Rapid Solar-Light Driven Superior Photocatalytic Degradation of Methylene Blue Using MoS2-ZnO Heterostructure Nanorods Photocatalyst
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterizations of ZnO Nanoparticles and MoS2-ZnO Heterostructure Nanorods
2.2. Photocatalytic Degradation of MB Based on MoS2-ZnO Heterostructure Nanorods
3. Results and Discussion
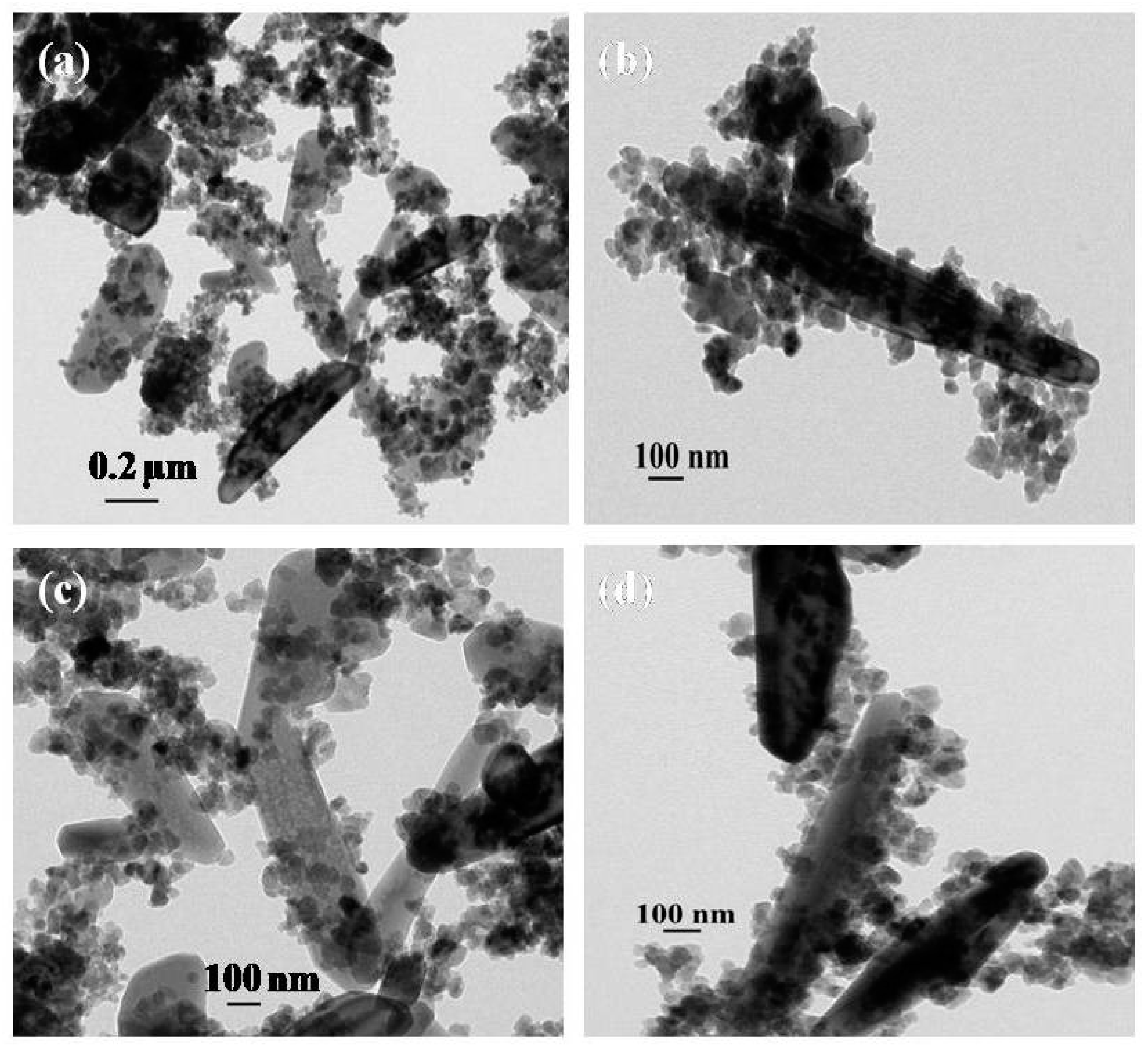
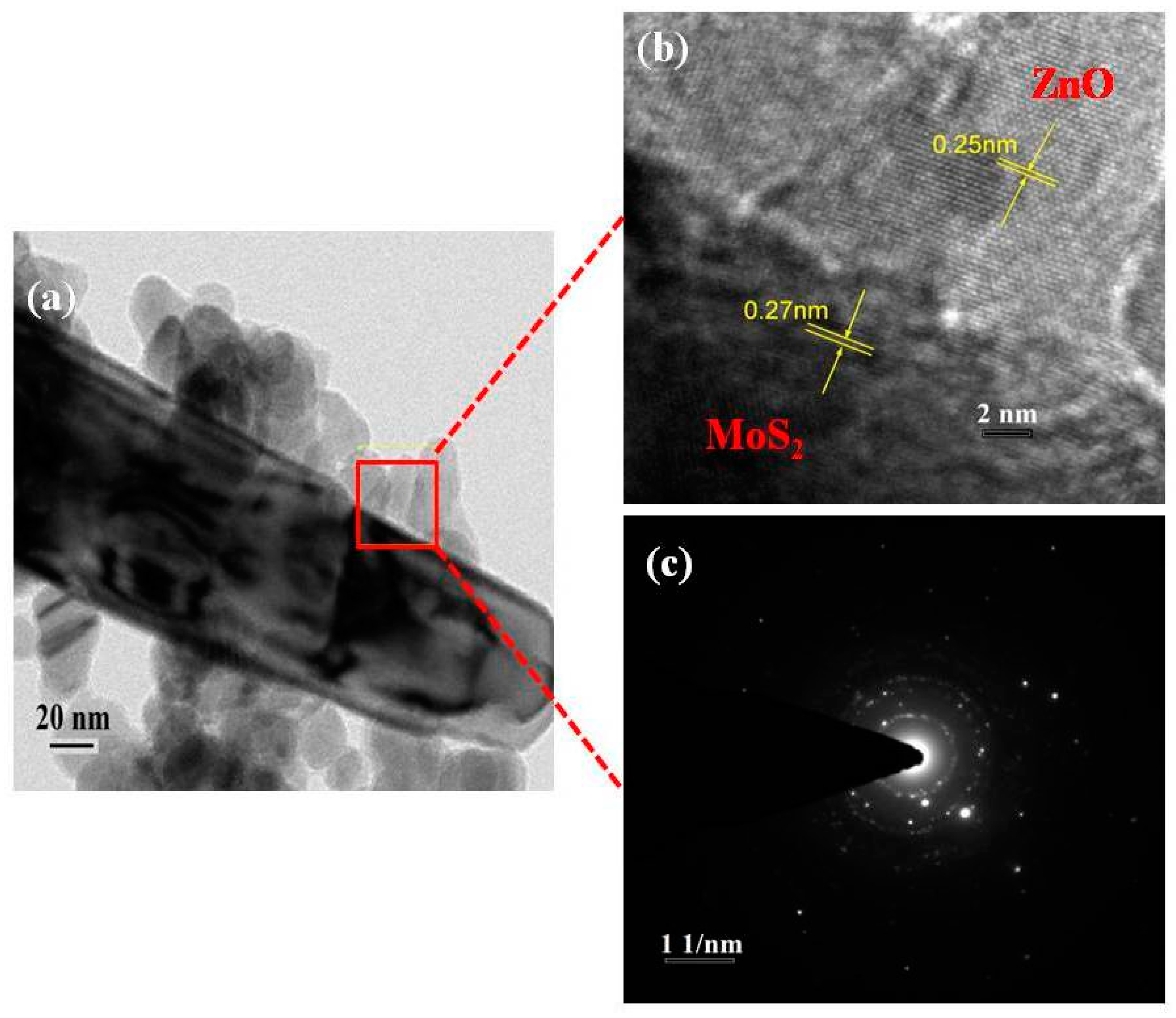
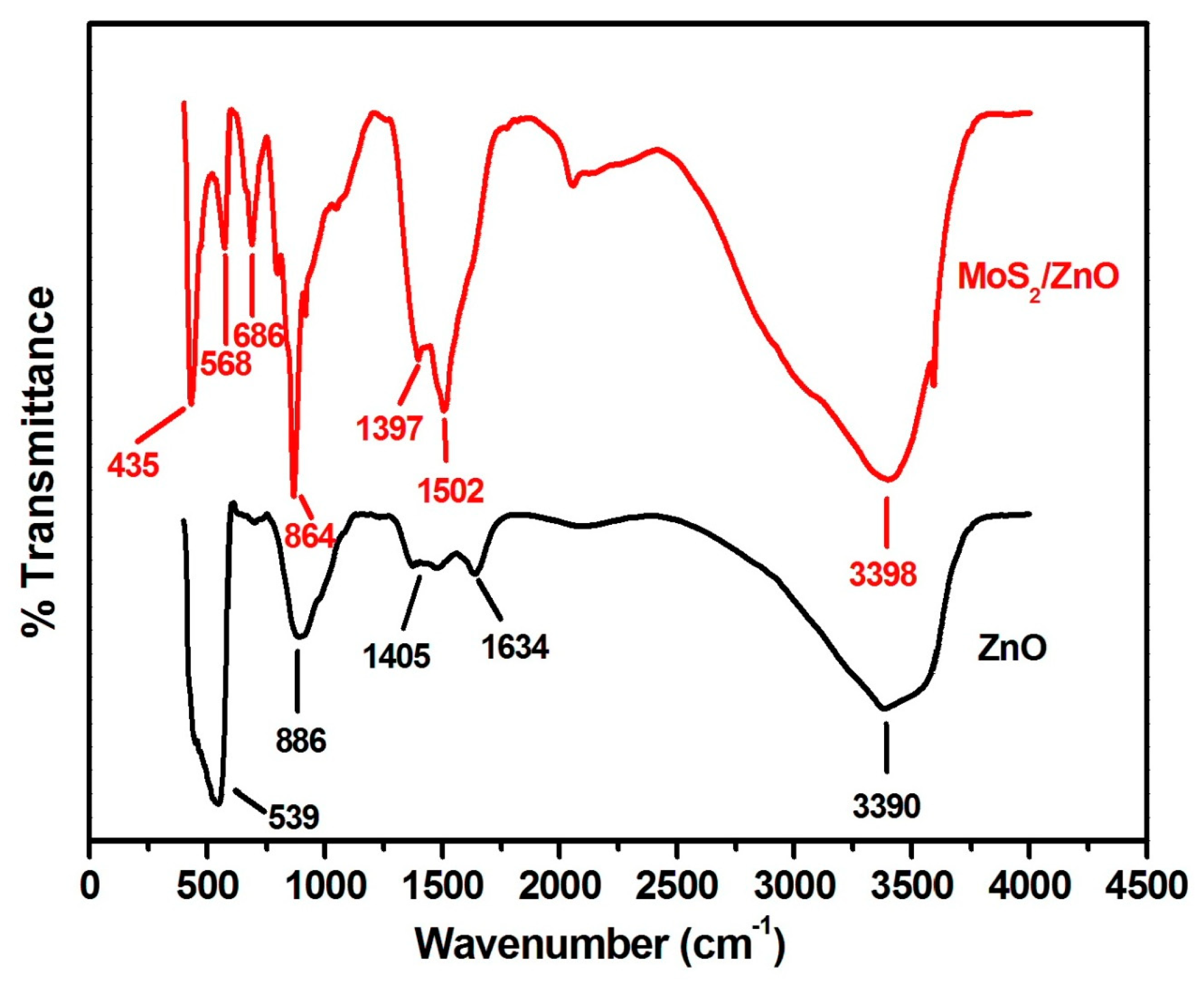
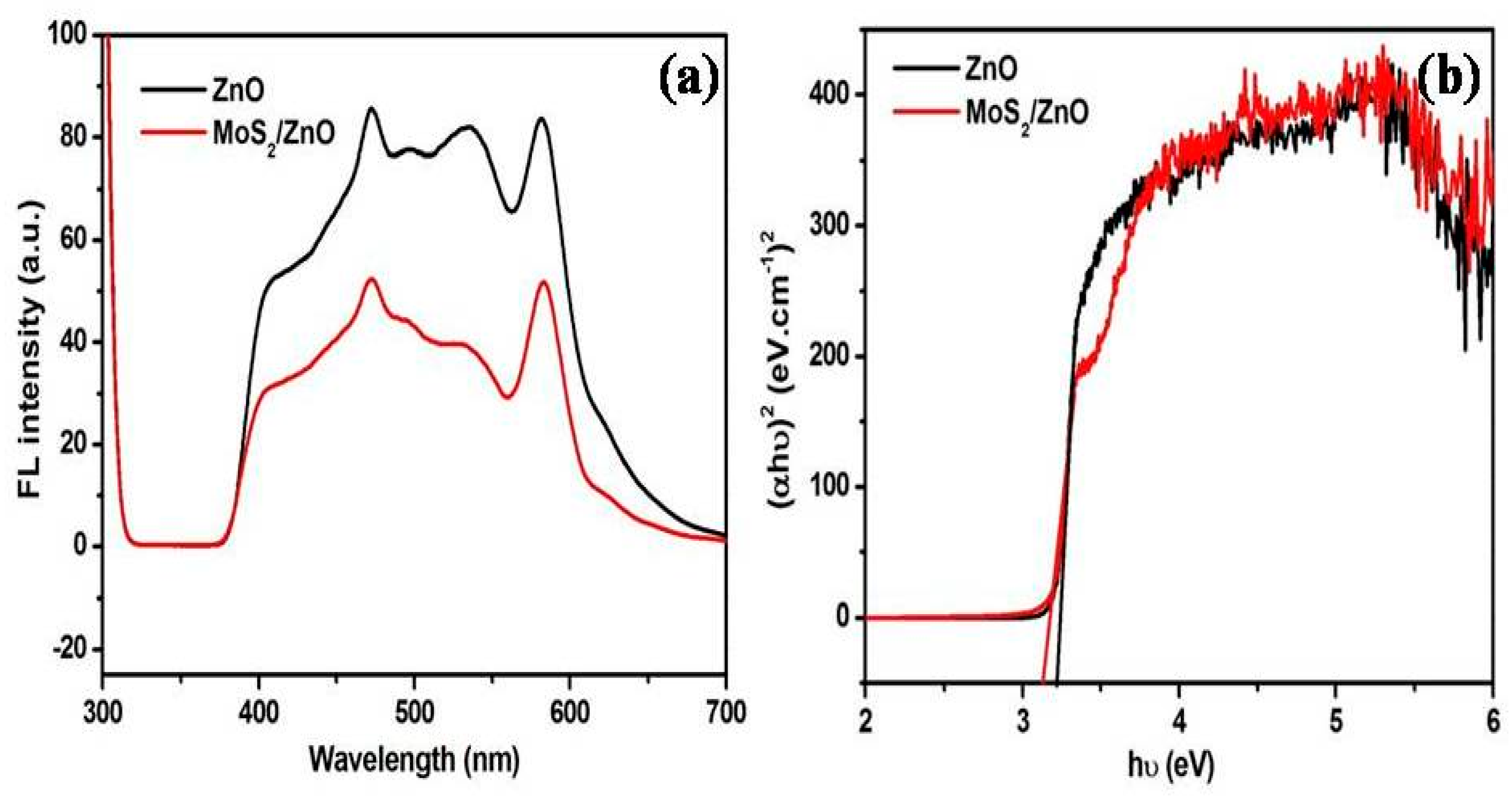
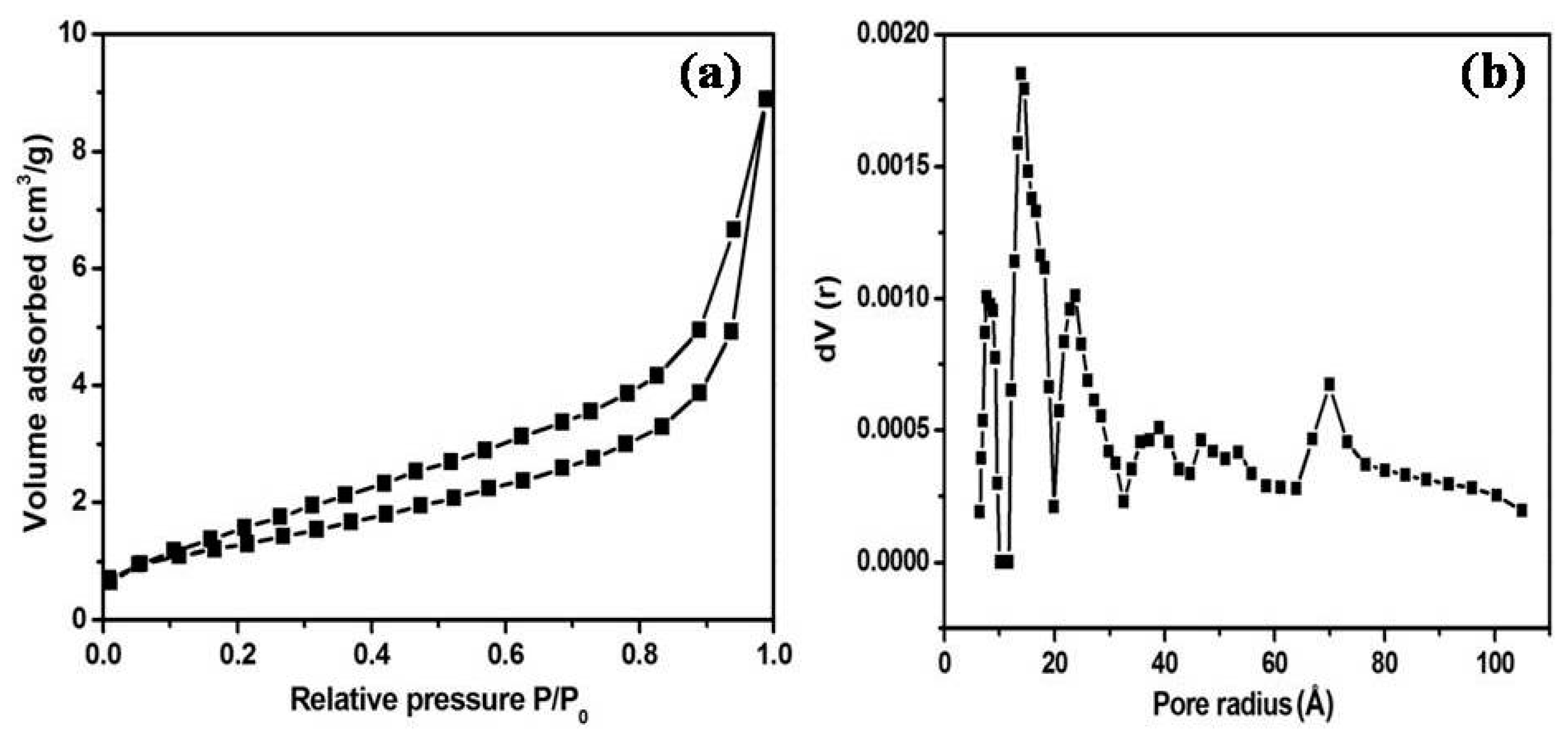
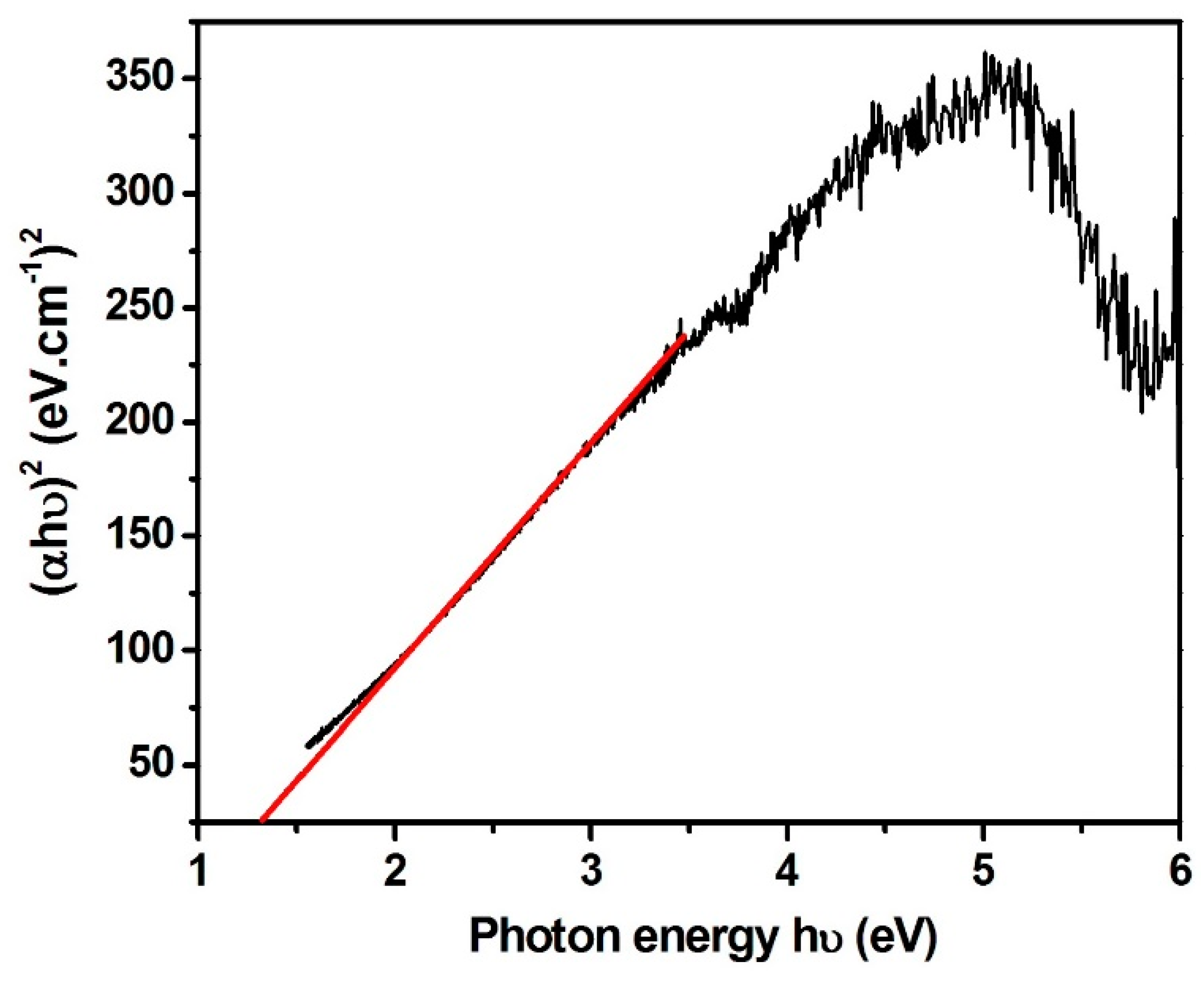
3.1. Characterization of As-Formed ZnO Nanoparticles and MoS2-ZnO Heterostructure Nanorods
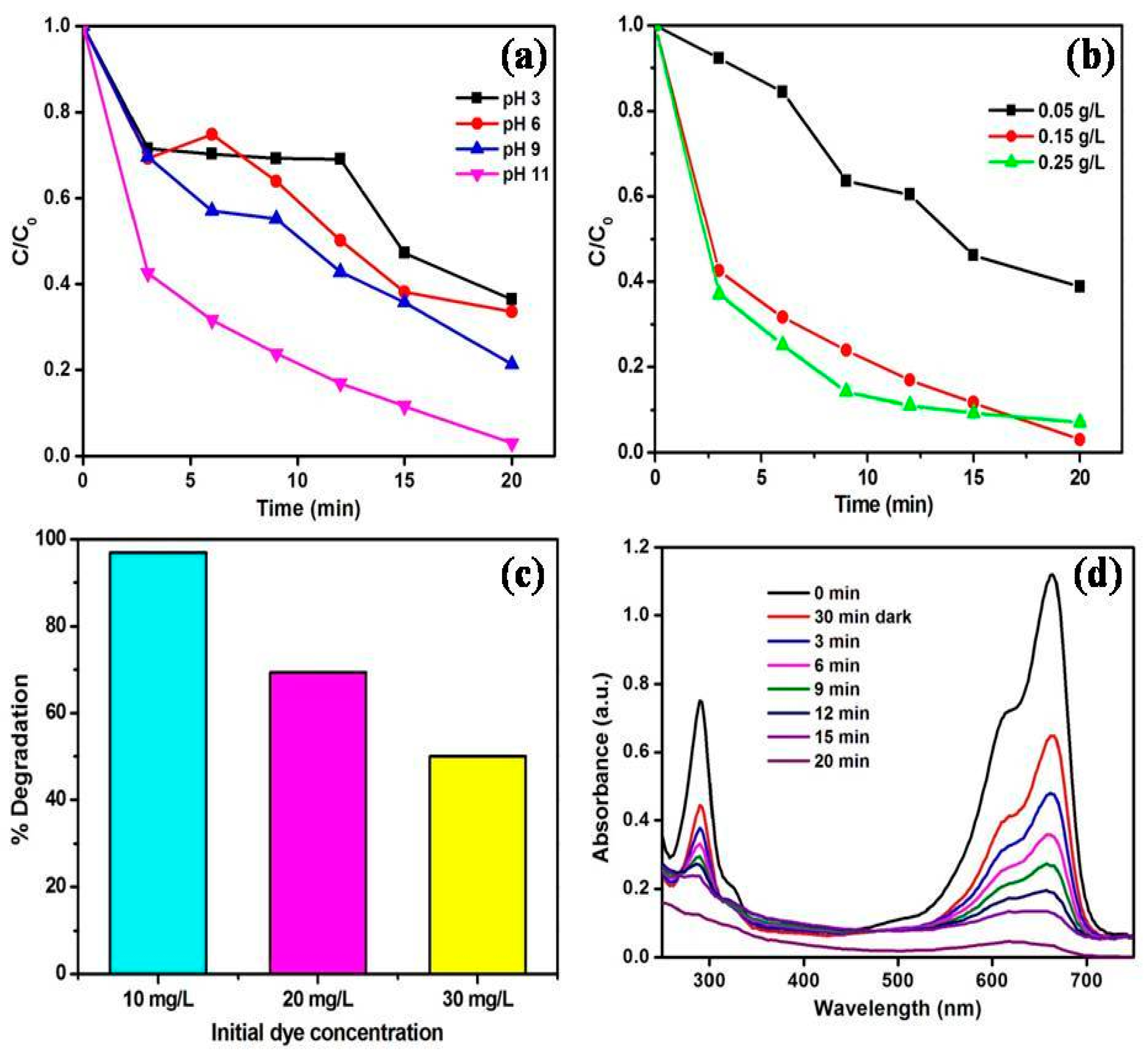
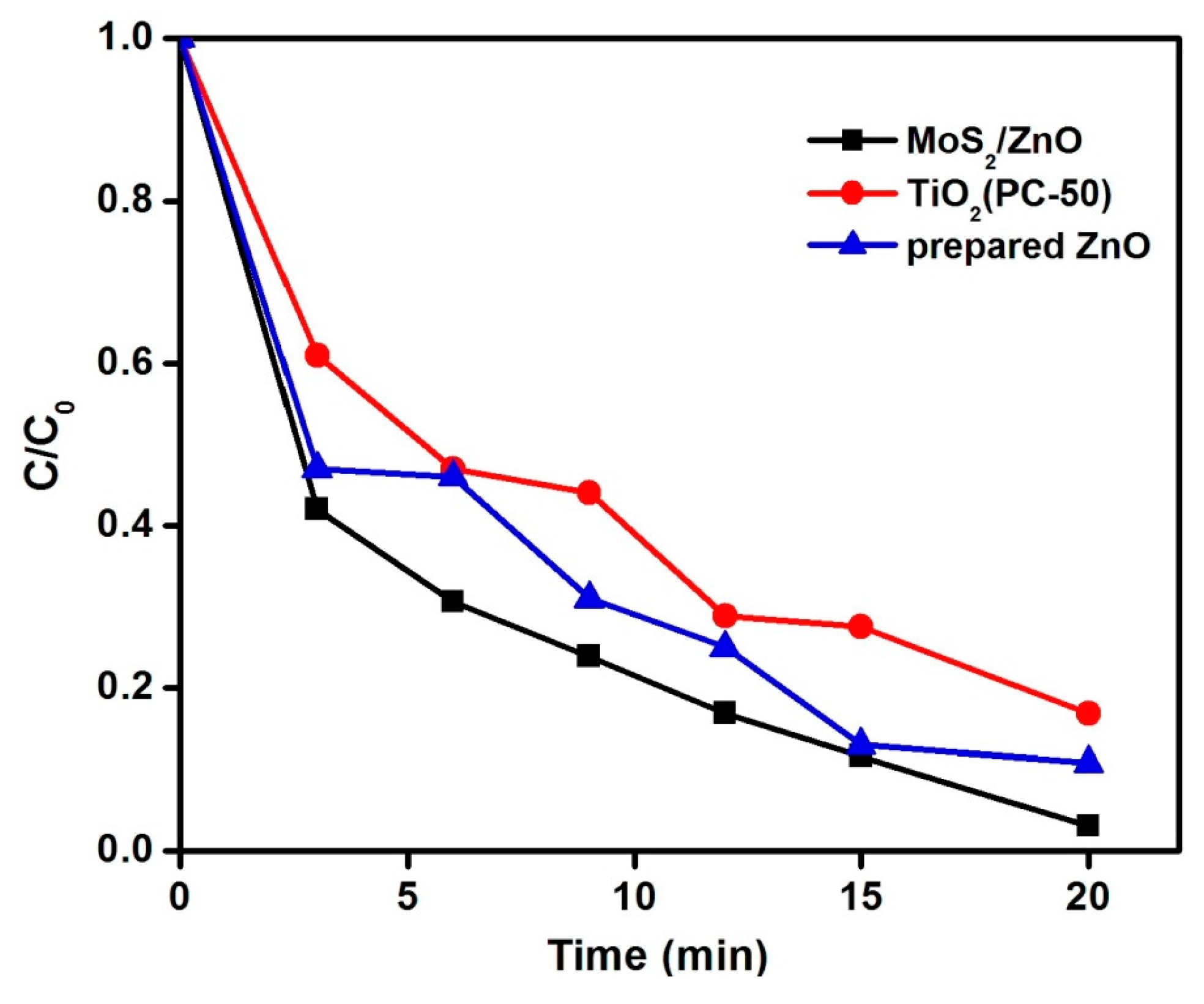
3.2. Investigation of MoS2/ZnO Heterostructure for the Removal of MB under Natural Sunlight
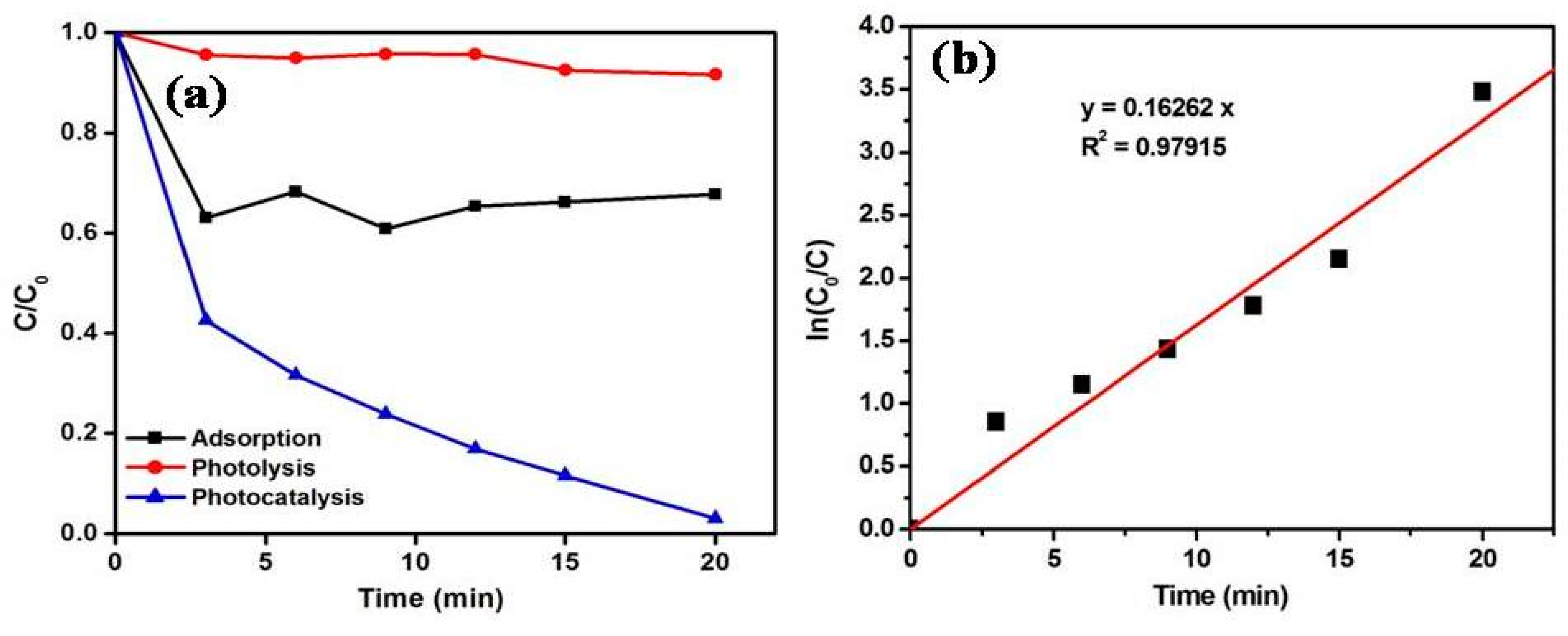
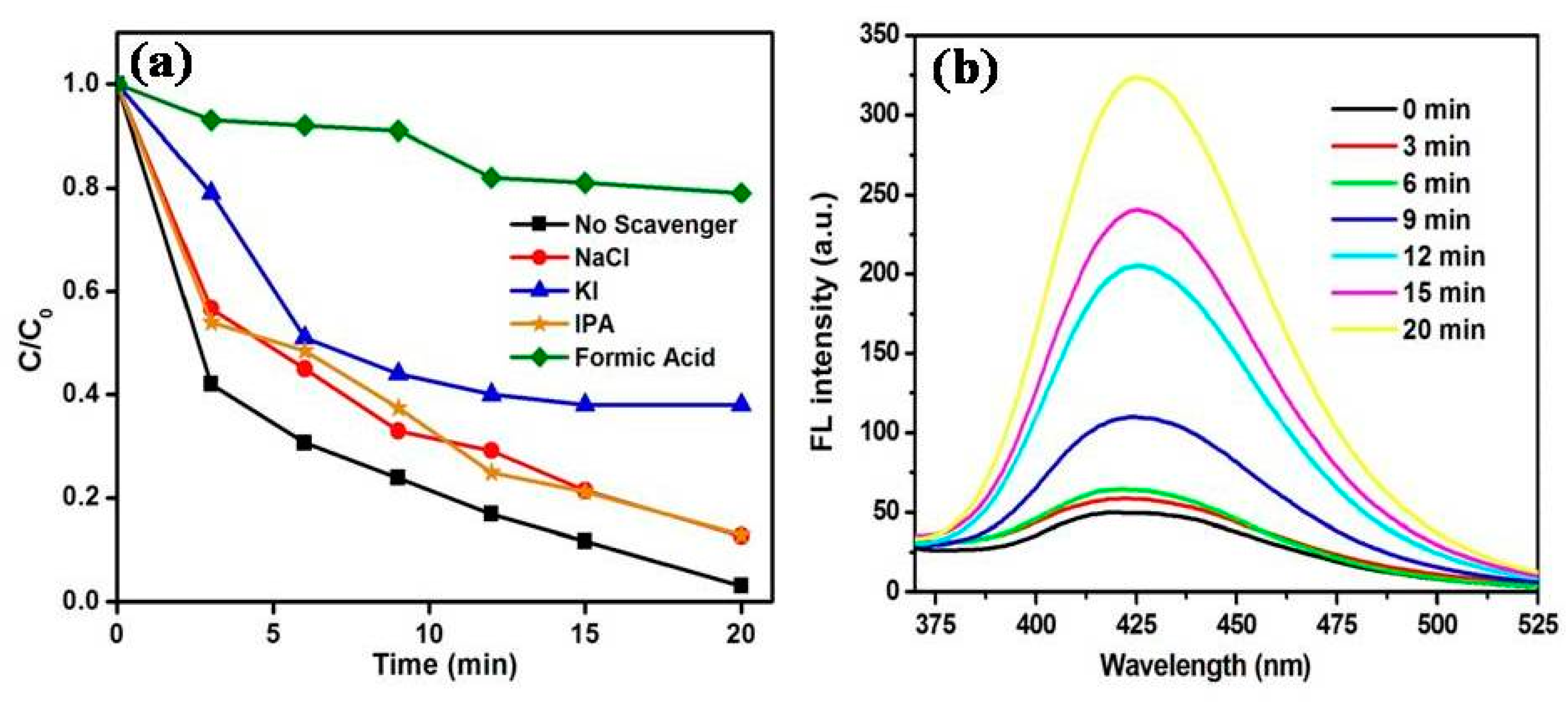
3.3. Role of Reactive Oxygen Species for the Removal of MB Using MoS2/ZnO Heterostructure
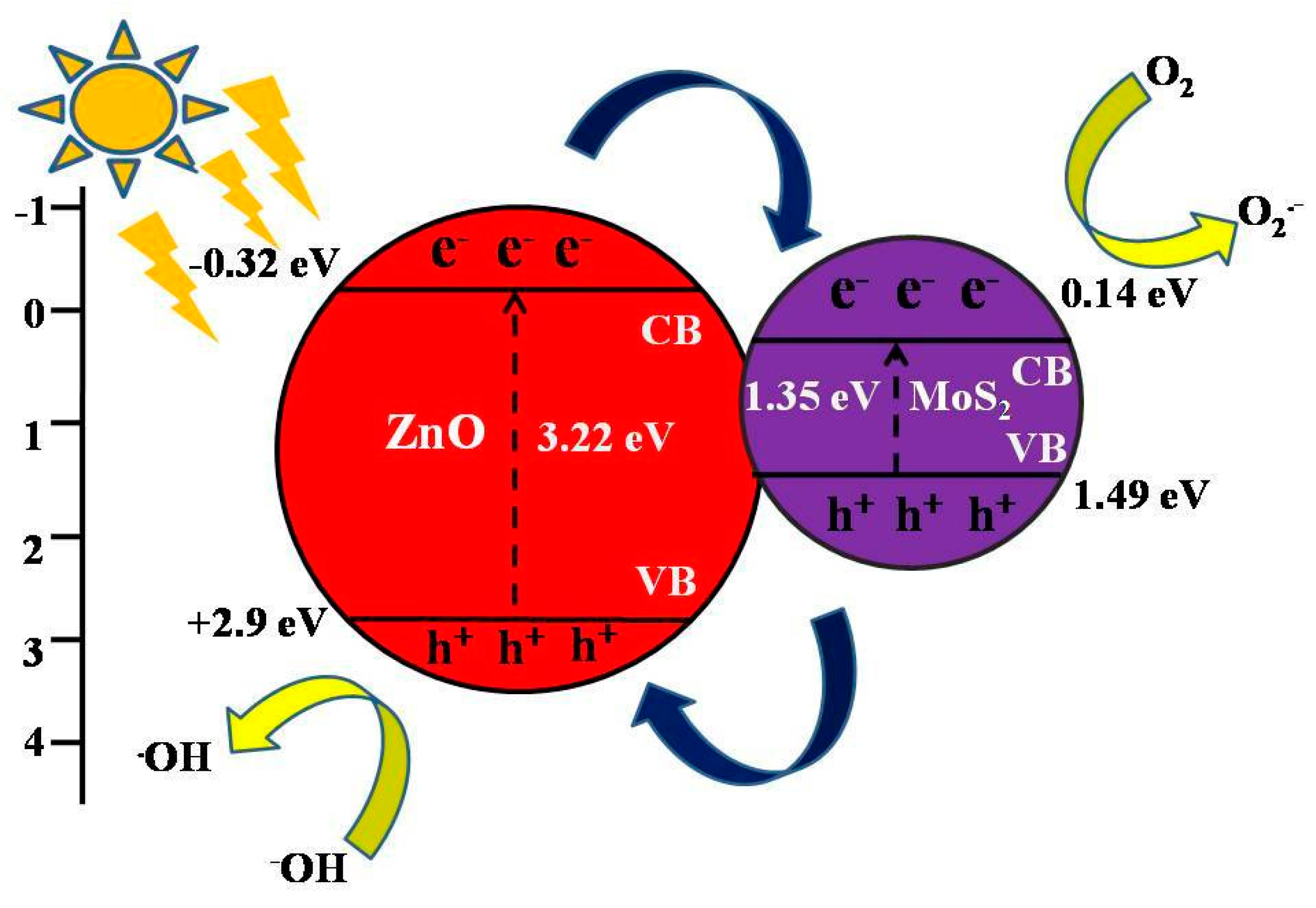
3.4. Proposed Photocatalytic Degradation Mechanism
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ngoc, X.D.M.; Yoon, J.; Kim, J.H.; Kim, I.T.; Son, H.B.; Bae, J.; Hur, J. Hybrid Hydrogel and Aerogel Membranes Based on Chitosan/Prussian Blue for Photo-Fenton-Based Wastewater Treatment Using Sunlight. Sci. Adv. Mater. 2017, 9, 1484–1487. [Google Scholar]
- Kaur, A.; Umar, A.; Anderson, W.A.; Kansal, S.K. Facile synthesis of CdS/TiO2 nanocomposite and their catalytic activity for ofloxacin degradation under visible illumination. J. Photochem. Photobiol. A Chem. 2018, 360, 34–43. [Google Scholar] [CrossRef]
- Zhu, J.; Tan, S. Polyacrylamide Worked as Adsorbents for Tetracycline-Polluted Water Treatment. J. Nanoelectron. Optoelectron. 2017, 12, 1364–1368. [Google Scholar] [CrossRef]
- Guan, W.; Tian, S. The Modified Chitosan for Dyeing Wastewater Treatment via Adsorption and Flocculation. Sci. Adv. Mater. 2017, 9, 1603–1609. [Google Scholar] [CrossRef]
- Kaur, M.; Mehta, S.K.; Kansal, S.K. Nitrogen doped graphene quantum dots: Efficient fluorescent chemosensor for the selective and sensitive detection of 2,4,6-trinitrophenol. Sens. Actuators B Chem. 2017, 245, 938–945. [Google Scholar] [CrossRef]
- Kaur, M.; Mehta, S.K.; Kansal, S.K. A fluorescent probe based on nitrogen doped graphene quantum dots for turn off sensing of explosive and detrimental water pollutant, TNP in aqueous medium. Spectrochim. Acta Part A 2017, 180, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Aitbara, A.; Djellabi, R.; Eulmi, A.; Hazourli, S. Electrocoagulation and Fenton Hybrid Processes for Dairy Water Purification: In Situ Generation of H2O2. Sens. Lett. 2017, 15, 992–997. [Google Scholar] [CrossRef]
- Dong, Y.; Xing, L.; Hu, F.; Umar, A.; Wu, X. Efficient removal of organic dyes molecules by grain-like α-Fe2O3 nanostructures under visible light irradiation. Vacuum 2018, 150, 35–40. [Google Scholar] [CrossRef]
- Kermanioryani, M.; Mutalib, M.I.A.; Kurnia, K.A.; Lethesh, K.C.; Krishnan, S.; Leveque, J.-M. Enhancement of ᴫ-ᴫ aromatic interactions between hydrophobic ionic liquids and methylene blue for an optimum removal efficiency and assessment of toxicity by microbiological method. J. Clean Prod. 2016, 137, 1149–1157. [Google Scholar] [CrossRef]
- Qada, E.N.E.; Allen, S.J.; Walker, G.M. Adsorption of Methylene Blue onto activated carbon produced from steam activated bituminous coal: A study of equilibrium adsorption isotherm. Chem. Eng. J. 2006, 124, 103–110. [Google Scholar] [CrossRef]
- Almeida, C.A.P.; Debacher, N.A.; Downs, A.J.; Cottet, L.; Mello, C.A.D. Removal of methylene blue from colored effluents by adsorption on montmorillonite clay. J. Colloid Interface Sci. 2009, 332, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Lv, Y.; Zeng, X.; Cao, D. ZIF-derived nitrogen-doped porous carbons as highly efficient adsorbents for removal of organic compounds from wastewater. Chem. Eng. J. 2017, 323, 502–511. [Google Scholar] [CrossRef]
- Liu, T.; Li, Y.; Du, Q.; Sun, J.; Jiao, Y.; Yang, G.; Wang, Z.; Xia, Y.; Zhang, W.; Wang, K.; et al. Adsorption of methylene blue from aqueous solution by graphene. Colloids Surf. B 2012, 90, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Xu, F.; Chen, M.; Xu, Z.; Zhu, Z. Adsorption behavior of methylene blue on carbon nanotubes. Bioresour. Technol. 2010, 101, 3040–3046. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Du, Q.; Liu, T.; Peng, X.; Wang, J.; Sun, J.; Wang, Y.; Wu, S.; Wang, Z.; Xia, Y.; et al. Comparative study of methylene blue dye adsorption onto activated carbon, graphene oxide, and carbon nanotubes. Chem. Eng. Res. Des. 2013, 91, 361–368. [Google Scholar] [CrossRef]
- Jung, E.; Moon, H.J.; Park, T.J.; Bong, K.W.; Yu, T. Facile Aqueous-Phase Synthesis of Magnetic Iron Oxide Nanoparticles to Enhance the Removal of Iodine from Water. Sci. Adv. Mater. 2017, 9, 1847–1853. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, P. Chitosan Modified by Polyacrylamide for Adsorptive Removal of Tetracycline from Wastewater. J. Nanoelectron. Optoelectron. 2017, 12, 1186–1190. [Google Scholar] [CrossRef]
- Yalcinkaya, F.; Yalcinkaya, B.; Hruza, J.; Hrabak, P. Effect of Nanofibrous Membrane Structures on the Treatment of Wastewater Microfiltration. Sci. Adv. Mater. 2017, 9, 747–757. [Google Scholar] [CrossRef]
- Kansal, S.K.; Sood, S.; Umar, A.; Mehta, S.K. Photocatalytic degradation of Eriochrome Black T dye using well-crystalline anatase TiO2 nanoparticles. J. Alloys Compd. 2013, 581, 392–397. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Liu, T.; Ai, Z.; Yang, S. Synthesis of Lanthanum Modified Titanium Pillared Montmorillonite and Its Application for Removal of Phosphate from Wastewater. Sci. Adv. Mater. 2017, 9, 673–681. [Google Scholar] [CrossRef]
- Shao, P.; Ren, Z.; Tian, J.; Gao, S.; Luo, X.; Shi, W.; Yan, B.; Li, J.; Cui, F. Silica hydrogel-mediated dissolution-recrystallization strategy for synthesis of ultrathin α-Fe2O3nanosheets with highly exposed (110) facets: A superior photocatalyst for degradation of bisphenol S. Chem. Eng. J. 2017, 323, 64–73. [Google Scholar] [CrossRef]
- Sood, S.; Umar, A.; Mehta, S.K.; Kansal, S.K. α-Bi2O3 nanorods: An efficient sunlight active photocatalyst for degradation of Rhodamine B and 2,4,6-trichlorophenol. Ceram. Int. 2015, 41, 3355–3364. [Google Scholar] [CrossRef]
- Jin, R.; Su, M.; Wang, J.; Zhang, P.; Cui, M.; Chen, Y.; Yang, H. Synthesis and enhanced photocatalytic activity of monodisperse flowerlike nanoarchitectures assembled from CdS nanoflakes with exposed {001} facets. Mater. Res. Bull. 2012, 47, 3070–3077. [Google Scholar] [CrossRef]
- Hu, J.-S.; Ren, L.-L.; Guo, Y.-G.; Luang, H.-P.; Cao, A.-M.; Wan, L.-J.; Bai, C.-L. Mass production and high photocatalytic activity of ZnS nanoporous nanoparticles. Angew. Chem. 2005, 117, 1295–1299. [Google Scholar] [CrossRef]
- Umar, A.; Hahn, Y.B. ZnO nanosheet networks and hexagonal nanodiscs grown on silicon substrate: Growth mechanism and structural and optical properties. Nanotechnology 2006, 17, 2174. [Google Scholar] [CrossRef]
- Chaudhary, S.; Umar, A.; Bhasin, K.K.; Baskoutas, S. Chemical Sensing applications of ZnO nanomaterials. Materials 2018, 11, 287. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Badran, R.I.; Umar, A. Fabrication of ZnO nanorods based p-n heterojunction diodes and their electrical behavior with temperature. J. Nanoelectron. Optoelectron. 2017, 12, 731–735. [Google Scholar] [CrossRef]
- Umar, A.; Khan, A.; Kumar, R.; Algarni, H. Ag-doped ZnO nanoparticles for enhanced ethanol gas sensing application. J. Nanosci. Nanotechnol. 2018, 18, 3557–3562. [Google Scholar] [CrossRef] [PubMed]
- Kanchana, S.; Chithra, M.J.; Ernest, S.; Pushpanathan, K. Violet emission from Fe doped ZnO nanoparticles synthesized by precipitation method. J. Lumin. 2016, 176, 6–14. [Google Scholar] [CrossRef]
- Lang, J.; Wang, J.; Zhang, Q.; Li, X.; Han, Q.; Wei, M.; Sui, Y.; Wang, D.; Yang, J. Chemical precipitation synthesis and significant enhancement in photocatalytic activity of Ce-doped ZnO nanoparticles. Ceram. Int. 2016, 42, 14175–14181. [Google Scholar] [CrossRef]
- Huo, P.; Zhou, M.; Tang, Y.; Liu, X.; Ma, C.; Yu, L.; Yan, Y. Incorporation of N-ZnO/CdS/Graphene oxide composite photocatalyst for enhanced photocatalytic activity under visible light. J. Alloys Compd. 2016, 670, 198–209. [Google Scholar] [CrossRef]
- Liu, H.; Lv, T.; Zhu, C.; Su, X.; Zhu, Z. Efficient synthesis of MoS2 nanoparticles modified TiO2nanobelts with enhanced visible-light-driven photocatalytic activity. J. Mol. Catal. A Chem. 2015, 396, 136–142. [Google Scholar] [CrossRef]
- Ebrahimiasl, S.; Seifi, R.; Nahli, R.E.; Zakaria, A. Ppy/Nanographene Modified Pencil Graphite Electrode Nanosensor for Detection and Determination of Herbicides in Agricultural Water. Sci. Adv. Mater. 2017, 9, 2045–2053. [Google Scholar] [CrossRef]
- Ding, Y.; Zhou, Y.; Nie, W.; Chen, P. MoS2–GO nanocomposites synthesized via a hydrothermal hydrogel method for solar light photocatalytic degradation of methylene blue. Appl. Surf. Sci. 2017, 357, 1606–1612. [Google Scholar] [CrossRef]
- Butanovs, E.; Kuzmin, A.; Butikova, J.; Vlassov, S.; Polyakov, B. Synthesis and characterization of ZnO/ZnS/MoS2 core-shell nanowires. J. Cryst. Growth. 2017, 459, 100–104. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, Z.; Dai, C.; Zhou, X.; Chen, W. Interfacial thermodynamics and kinetics of sorption of diclofenac on prepared high performance flower-like MoS2. J. Colloid Interface Sci. 2016, 481, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Xu, J.; Luo, L. r-Graphene Oxide/Fe3O4/Carbon Nanotube Composites for the Adsorption of Copper and Methyl Testosterone in Wastewater. Sci. Adv. Mater. 2017, 9, 2082–2089. [Google Scholar] [CrossRef]
- Zhang, S.; Tang, F.; Liu, J.; Che, W.; Su, H.; Liu, W.; Huang, Y.; Jiang, Y.; Yao, T.; Liu, Q.; et al. MoS2-coated ZnO nanocomposite as an active heterostructure photocatalyst for hydrogen evolution. Radiat. Phys. Chem. 2017, 137, 104–107. [Google Scholar] [CrossRef]
- Yuan, Y.-J.; Tu, J.-R.; Ye, Z.-J.; Lu, H.-W.; Ji, Z.-G.; Hu, B.; Li, Y.-H.; Cao, D.-P.; Yu, Z.-T.; Zou, Z.-G. Visible-light-driven hydrogen production from water in a noble metal-free system catalyzed by zinc porphyrin sensitized MoS2/ZnO. Dyes Pigm. 2015, 123, 285–292. [Google Scholar] [CrossRef]
- Yan, H.; Song, P.; Zhang, S.; Yang, Z.; Wang, Q. Facile synthesis, characterization and gas sensing performance of ZnO nanoparticles-coated MoS2nanosheets. J. Alloys Compd. 2016, 662, 118–125. [Google Scholar] [CrossRef]
- Ze, L.; Gong, Y.; Li, X.; Zhang, Y. MoS2-modified ZnO quantum dots nanocomposite: Synthesis and ultrafast humidity response. Appl. Surf. Sci. 2017, 399, 330–336. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, S.; Li, H.; Wang, X. A highly efficient sunlight driven ZnO nanosheets photocatalyst: Synergetic effect of P-doping and MoS2 atomic layer loading. ChemCatChem 2014, 6, 2522–2526. [Google Scholar] [CrossRef]
- Lamba, R.; Umar, A.; Mehta, S.K.; Anderson, W.A.; Kansal, S.K. Visible-light-driven photocatalytic properties of self-assembled cauliflower-like AgCl/ZnO hierarchical nanostructures. J. Mol. Catal. A Chem. 2015, 408, 189–201. [Google Scholar] [CrossRef]
- Xiao, P.; Kim, J.H.; Seo, S. Simple Fabrication of Highly Sensitive Photodetectors Using MoS2 Nanoparticles and Ag Nanowires. Sci. Adv. Mater. 2017, 9, 1626–1630. [Google Scholar] [CrossRef]
- Lamba, R.; Umar, A.; Mehta, S.K.; Kansal, S.K. Well-crystalline porous ZnO-SnO2 nanosheets: An effective visible-light driven photocatalyst and highly sensitive smart sensor material. Talanta 2015, 131, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Lamba, R.; Umar, A.; Mehta, S.K.; Kansal, S.K. Enhanced visible light driven photocatalytic application of Ag2O decorated ZnO nanorods heterostructures. Sep. Purif. Technol. 2017, 183, 341–349. [Google Scholar] [CrossRef]
- Feng, X.; Tang, Q.; Zhou, J.; Fang, J.; Ding, P.; Sun, L.; Shi, L. Novel mixed-solvothermal synthesis of MoS2nanosheets with controllable morphologies. Cryst. Res. Technol. 2013, 48, 363–368. [Google Scholar] [CrossRef]
- Lamba, R.; Umar, A.; Mehta, S.K.; Kansal, S.K. ZnO doped SnO2 nanoparticles heterojunction photo-catalyst for environmental remediation. J. Alloys Compd. 2015, 653, 327–333. [Google Scholar] [CrossRef]
- Lamba, R.; Umar, A.; Mehta, S.K.; Kansal, S.K. CeO2-ZnO hexagonal nanodisks: Efficient material for the degradation of direct blue 15 dye and its simulated dye bath effluent under solar light. J. Alloys Compd. 2015, 620, 67–73. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, V.; Bhattacharyya, K.; Krishnan, V. Synergetic effect of MoS2-RGO doping to enhance the photocatalytic performance of ZnO nanoparticles. New J. Chem. 2016, 40, 5185–5197. [Google Scholar] [CrossRef]
- Wang, H.; Wen, F.; Li, X.; Gan, X.; Yang, Y.; Chen, P.; Zhang, Y. Cerium-doped MoS2 nanostructures: Efficient visible photocatalysis for Cr(VI) removal. Sep. Purif. Technol. 2016, 170, 190–198. [Google Scholar] [CrossRef]
- Lamba, R.; Umar, A.; Mehta, S.K.; Kansal, S.K. Sb2O3-ZnO nanospindles: A potential material for photocatalytic and sensing applications. Ceram. Int. 2015, 41, 5429–5438. [Google Scholar] [CrossRef]
- Georgekutty, R.; Seery, M.K.; Pillai, S.C. A highly efficient Ag-ZnO photocatalyst: Synthesis, properties, and mechanism. J. Phys. Chem. C 2008, 112, 13563–13570. [Google Scholar] [CrossRef]
- Trevizo, A.S.; Madrid, P.A.; Ruiz, P.P.; Flores, W.A.; Yoshida, M.M. Optical band gap estimation of ZnO nanorods. Mater. Res. 2016, 19, 33–38. [Google Scholar] [CrossRef]
- Ma, C.; Zhou, Z.; Wei, H.; Yang, Z.; Wang, Z.; Zhang, Y. Rapid large-scale preparation of ZnO nanowires for photocatalytic application. Nanoscale Res. Lett. 2011, 6, 536. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Sun, H.; Zhang, L.; Zhao, L.; Lian, J.; Jiang, Q. High efficient photo-Fenton catalyst of α-Fe2O3/MoS2 hierarchical nanoheterostructures: Reutilization for supercapacitors. Sci. Rep. 2016, 6, 31591. [Google Scholar] [CrossRef] [PubMed]
- Ong, W.-J.; Voon, S.-Y.; Tan, L.-L.; Goh, B.T.; Yong, S.-T.; Chai, S.-P. Enhanced daylight-induced photocatalytic activity of solvent exfoliated graphene (SEG)/ZnO hybrid nanocomposites toward degradation of Reactive Black 5. Ind. Eng. Chem. Res. 2014, 53, 17333–17344. [Google Scholar] [CrossRef]
- Ritika Kaur, M.; Umar, A.; Mehta, S.K.; Singh, S.; Kansal, S.K. Visible-light photocatalytic degradation of organic pollutants using molybdenum disulfide (MoS2) microtubes. Nanosci. Nanotechnol. Lett. 2017, 9, 1966–1974. [Google Scholar] [CrossRef]
- Wang, L.; Chai, Y.; Ren, J.; Ding, J.; Liu, Q.; Dai, W.-L. Ag3PO4 nanoparticles loaded on 3D flower-like spherical MoS2: A highly efficient hierarchical heterojunction photocatalyst. Dalton Trans. 2015, 44, 14625–14634. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.-C.; Li, X.-Y. Synthesis of MoS2/g-C3N4 as a solar light-responsive photocatalyst for organic degradation. Catal. Commun. 2014, 49, 63–67. [Google Scholar] [CrossRef]
- Tian, Q.; Wu, W.; Yang, S.; Liu, J.; Yao, W.; Ren, F.; Jiang, C. Zinc oxide coating effect for the dye removal and photocatalytic mechanisms of flower-like MoS2 nanoparticles. Nanoscale Res. Lett. 2017, 12, 221. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, G.P.; Adhikari, S.P.; Ko, S.; Kim, H.J.; Park, C.H.; Kim, C.S. Facile synthesis of ZnO flowers modified graphene like MoS2 sheets for enhanced visible-light-driven photocatalytic activity and antibacterial properties. J. Alloys Compd. 2016, 682, 208–215. [Google Scholar] [CrossRef]
- Jo, W.-K.; Lee, J.Y.; Selvam, N.C.S. Synthesis of MoS2 nanosheets loaded ZnO–g-C3N4 nanocomposites for enhanced photocatalytic applications. Chem. Eng. J. 2016, 289, 306–318. [Google Scholar] [CrossRef]


| Catalyst | Target Pollutant | Light Source | Reaction Time | Degradation (%) | Reference |
|---|---|---|---|---|---|
| MoS2-RGO doped ZnO (1 wt % of MoS2-RGO in ZnO) | MB and carbendazim | Natural solar light | 60 min | 98% for MB and 97% for carbendazim | [50] |
| MoS2/ZnO | Rhodamine B | Simulated solar light | 90 min | 91.4% | [61] |
| MoS2/ZnO | Phenol red | UV and visible light | 50 min under UV light | 93% | [62] |
| 80 min under visible light | 90% | ||||
| P-doped ZnO nanosheets decorated MoS2 | MB | Natural solar light | 6 min for MB | 95% | [42] |
| ZnO-g-C3N4 (50%)/MoS2 (1%) | MB and atrazine | UV-visible light | 30 min for MB | 99.5% for MB | [63] |
| 300 min for atrazine | 84.9% for atrazine | ||||
| MoS2/ZnO | MB | Natural solar light | 20 min | 97% | This work |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ritika; Kaur, M.; Umar, A.; Mehta, S.K.; Singh, S.; Kansal, S.K.; Fouad, H.; Alothman, O.Y. Rapid Solar-Light Driven Superior Photocatalytic Degradation of Methylene Blue Using MoS2-ZnO Heterostructure Nanorods Photocatalyst. Materials 2018, 11, 2254. https://doi.org/10.3390/ma11112254
Ritika, Kaur M, Umar A, Mehta SK, Singh S, Kansal SK, Fouad H, Alothman OY. Rapid Solar-Light Driven Superior Photocatalytic Degradation of Methylene Blue Using MoS2-ZnO Heterostructure Nanorods Photocatalyst. Materials. 2018; 11(11):2254. https://doi.org/10.3390/ma11112254
Chicago/Turabian StyleRitika, Manjot Kaur, Ahmad Umar, Surinder Kumar Mehta, Surinder Singh, Sushil Kumar Kansal, H. Fouad, and Othman Y. Alothman. 2018. "Rapid Solar-Light Driven Superior Photocatalytic Degradation of Methylene Blue Using MoS2-ZnO Heterostructure Nanorods Photocatalyst" Materials 11, no. 11: 2254. https://doi.org/10.3390/ma11112254
APA StyleRitika, Kaur, M., Umar, A., Mehta, S. K., Singh, S., Kansal, S. K., Fouad, H., & Alothman, O. Y. (2018). Rapid Solar-Light Driven Superior Photocatalytic Degradation of Methylene Blue Using MoS2-ZnO Heterostructure Nanorods Photocatalyst. Materials, 11(11), 2254. https://doi.org/10.3390/ma11112254









