Corrosion of Hydrogen Storage Metal Alloy LaMm-Ni4.1Al0.3Mn0.4Co0.45 in the Aqueous Solutions of Alkali Metal Hydroxides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Working Electrode
2.2. Electrolytes
2.3. Electrochemical Setup and Techniques
2.4. Other Techniques
3. Results and Discussion
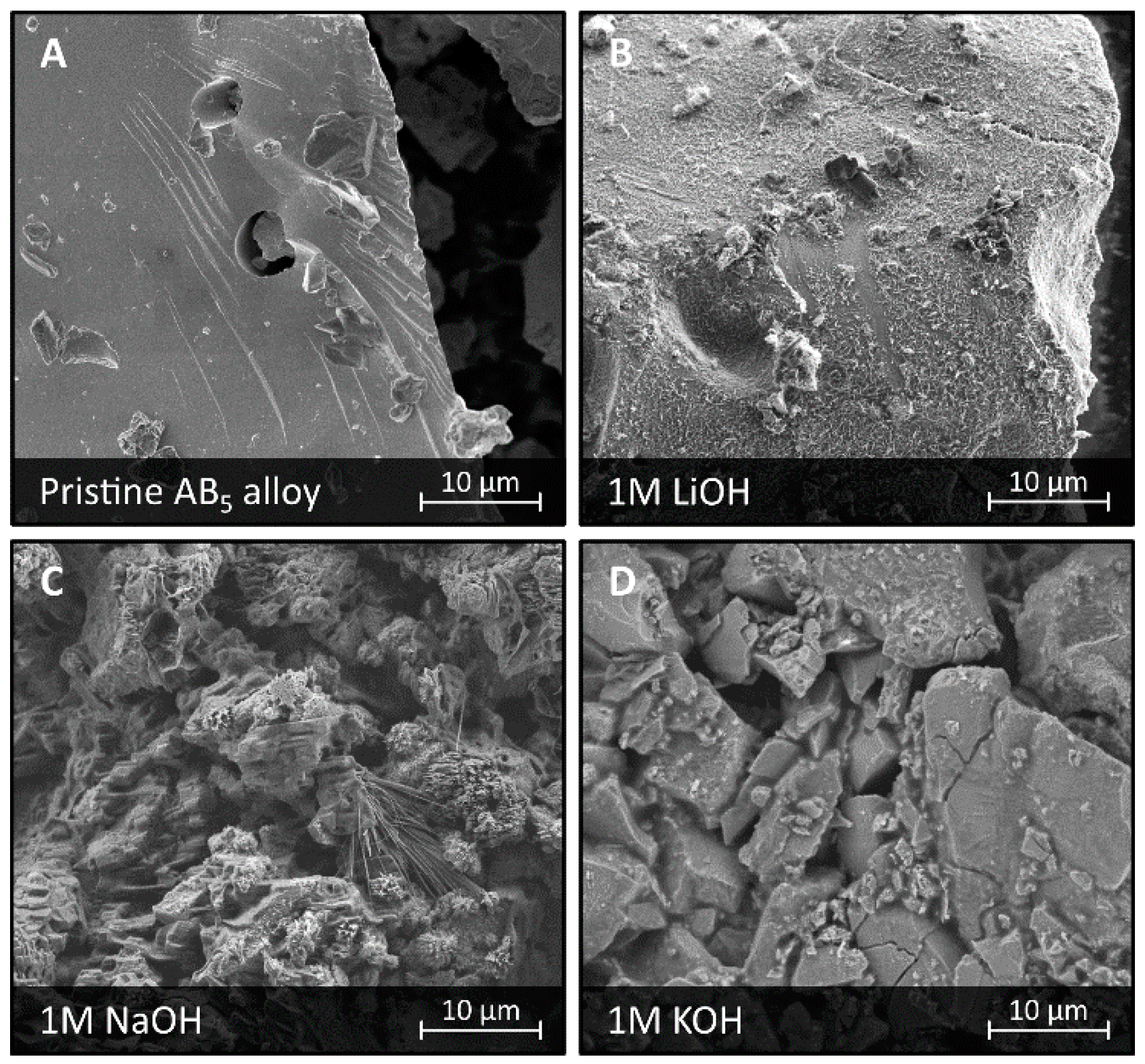
3.1. Corrosion in LiOH, NaOH, and KOH Solutions
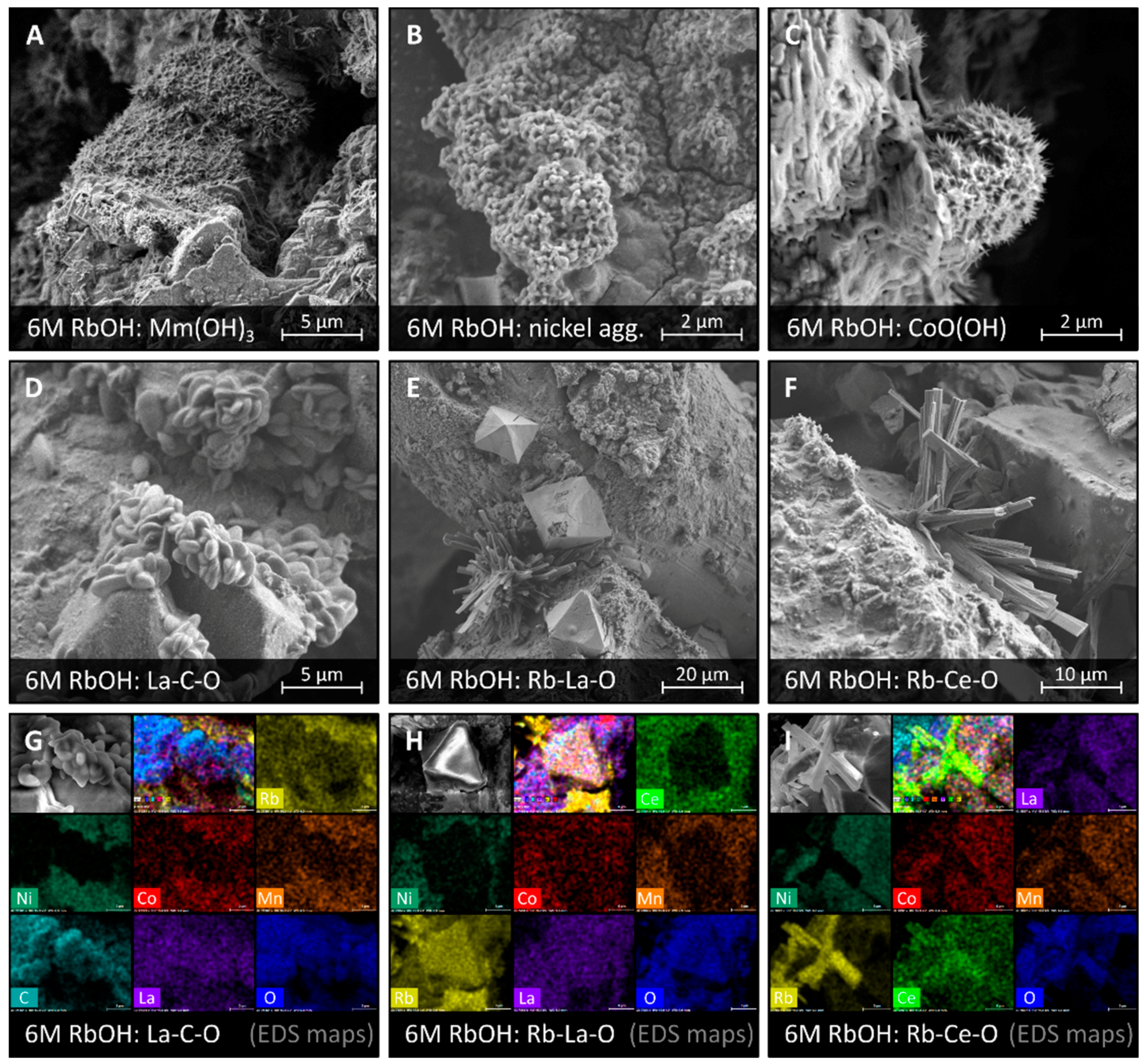
3.2. Corrosion in RbOH Solutions
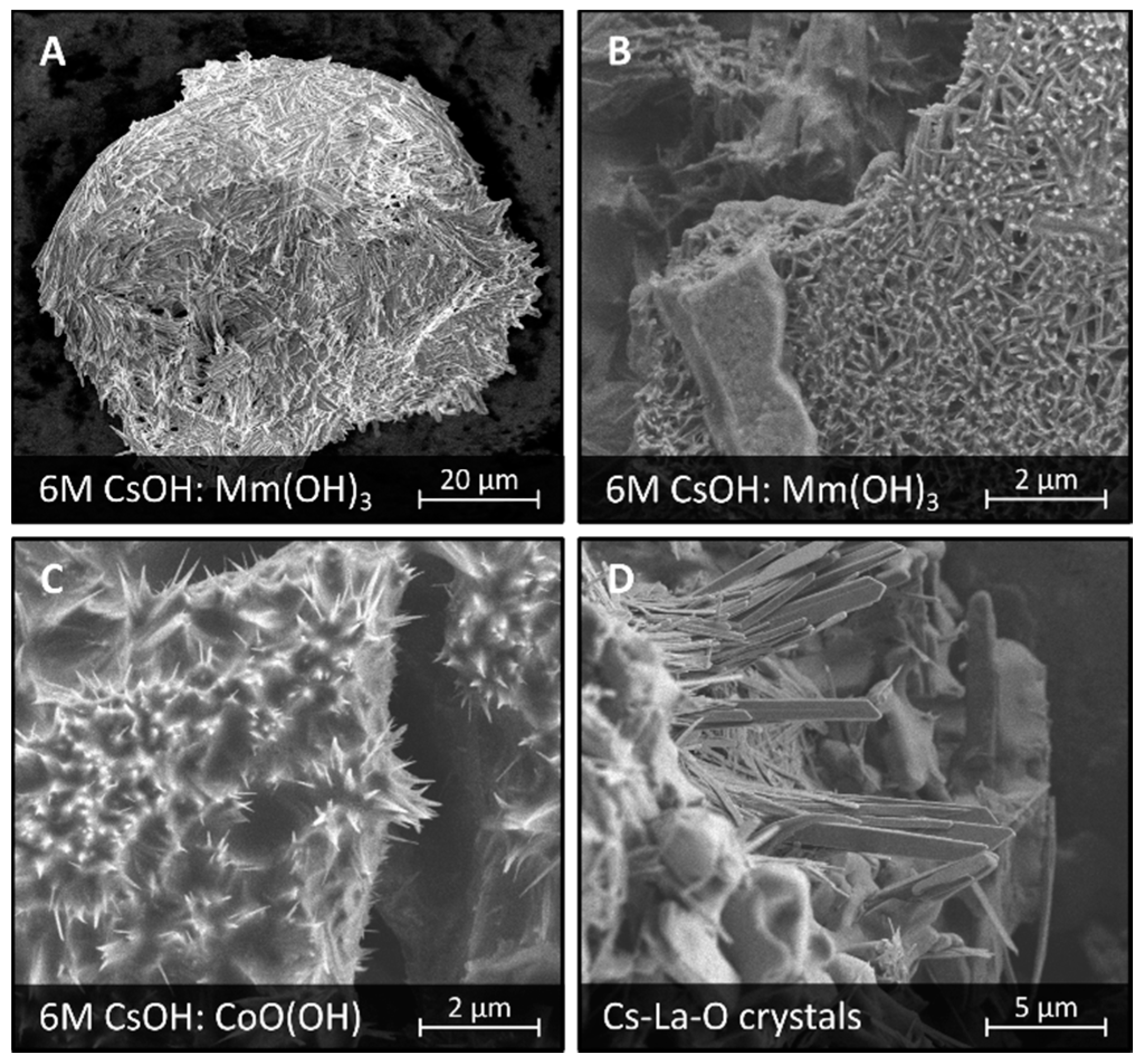
3.3. Corrosion in CsOH Solutions
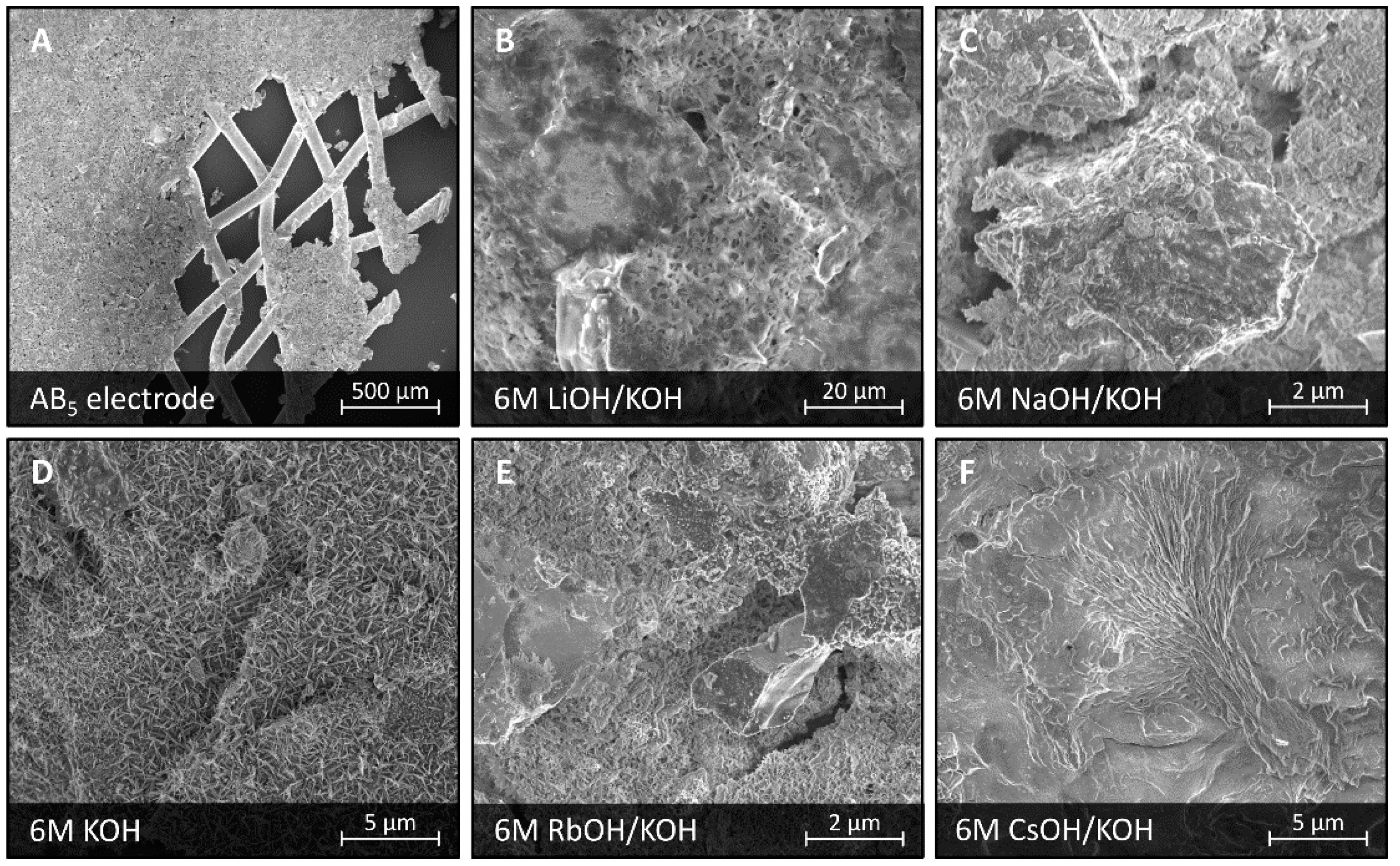
3.4. Corrosion in Bimetallic Mixed Electrolytes Based on KOH
4. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Linden, D.; Reddy, T.B. Handbook of Batteries, 3rd ed.; McGraw-Hill: New York, NY, USA, 2002. [Google Scholar]
- Fetcenko, M.A.; Ovshinsky, S.R.; Reichman, B.; Young, K.; Fierro, C.; Koch, J.; Zallen, A.; Mays, W.; Ouchi, T. Recent advances in NiMH battery technology. J. Power Sources 2007, 165, 544–551. [Google Scholar] [CrossRef]
- Pillot, C. Main trends for the rechargeable battery market worldwide 2007–2015. Available online: http://www.avem.fr/docs/pdf/AvicenneDiapoXining.pdf (accessed on 27 November 2018).
- Kleperis, J.; Wójcik, G.; Czerwiński, A.; Skowroński, J.; Kopczyk, M.; Bełtowska-Brzezińska, M. Electrochemical behavior of metal hydrides. J. Solid State Electrochem. 2001, 7, 229–249. [Google Scholar] [CrossRef]
- Nishimura, K.; Takasaki, T.; Sakai, T. Introduction of large-sized nickel-metal hydride battery GIGACELL® for industrial applications. J. Alloys Compd. 2013, 580, S353–S358. [Google Scholar] [CrossRef]
- Van Mal, H.H. Stability of Ternary Hydrides and Some Applications. Ph.D. Thesis, Technische Hogenschool, Delft, The Netherlands, May 1976. [Google Scholar]
- Willems, J.J.G. Metal Hydride Electrodes Stability of LaNi5-related Compounds. Philips J. Res. 1984. [Google Scholar] [CrossRef]
- Sakai, T.; Oguro, K.; Miyamura, H.; Kuriyama, N.; Kato, A.; Ishikawa, H. Some factors affecting the cycle lives of LaNi5-based alloy electrodes of hydrogen batteries. J. Less Common. Metals 1990, 161, 193–202. [Google Scholar] [CrossRef]
- Wallace, W.E.; Karlicek, R.F.; Imamura, H. Mechanism of Hydrogen Absorption by LaNi5. J. Phys. Chem. 1979, 3, 1708–1712. [Google Scholar] [CrossRef]
- Boonstra, A.H.; Lippits, G.J.M.; Bernards, T.N.M. Degradation Processes in a LaNi5 Electrode. J. Less Common. Metals 1989, 155, 119–131. [Google Scholar] [CrossRef]
- Notten, P.H.L.; Einerhand, R.E.F.; Daams, J.L.C. How to achieve long-term electrochemical cycling stability with hydride-forming electrode materials. J. Alloys Compd. 1995, 231, 604–610. [Google Scholar] [CrossRef]
- Raekelboom, E.; Cuervas, F.; Knosp, B.; Percheron-Guegan, A. Influence of the stoichiometry on the H-desorption rates measured in solid–gas phase and electrochemical cell for air-exposed LaNi5+x-type alloys. J. Alloys Compd. 2005, 404–406, 347–350. [Google Scholar] [CrossRef]
- Bala, H.; Dymek, M.; Adamczyk, L.; Giza, K.; Drulis, H. Hydrogen diffusivity, kinetics of H2O/H2 charge transfer and corrosion properties of LaNi5-powder, composite electrodes in 6 M KOH solution. J. Solid State Electrochem. 2014, 18, 3039–3048. [Google Scholar] [CrossRef]
- Cao, J.; Zhao, Y.; Zhang, L.; Jia, Z.; Wang, W.; Dong, Z.; Han, S.; Li, Y. Effect and mechanism of Mg on crystal structures and electrochemical cyclic stability of Ce2Ni7-type La–Mg–Ni-based hydrogen storage alloys. Int. J. Hydorgen Energy 2018, 43, 17800–17808. [Google Scholar] [CrossRef]
- Maurel, F.; Knosp, B.; Backhaus-Ricoult, M. Characterization of Corrosion Products of AB5-Type Hydrogen Storage Alloys for Nickel-Metal Hydride Batteries. J. Electrochem. Soc. 2000, 147, 78–86. [Google Scholar] [CrossRef]
- Morishita, S.; Kondo, Y.; Ohya, Y.; Towata, S.I. Quantitative Analysis of Corrosion Layer on LaNi5 Based Hydrogen Storage Alloys. Nippon Kagaku Kaishi 2001, 8, 469–474. [Google Scholar] [CrossRef]
- Kaiya, H.; Ookawa, T. Improvement in cycle life performance of high capacity nickel-metal hydride battery. J. Alloys Comp. 1995, 231, 598–603. [Google Scholar] [CrossRef]
- Maurel, F.; Leblanc, P.; Knosp, B.; Backhaus-Ricoult, M. Effect of yttrium on the corrosion of AB5-type alloys for nickel–metal hydride batteries. J. Alloys Compd. 2000, 309, 88–94. [Google Scholar] [CrossRef]
- Casini, J.C.S.; Saeku, M.J.; Guo, Z.; Liu, H.K.; Nunes de Faria, R.; Takiishi, H. Effect of Sn and Cu on Corrosion Resistance of LaMgAlMnCoNi Type Alloys. Mater. Sci. Forum 2017, 899, 353–357. [Google Scholar] [CrossRef]
- Wang, C.C.; Zhou, Y.T.; Yang, C.C.; Jiang, Q. Clarifying the capacity deterioration mechanism sheds light on the design of ultra-long-life hydrogen storage alloys. Chem. Eng. J. 2018, 352, 325–332. [Google Scholar] [CrossRef]
- Karwowska, M.; Jaroń, T.; Fijalkowski, K.J.; Leszczyński, P.J.; Rogulski, Z.; Czerwinski, A. Influence of electrolyte composition and temperature on behaviour of AB5 hydrogen storage alloy used as negative electrode in Ni–MH batteries. J. Power Sources 2014, 263, 304–309. [Google Scholar] [CrossRef]
- Karwowska, M.; Fijalkowski, K.J.; Czerwinski, A. Comparative study of hydrogen electrosorption from alkali metals electrolytes and hydrogen sorption from gas phase in AB5 alloy. Electrochim. Acta 2017, 252, 381–386. [Google Scholar] [CrossRef]
- Nowotny, H.N. Die Kristallstrukturen von Ni5Ce, Ni5Ca, Cu5La, Cu5Ca, Zn5La, Zn5Ca, Ni2Ce, MgCe, MgLa und MgSr. Z. Metallkd. 1942, 34, 247–253. [Google Scholar]
- Kisi, E.H.; Buckley, C.E.; Gray, E.M. The hydrogen activation of LaNi5. J. Alloys Compd. 1992, 185, 369–384. [Google Scholar] [CrossRef]
- Czerwiński, A.; Marassi, R. The absorption of hydrogen and deuterium in thin palladium electrodes: Part II: Basic solutions. J. Electroanal. Chem. 1992, 322, 373–381. [Google Scholar] [CrossRef]
- Geng, M.; Northwood, D.O. The characteristics of the negative electrode of a nickel-metal hydride battery. Int. J. Hydrogen Energy 1996, 21, 887–890. [Google Scholar] [CrossRef]
- Ise, T.; Murata, T.; Hirota, Y.; Nogami, M.; Nakahori, S. The effect of particle size on the electrochemical properties of hydrogen absorbing alloy electrodes. J. Alloys Compd. 2000, 298, 310–318. [Google Scholar] [CrossRef]
- Kim, D.M.; Lee, H.; Cho, K.; Lee, J.Y. Effect of Cu powder as an additive material on the inner pressure of a sealed-type Ni-MH rechargeable battery using a Zr-based alloy as an anode. J. Alloys Compd. 1999, 282, 261–267. [Google Scholar] [CrossRef]
- Naito, K.; Matsunami, T.; Okuno, K.; Matsuoka, M.; Iwakura, C. Factors affecting the characteristics of the negative electrodes for nickel-hydrogen batteries. J. Appl. Electrochem. 1993, 23, 1051–1055. [Google Scholar] [CrossRef]
- Rogulski, Z.; Dłubak, J.; Karwowska, M.; Krebs, M.; Pytlik, M.; Schmalz, E.; Gumkowska, A.; Czerwiński, A. Studies on metal hydride electrodes containing no binder additives. J. Power Sources 2010, 195, 7517–7523. [Google Scholar] [CrossRef]
- Inorganic Crystal Structure Database (ICSD) by Fachinformationszentrum Karlsruhe. Available online: https://icsd.fiz-karlsruhe.de (accessed on 17 September 2018).
- Brunn, H.; Hoppe, R. Neue ternäre Oxide der Seltenen Erden: Über RbSEO2 (SE = La, Nd, Sm, Eu, Gd) sowie CsNdO2. Z. Anorg. Allg. Chem. 1975, 417, 213–220. [Google Scholar] [CrossRef]
- Brunn, H.; Hoppe, R. Die Kristallstruktur von Cs2PrO3, sowie über Cs2CeO3, Cs2TbO3, Rb2CeO3 und Rb2TbO3. Z. Anorg. Allg. Chem. 1977, 433, 189–199. [Google Scholar] [CrossRef]
- Hoppe, R.; Brunn, H. Zur Kenntnis neuer ternäre Oxide der Seltenen Erden vom Typ CsSEO2 (SE = La, Pr, Lu). Rev. Chim. Miner. 1976, 13, 41–54. [Google Scholar]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karwowska, M.; Fijalkowski, K.J.; Czerwiński, A.A. Corrosion of Hydrogen Storage Metal Alloy LaMm-Ni4.1Al0.3Mn0.4Co0.45 in the Aqueous Solutions of Alkali Metal Hydroxides. Materials 2018, 11, 2423. https://doi.org/10.3390/ma11122423
Karwowska M, Fijalkowski KJ, Czerwiński AA. Corrosion of Hydrogen Storage Metal Alloy LaMm-Ni4.1Al0.3Mn0.4Co0.45 in the Aqueous Solutions of Alkali Metal Hydroxides. Materials. 2018; 11(12):2423. https://doi.org/10.3390/ma11122423
Chicago/Turabian StyleKarwowska, Malgorzata, Karol J. Fijalkowski, and Andrzej A. Czerwiński. 2018. "Corrosion of Hydrogen Storage Metal Alloy LaMm-Ni4.1Al0.3Mn0.4Co0.45 in the Aqueous Solutions of Alkali Metal Hydroxides" Materials 11, no. 12: 2423. https://doi.org/10.3390/ma11122423
APA StyleKarwowska, M., Fijalkowski, K. J., & Czerwiński, A. A. (2018). Corrosion of Hydrogen Storage Metal Alloy LaMm-Ni4.1Al0.3Mn0.4Co0.45 in the Aqueous Solutions of Alkali Metal Hydroxides. Materials, 11(12), 2423. https://doi.org/10.3390/ma11122423






