Thermal Shock Performance of DBA/AMB Substrates Plated by Ni and Ni–P Layers for High-Temperature Applications of Power Device Modules
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Thermal Shock Behavior
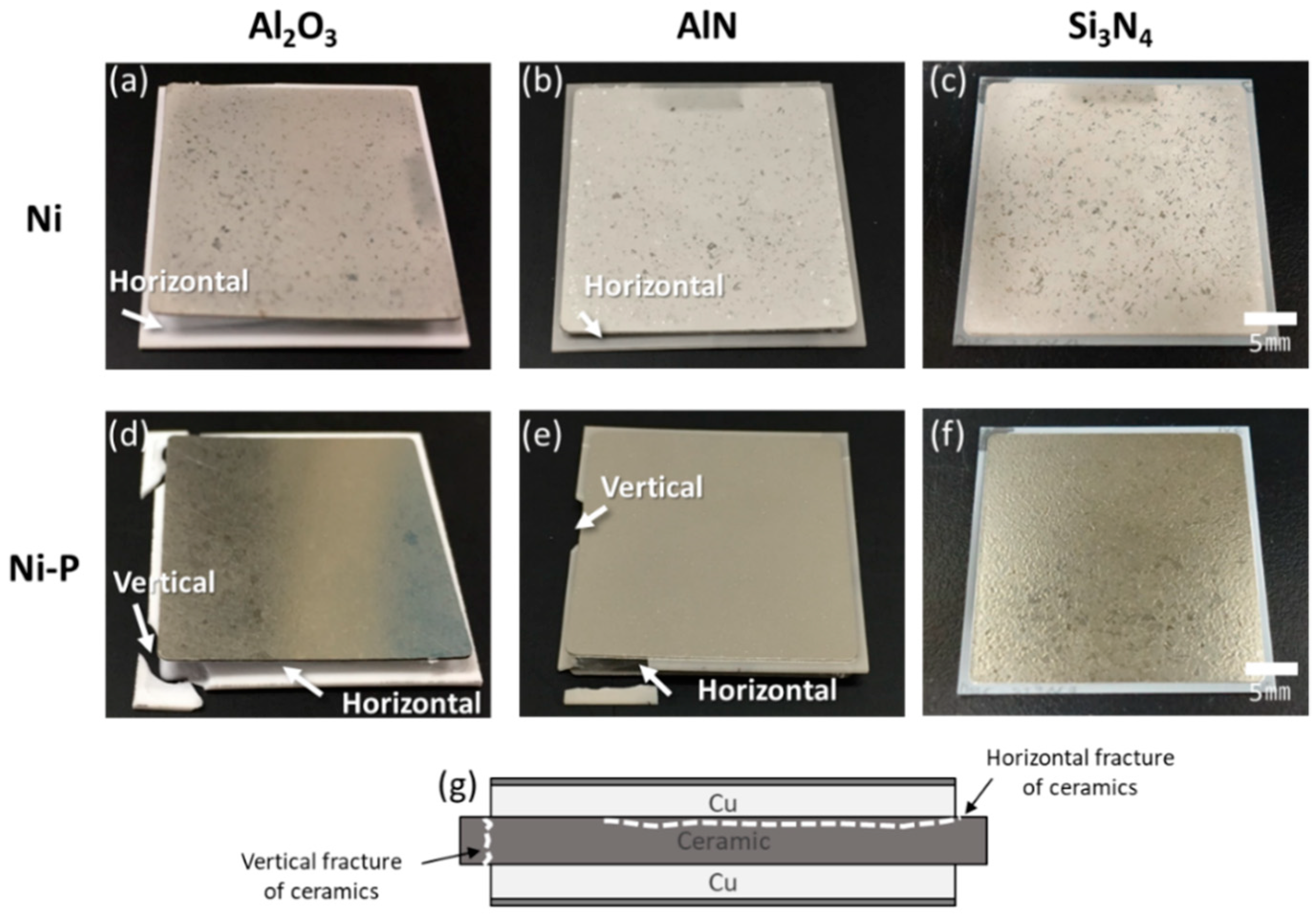
3.1.1. Active Metal Brazing (AMB)
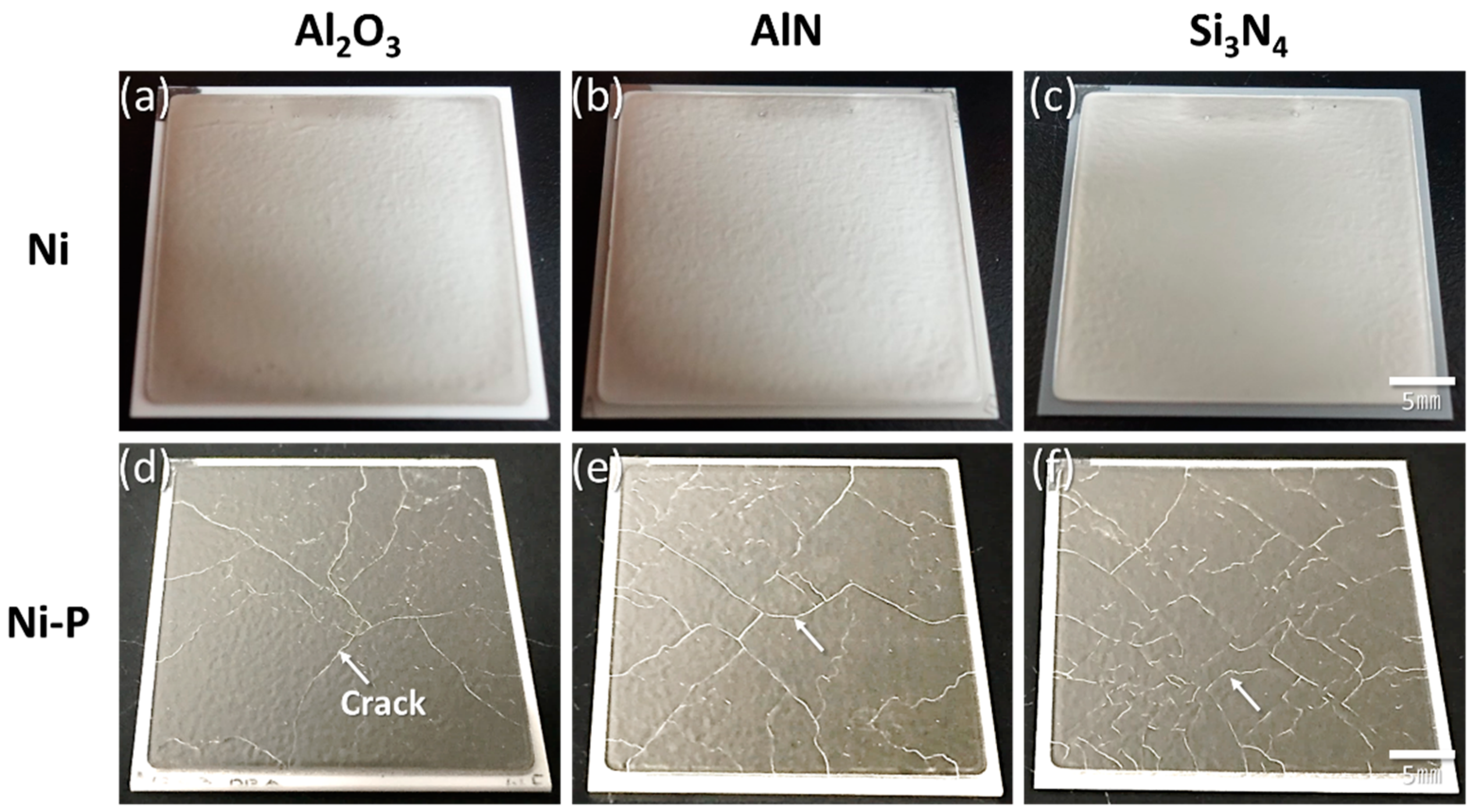
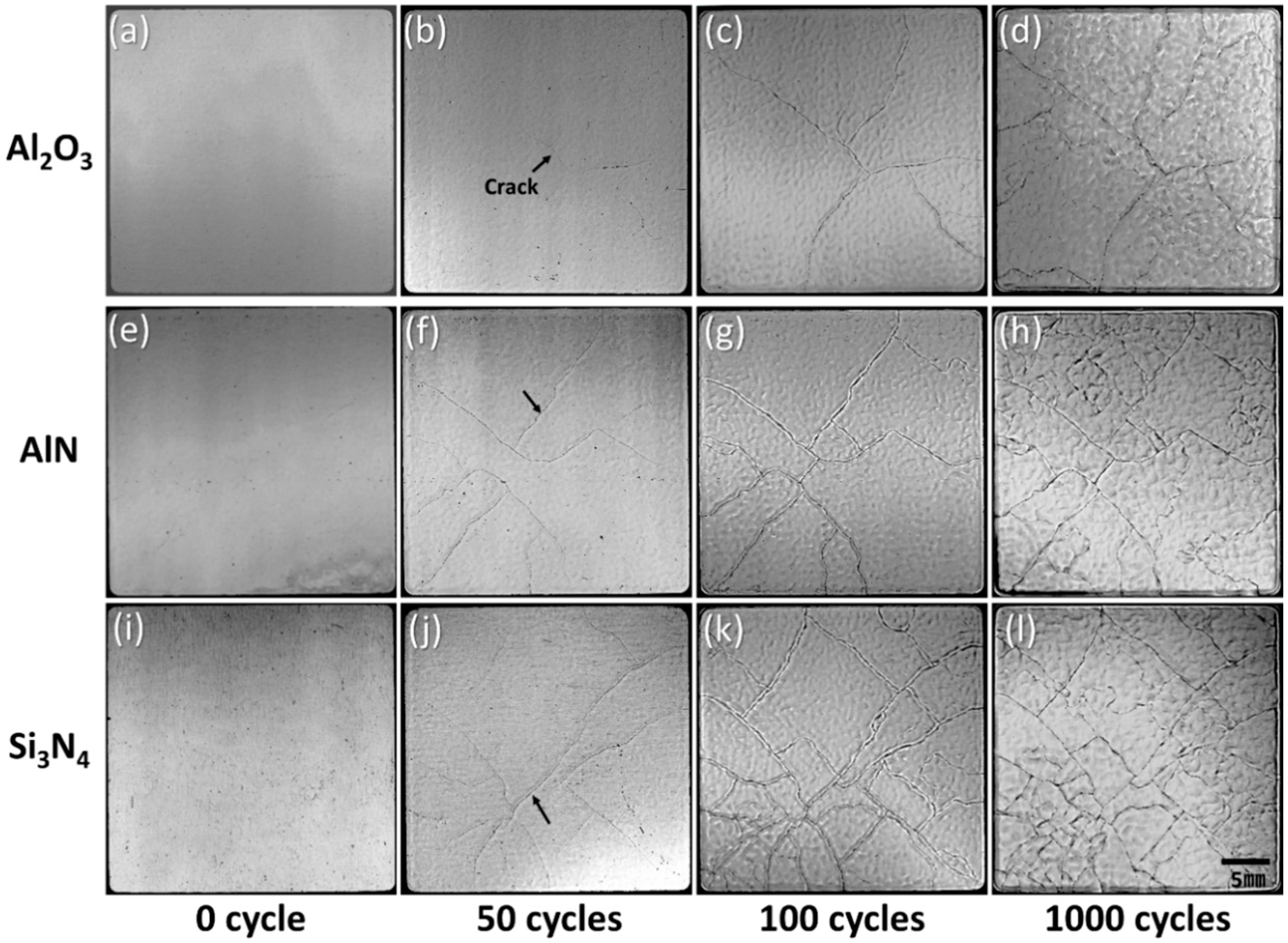
3.1.2. Direct Bonded Aluminum (DBA)
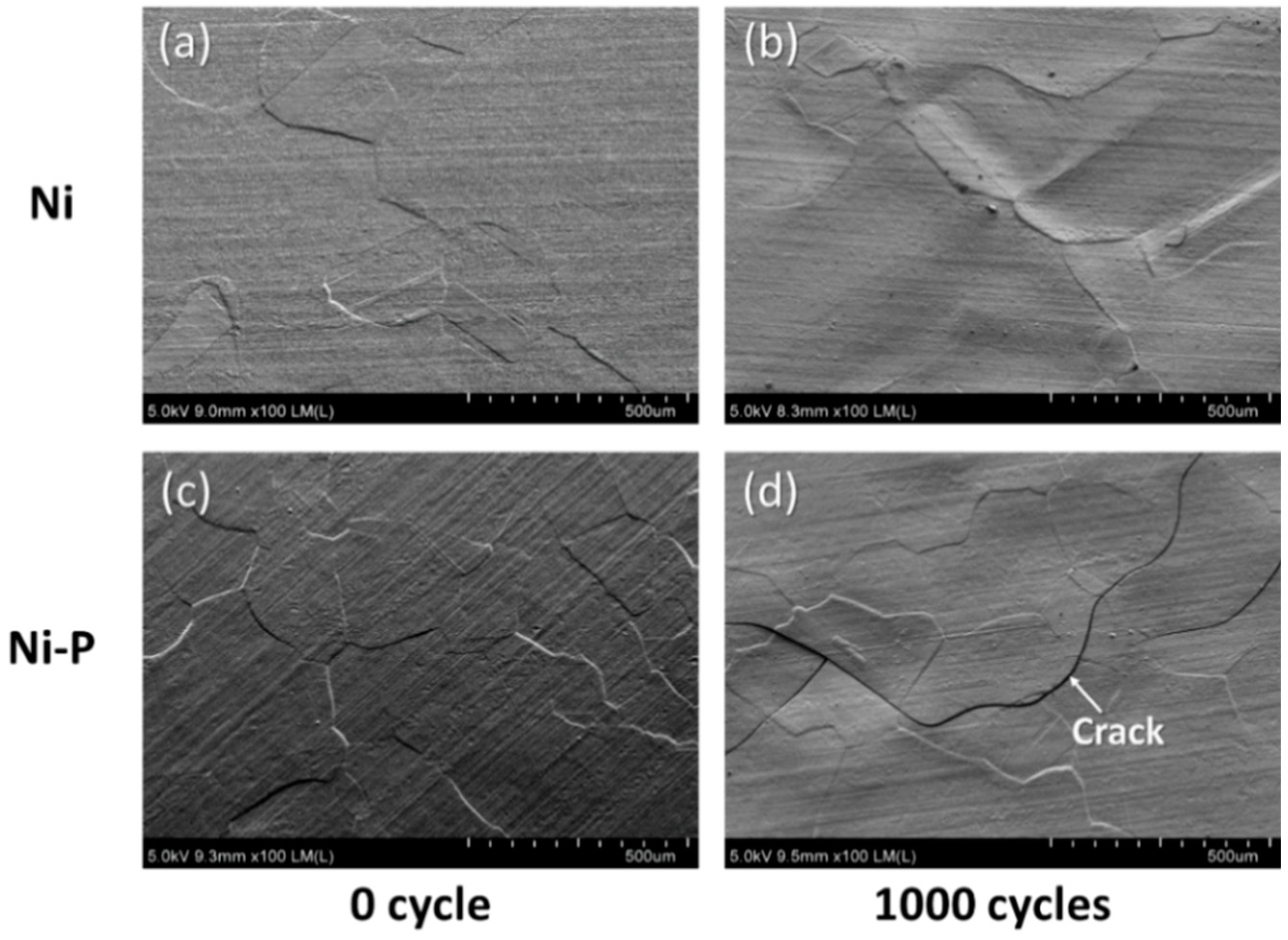
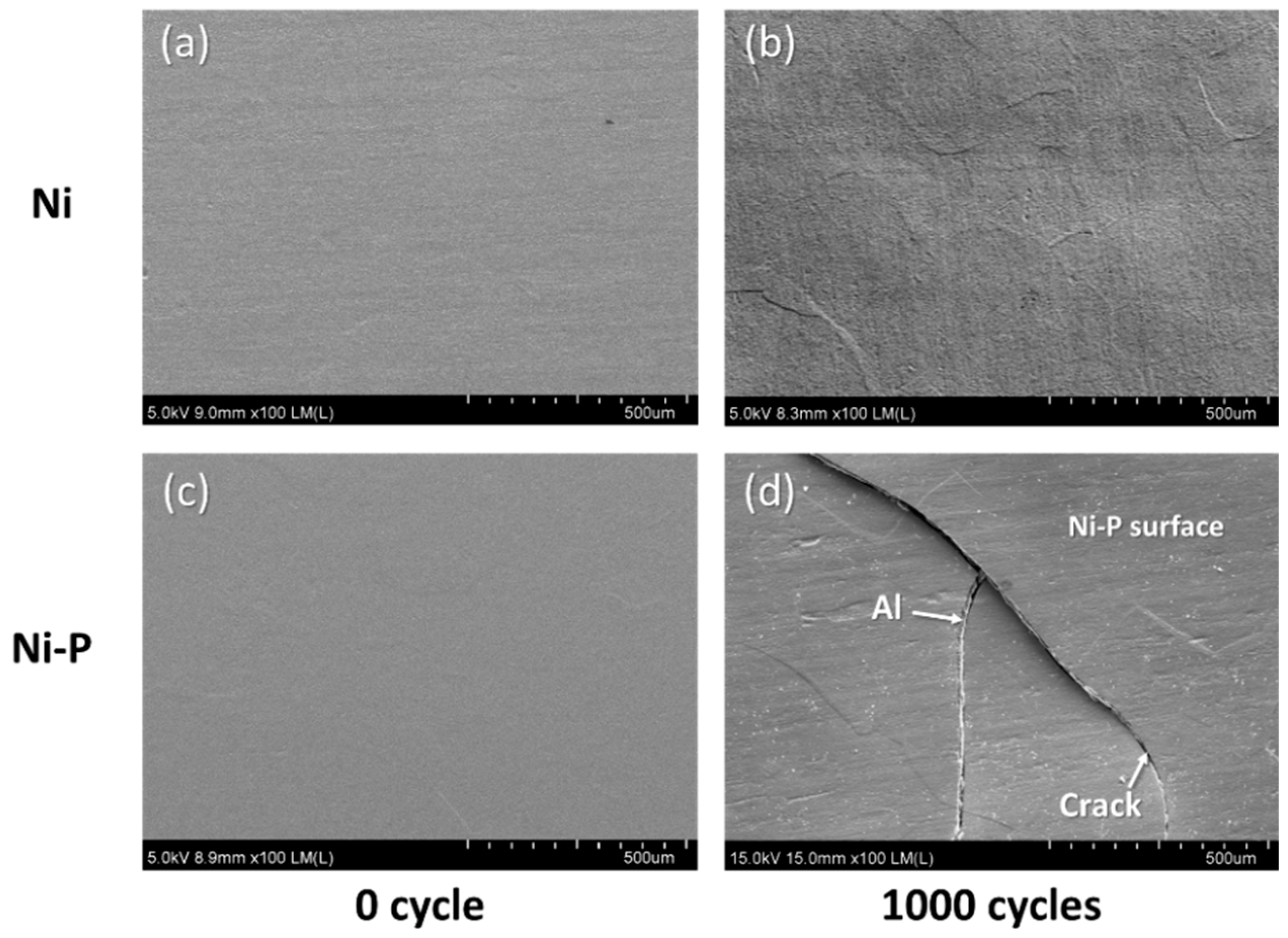
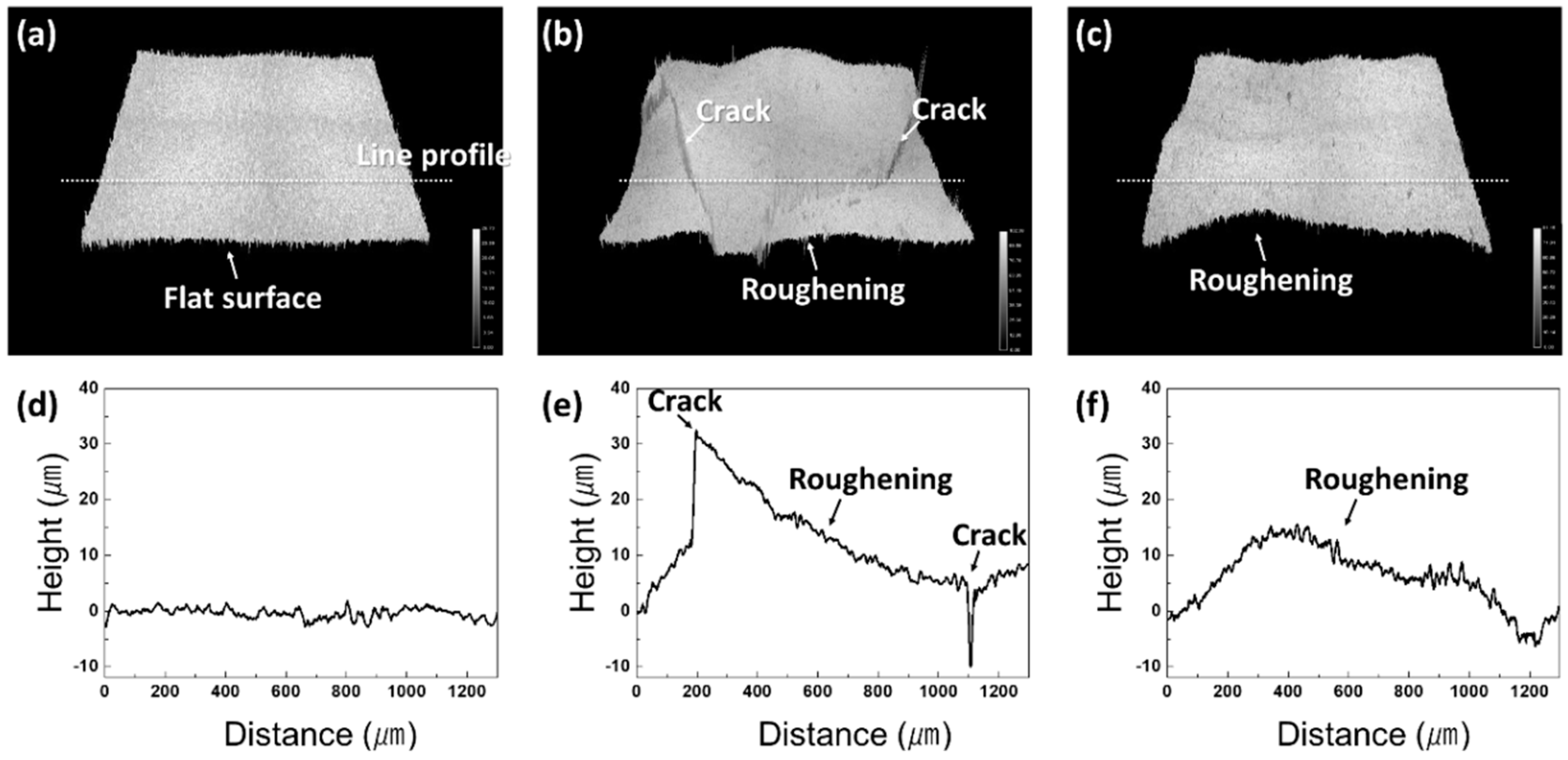
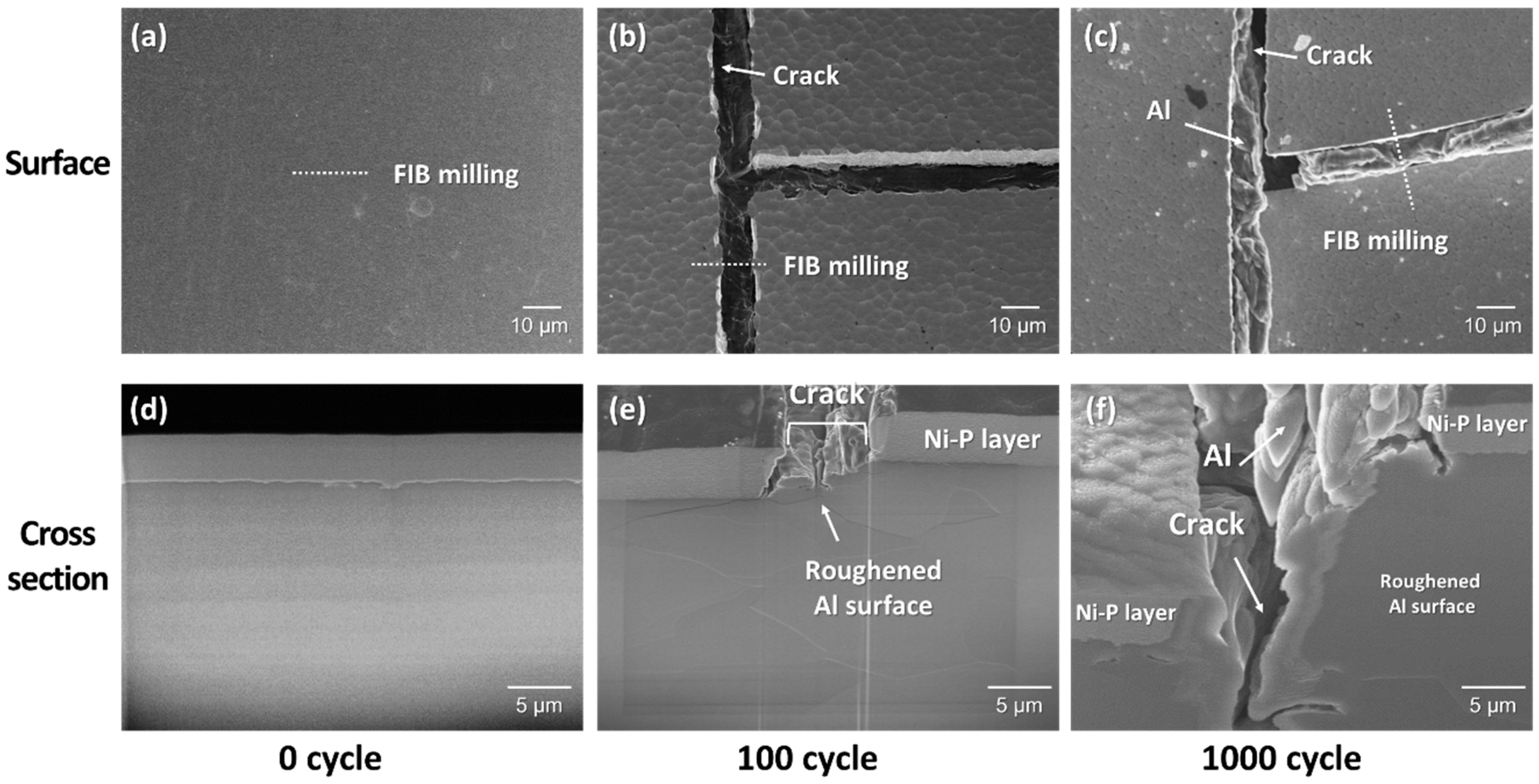
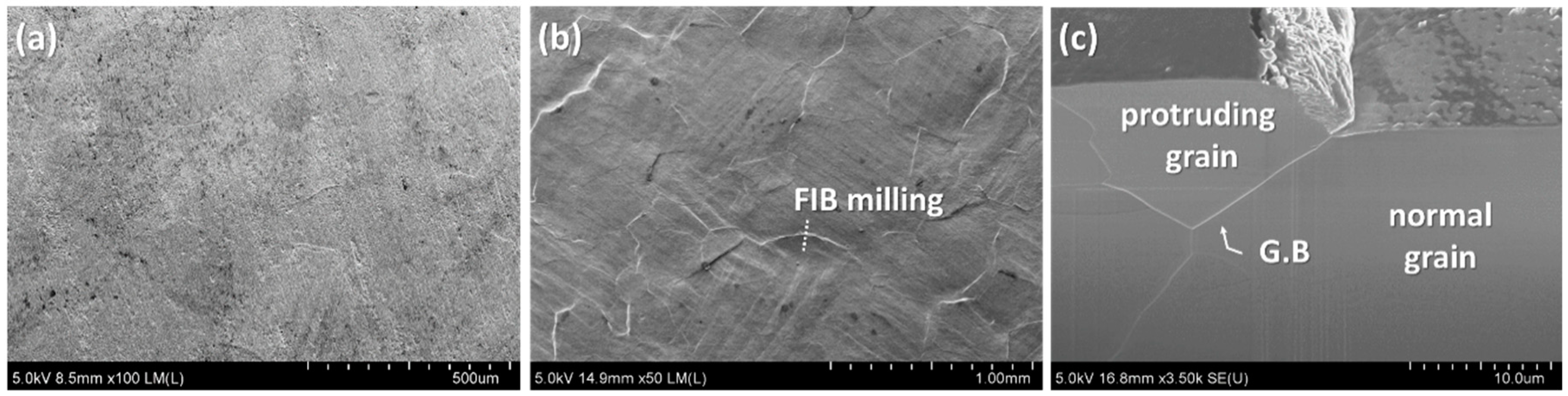
3.2. Cracking Mechanism of the Ni–P Layer
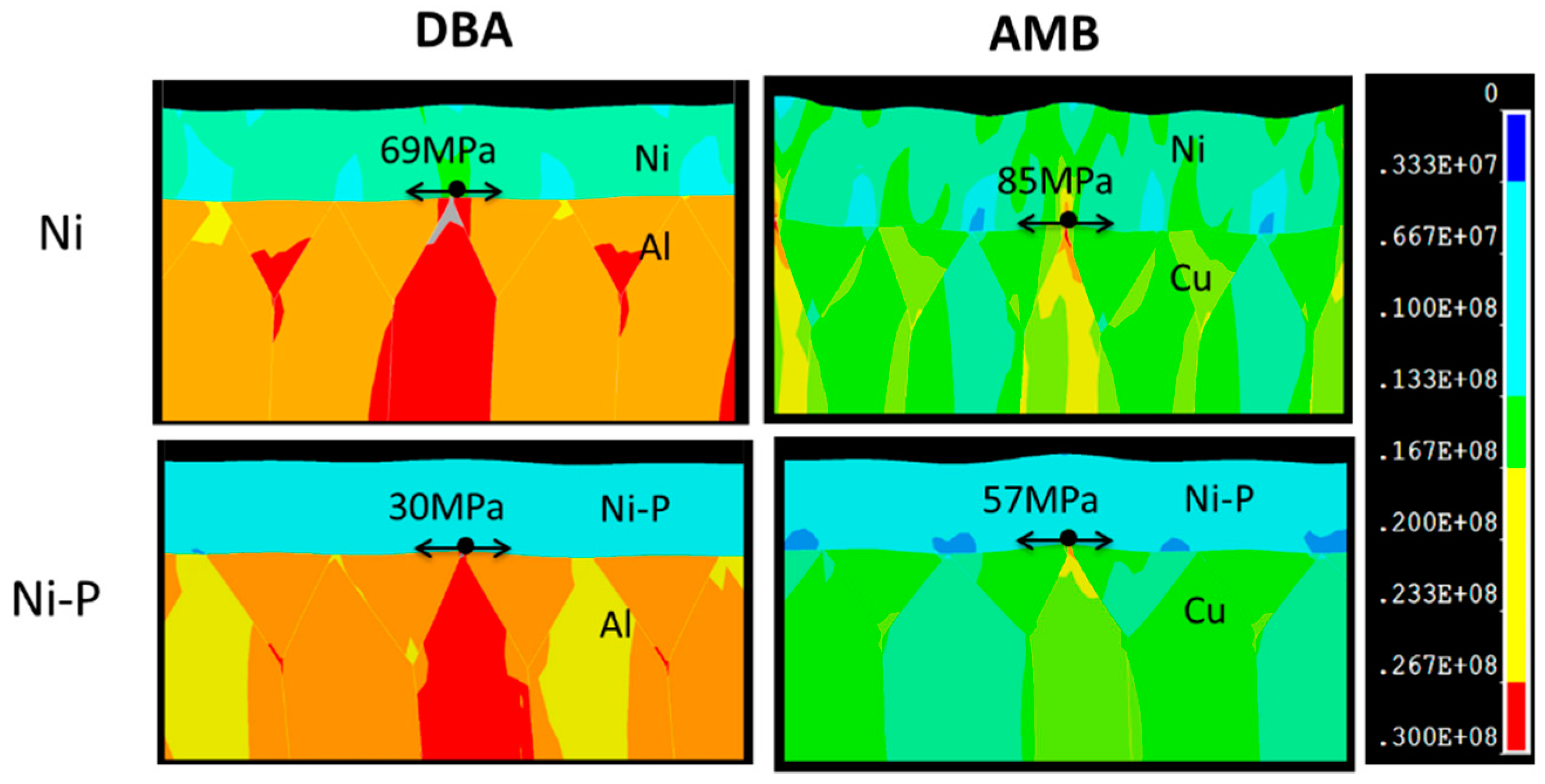
3.3. FEM Simulation
4. Conclusions
- (1)
- AMB substrates with AlN and Al2O3 fractured after a thermal shock test of 10 cycles between −50 and 250 °C, while AMB with Si3N4 survived after a thermal shock test of 1000 cycles. On the other hand, all DBA substrates were not fractured until 1000 cycles. However, after 1000 cycles, the Ni–P electroless plating layer on the surviving DBA/AMB substrates became rough and cracked, regardless of the ceramic and metal type of substrates after 1000 cycles, while the Ni electroplating was not cracked. This is because the ductile Ni layer had a higher fracture toughness than the Ni–P layer, which could suppress crack generation by absorbing cracking energy.
- (2)
- Beneath the cracked Ni–P layer on the DBA substrates, GBS deformation of Al was observed. It is considered that such deformation resulted in the cracking and roughening of the Ni–P layer. The thermal stress distribution simulation indicated that the tensile stress was concentrated at the interface between the polycrystalline grain layer and the plated Ni–P layer, along with the grain boundary. The results supported the cracking mechanism of the Ni–P layer by the GBS deformation of the metal.
- (3)
- The Ni-plated AMB substrates with Si3N4 as well as the Ni-plated DBA substrates with Al2O3, AlN, and Si3N4 are promising material designs for high-temperature power substrates based on the proper ductility of the electroplated Ni layer.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Flack, T.J.; Pushpakaran, B.N.; Bayne, S.B. GaN Technology for Power Electronic Applications: A Review. J. Electron. Mater. 2016, 45, 2673–2682. [Google Scholar] [CrossRef] [Green Version]
- Chow, T.P. High-voltage SiC and GaN power devices. Microelectron. Eng. 2006, 83, 112–122. [Google Scholar] [CrossRef]
- Khazaka, R.; Mendizabal, L.; Henry, D.; Hanna, R. Survey of high-temperature reliability of power electronics packaging components. IEEE Trans. Power Electron. 2015, 30, 2456–2464. [Google Scholar] [CrossRef]
- Hamilton, D.; Mills, L.; Bowen, J.; Jennings, M.; Mawby, P. High temperature reliability of power module substrates. In Proceedings of the PCIM Europe 2015, International Exhibition and Conference for Power Electronics, Intelligent Motion, Renewable Energy and Energy Management, Nuremberg, Germany, 19–20 May 2015; pp. 1–7. [Google Scholar]
- Lin, C.Y.; Tuan, W.H. Direct bonding of aluminum to alumina for thermal dissipation purposes. Int. J. Appl. Ceram. Technol. 2016, 13, 170–176. [Google Scholar] [CrossRef]
- Hromadka, K.; Stulik, J.; Reboun, J.; Hamacek, A. DBC technology for low cost power electronic substrate manufacturing. Procedia Eng. 2014, 69, 1180–1183. [Google Scholar] [CrossRef]
- Lindemann, A.; Strauch, G. Properties of direct aluminium bonded substrates for power semiconductor components. IEEE Trans. Power Electron. 2007, 22, 384–391. [Google Scholar] [CrossRef]
- Tsai, M.Y.; Huang, P.S.; Lin, C.H.; Wu, C.T.; Hu, S.C. Mechanical design and analysis of direct-plated-copper aluminum nitride substrates for enhancing thermal reliability. Microelectron. Reliab. 2015, 55, 2589–2595. [Google Scholar] [CrossRef]
- Xu, L.; Wang, M.; Zhou, Y.; Qian, Z.; Liu, S. An optimal structural design to improve the reliability of Al2O3-DBC substrates under thermal cycling. Microelectron. Reliab. 2016, 56, 101–108. [Google Scholar] [CrossRef]
- Fei, M.; Fu, R.; Agathopoulos, S.; Fang, J.; Wang, C.; Zhu, H. A preparation method for Al/AlN ceramics substrates by using a CuO interlayer. Mater. Des. 2017, 130, 373–380. [Google Scholar] [CrossRef]
- Zhang, P.; Fu, R.; Tang, Y.; Cao, B.; Fei, M.; Yang, Y. Morphology of thick film metallization on aluminum nitride ceramics and composition of interface layer. Ceram. Int. 2015, 41, 13381–13388. [Google Scholar] [CrossRef]
- Lei, T.G.; Calata, J.N.; Ngo, K.D.T.; Lu, G.Q. Effects of large-temperature cycling range on direct bond aluminum substrate. IEEE Trans. Device Mater. Reliab. 2009, 9, 563–568. [Google Scholar] [CrossRef]
- Gu, C.D.; You, Y.H.; Yu, Y.L.; Qu, S.X.; Tu, J.P. Microstructure, nanoindentation, and electrochemical properties of the nanocrystalline nickel film electrodeposited from choline chloride-ethylene glycol. Surf. Coat. Technol. 2011, 205, 4928–4933. [Google Scholar] [CrossRef]
- Rashidi, A.M.; Amadeh, A. Effect of Electroplating Parameters on Microstructure of Nanocrystalline Nickel Coatings. J. Mater. Sci. Technol. 2010, 26, 82–86. [Google Scholar] [CrossRef]
- Loto, C.A. Electroless Nickel Plating – A Review. Silicon 2016, 8, 177–186. [Google Scholar] [CrossRef]
- Krishnan, K.H.; John, S.; Srinivasan, K.N.; Praveen, J.; Ganesan, M.; Kavimani, P.M. An Overall Aspect of Electroless Ni–P Depositions—A Review Article. Metall. Mater. Trans. 2006, 37A, 1917–1926. [Google Scholar] [CrossRef]
- Keong, K.G.; Sha, W.; Malinov, S. Crystallisation kinetics and phase transformation behaviour of electroless nickel-phosphorus deposits with high phosphorus content. J. Alloys Compd. 2002, 334, 192–199. [Google Scholar] [CrossRef]
- Alam, M.O.; Chan, Y.C.; Hung, K.C. Reliability study of the electroless Ni–P layer against solder alloy. Microelectron. Reliab. 2002, 42, 1065–1073. [Google Scholar] [CrossRef]
- Färber, B.; Cadel, E.; Menand, A.; Schmitz, G.; Kirchheim, R. Phosphorus segregation in nanocrystalline Ni-3.6 at.% P alloy investigated with the tomographic atom probe (TAP). Acta Mater. 2000, 48, 789–796. [Google Scholar] [CrossRef]
- Mai, Q.X.; Daniels, R.D.; Harpalani, H.B. Structural changes induced by heating in electroless nickel-phosphorus alloys. Thin Solid Films 1988, 166, 235–247. [Google Scholar] [CrossRef]
- Pietranico, S.; Pommier, S.; Lefebvre, S.; Pattofatto, S. Thermal fatigue and failure of electronic power device substrates. Int. J. Fatigue 2009, 31, 1911–1920. [Google Scholar] [CrossRef] [Green Version]
- Dupont, L.; Khatir, Z.; Lefebvre, S.; Bontemps, S. Effects of metallization thickness of ceramic substrates on the reliability of power assemblies under high temperature cycling. Microelectron. Reliab. 2006, 46, 1766–1771. [Google Scholar] [CrossRef]
- Ning, Z.; He, Y.; Gao, W. Mechanical attrition enhanced Ni electroplating. Surf. Coatings Technol. 2008, 202, 2139–2146. [Google Scholar] [CrossRef]
- MIL-STD-883E: Test Method Standard for Microcircuits; Department of Defense: Columbus, OH, USA, 1996.
- AQG 324: Qualification of Power Modules for Use in Power Electronics Converter Units in Motor Vehicles; European Center for Power Electronics: Nuremberg, Germany, 2018.
- Fukumoto, A.; Berry, D.; Ngo, K.D.T.; Lu, G.Q. Effects of extreme temperature swings (−55 °C to 250 °C) on silicon nitride active metal brazing substrates. IEEE Trans. Device Mater. Reliab. 2014, 14, 751–756. [Google Scholar] [CrossRef]
- Basavalingappa, A.; Shen, M.Y.; Lloyd, J.R. Effect of texture and elastic anisotropy of copper microstructure on reliability. Mech. Adv. Mater. Mod. Process. 2017, 3, 6. [Google Scholar] [CrossRef]
- Liang, H.; Woo, C.; Huang, H.; Ngan, A.H.W.; Yu, T.X. Surfaces during Nanoindentation. Comput. Model. Eng. Sci. 2004, 6, 105–114. [Google Scholar]
- Komanduri, R.; Chandrasekaran, N.; Raff, L.M.M.D. Simulation of nanometric cutting of single crystal aluminum-effect of crystal orientation and direction of cutting. Wear 2000, 242, 60–88. [Google Scholar] [CrossRef]
- Seigo, K.; Sato, M. Properties of Electroless Nickel Deposit. J. Surf. Finish. Soc. Jpn. 2014, 64, 118–122. [Google Scholar] [CrossRef]
- Dai, W.; Oropeza, C.; Lian, K.; Wang, W. Experiment design and UV-LIGA microfabrication technology to study the fracture toughness of Ni microstructures. Microsyst. Technol. 2006, 12, 306–314. [Google Scholar] [CrossRef]
- Zhou, Q.J.; He, J.Y.; Sun, D.B.; Chu, W.Y.; Qiao, L.J. Deformation and fracture of nickel phosphorus coatings. Scr. Mater. 2006, 54, 603–608. [Google Scholar] [CrossRef]
- Roman, A.; Chicot, D.; Lesage, J. Indentation tests to determine the fracture toughness of nickel phosphorus coatings. Surf. Coat. Technol. 2002, 155, 161–168. [Google Scholar] [CrossRef]
- Bozzini, B.; Boniardi, M. Fracture toughness of supported Ni–P films prepared by autocatalytic chemical deposition. Z. Met. 1997, 88, 493–497. [Google Scholar]
- Molteni, C.; Marzari, N.; Payne, M.C.; Heine, V. Sliding Mechanisms in Aluminum Grain Boundaries. Phys. Rev. Lett. 1997, 79, 869–872. [Google Scholar] [CrossRef]
- Ritchie, R.O.; Thompson, A.W. On macroscopic and microscopic analyses for crack initiation and crack growth toughness in ductile alloys. Metall. Trans. A 1985, 16, 233–248. [Google Scholar] [CrossRef] [Green Version]
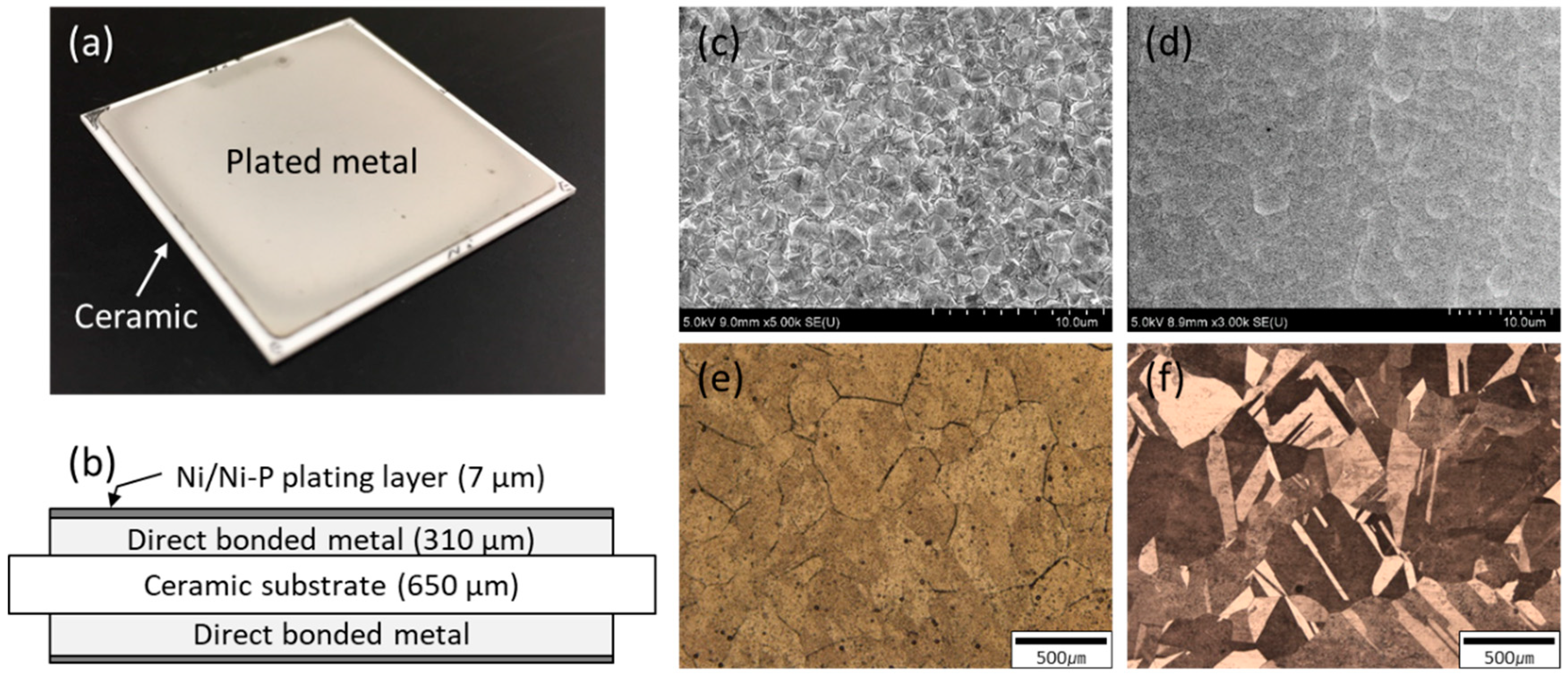



| Substrates | Metallization (Thickness: 7 μm) | ||
|---|---|---|---|
| Type | Metal (30 × 30 × 0.31 mm) | Ceramic (32 × 32 × 0.65 mm) | |
| DBA | Al | Al2O3 | Ni electroplating or Ni–P electroless plating |
| AlN | Ni electroplating or Ni–P electroless plating | ||
| Si3N4 | Ni electroplating or Ni–P electroless plating | ||
| AMB | Cu | Al2O3 | Ni electroplating or Ni–P electroless plating |
| AlN | Ni electroplating or Ni–P electroless plating | ||
| Si3N4 | Ni electroplating or Ni–P electroless plating | ||
| Materials | Young’s Modulus (GPa) | Poisson’s Ratio | Coefficient of Thermal Expansion (µm·m−1·K−1) | Yield Strength (GPa) | Tensile Strength (MPa) | Fracture Toughness (MPa·m1/2) |
|---|---|---|---|---|---|---|
| Cu (001) [27,28] | 75.7 | 0.28 | 17.7 | 0.7 | - | |
| Cu (110) [27,28] | 101.8 | 0.3 | 17.7 | 0.93 | - | |
| Cu (111) [27,28] | 123.4 | 0.3 | 17.7 | 1.2 | - | |
| Al (001) [29] | 63.7 | 0.34 | 21.3 | 0.59 | - | |
| Al (110) [29] | 72.59 | 0.34 | 21.3 | 0.52 | - | |
| Al (111) [29] | 76.1 | 0.34 | 21.3 | 0.51 | - | |
| Al2O3 | 280 | 0.23 | 7.9 | - | - | 4 |
| AlN | 320 | 0.24 | 4.6 | - | - | 3 |
| Si3N4 | 290 | 0.27 | 2.9 | - | - | 7 |
| Ni [30,31] | 220 | 0.31 | 14.1 | 0.08 | 500–1000 | 53 |
| Ni–P [30,32,33,34] | 50 | 0.31 | 13.0 | 0.23 | 50–150 | 1.1–2.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choe, C.; Chen, C.; Noh, S.; Suganuma, K. Thermal Shock Performance of DBA/AMB Substrates Plated by Ni and Ni–P Layers for High-Temperature Applications of Power Device Modules. Materials 2018, 11, 2394. https://doi.org/10.3390/ma11122394
Choe C, Chen C, Noh S, Suganuma K. Thermal Shock Performance of DBA/AMB Substrates Plated by Ni and Ni–P Layers for High-Temperature Applications of Power Device Modules. Materials. 2018; 11(12):2394. https://doi.org/10.3390/ma11122394
Chicago/Turabian StyleChoe, Chanyang, Chuantong Chen, Seungjun Noh, and Katsuaki Suganuma. 2018. "Thermal Shock Performance of DBA/AMB Substrates Plated by Ni and Ni–P Layers for High-Temperature Applications of Power Device Modules" Materials 11, no. 12: 2394. https://doi.org/10.3390/ma11122394
APA StyleChoe, C., Chen, C., Noh, S., & Suganuma, K. (2018). Thermal Shock Performance of DBA/AMB Substrates Plated by Ni and Ni–P Layers for High-Temperature Applications of Power Device Modules. Materials, 11(12), 2394. https://doi.org/10.3390/ma11122394






