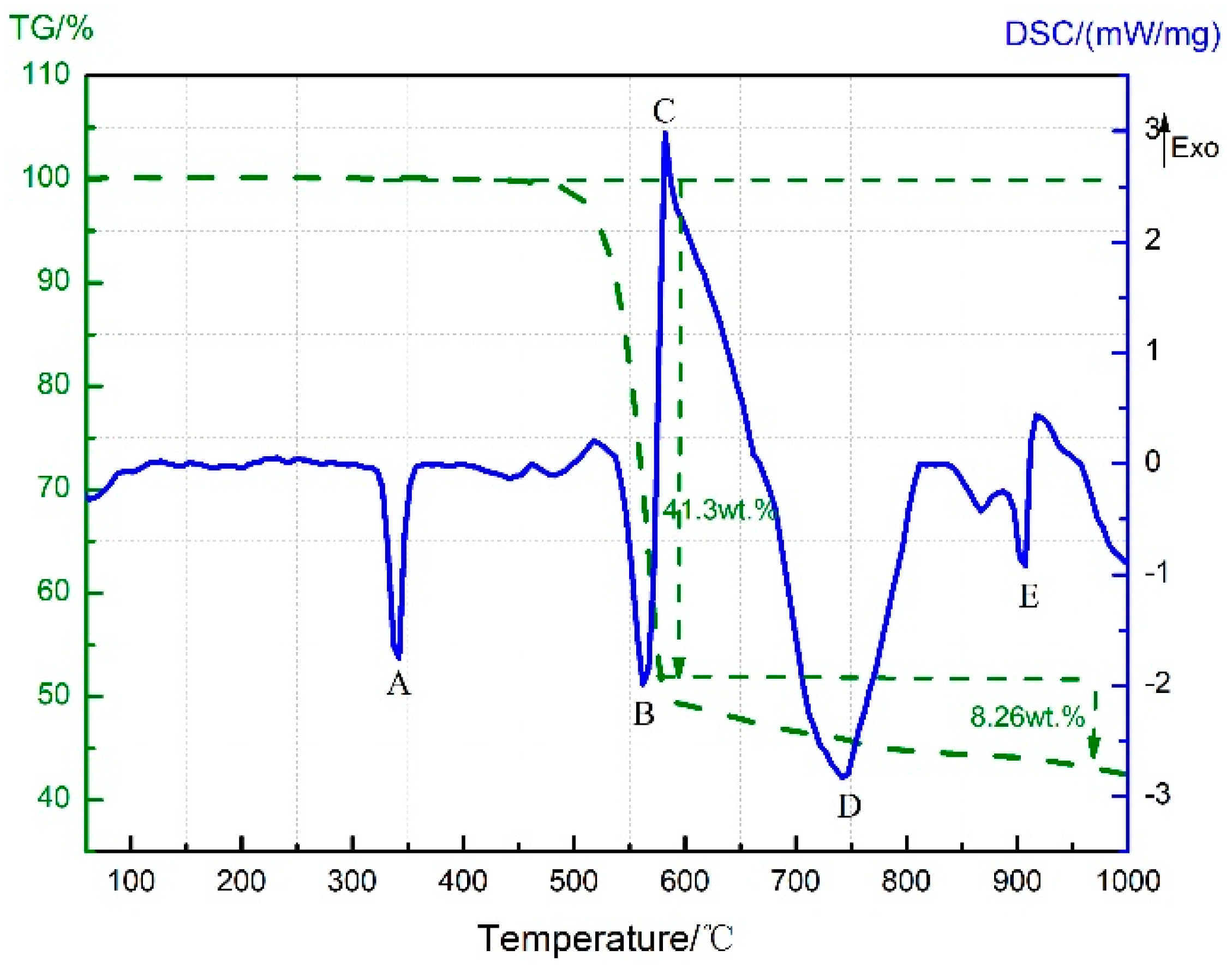
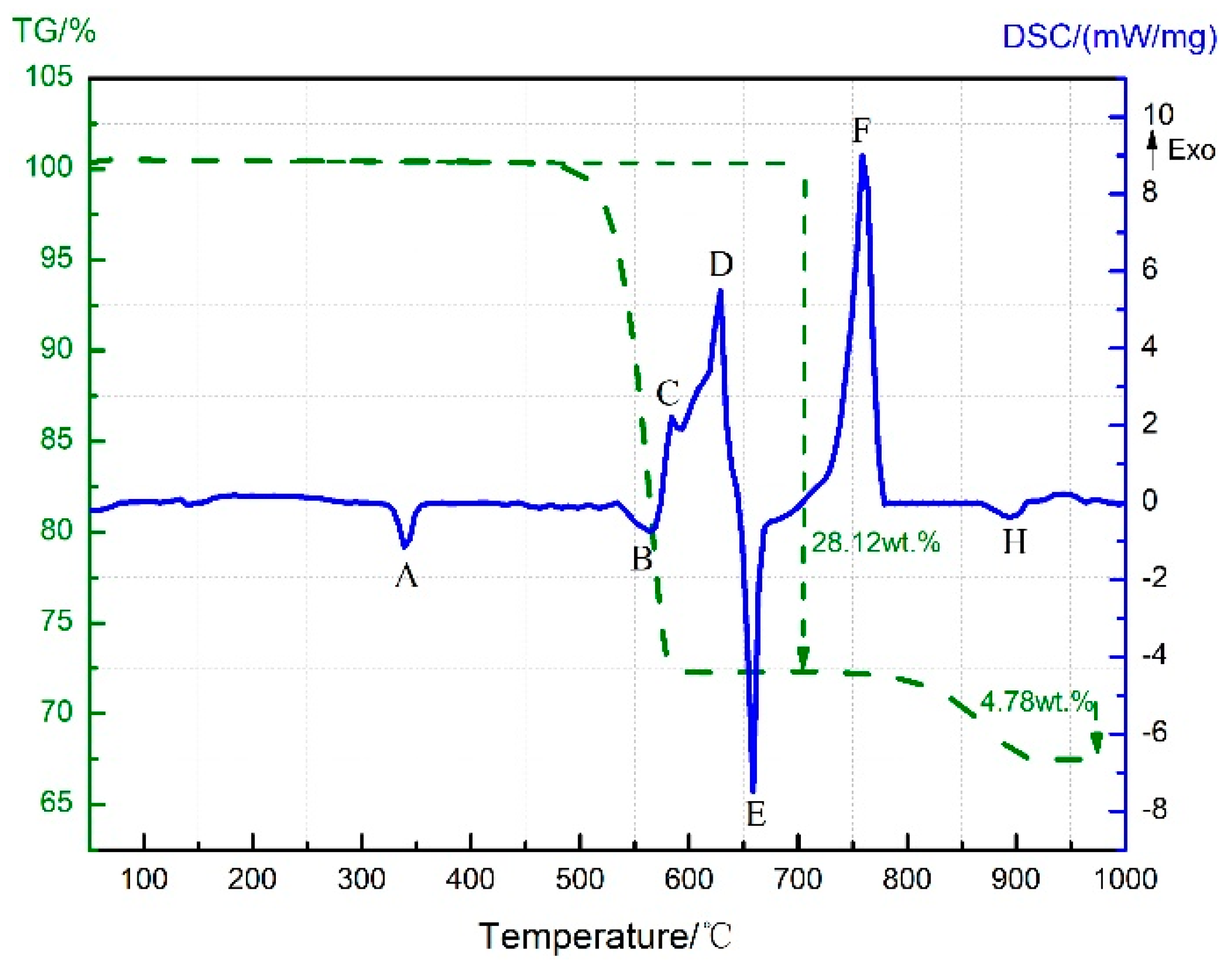
3.4. Thermal Decomposition and Thermal Reaction Process of PTFE/Al/MnO2
4. Conclusions
- PTFE oxidized Al into AlF3 in the PTFE/Al sample but were reduced into CO2 by Mn oxides (MnO2 or Mn2O3) in the PTFE/MnO2 sample. PTFE acted as both an oxidizer and a reducer in the PTFE/Al/MnO2 sample.
- The reaction process of the PTFE/Al/MnO2 sample could be divided into two stages. In the first stage, PTFE and partial MnO2 decomposed. C2F4, the decomposition product of PTFE, could oxidize Al into AlF3 and get reduced into C. In addition, it would reduce Mn oxides (MnO2 or Mn2O3) into MnF2 and get oxidized into CO2. In the meantime, Al reacted with MnO2 to generate Mn3O4 and Al2O3. In the second stage, the constantly increasing temperature let excessive Al reduce Mn2O3/Mn3O4/MnF2 into MnAl2O4 and Mn, with AlF3 and Al2O3 produced simultaneously.
- In the PTFE/Al/MnO2 sample, the mutual reaction between each two components could promote the overall reaction. Compared with the other three systems, the ternary system exhibited a shorter reaction time, a faster energy release, and a better exothermic performance.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, X.F.; Shi, A.S.; Qiao, L.; Zhang, J.; Zhang, Y.G.; Guan, Z.W. Experimental study on impact-initiated characters of multifunctional energetic structural materials. J. Appl. Phys. 2013, 113, 083508. [Google Scholar] [CrossRef]
- Wang, Y. Application of Nanometer Materials and Nanocomposites in Thermites. Ph.D. Thesis, Nanjing University of Science and Technology, Nanjing, China, August 2008. [Google Scholar]
- Osborne, D.T.; Pantoya, M.L. Effect of Al particle size on the thermal degradation of Al/Teflon mixtures. Conbust. Sci. Technol. 2007, 179, 1467–1480. [Google Scholar] [CrossRef]
- Kubota, N.; Serizawa, C. Combustion of magnesium/polytetrafluoroethylene. J. Propuls. Power 1987, 9, 539–545. [Google Scholar] [CrossRef]
- Watson, K.W.; Pantoya, M.L.; Levitas, V.I. Fast reactions with nano-and micrometer aluminum: A study on oxidation versus fluorination. Combust. Flame 2008, 155, 619–634. [Google Scholar] [CrossRef]
- Glavier, L.; Taton, G.; Ducéré, J.M.; Baijot, V.; Pinon, S.; Calais, T.; Rossi, C. Nanoenergetics as pressure generator for nontoxic impact primers: comparison of Al/Bi2O3, Al/CuO, Al/MoO3 nanothermites and Al/PTFE. Combust. Flame 2015, 162, 1813–1820. [Google Scholar] [CrossRef]
- Ye, W.J.; Wang, T.; Yu, Y.H. Research Progress of Fluoropolymer-Matrix Energetic Reactive Materials. Aerosp. Mater. Technol. 2012, 42, 19–23. [Google Scholar]
- Li, X.Y. Preparation and Reactivity of Fluoro-Nanothermite. Ph.D. Thesis, Nanjing University of Science and Technology, Nanjing, China, March 2016. [Google Scholar]
- Puts, G.J.; Crouse, P.L. The influence of inorganic materials on the pyrolysis of polytetrafluoroethylene. Part 1: The sulfates and fluorides of Al, Zn, Cu, Ni, Co, Fe and Mn. J. Fluor. Chem. 2014, 168, 260–267. [Google Scholar] [CrossRef]
- Л.П.Орленкo. Explosion Physics; Science Press: Beijing, China, 2011. [Google Scholar]
- Osborne, D.T. The Effects of Fuel Particle Size on the Reaction of Al/Teflon Mixtures. Ph.D. Thesis, Texas Tech University, Lubbock, TX, USA, May 2006. [Google Scholar]
- Cudzilo, S.; Szala, M.; Huczko, A.; Bystrejewski, M. Combustion reactions of poly(carbon monofluoride), (CF)n, with different reductants and characterization of the products. Propellants Explos. Pyrotech. 2007, 32, 149–154. [Google Scholar] [CrossRef]
- Zhou, X. Highly exothermic and superhydrophobic Mg/fluorocarbon core/shell nanoenergetic arrays. ACS Appl. Mater. Interfaces 2014, 6, 10497–10505. [Google Scholar] [CrossRef] [PubMed]
- Mccollum, J.; Pantoya, M.L.; Iacono, S.T. Activating Aluminum Reactivity with Fluoropolymer Coatings for Improved Energetic Composite Combustion. ACS Appl. Mater. Interfaces 2015, 7, 18742. [Google Scholar] [CrossRef] [PubMed]
- Lide, D.R. CRC Handbook of Chemistry and Physics 1999-2000: A Ready-Reference Book of Chemical and Physical Data. CRC Handb. Chem. Phys. 1999, 15, 504. [Google Scholar]
- Chernenko, E.V.; Afanas’Eva, L.F.; Lebedeva, V.A.; Rozenband, V.I. Inflammability of mixtures of metal oxides with aluminum. Combust. Explos. Shock Waves 1988, 24, 639–646. [Google Scholar] [CrossRef]
- Terayama, K.; Ikeda, M. Study on Thermal Decomposition of MnO2 and Mn2O3 by Thermal Analysis. Mater. Trans. Jim 1983, 24, 754–758. [Google Scholar] [CrossRef]
- Hu, X.L. Study on the preparation and kinetics of Mn3O4 from the deco potion of MnO2. Ph.D. Thesis, Guangxi University, Nanning, China, May 2007. [Google Scholar]
- Schoenitz, M.; Umbrajkar, S.M.; Dreizin, E.L. Kinetic Analysis of Thermite Reactions in Al-MoO3 Nanocomposites. J. Propuls. Power 2012, 23, 683–687. [Google Scholar] [CrossRef]

| Sample | Composition (wt.%) | ||
|---|---|---|---|
| PTFE | Al | MnO2 | |
| Al/MnO2 | \ | 30 | 70 |
| PTFE/Al | 51 | 49 | \ |
| PTFE/MnO2 | 42 | \ | 58 |
| PTFE/Al/MnO2 | 30 | 29 | 41 |
| Temperature/°C | <450 | 450–590 | 590–619 | 619–1000 |
|---|---|---|---|---|
| Products | PTFE and Al | Al, AlF3, C, and C2F4 (g) | Al, AlF3, Al4C3, and C2F4 (g) | Al, AlF3, and Al4C3 |
| Temperature Range | Energy Release (J/g) | |||
|---|---|---|---|---|
| PTFE/Al | PTFE/MnO2 | Al/MnO2 | PTFE/Al/MnO2 | |
| 500–650 °C | 20.32 | 43.11 | 94.41 | 176.12 |
| 700–1000 °C | \ | \ | 129.06 | 209.14 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Huang, J.; Fang, X.; Li, Y.; Yu, Z.; Gao, Z.; Wu, S.; Yang, L.; Wu, J.; Kui, J. Thermal Decomposition and Thermal Reaction Process of PTFE/Al/MnO2 Fluorinated Thermite. Materials 2018, 11, 2451. https://doi.org/10.3390/ma11122451
Zhang J, Huang J, Fang X, Li Y, Yu Z, Gao Z, Wu S, Yang L, Wu J, Kui J. Thermal Decomposition and Thermal Reaction Process of PTFE/Al/MnO2 Fluorinated Thermite. Materials. 2018; 11(12):2451. https://doi.org/10.3390/ma11122451
Chicago/Turabian StyleZhang, Jun, Junyi Huang, Xiang Fang, Yuchun Li, Zhongshen Yu, Zhenru Gao, Shuangzhang Wu, Li Yang, Jiaxiang Wu, and Jiaying Kui. 2018. "Thermal Decomposition and Thermal Reaction Process of PTFE/Al/MnO2 Fluorinated Thermite" Materials 11, no. 12: 2451. https://doi.org/10.3390/ma11122451
APA StyleZhang, J., Huang, J., Fang, X., Li, Y., Yu, Z., Gao, Z., Wu, S., Yang, L., Wu, J., & Kui, J. (2018). Thermal Decomposition and Thermal Reaction Process of PTFE/Al/MnO2 Fluorinated Thermite. Materials, 11(12), 2451. https://doi.org/10.3390/ma11122451





