Local Pressure of Supercritical Adsorbed Hydrogen in Nanopores
Abstract
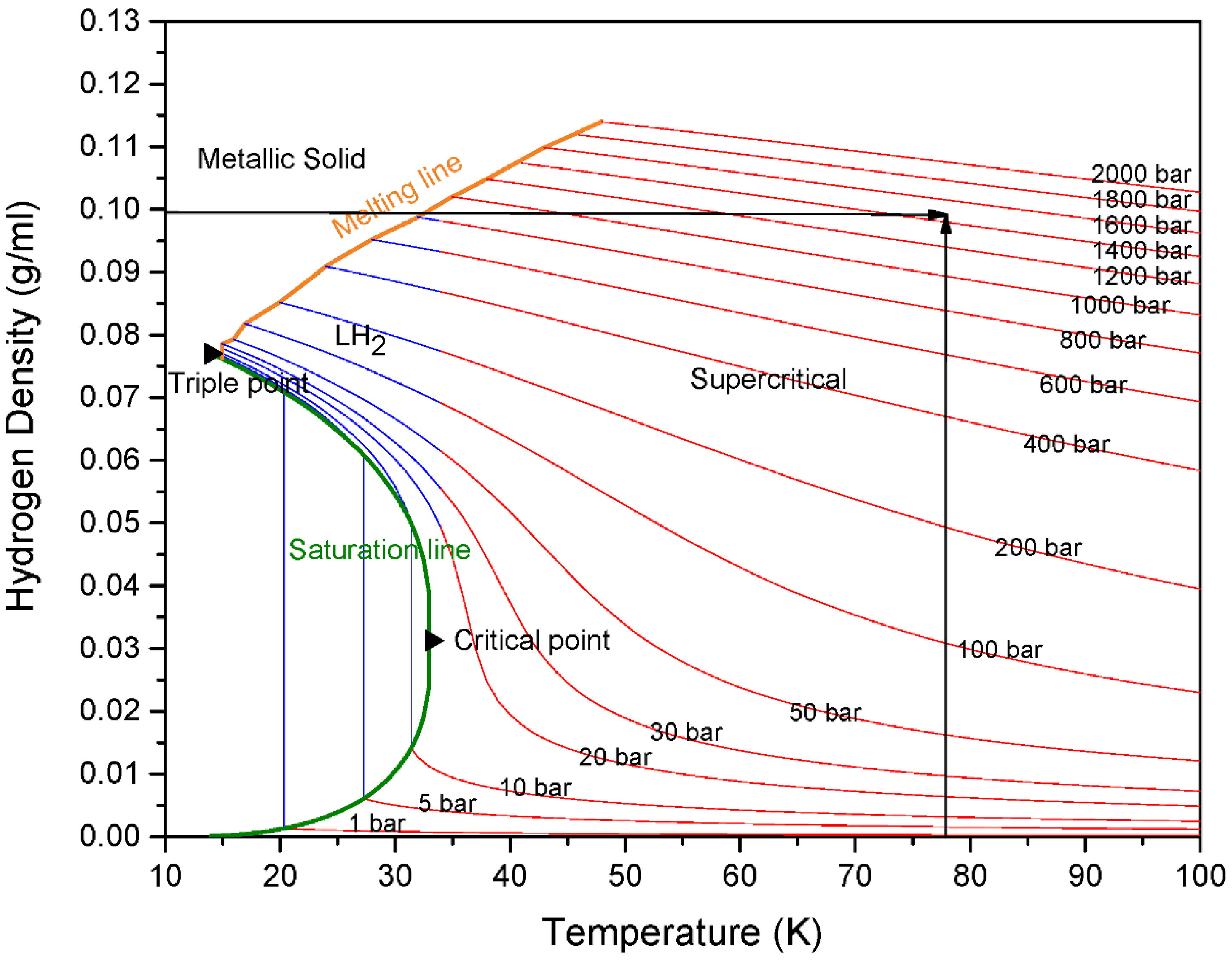
:1. Introduction
2. Material and Methods
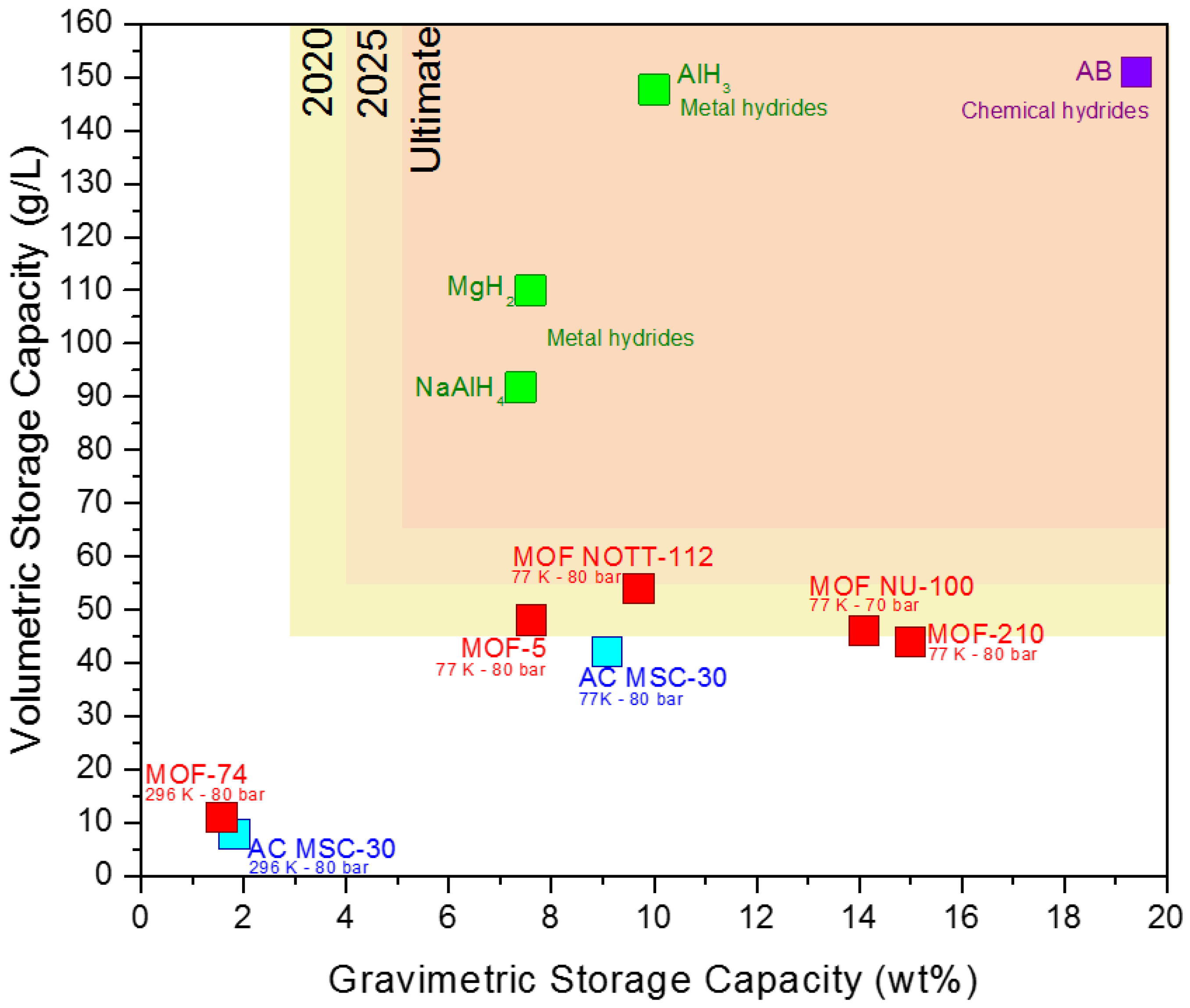
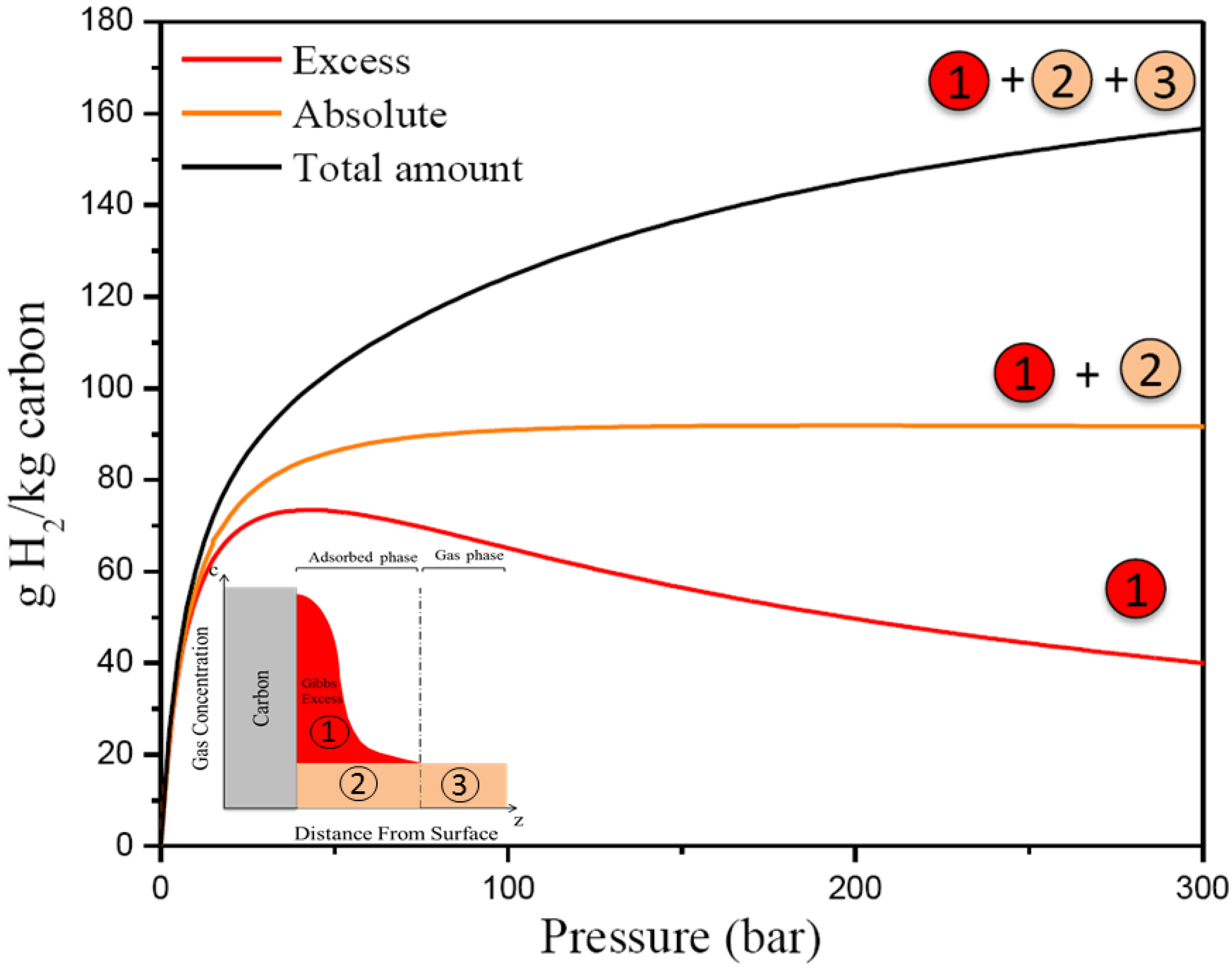
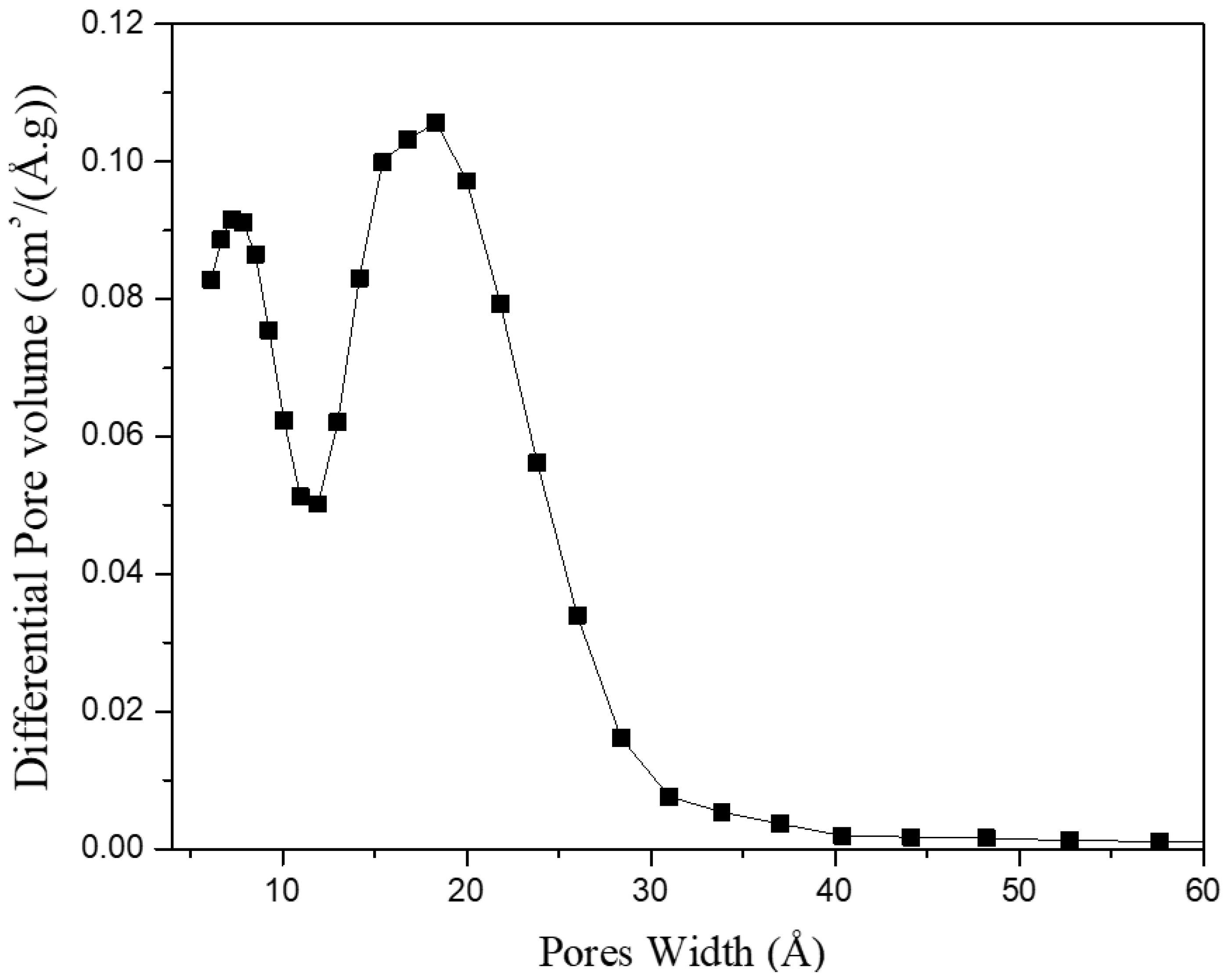
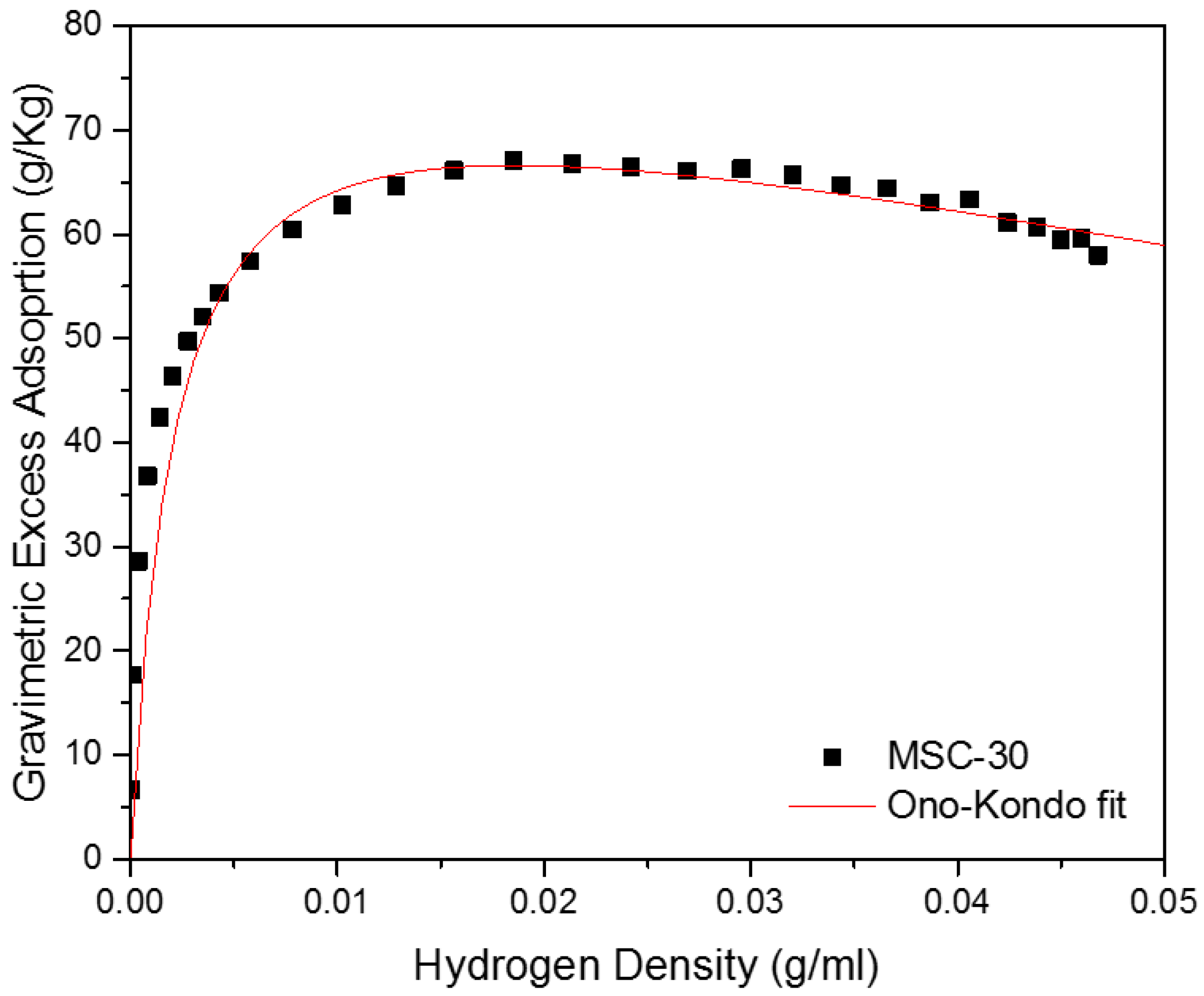
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ley, M.B.; Meggouh, M.; Moury, R.; Peinecke, K.; Felderhoff, M. Development of hydrogen storage tank systems based on complex metal hydrides. Materials 2015, 8, 5891–5921. [Google Scholar] [CrossRef] [PubMed]
- Romanos, J.; Barakat, F.; Dargham, S.A. Nanoporous Graphene Monolith for Hydrogen Storage. Mater. Today Proc. 2018, 5, 17478–17483. [Google Scholar] [CrossRef]
- Yan, Y.; Lin, X.; Yang, S.; Blake, A.J.; Dailly, A.; Champness, N.R. Exceptionally high hydrogen storage by a metal–organic polyhedral framework. Chem. Commun. 2009, 1025–1027. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yaping, Z.; Yan, S. Enhanced storage of hydrogen at the temperature of liquid nitrogen. Int. J. Hydrogen Energy 2004, 29, 319–322. [Google Scholar]
- Furukawa, H.; Ko, N.; Go, Y.B.; Aratani, N.; Choi, S.B.; Choi, E. Ultrahigh Porosity in Metal-Organic Frameworks. Science 2010, 329, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Suh, M.P.; Park, H.J.; Prasad, T.K.; Lim, D.W. Hydrogen storage in metal–organic frameworks. Chem. Rev. 2011, 112, 782–835. [Google Scholar] [CrossRef] [PubMed]
- Farha, O.K.; Yazaydın, A.Ö.; Eryazici, I.; Malliakas, C.D.; Hauser, B.G.; Kanatzidis, M.G. De novo synthesis of a metal–organic framework material featuring ultrahigh surface area and gas storage capacities. Nature 2010, 2, 944–948. [Google Scholar] [CrossRef] [PubMed]
- Bénard, P.; Chahine, R. Storage of hydrogen by physisorption on carbon and nanostructured materials. Scr. Mater. 2007, 56, 803–808. [Google Scholar] [CrossRef]
- Nishihara, H.; Hou, P.X.; Li, L.X.; Ito, M.; Uchiyama, M.; Kaburagi, T. High-Pressure Hydrogen Storage in Zeolite-Templated Carbon. J. Phys. Chem. C 2009, 113, 3189–3196. [Google Scholar] [CrossRef]
- Kuchta, B.; Firlej, L.; Mohammadhosseini, A.; Beckner, M.; Romanos, J.; Pfeifer, P. Open carbon frameworks-a search for optimal geometry for hydrogen storage. J. Mol. Model. 2013, 19, 4079–4087. [Google Scholar] [CrossRef] [PubMed]
- Bult, J.B.; Lee, J.; O’Neill, K.; Engtrakul, C.; Hurst, K.E.; Zhao, Y.; Simpson, L.J.; Parilla, P.A.; Gennett, T.; Blackburn, J.L. Manipulation of Hydrogen Binding Energy and Desorption Kinetics by Boron Doping of High Surface Area Carbon. J. Phys. Chem. C 2012, 116, 26138–26143. [Google Scholar] [CrossRef]
- Sevilla, M.; Mokaya, R. Energy storage applications of activated carbons: supercapacitors and hydrogen storage. Energy Environ. Sci. 2014, 7, 1250–1280. [Google Scholar] [CrossRef] [Green Version]
- Govind, S.; Abdelhamid, S. Activated carbon with optimum pore size distribution for hydrogen storage. Carbon 2016, 99, 289–294. [Google Scholar]
- Yang, S.J.; Im, J.H.; Nishihara, H.; Jung, H.; Lee, K.; Kyotani, T.; Park, C.R. General Relationship between Hydrogen Adsorption Capacities at 77 and 298 K and Pore Characteristics of the Porous Adsorbents. J. Phys. Chem. C 2012, 116, 10529–10540. [Google Scholar] [CrossRef]
- Aboud, M.A.; Alothman, Z.; Habila, M.; Zlotea, C.; Latroche, M.; Cuevas, F. Hydrogen Storage in Pristine and d10-Block Metal-Anchored Activated Carbon Made from Local Wastes. Energies 2015, 8, 3578–3590. [Google Scholar] [CrossRef] [Green Version]
- DOE. Revised Hydrogen Storage Targets; United Stated Department of Energy: Washington, DC, USA, 2017.
- Kuchta, B.; Firlej, L.; Pfeifer, P.; Wexler, C. Numerical estimation of hydrogen storage limits in carbon-based nanospaces. Carbon 2010, 48, 223–231. [Google Scholar] [CrossRef]
- Kuchta, B.; Firlej, L.; Mohammadhosseini, A.; Boulet, P.; Beckner, M.; Romanos, J.; Pfeifer, P. Hypothetical High-Surface-Area Carbons with Exceptional Hydrogen Storage Capacities: Open Carbon Frameworks. J. Am. Chem. Soc. 2012, 134, 15130–15137. [Google Scholar] [CrossRef] [PubMed]
- Romanos, J.; Beckner, M.; Stalla, D.; Tekeei, A.; Suppes, G.; Jalisatgi, S.; Lee, M.; Hawthorne, F.; Robertson, J.D.; Firlej, L.; et al. Infrared study of boron–carbon chemical bonds in boron-doped activated carbon. Carbon 2013, 54, 208–214. [Google Scholar] [CrossRef]
- Mason, J.A.; Veenstra, M.; Long, J.R. Evaluating metal-organic frameworks for natural gas storage. Chem. Sci. 2014, 5, 32–51. [Google Scholar] [CrossRef]
- Firlej, L.; Beckner, M.; Romanos, J.; Pfeifer, P.; Kuchta, B. Different Approach to Estimation of Hydrogen- Binding Energy in Nanospace-Engineered Activated Carbons. J. Phys. Chem. C 2014, 118, 955–961. [Google Scholar] [CrossRef]
- Aranovich, G.L.; Donohue, M.D. Adsorption isotherms for microporous adsorbents. Carbon 1995, 33, 1369–1375. [Google Scholar] [CrossRef]
- Bénard, P.; Chahine, R. Modeling of High-Pressure Adsorption Isotherms above the Critical Temperature on Microporous Adsorbents: Application to Methane. Langmuir 1997, 13, 808–813. [Google Scholar] [CrossRef]
- Otowa, T.; Tanibata, R.; Itoh, M. Production and adsorption characteristics of MAXSORB. Gas Sep. Purif. 1993, 7, 241–245. [Google Scholar] [CrossRef]
- Alexander, V.N.; Yangzheng, L.; Peter, I.R.; Matthias, T. Quenched solid density functional theory and pore size analysis of micro-mesoporous carbons. Carbon 2009, 47, 1617–1628. [Google Scholar]
- Cimino, R.T.; Kowalczyk, P.; Ravikovitch, P.I.; Neimark, A.V. Determination of Isosteric Heat of Adsorption by Quenched Solid Density Functional Theory. Langmuir 2017, 33, 1769–1779. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Bai, S.; Su, W.; Yang, J.; Zhou, Y. Comparative Study of the Excess versus Absolute Adsorption of CO2 on Superactivated Carbon for the Near-Critical Region. Langmuir 2003, 19, 2683–2690. [Google Scholar] [CrossRef]
- Ronny, P. Interpretation of net and excess adsorption isotherms in microporous adsorbents. Microporous Mesoporous Mater. 2014, 187, 40–52. [Google Scholar]
- Beckner, M.; Dailly, A. A pilot study of activated carbon and metal–organic frameworks for methane storage. Appl. Energy 2016, 162, 506–514. [Google Scholar] [CrossRef]
- Nakai, K.; Sonoda, J.; Iegami, H.; Naono, H. High Precision Volumetric Gas Adsorption Apparatus. Adsorption 2005, 11, 227–230. [Google Scholar] [CrossRef]
- Policicchio, A.; Maccallini, E.; Kalantzopoulos, G.; Cataldi, U.; Abate, S.; Desiderio, G. Volumetric apparatus for hydrogen adsorption and diffusion measurements: Sources of systematic error and impact of their experimental resolutions. Rev. Sci. Instrum. 2013, 84, 103907. [Google Scholar] [CrossRef] [PubMed]
- Sudibandriyo, M.; Mohammad, S.A.; Robinson, R.L.; Gasem, K.A. Ono-Kondo lattice model for high-pressure adsorption. Fluid Phase Equilib. 2010, 299, 238–251. [Google Scholar] [CrossRef]
- Purewal, J.J.; Kabbour, H.; Vajo, J.J.; Ahn, C.C.; Fultz, B. Pore size distribution and supercritical hydrogen adsorption in activated carbon fibers. Nanotechnology 2009, 20, 204012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmitz, B.; Müller, U.; Trukhan, N.; Schubert, M.; Férey, G.; Hirscher, M. Heat of Adsorption for Hydrogen in Microporous High-Surface-Area Materials. Chem. Phys. Chem. 2008, 9, 2181–2184. [Google Scholar] [CrossRef] [PubMed]
- Lemmon, E.W. Thermophysical Properties of Fluids; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Long, Y.; Palmer, J.C.; Coasne, B.; Sliwinska-Bartkowiak, M.; Jackson, G.; Müller, E.; Gubbins, K. On the molecular origin of high-pressure effects in nanoconfinement: The role of surface chemistry and roughness. J. Chem. Phys. 2013, 139, 144701. [Google Scholar] [PubMed] [Green Version]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romanos, J.; Abou Dargham, S.; Roukos, R.; Pfeifer, P. Local Pressure of Supercritical Adsorbed Hydrogen in Nanopores. Materials 2018, 11, 2235. https://doi.org/10.3390/ma11112235
Romanos J, Abou Dargham S, Roukos R, Pfeifer P. Local Pressure of Supercritical Adsorbed Hydrogen in Nanopores. Materials. 2018; 11(11):2235. https://doi.org/10.3390/ma11112235
Chicago/Turabian StyleRomanos, Jimmy, Sara Abou Dargham, Roy Roukos, and Peter Pfeifer. 2018. "Local Pressure of Supercritical Adsorbed Hydrogen in Nanopores" Materials 11, no. 11: 2235. https://doi.org/10.3390/ma11112235
APA StyleRomanos, J., Abou Dargham, S., Roukos, R., & Pfeifer, P. (2018). Local Pressure of Supercritical Adsorbed Hydrogen in Nanopores. Materials, 11(11), 2235. https://doi.org/10.3390/ma11112235





