Study on Adsorption Mechanism and Failure Characteristics of CO2 Adsorption by Potassium-Based Adsorbents with Different Supports
Abstract
1. Introduction
2. Research Object and Method
2.1. Preparation and Characterization of Samples
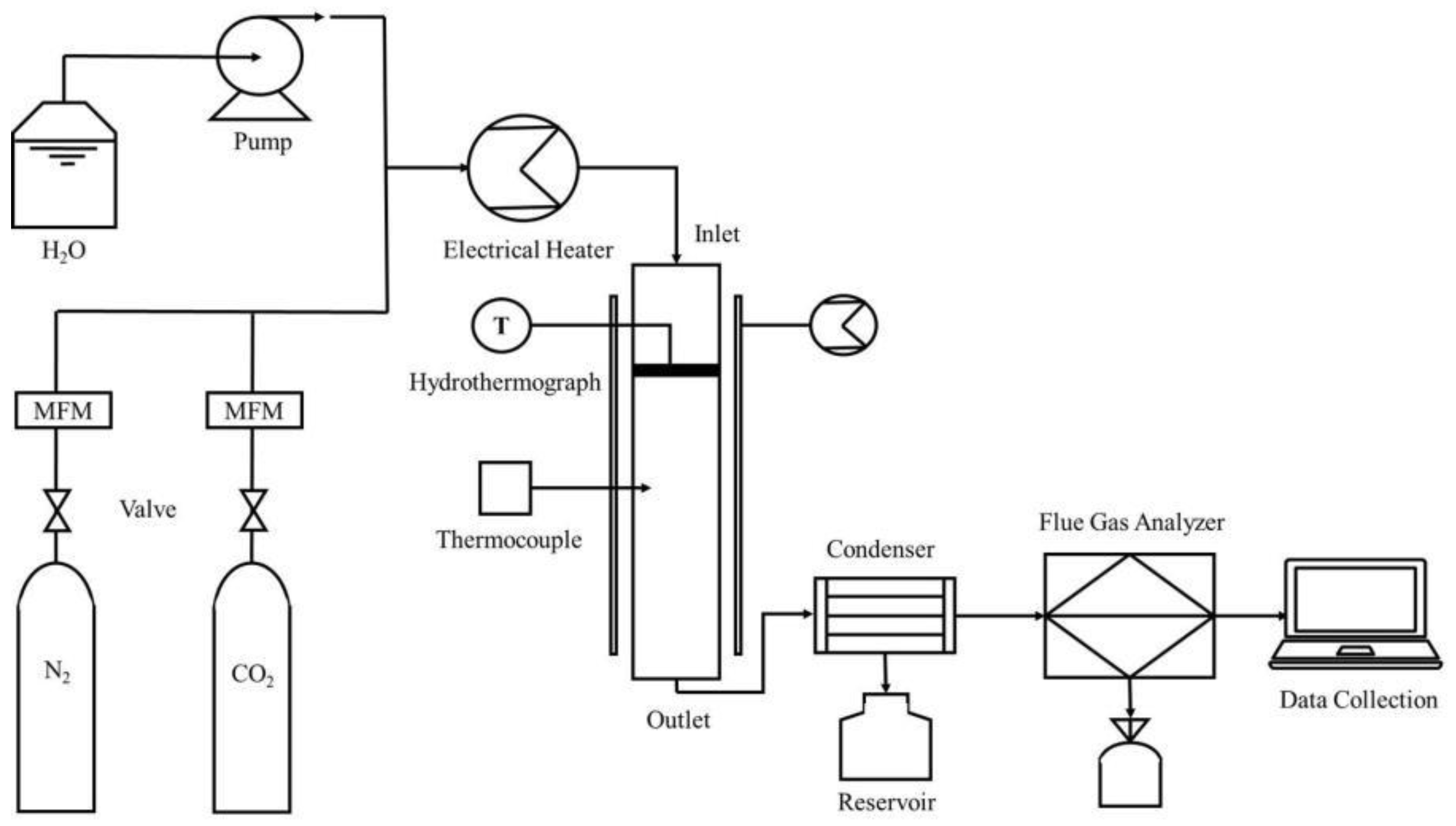
2.2. Fixed Bed CO2 Adsorption Experiment System
3. Results and Discussion
3.1. Effect of Loading and Adsorption Conditions on Carbonation Reaction
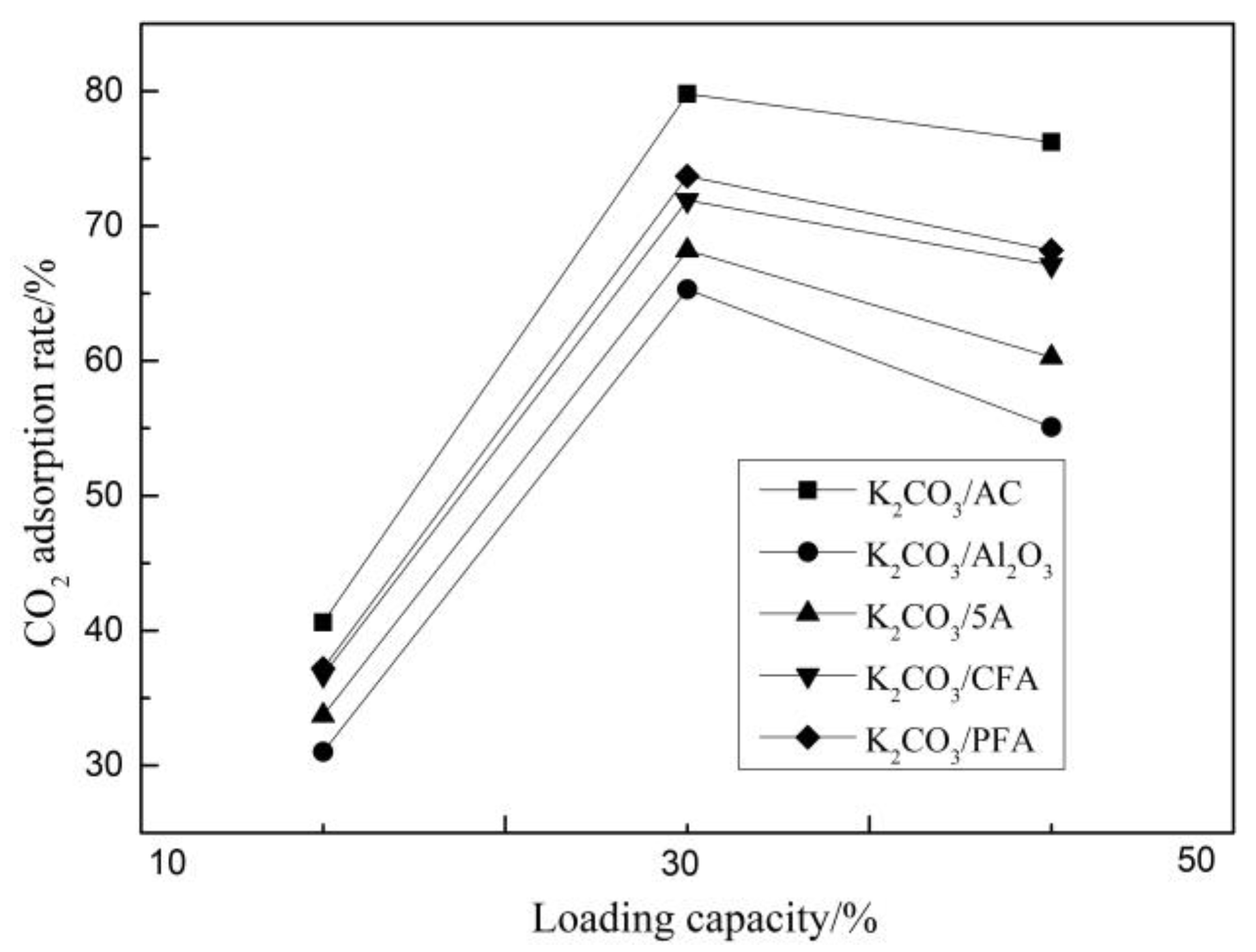
3.1.1. Carbonation Reaction Characteristics of Adsorbents under Different Load Conditions
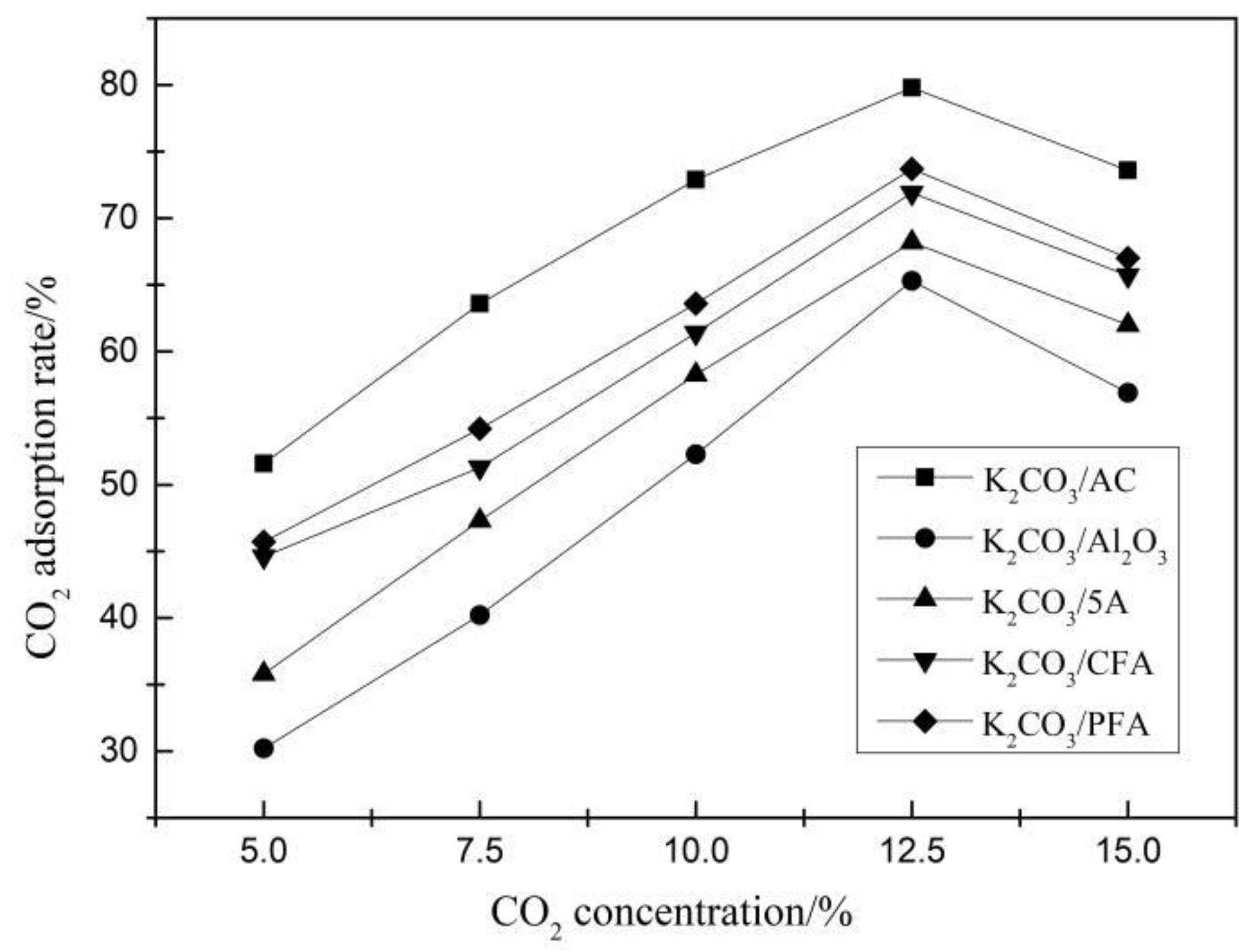
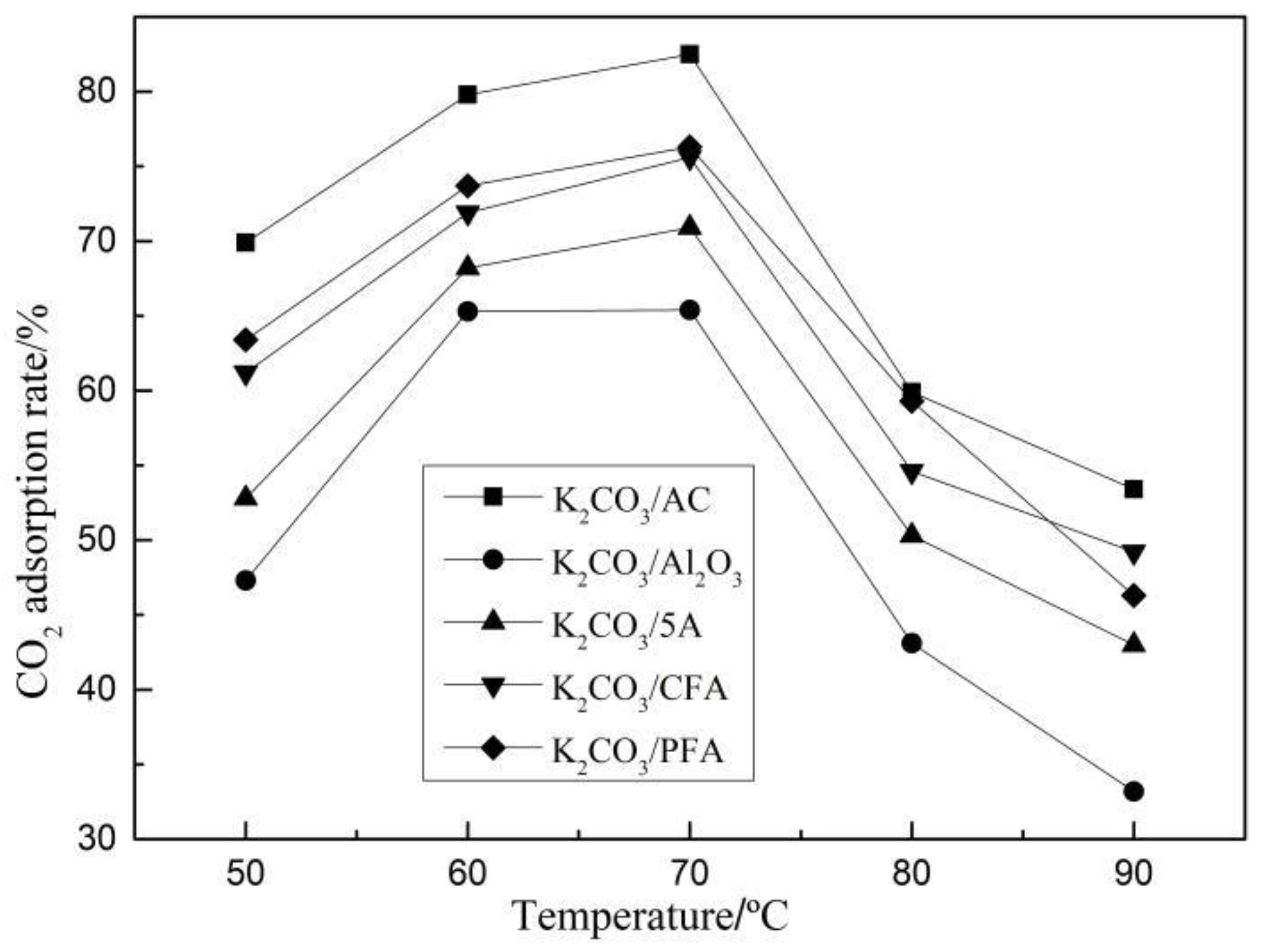
3.1.2. Carbonation Reaction Characteristics of Adsorbents under Different Adsorption Conditions
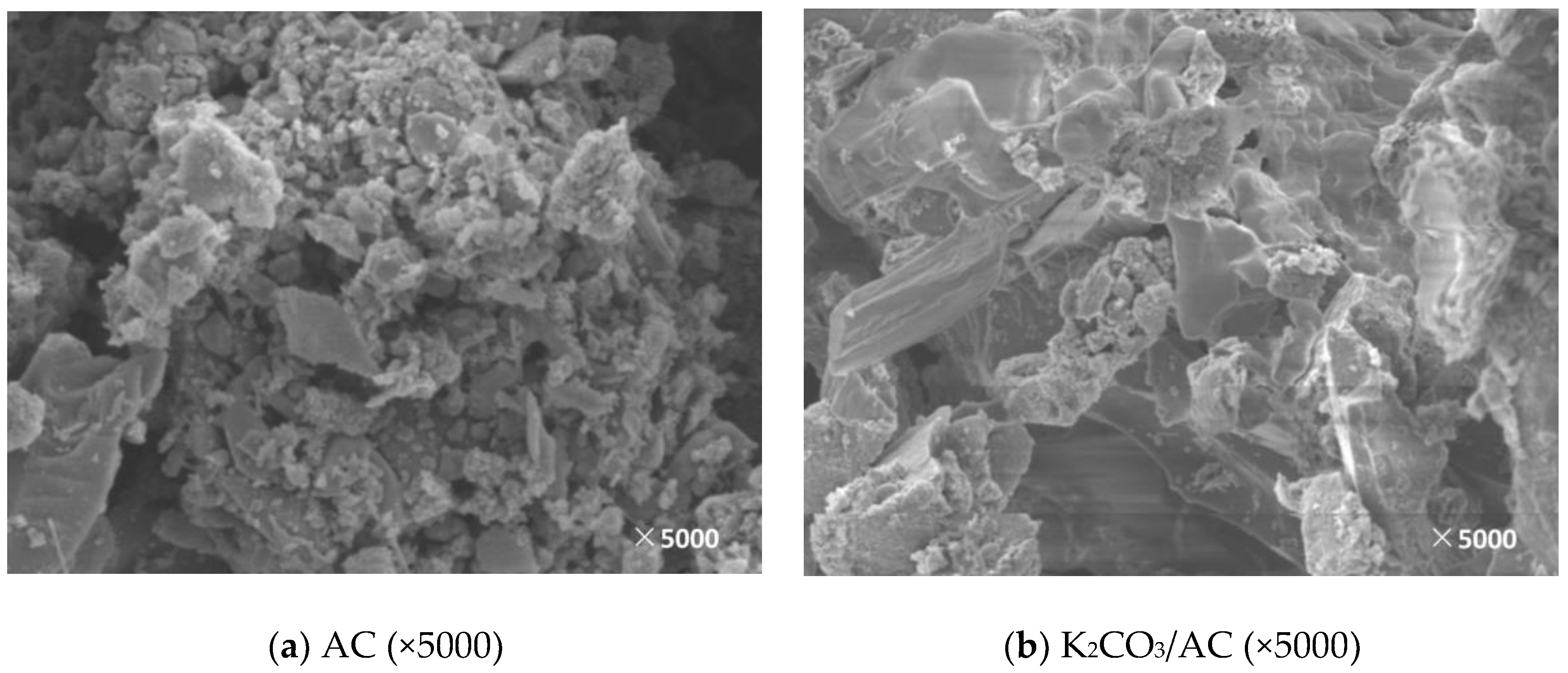
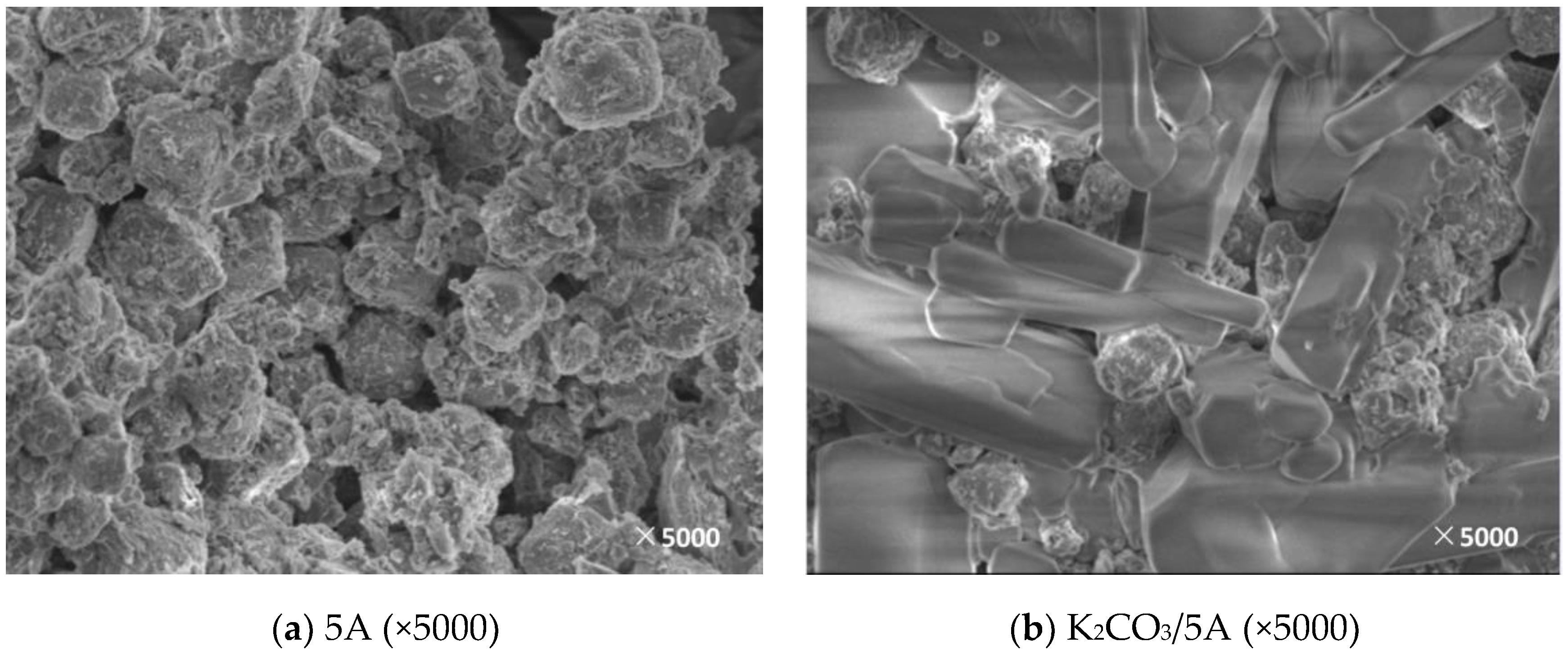
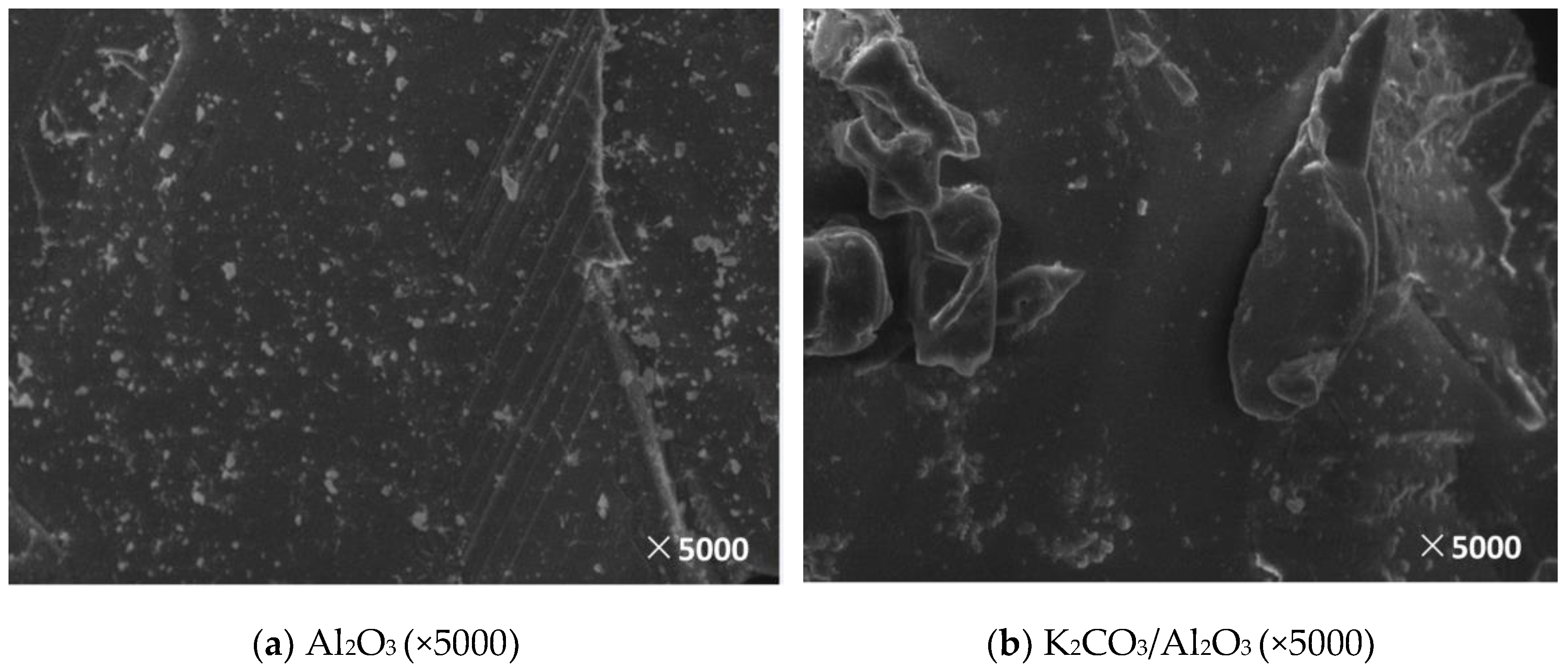
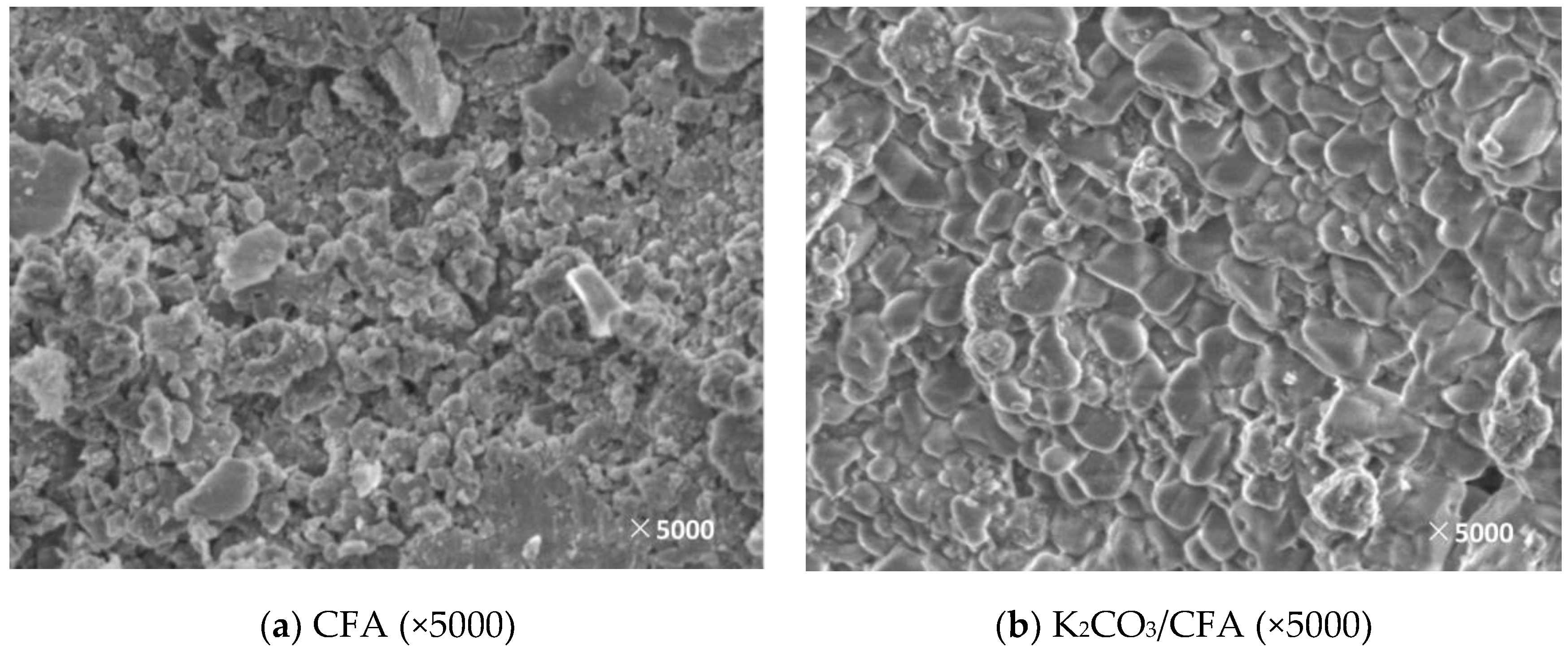
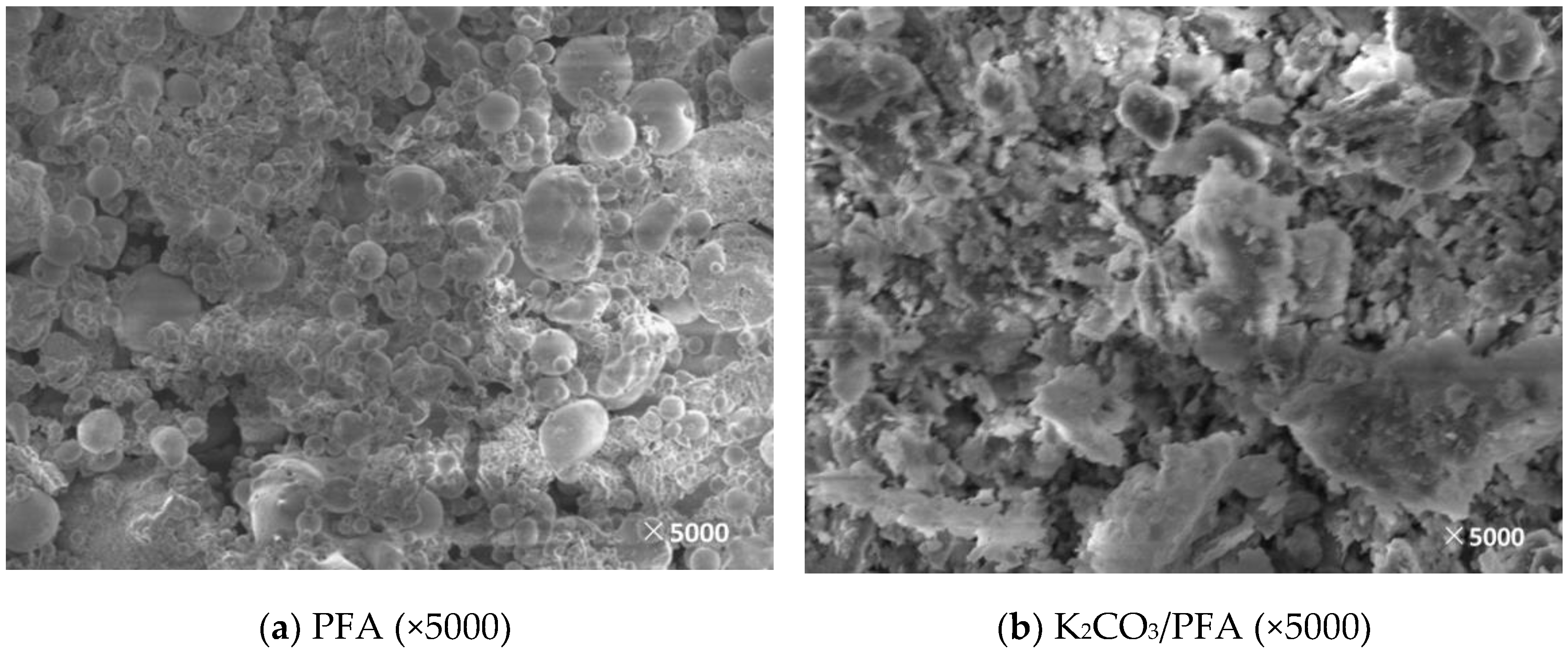
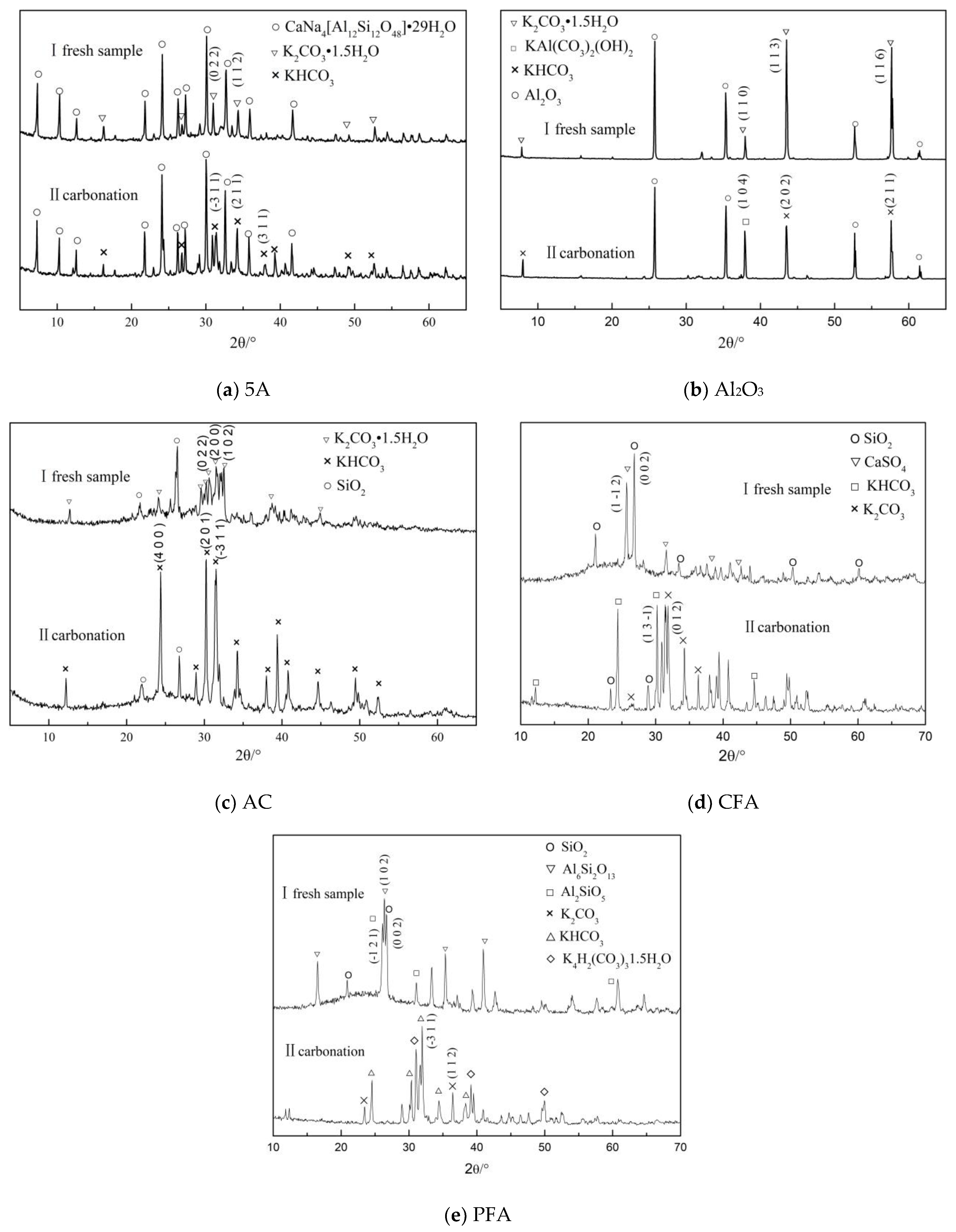
3.2. Microscopic Characteristics
3.2.1. Pore Structure
3.2.2. Surface Morphology
3.2.3. Lattice Structure
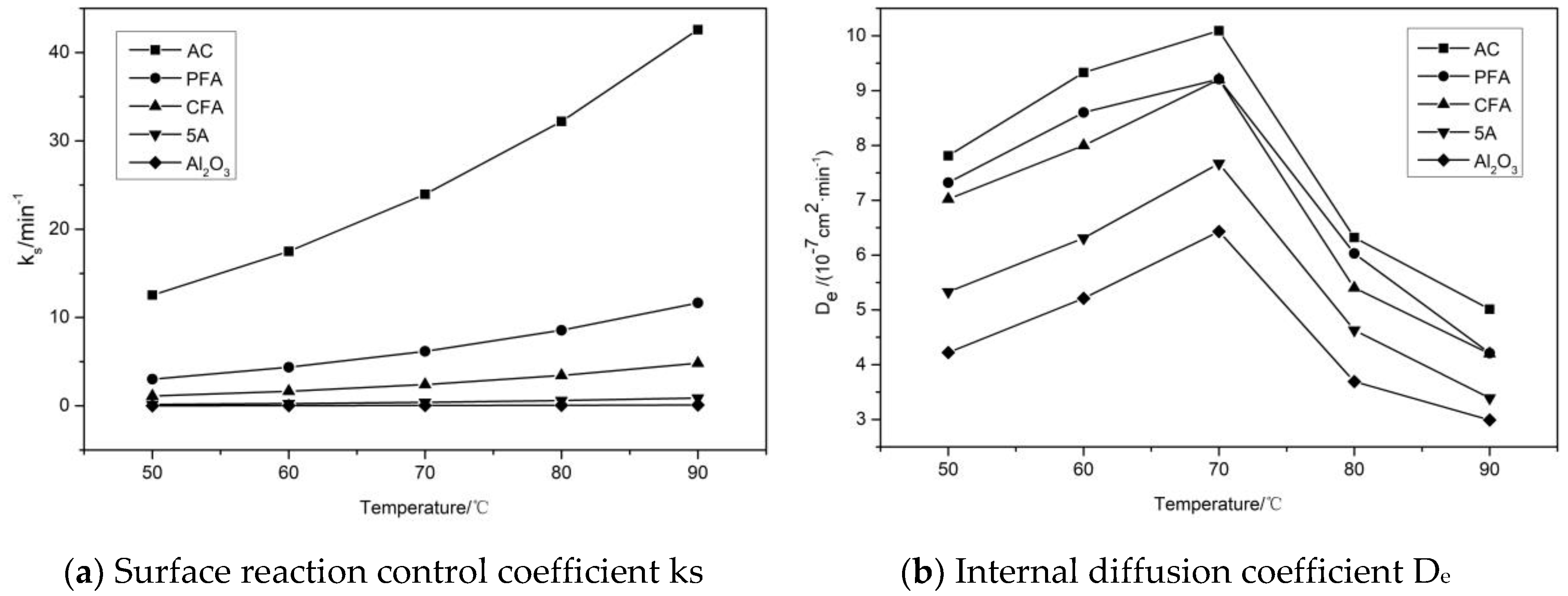
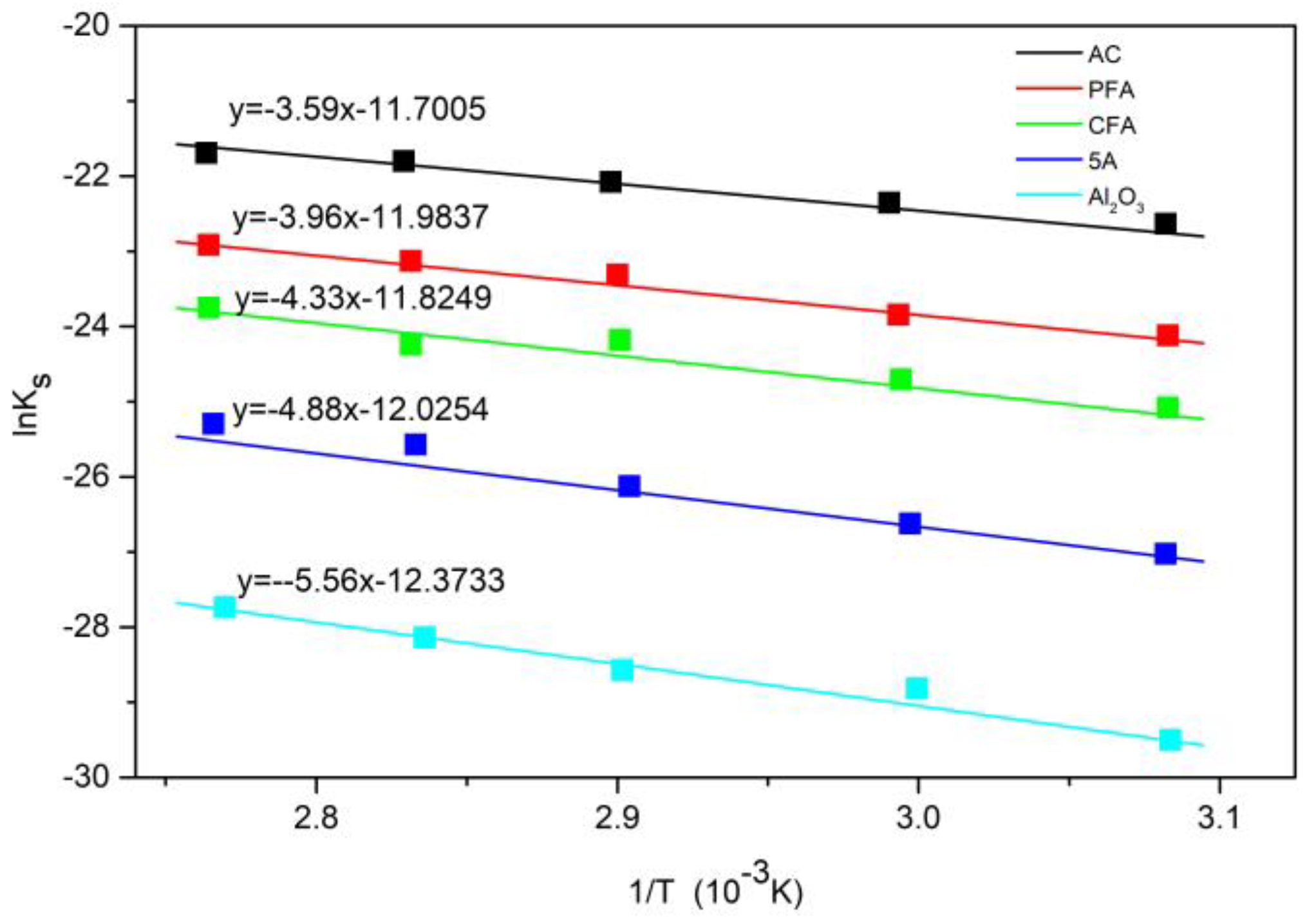
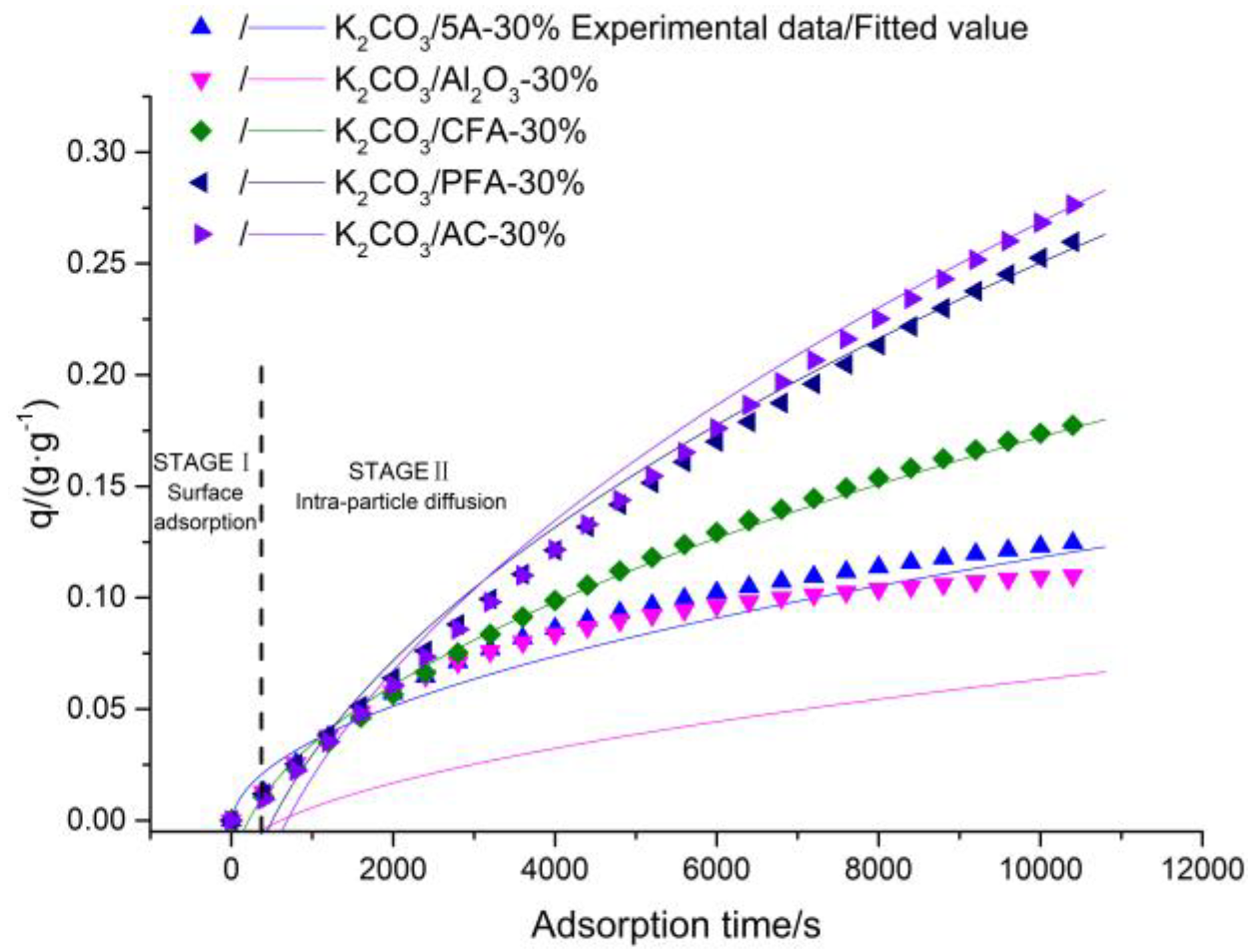
3.3. Reaction and Adsorption Kinetics
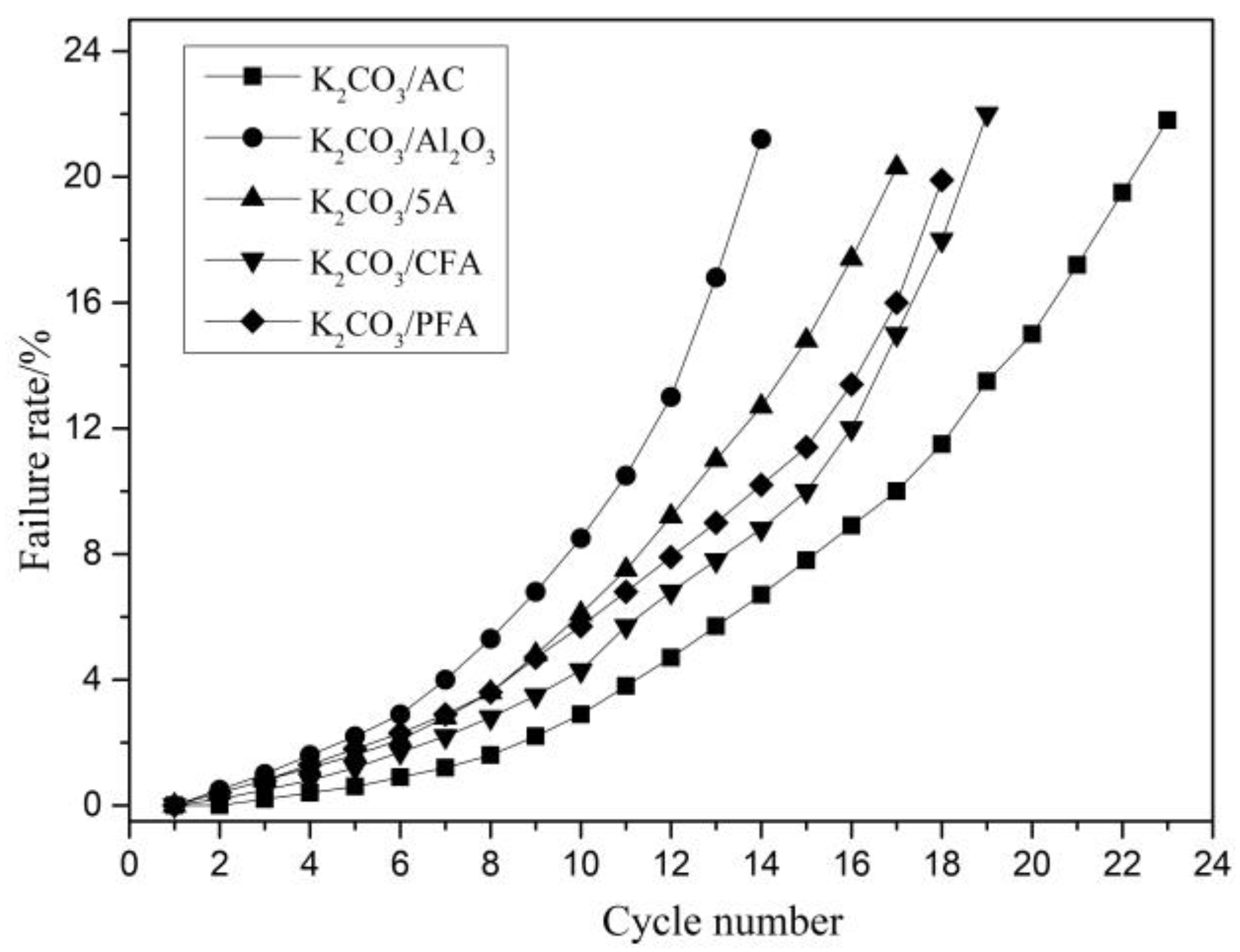
3.4. Study on the Effect of Flue Gas Composition on the Failure Characteristics of Adsorbents
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Le Quéré, C.; Andrew, R.M.; Canadell, J.G.; Sitch, S.; Korsbakken, J.I.; Peters, G.P.; Manning, A.C.; Boden, T.A.; Tans, P.P.; Houghton, R.A.; et al. Global Carbon Budget 2016. Earth Syst. Sci. Data 2016, 8, 605–649. [Google Scholar] [CrossRef]
- Pardemann, R.; Meyer, B. Pre-Combustion Carbon Capture. In Handbook of Clean Energy Systems; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015. [Google Scholar]
- Hu, J.; Galvita, V.V.; Poelman, H.; Marin, G.B. Advanced chemical looping materials for CO2 utilization: A review. Materials 2018, 11, 1187. [Google Scholar] [CrossRef] [PubMed]
- Thiruvenkatachari, R.; Su, S.; An, H.; Yu, X.X. Post combustion CO2 capture by carbon fibre monolithic adsorbents. Prog. Energy Combust. Sci. 2009, 35, 438–455. [Google Scholar] [CrossRef]
- Bhown, A.S.; Freeman, B.C. Analysis and status of post-combustion carbon dioxide capture technologies. Environ. Sci. Technol. 2011, 45, 8624–8632. [Google Scholar] [CrossRef] [PubMed]
- Bu, C.; Gómez-Barea, A.; Leckner, B.; Chen, X.; Pallarès, D.; Liu, D.; Lu, P. Oxy-fuel conversion of sub-bituminous coal particles in fluidized bed and pulverized combustors. Proc. Combust. Inst. 2017, 36, 3331–3339. [Google Scholar] [CrossRef]
- Buhre, B.J.; Elliott, L.K.; Sheng, C.D.; Gupta, R.P.; Wall, T.F. Oxy-fuel combustion technology for coal-fired power generation. Prog. Energy Combust. Sci. 2005, 31, 283–307. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, H.; Song, F.; Huang, T.; Ji, J.; Zhong, Q.; Chu, W.; Xu, Q. UiO-66-NH2/GO composite: Synthesis, characterization and CO2 adsorption performance. Materials 2018, 11, 589. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Wang, W.; Li, L.; Wang, Z.; Wang, S.; Ding, H.; Zhang, Z.; Sun, L.; Wang, W. Effective capture of carbon dioxide using hydrated sodium carbonate powders. Materials 2018, 11, 183. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Li, Y.; Liu, H.; Wu, S.; Lu, C. CO2 capture performance of calcium-based sorbent doped with manganese salts during calcium looping cycle. Appl. Energy 2012, 89, 368–373. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, P.; Duan, Y.; Zhao, C. CO2 capture of calcium based sorbents developed by sol–gel technique in the presence of steam. Chem. Eng. J. 2016, 295, 218–226. [Google Scholar] [CrossRef]
- Sánchez-Zambrano, K.S.; Lima Duarte, L.; Soares Maia, D.A.; Vilarrasa-García, E.; Bastos-Neto, M.; Rodríguez-Castellón, E.; Silva de Azevedo, D.C. CO2 capture with mesoporous silicas modified with amines by double functionalization: Assessment of adsorption/desorption cycles. Materials 2018, 11, 887. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.-C.; Chen, Y.-J.; Wu, C.-Y. Effect of relative humidity on adsorption breakthrough of CO2 on activated carbon fibers. Materials 2017, 10, 1296. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Harrison, D.P.; Gupta, R.P.; Green, D.A.; McMichael, W.J. Carbon dioxide capture using dry sodium-based sorbents. Energy Fuels 2004, 18, 569–575. [Google Scholar] [CrossRef]
- Querejeta, N.; Plaza, M.G.; Rubiera, F.; Pevida, C. Water vapor adsorption on biomass based carbons under post-combustion CO2 capture conditions: Effect of post-treatment. Materials 2016, 9, 359. [Google Scholar] [CrossRef] [PubMed]
- Ryu, C.K.; Lee, J.B.; Eom, T.H.; Baek, J.I.; Eom, H.M.; Yi, C.K. CO2 capture from flue gas using dry regenerable sorbents. In Proceedings of the 8th International Conference on Greenhouse Gas Control Technology, Trondheim, Norway, 19–22 June 2006. [Google Scholar]
- Zhao, C.; Chen, X.; Zhao, C. Characteristics of regeneration reaction of dry potassium-based sorbent for CO2 capture. J. Eng. Thermophys. 2009, 30, 2145–2148. [Google Scholar]
- Zhou, L.; Fan, J.; Shang, X. CO2 Capture and separation properties in the ionic liquid 1-n-Butyl-3-methylimidazolium nonafluorobutylsulfonate. Materials 2014, 7, 3867–3880. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Chen, X.; Zhao, C. Carbonation reaction characteristics of dry potassium-based sorbent for CO2 capture. J. Chem. Ind. Eng. 2008, 59, 2328–2333. [Google Scholar] [CrossRef]
- Zhao, C.; Chen, X.; Anthony, E.J.; Jiang, X.; Duan, L.; Wu, Y.; Dong, W.; Zhao, C. Capturing CO2 in flue gas from fossil fuel-fired power plants using dry regenerable alkali metal-based sorbent. Prog. Energy Combust. Sci. 2013, 39, 515–534. [Google Scholar] [CrossRef]
- Li, Y.; Sun, N.; Li, L.; Zhao, N.; Xiao, F.; Wei, W.; Sun, Y.; Huang, W. Grafting of amines on ethanol-extracted SBA-15 for CO2 adsorption. Materials 2013, 6, 981–999. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Guo, Y.; Li, C.; Lu, S. Removal of low concentration CO2, at ambient temperature using several potassium-based sorbents. Appl. Energy 2014, 124, 241–247. [Google Scholar] [CrossRef]
- Lee, S.C.; Choi, B.Y.; Lee, S.J.; Jung, S.Y.; Ryu, C.K.; Kim, J.C. CO2 absorption and regeneration using Na and K based sorbents. Stud. Surf. Sci. Catal. 2004, 153, 527–530. [Google Scholar] [CrossRef]
- Zhao, C.; Chen, X.; Zhao, C.; Wu, Y.; Dong, W. K2CO3/Al2O3 for Capturing CO2 in Flue Gas from Power Plants. Part 3: CO2 Capture Behaviors of K2CO3/Al2O3 in a Bubbling Fluidized-Bed Reactor. Energy Fuels 2012, 26, 3062–3068. [Google Scholar] [CrossRef]
- Fan, B.G.; Jia, L.; Li, B.; Yao, Y.X.; Huo, R.P.; Zhao, R.; Qiao, X.L.; Jin, Y. Study on the effects of the pyrolysis atmosphere on the elemental mercury adsorption characteristics and mechanism of biomass char. Energy Fuels 2018, 32, 6869–6878. [Google Scholar] [CrossRef]
- Jia, L.; Fan, B.G.; Li, B.; Yao, Y.X.; Huo, R.P.; Zhao, R.; Qiao, X.L.; Jin, Y. Effects of pyrolysis mode and particle size on the microscopic characteristics and mercury adsorption characteristics of biomass Char. Bioresources 2018, 13, 5450–5471. [Google Scholar]
- Alghamdi, A.A.; Alshahrani, A.F.; Khdary, N.H.; Alharthi, F.A.; Alattas, H.A.; Adil, S.F. Enhanced CO2 Adsorption by Nitrogen-Doped Graphene Oxide Sheets (N-GOs) prepared by employing polymeric precursors. Materials 2018, 11, 578. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, P. Fractals in Surface Science: Scattering and Thermodynamics of Adsorbed Films. In Chemistry and Physics of Solid Surfaces VII; Springer: Berlin/Heidelberg, Germany, 1988; pp. 283–305. [Google Scholar]
- Ji, C.; Huang, X.; Li, L.; Xiao, F.; Zhao, N.; Wei, W. Pentaethylenehexamine-loaded hierarchically porous silica for CO2 adsorption. Materials 2016, 9, 835. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.G.; Jia, L.; Li, B.; Huo, R.P.; Yao, Y.X.; Han, F.; Qiao, X.L.; Jin, Y. Study on desulfurization performances of magnesium slag with different hydration modification. J. Mater. Cycles Waste Manag. 2018, 20, 1771–1780. [Google Scholar] [CrossRef]
- Jia, L.; Fan, B.G.; Huo, R.P.; Li, B.; Yao, Y.X.; Han, F.; Qiao, X.L.; Jin, Y. Study on quenching hydration reaction kinetics and desulfurization characteristics of magnesium slag. J. Clean. Prod. 2018, 190, 12–23. [Google Scholar] [CrossRef]
| Theoretical Loading (%) | Actual Loading (%) | ||||
|---|---|---|---|---|---|
| AC | Al2O3 | 5A | CFA | PFA | |
| 10 | 9.4 | 7.7 | 8.4 | 8.5 | 8.9 |
| 30 | 28.6 | 21.2 | 24.3 | 26.2 | 27.5 |
| 50 | 44.3 | 32.5 | 37.5 | 39.6 | 41.3 |
| Theoretical Loading (%) | Actual Loading (%) | ||||
|---|---|---|---|---|---|
| AC | Al2O3 | 5A | CFA | PFA | |
| 10 | 9.63 | 7.68 | 8.45 | 8.64 | 8.91 |
| 30 | 28.72 | 21.32 | 24.24 | 26.27 | 27.65 |
| 50 | 44.32 | 32.45 | 37.73 | 39.81 | 41.36 |
| Samples | BET Specific Surface Area m2·g−1 | Cumulative Pore Volume cm3·g−1 | Fractal Dimension | Pore Richness Z | Relative Pore Volume % | |
|---|---|---|---|---|---|---|
| Micropore and Mesopore | Macropore | |||||
| K2CO3 | 0.78 | 0.0073 | 2.0987 | 106.849 | 99.22 | 0.78 |
| AC | 493.87 | 0.3738 | 2.9050 | 1321.215 | 95.56 | 4.44 |
| K2CO3/AC-30% | 200.94 | 0.1630 | 2.9009 | 1232.761 | 96.84 | 3.16 |
| Al2O3 | 18.54 | 0.0445 | 2.4512 | 416.629 | 93.65 | 6.35 |
| K2CO3/Al2O3-30% | 7.13 | 0.0275 | 2.5325 | 259.27 | 98.46 | 1.54 |
| 5A | 44.75 | 0.1012 | 2.7958 | 443.061 | 92.61 | 7.39 |
| K2CO3/5A-30% | 8.53 | 0.0325 | 2.7789 | 262.403 | 96.00 | 4.00 |
| CFA | 80.85 | 0.0194 | 2.8775 | 4167.371 | 96.31 | 3.69 |
| K2CO3/CFA-30% | 47.02 | 0.0119 | 2.8543 | 3950.847 | 95.44 | 4.56 |
| PFA | 99.70 | 0.0257 | 2.8733 | 3879.494 | 93.81 | 6.19 |
| K2CO3/PFA-30% | 73.17 | 0.0199 | 2.8783 | 3676.935 | 93.35 | 6.65 |
| Sorbents (30% Loading) | Pseudo-First Order Kinetic Equation | Pseudo-Second Order Kinetic Equation | Intra-Particle Diffusion Kinetic Equation | Elovich Equation | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | k1 | qe | R2 | k2 | qe | R2 | kid | c | R2 | α | β | |
| K2CO3/5A | 0.9931 | 2.38 × 10−4 | 1379 | 0.9985 | 2.25 × 10−9 | 1793 | 0.9728 | 12.0765 | −26 | 0.9973 | 0.3901 | 2.01 × 10−3 |
| K2CO3/Al2O3 | 0.9975 | 3.31 × 10−5 | 1138 | 0.9956 | 1.75 × 10−9 | 1367 | 0.8789 | 8.4099 | −208 | 0.9712 | 0.3784 | 3.15 × 10−3 |
| K2CO3/CFA | 0.9994 | 6.28 × 10−4 | 2429 | 0.9999 | 2.59 × 10−7 | 3605 | 0.9969 | 20.1509 | −294 | 0.9995 | 0.5409 | 3.31 × 10−4 |
| K2CO3/PFA | 0.9998 | 7.09 × 10−4 | 5524 | 0.9999 | 5.12 × 10−7 | 8386 | 0.9962 | 32.3145 | −727 | 0.9993 | 0.5851 | 7.92 × 10−4 |
| K2CO3/AC | 0.9995 | 7.39 × 10−4 | 6404 | 0.9999 | 6.46 × 10−7 | 10199 | 0.9948 | 36.4391 | −957 | 0.9991 | 0.8879 | 2.54 × 10−4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, B.-g.; Jia, L.; Wang, Y.-l.; Zhao, R.; Mei, X.-s.; Liu, Y.-y.; Jin, Y. Study on Adsorption Mechanism and Failure Characteristics of CO2 Adsorption by Potassium-Based Adsorbents with Different Supports. Materials 2018, 11, 2424. https://doi.org/10.3390/ma11122424
Fan B-g, Jia L, Wang Y-l, Zhao R, Mei X-s, Liu Y-y, Jin Y. Study on Adsorption Mechanism and Failure Characteristics of CO2 Adsorption by Potassium-Based Adsorbents with Different Supports. Materials. 2018; 11(12):2424. https://doi.org/10.3390/ma11122424
Chicago/Turabian StyleFan, Bao-guo, Li Jia, Yan-lin Wang, Rui Zhao, Xue-song Mei, Yan-yan Liu, and Yan Jin. 2018. "Study on Adsorption Mechanism and Failure Characteristics of CO2 Adsorption by Potassium-Based Adsorbents with Different Supports" Materials 11, no. 12: 2424. https://doi.org/10.3390/ma11122424
APA StyleFan, B.-g., Jia, L., Wang, Y.-l., Zhao, R., Mei, X.-s., Liu, Y.-y., & Jin, Y. (2018). Study on Adsorption Mechanism and Failure Characteristics of CO2 Adsorption by Potassium-Based Adsorbents with Different Supports. Materials, 11(12), 2424. https://doi.org/10.3390/ma11122424





