Effects of a Carbon Nanotube Additive on the Corrosion-Resistance and Heat-Dissipation Properties of Plasma Electrolytic Oxidation on AZ31 Magnesium Alloy
Abstract
1. Introduction
2. Experimental Procedure
3. Results and Discussion
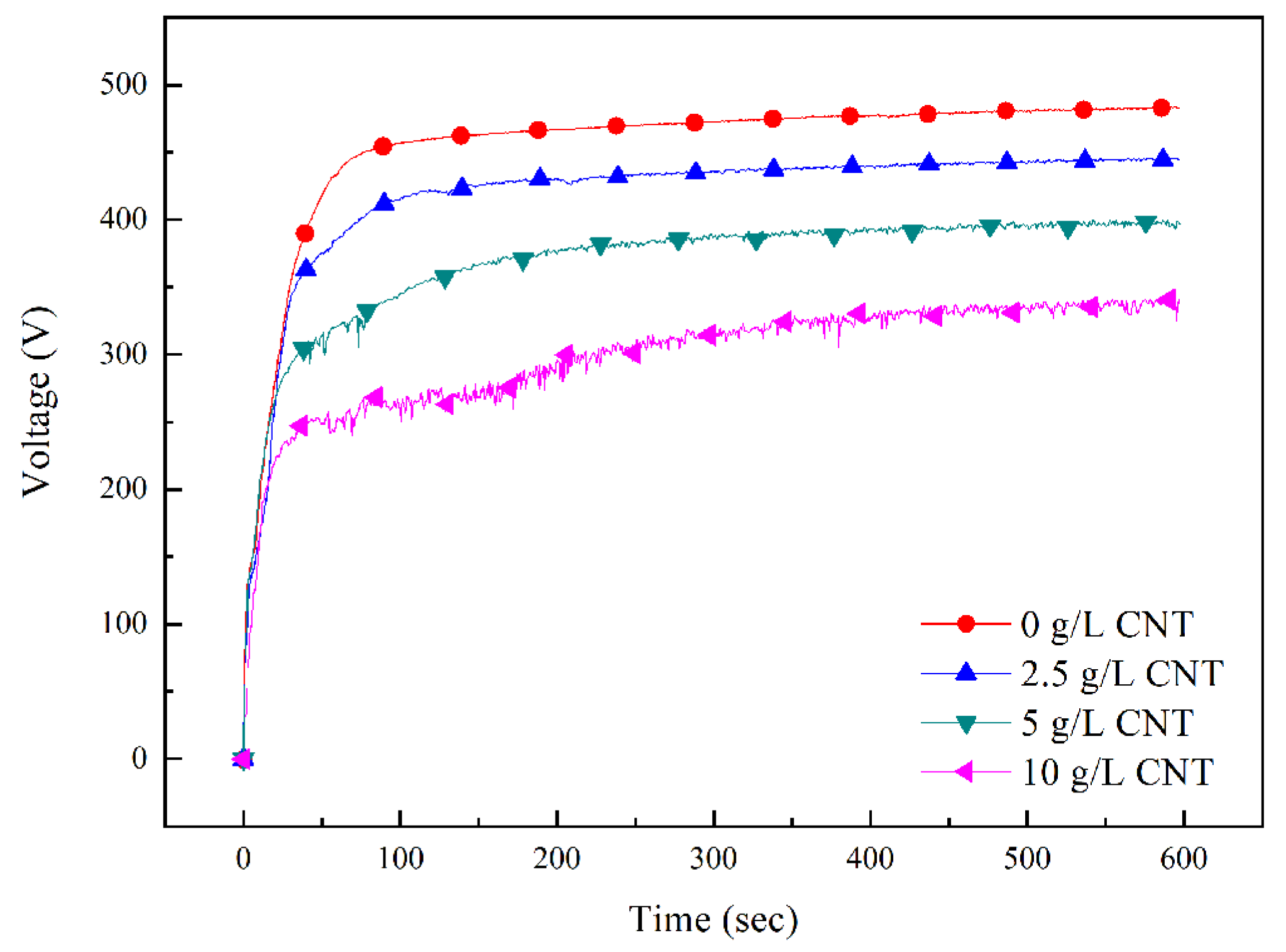
3.1. Plasma Electrolytic Oxidation Process
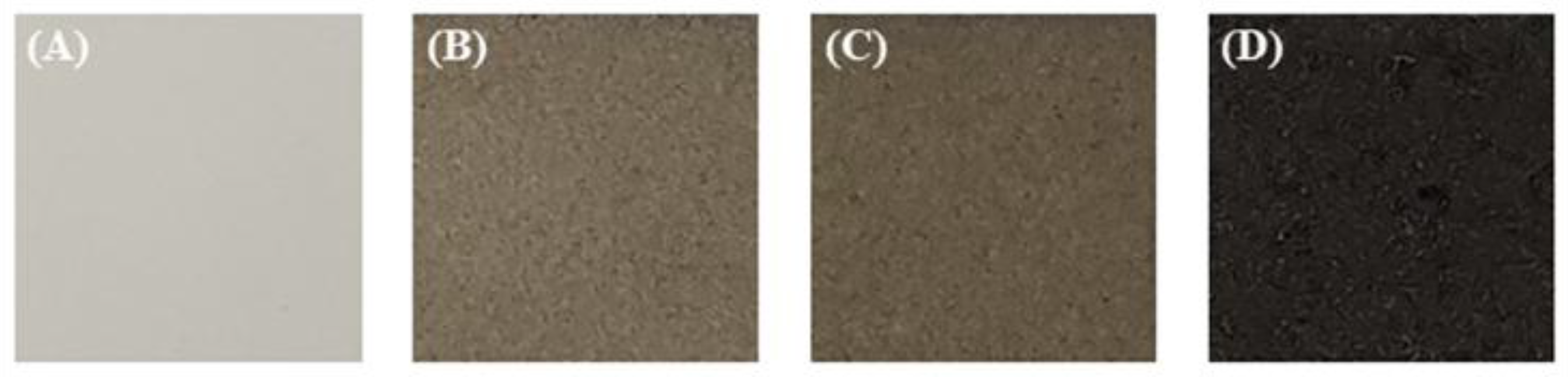
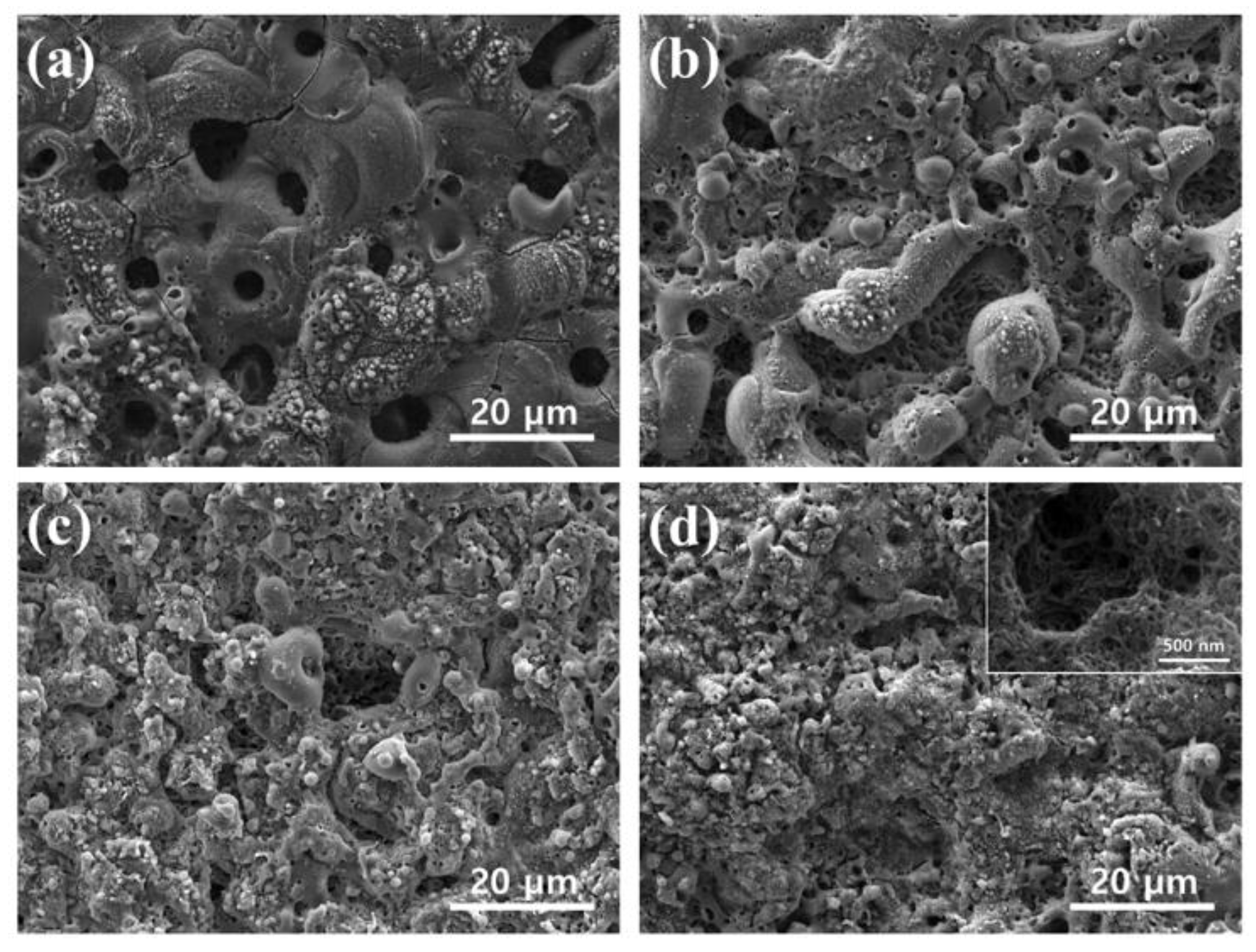
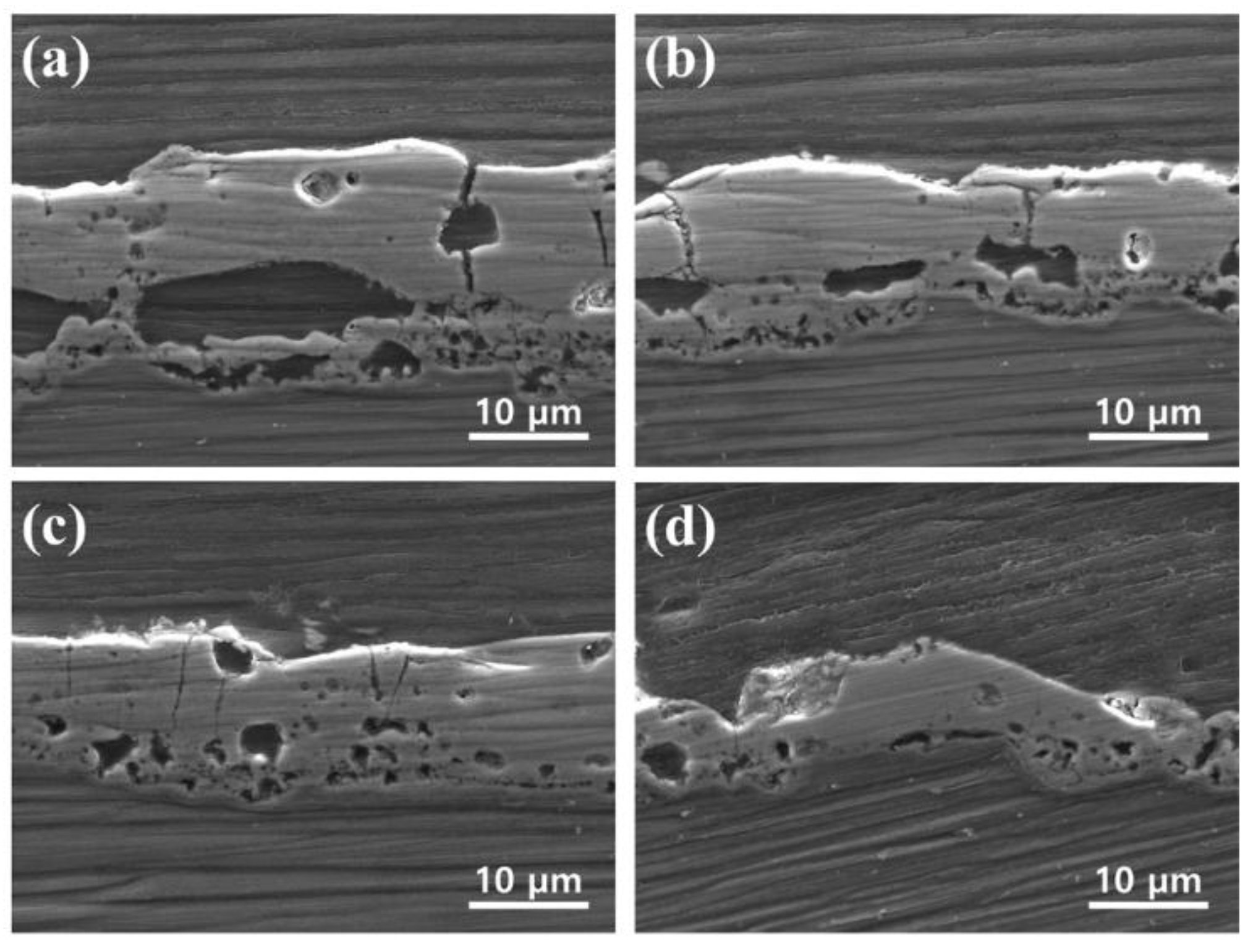
3.2. Morphology and Structure of PEO Coatings
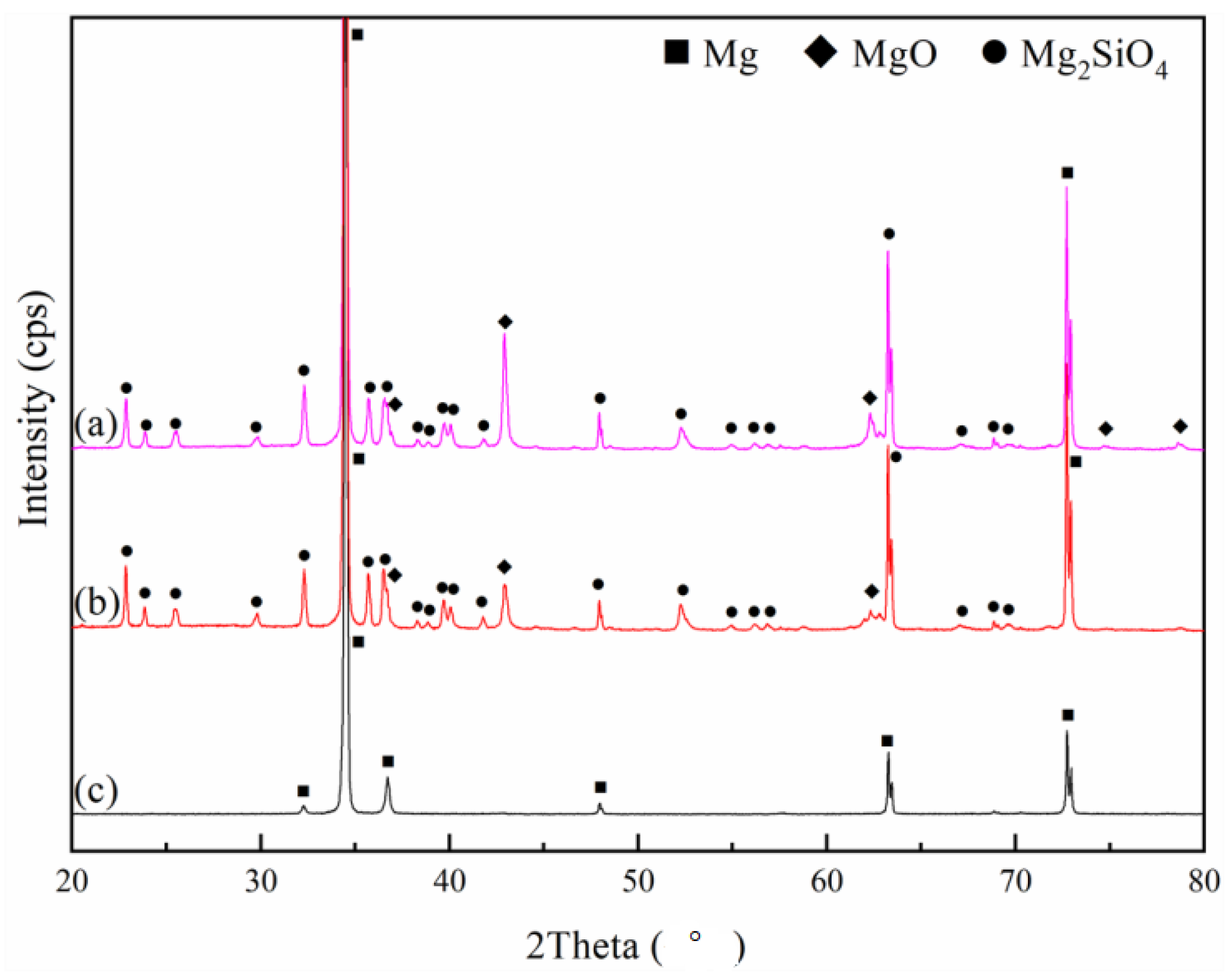
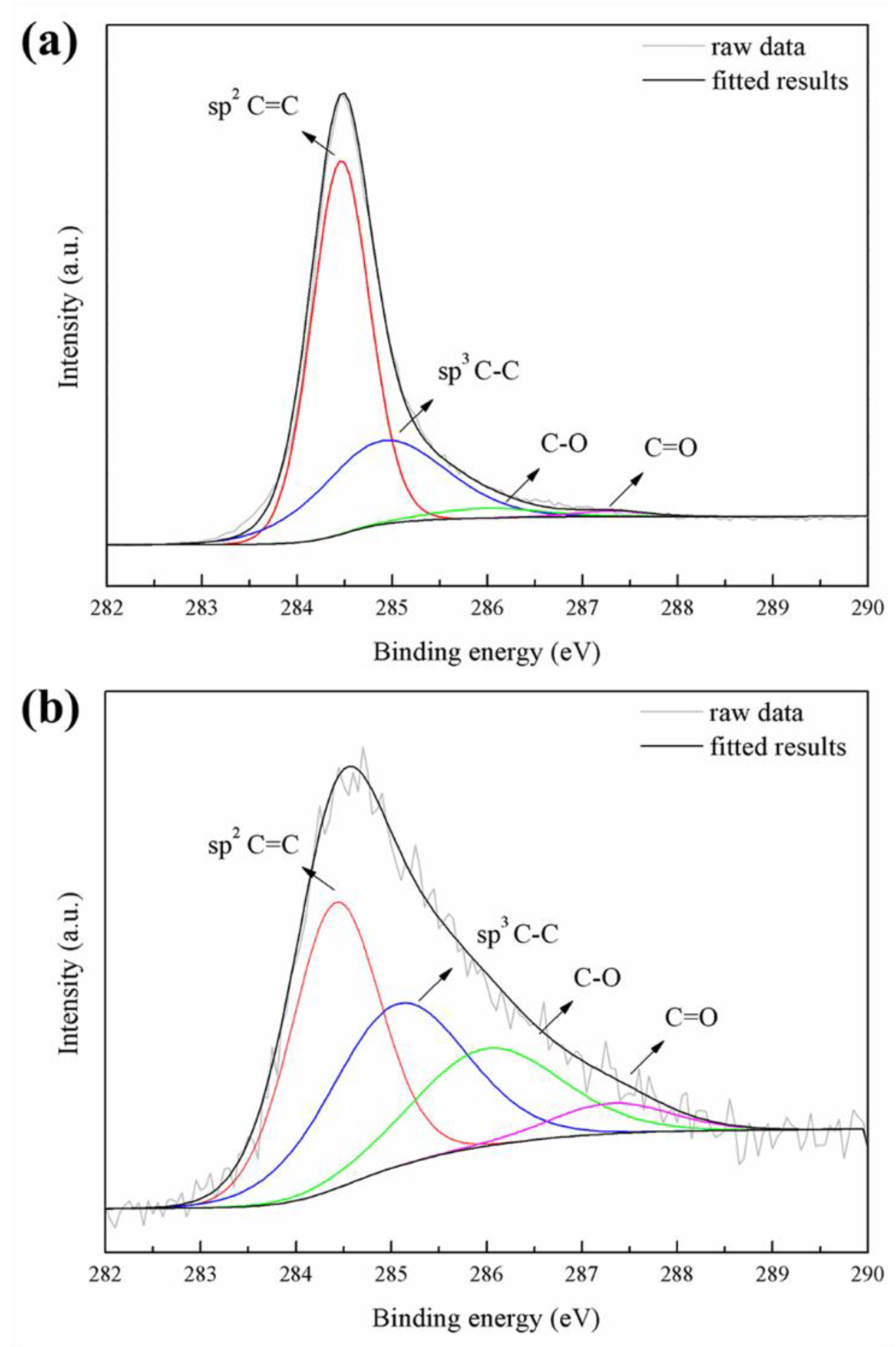
3.3. Phase Analysis and Chemical State of PEO Coatings
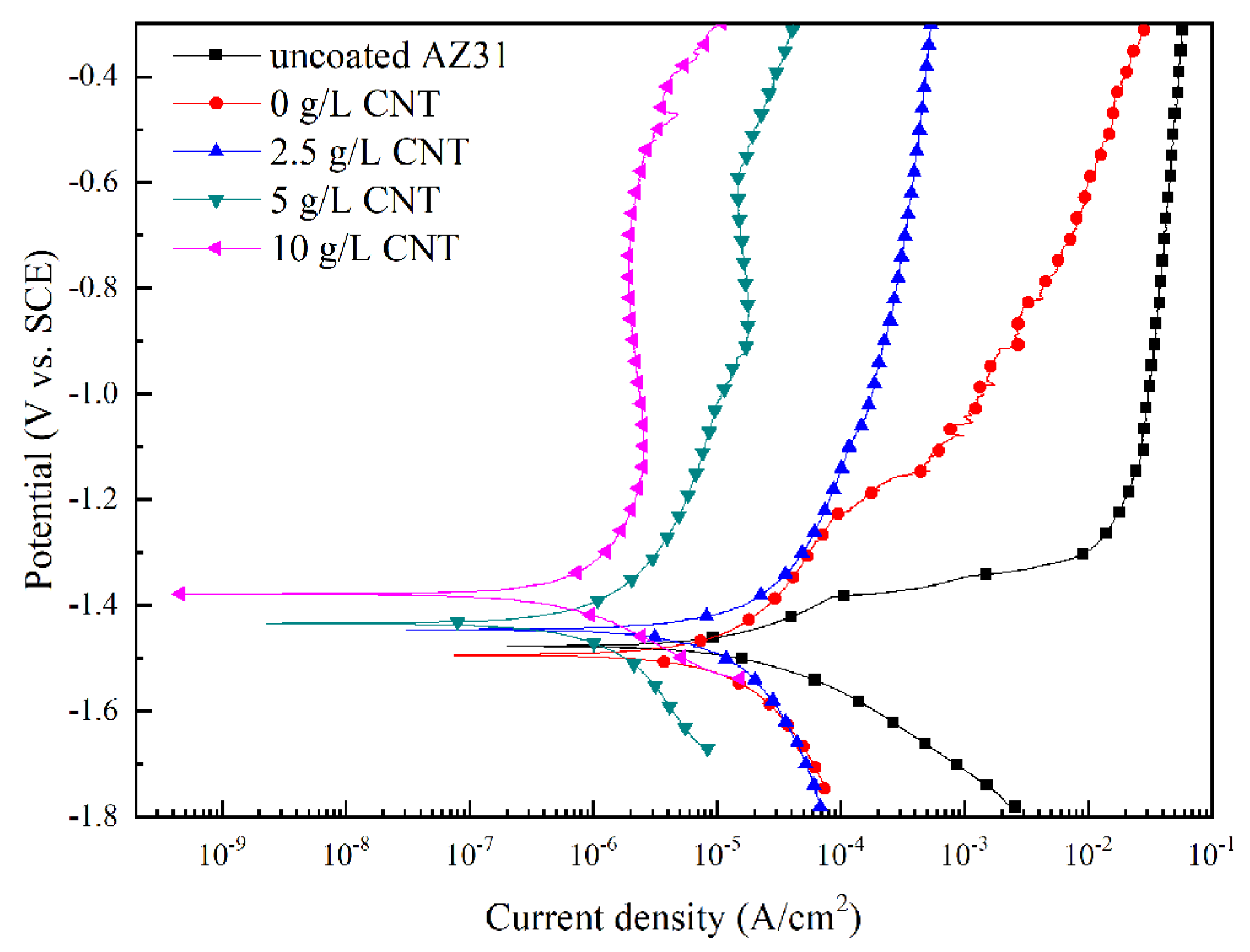
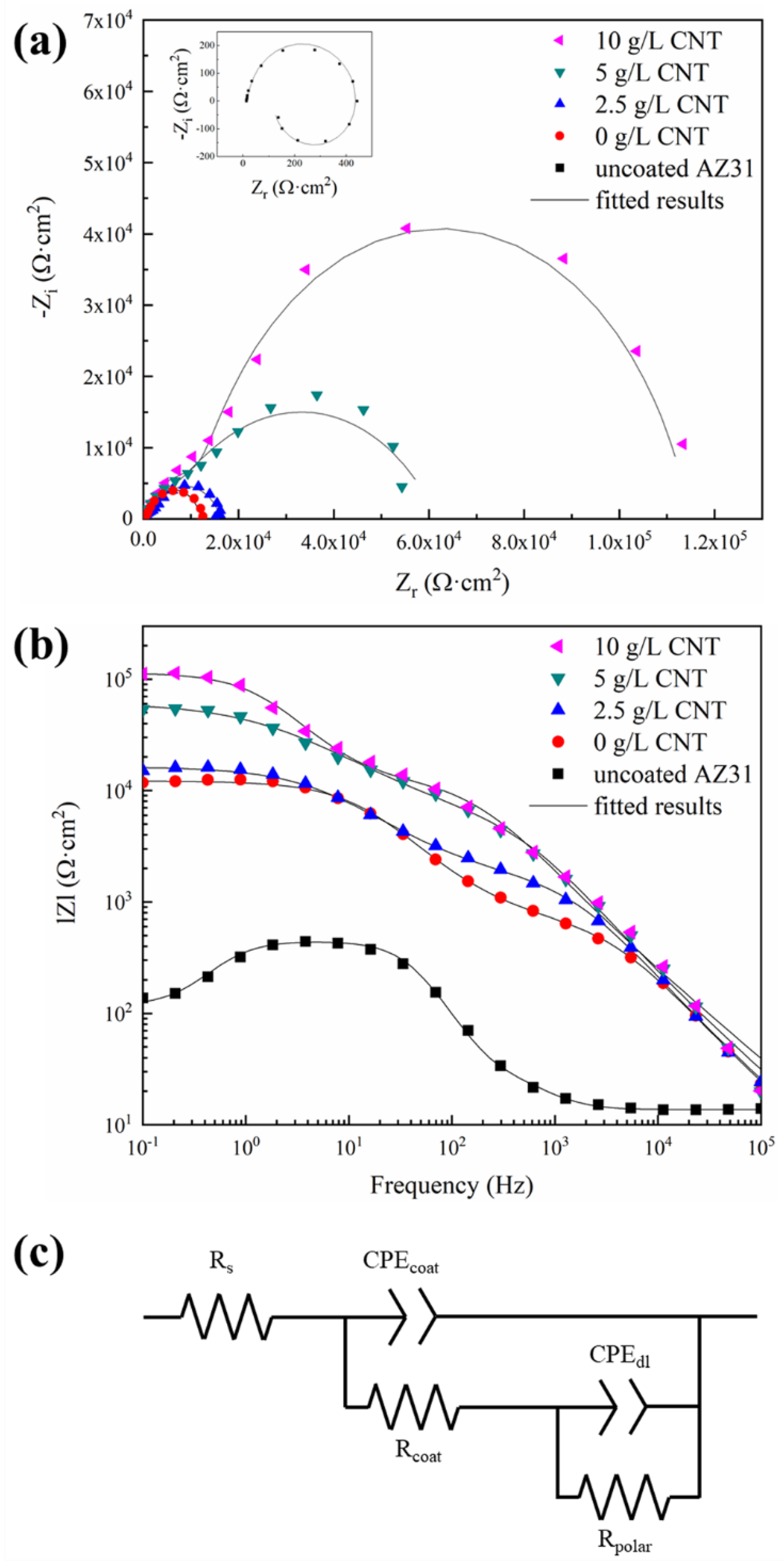
3.4. Corrosion Behavior of PEO Coatings
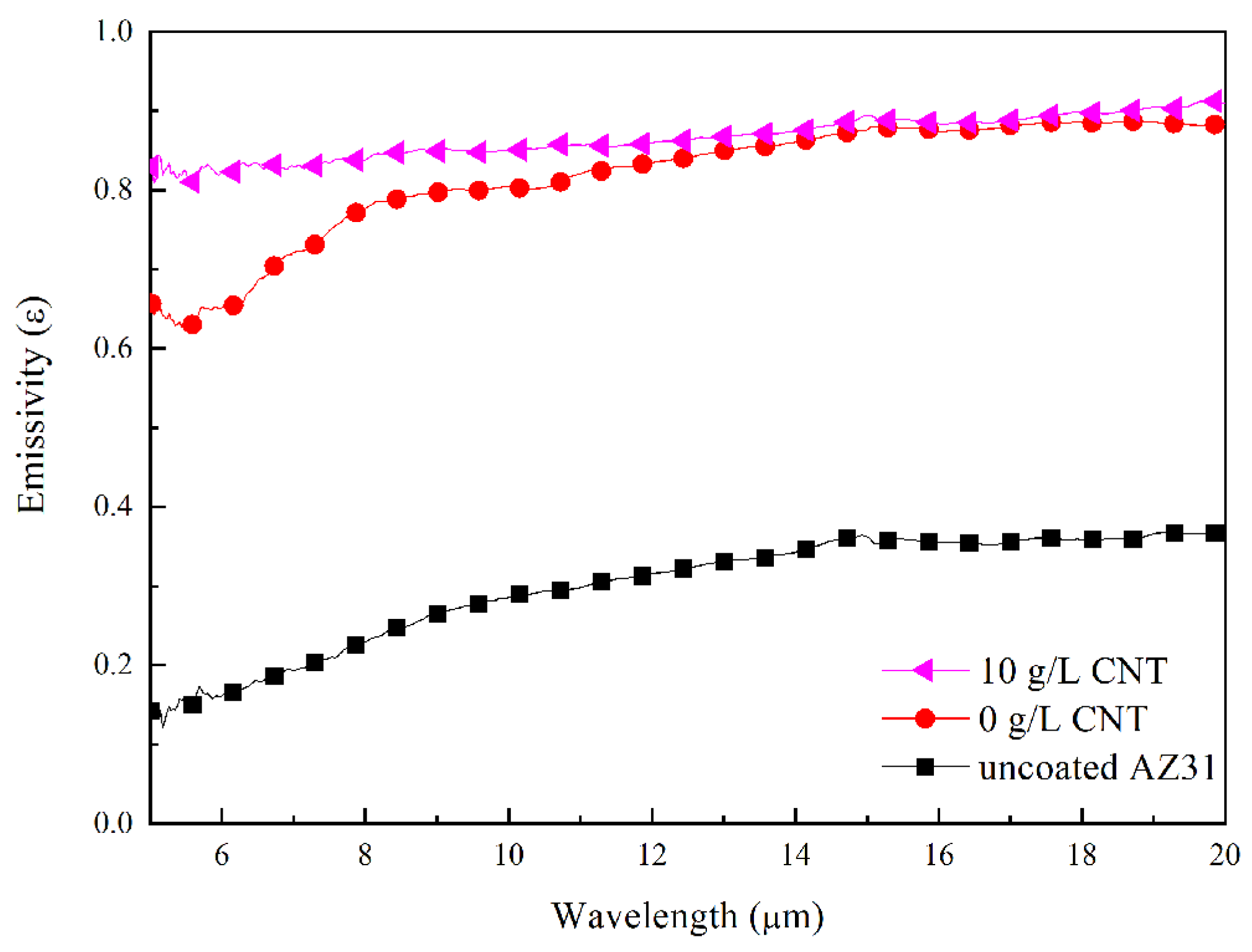
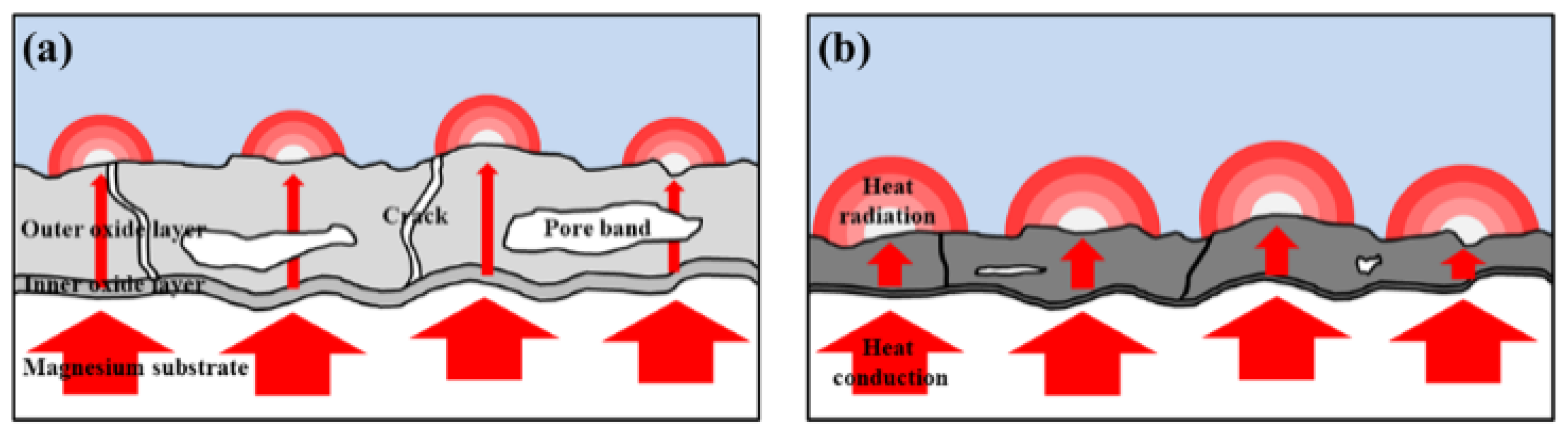
3.5. Heat-Dissipation Property of PEO Coatings
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mordike, B.L.; Ebert, T. Magnesium: Properties—Applications—Potential. Mater. Sci. Eng. A 2001, 302, 37–45. [Google Scholar] [CrossRef]
- Shu, D.W.; Ahmad, I. Magnesium Alloys: An Alternative for Aluminium in Structural Applications. Adv. Mater. Res. 2011, 168, 1631–1635. [Google Scholar] [CrossRef]
- Hollstein, F.; Wiedemann, R.; Scholz, J. Characteristics of PVD-coatings on AZ31hp magnesium alloys. Surf. Coat. Technol. 2003, 162, 261–268. [Google Scholar] [CrossRef]
- Gray, J.E.; Luan, B. Protective coatings on magnesium and its alloys—A critical review. J. Alloys Compd. 2002, 336, 88–113. [Google Scholar] [CrossRef]
- Blawert, C.; Dietzel, W.; Ghali, E.; Song, G. Anodizing Treatments for Magnesium Alloys and Their Effect on Corrosion Resistance in Various Environments. Adv. Eng. Mater. 2006, 8, 511–533. [Google Scholar] [CrossRef]
- Yerokhin, A.L.; Nie, X.; Leyland, A.; Matthews, A.; Dowey, S.J. Plasma electrolysis for surface engineering. Surf. Coat. Technol. 1999, 122, 73–93. [Google Scholar] [CrossRef]
- Arrabal, R.; Matykina, E.; Hashimoto, T.; Skeldon, P.; Thompson, G. Characterization of AC PEO coatings on magnesium alloys. Surf. Coat. Technol. 2009, 203, 2207–2220. [Google Scholar] [CrossRef]
- Ghasemi, A.; Raja, V.S.; Blawert, C.; Dietzel, W.; Kainer, K. Study of the structure and corrosion behavior of PEO coatings on AM50 magnesium alloy by electrochemical impedance spectroscopy. 2008, 202, 3513–3518. [Google Scholar]
- Shim, G. Factors Influencing Plasma Electrolytic Oxidation (PEO) Coatings on Magnesium Alloys: A Review. J. Korean Inst. Met. Mater. 2017, 55, 296–307. [Google Scholar] [CrossRef]
- Bergman, T.L.; Incropera, F.P. Fundamentals of Heat and Mass Transfer; Wiley: Hoboken, NJ, USA, 2011. [Google Scholar]
- Hamada, T.; Suzuki, T.; Ishiwa, M. Backlight unit and liquid crystal display device having the same. U.S. Patent 7,488,104, 2009. [Google Scholar]
- Yu, S.-H.; Jang, D.; Lee, K.-S. Effect of radiation in a radial heat sink under natural convection. Int. J. Heat Mass Transf. 2012, 55, 505–509. [Google Scholar] [CrossRef]
- Tsai, W.-Y.; Huang, G.-R.; Wang, K.-K.; Chen, C.-F.; Huang, J. High Thermal Dissipation of Al Heat Sink When Inserting Ceramic Powders by Ultrasonic Mechanical Coating and Armoring. Materials 2017, 10, 454. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Choi, D.-H.; Lee, K.-S. Numerical Shape Optimization for High Performance of a Heat Sink with Pin-Fins. Numer. Heat Transf. A-Appl. 2004, 46, 909–927. [Google Scholar] [CrossRef]
- Chu, K.; Wu, Q.; Jia, C.; Liang, X.; Nie, J.; Tian, W.; Gai, G.; Guo, H. Fabrication and effective thermal conductivity of multi-walled carbon nanotubes reinforced Cu matrix composites for heat sink applications. Compos. Sci. Technol. 2010, 70, 298–304. [Google Scholar] [CrossRef]
- Bornoff, R.; Parry, J. An additive design heatsink geometry topology identification and optimisation algorithm. In Proceedings of the 2015 31st Thermal Measurement, Modeling and Management Symposium (SEMI-THERM), San Jose, CA, USA, 15–19 March 2015; pp. 303–308. [Google Scholar]
- Tiihonen, T. Stefan-Boltzmann Radiation on Non-convex Surfaces. Math. Methods Appl. Sci. 1998, 20, 47–57. [Google Scholar] [CrossRef]
- Mi, J.; Grant, P.S. Modelling the shape and thermal dynamics of Ni superalloy rings during spray forming. Part 2: Thermal modelling—Heat flow and solidification. Acta Mater. 2008, 56, 1597–1608. [Google Scholar] [CrossRef]
- Siegel, R. Thermal Radiation Heat Transfer, 4th ed.; Taylor and Francis: Didcot, UK, 2001. [Google Scholar]
- Mizuno, K.; Ishii, J.; Kishida, H.; Hayamizu, Y.; Yasuda, S.; Futaba, D.N.; Yumura, M.; Hata, K. A black body absorber from vertically aligned single-walled carbon nanotubes. Proc. Natl. Acad. Sci. USA 2009, 106, 6044. [Google Scholar] [CrossRef]
- Lee, J.; Kim, D.; Choi, C.-H.; Chung, W. Nanoporous anodic alumina oxide layer and its sealing for the enhancement of radiative heat dissipation of aluminum alloy. Nano Energy 2017, 31, 504–513. [Google Scholar] [CrossRef]
- Singh, B.; Nayak, S.; Samal, S.; Besra, L.; Bhattacharjee, S. Characterization and Dispersion of Multiwalled Carbon Nanotubes (MWCNTs) in Aqueous Suspensions: Surface Chemistry Aspects. J. Dispers. Sci. Technol. 2012, 33, 1021–1029. [Google Scholar] [CrossRef]
- Lee, K.M.; Ko, Y.G.; Shin, D.H. Incorporation of carbon nanotubes into micro-coatings film formed on aluminum alloy via plasma electrolytic oxidation. Mater. Lett. 2011, 65, 2269–2273. [Google Scholar] [CrossRef]
- Barati Darband, G.; Aliofkhazraei, M.; Hamghalam, P.; Valizade, N. Plasma electrolytic oxidation of magnesium and its alloys: Mechanism, properties and applications. J. Magnes. Alloys 2017, 5, 74–132. [Google Scholar] [CrossRef]
- Liang, J.; Guo, B.; Tian, J.; Liu, H.; Zhou, J.; Liu, W.; Xu, T. Effects of NaAlO2 on structure and corrosion resistance of microarc oxidation coatings formed on AM60B magnesium alloy in phosphate-KOH electrolyte. Surf. Coat. Technol. 2005, 199, 121–126. [Google Scholar] [CrossRef]
- Weiping, L.; Liqun, Z.; Yihong, L. Electrochemical oxidation characteristic of AZ91D magnesium alloy under the action of silica sol. Surf. Coat. Technol. 2006, 201, 1085–1092. [Google Scholar] [CrossRef]
- Curran, J.A.; Clyne, T.W. Porosity in plasma electrolytic oxide coatings. Acta Mater. 2006, 54, 1985–1993. [Google Scholar] [CrossRef]
- Khaselev, O.; Weiss, D.; Yahalom, J. Anodizing of pure magnesium in KOH-aluminate solutions under sparking. J. Electrochem. Soc. 1999, 146, 1757–1761. [Google Scholar] [CrossRef]
- Duan, H.; Yan, C.; Wang, F. Effect of electrolyte additives on performance of plasma electrolytic oxidation films formed on magnesium alloy AZ91D. Electrochimi. Acta 2007, 52, 3785–3793. [Google Scholar] [CrossRef]
- Lv, G.; Gu, W.; Chen, H.; Feng, W.; Khosa, M.L.; Li, L.; Niu, E.; Zhang, G.; Yang, S.-Z. Characteristic of ceramic coatings on aluminum by plasma electrolytic oxidation in silicate and phosphate electrolyte. Appl. Surf. Sci. 2006, 253, 2947–2952. [Google Scholar] [CrossRef]
- Lu, X.; Blawert, C.; Scharnagl, N.; Kainer, K.U. Influence of incorporating Si3N4 particles into the oxide layer produced by plasma electrolytic oxidation on AM50 Mg alloy on coating morphology and corrosion properties. J. Magnes. Alloys 2013, 1, 267–274. [Google Scholar] [CrossRef]
- Lu, X.; Sah, S.P.; Scharnagl, N.; Störmer, M.; Starykevich, M.; Mohedano, M.; Blawert, C.; Zheludkevich, M.L.; Kainer, K.U. Degradation behavior of PEO coating on AM50 magnesium alloy produced from electrolytes with clay particle addition. Surf. Coat. Technol. 2015, 269, 155–169. [Google Scholar] [CrossRef]
- Song, G.-L. Corrosion of Magnesium Alloys; Woodhead Publishing: Philadelphia, PA, USA, 2011. [Google Scholar]
- Stachurski, Z.H. On Structure and Properties of Amorphous Materials. Materials 2011, 4, 1564–1598. [Google Scholar] [CrossRef]
- Durdu, S.; Aytac, A.; Usta, M. Characterization and corrosion behavior of ceramic coating on magnesium by micro-arc oxidation. J. Alloys Compd. 2011, 509, 8601–8606. [Google Scholar]
- Hussein, R.; Nie, X.; Northwood, D. Plasma electrolytic oxidation coatings on Mg-alloys for improved wear and corrosion resistance. In Corrosion: Material Performance and Cathodic Protection; WIT Press: Ashurst, UK, 2017; pp. 133–147. [Google Scholar]
- Smith, G.C. Evaluation of a simple correction for the hydrocarbon contamination layer in quantitative surface analysis by XPS. J. Electron Spectrosc. Relat. Phenom. 2005, 148, 21–28. [Google Scholar] [CrossRef]
- Ago, H.; Kugler, T.; Cacialli, F.; Salaneck, W.R.; Shaffer, M.S.P.; Windle, A.H.; Friend, R.H. Work Functions and Surface Functional Groups of Multiwall Carbon Nanotubes. J. Phys. Chem. B 1999, 103, 8116–8121. [Google Scholar] [CrossRef]
- Hong, C.-E.; Lee, J.-H.; Kalappa, P.; Advani, S.G. Effects of oxidative conditions on properties of multi-walled carbon nanotubes in polymer nanocomposites. Compos. Sci. Technol. 2007, 67, 1027–1034. [Google Scholar] [CrossRef]
- Hirschorn, B.; Orazem, M.E.; Tribollet, B.; Vivier, V.; Frateur, I.; Musiani, M. Determination of effective capacitance and film thickness from constant-phase-element parameters. Electrochim. Acta 2010, 55, 6218–6227. [Google Scholar] [CrossRef]
- Huang, Z.; Zhou, W.; Tang, X.; Zhu, D.; Luo, F. Effects of substrate roughness on infrared-emissivity characteristics of Au films deposited on Ni alloy. Thin Solid Films 2011, 519, 3100–3106. [Google Scholar] [CrossRef]
- Cverna, F.; ASM International; Materials Properties Database Committee. ASM Ready Reference; ASM International: Materials Park, OH, USA, 2002. [Google Scholar]
- Kim, Y.S.; Yang, H.W.; Shin, K.R.; Ko, Y.G.; Shin, D.H. Heat dissipation properties of oxide layers formed on 7075 Al alloy via plasma electrolytic oxidation. Surf. Coat. Technol. 2015, 269, 114–118. [Google Scholar] [CrossRef]
- Slifka, A.; Filla, B.; Phelps, J. Thermal Conductivity of Magnesium Oxide from Absolute, Steady-State Measurements. J. Res. Natl. Inst. Stand. Technol. 1998, 103, 357–363. [Google Scholar] [CrossRef]
- Han, Z.; Fina, A. Thermal conductivity of carbon nanotubes and their polymer nanocomposites: A review. Prog. Polym. Sci. 2011, 36, 914–944. [Google Scholar] [CrossRef]



| Sample | CNT (g/L) | KOH (g/L) | KF (g/L) | Na2SiO3 (g/L) | pH | Conductivity |
|---|---|---|---|---|---|---|
| 0 g/L CNT | 0 | 2 | 2 | 6 | 12.3 | 13.7 |
| 2.5 g/L CNT | 2.5 | 2 | 2 | 6 | 12.3 | 14.8 |
| 5 g/L CNT | 5 | 2 | 2 | 6 | 12.3 | 16.4 |
| 10 g/L CNT | 10 | 2 | 2 | 6 | 12.3 | 18.1 |
| Sample | Potential (V) | Current (A/cm2) | ba (mV) | bc (mV) |
|---|---|---|---|---|
| uncoated AZ31 | −1.481 | 1.12 × 10−5 | 94 | 82 |
| 0 g/L CNT | −1.493 | 7.14 × 10−6 | 128 | 118 |
| 2.5 g/L CNT | −1.445 | 3.67 × 10−6 | 55 | 100 |
| 5 g/L CNT | −1.473 | 1.43 × 10−6 | 132 | 108 |
| 10 g/L CNT | −1.376 | 4.80 × 10−7 | 146 | 111 |
| Sample | Rcoat (Ω⋅cm2) | CPEcoat (Ω⋅−1⋅sn⋅cm−2) | ncoat | Rpolar (Ω⋅cm2) | CPEdl (Ω⋅−1⋅sn⋅cm−2) | ndl |
|---|---|---|---|---|---|---|
| 0 g/L CNT | 9.47 × 102 | 6.37 × 10−8 | 0.79 | 1.13 × 104 | 2.99 × 10−9 | 0.78 |
| 2.5 g/L CNT | 2.09 × 103 | 5.25 × 10−8 | 0.80 | 1.43 × 104 | 3.23 × 10−9 | 0.69 |
| 5 g/L CNT | 7.60 × 103 | 3.60 × 10−8 | 0.81 | 5.43 × 105 | 1.04 × 10−9 | 0.60 |
| 10 g/L CNT | 1.60 × 104 | 3.97 × 10−8 | 0.78 | 9.87 × 105 | 3.21 × 10−9 | 0.86 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, M.; Chung, W. Effects of a Carbon Nanotube Additive on the Corrosion-Resistance and Heat-Dissipation Properties of Plasma Electrolytic Oxidation on AZ31 Magnesium Alloy. Materials 2018, 11, 2438. https://doi.org/10.3390/ma11122438
Hwang M, Chung W. Effects of a Carbon Nanotube Additive on the Corrosion-Resistance and Heat-Dissipation Properties of Plasma Electrolytic Oxidation on AZ31 Magnesium Alloy. Materials. 2018; 11(12):2438. https://doi.org/10.3390/ma11122438
Chicago/Turabian StyleHwang, Myungwon, and Wonsub Chung. 2018. "Effects of a Carbon Nanotube Additive on the Corrosion-Resistance and Heat-Dissipation Properties of Plasma Electrolytic Oxidation on AZ31 Magnesium Alloy" Materials 11, no. 12: 2438. https://doi.org/10.3390/ma11122438
APA StyleHwang, M., & Chung, W. (2018). Effects of a Carbon Nanotube Additive on the Corrosion-Resistance and Heat-Dissipation Properties of Plasma Electrolytic Oxidation on AZ31 Magnesium Alloy. Materials, 11(12), 2438. https://doi.org/10.3390/ma11122438




