Combining the Photocatalysis and Absorption Properties of Core-Shell Cu-BTC@TiO2 Microspheres: Highly Efficient Desulfurization of Thiophenic Compounds from Fuel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
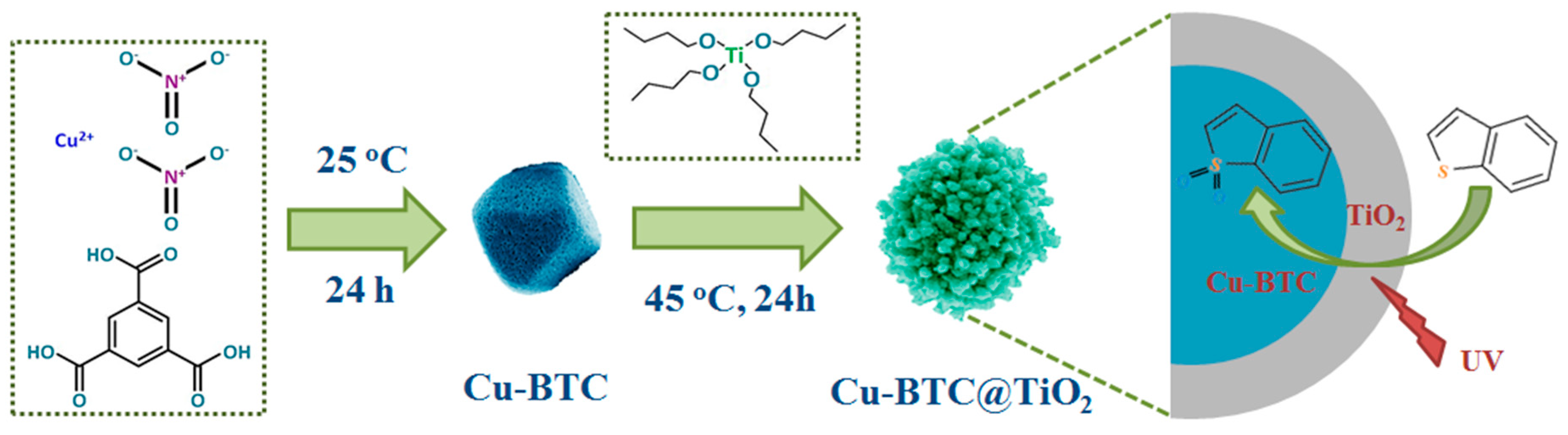
2.2. Synthesis
2.3. Characterization
2.4. Measurement of Desulfurization Performance
3. Results and Discussion
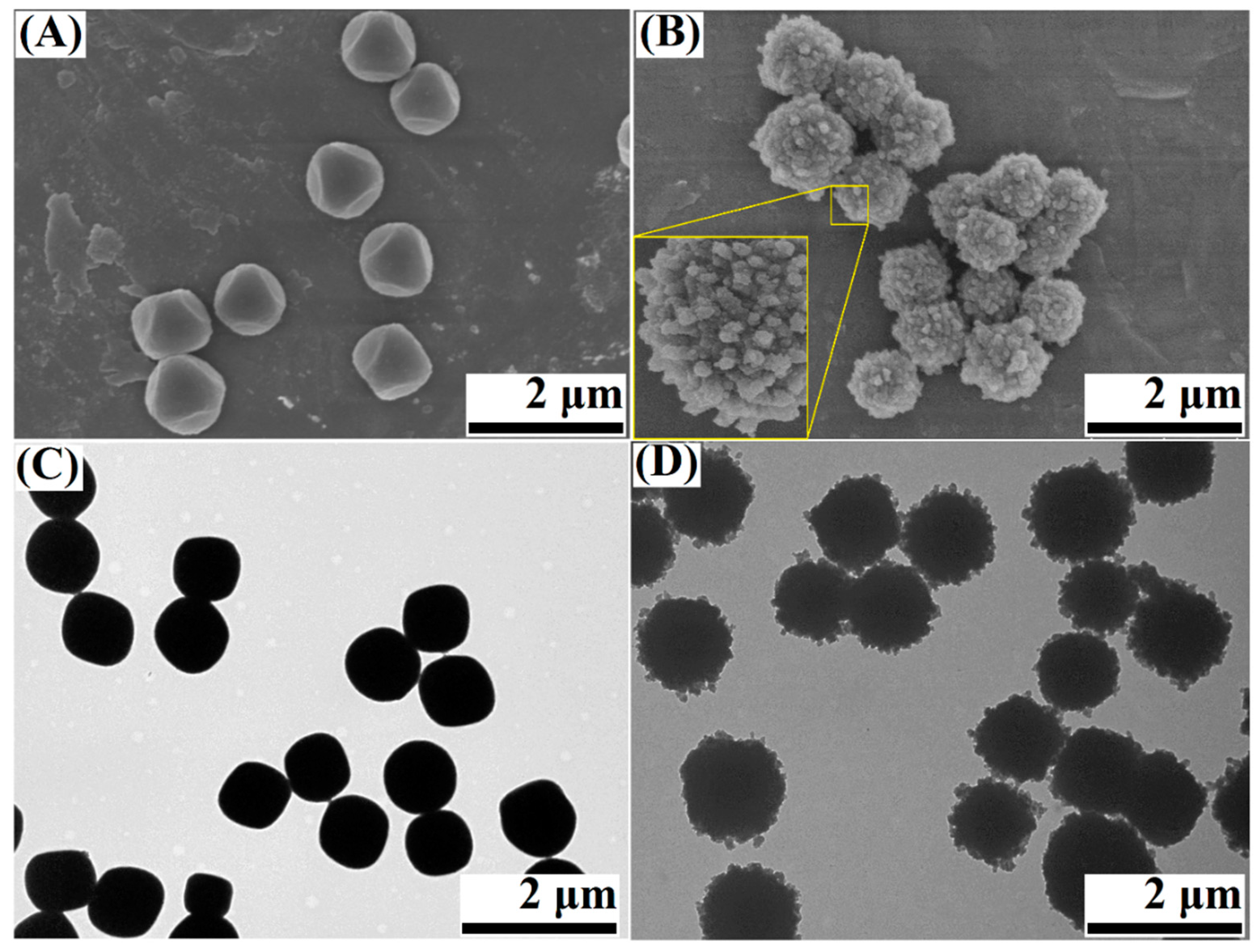
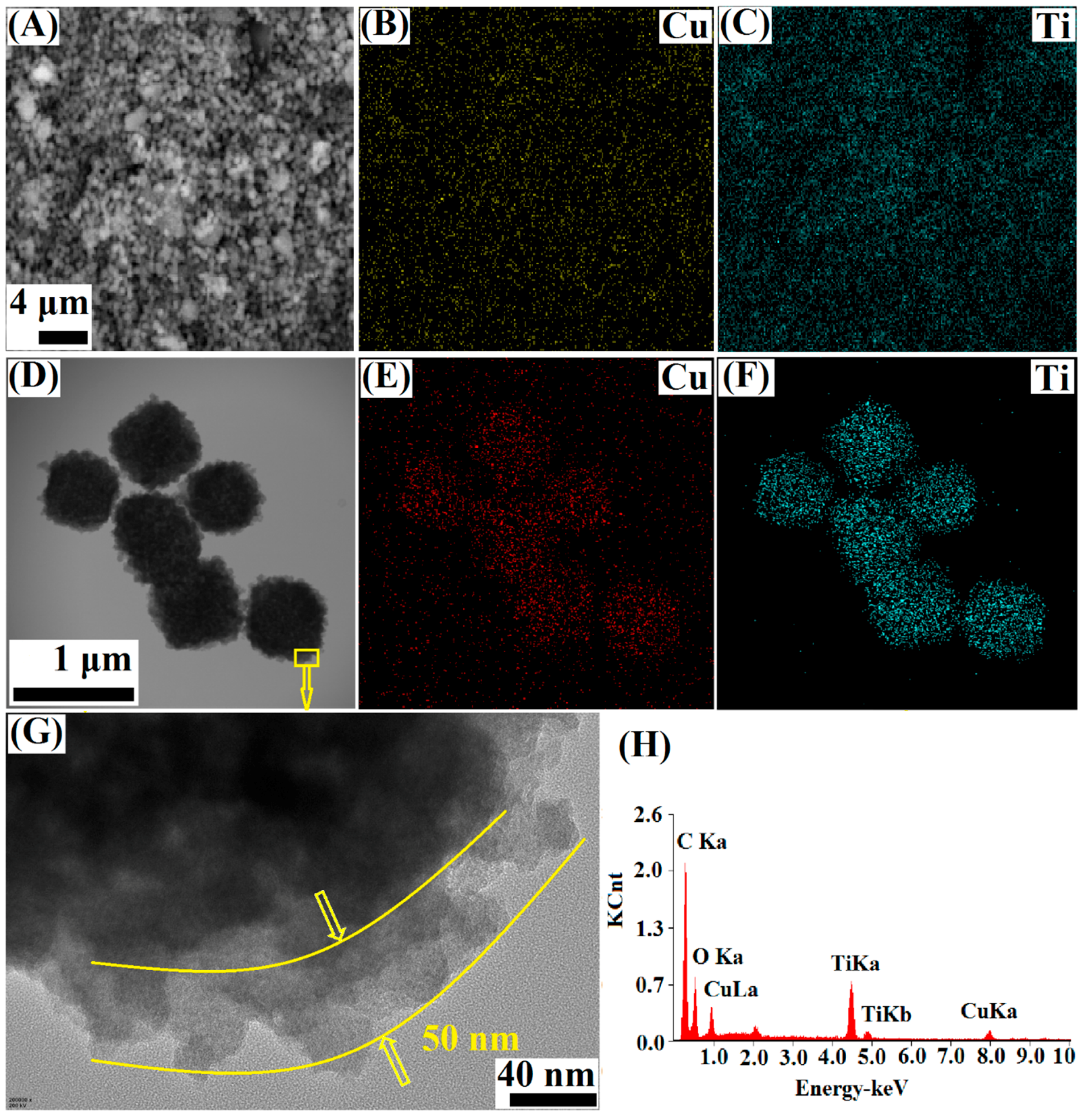
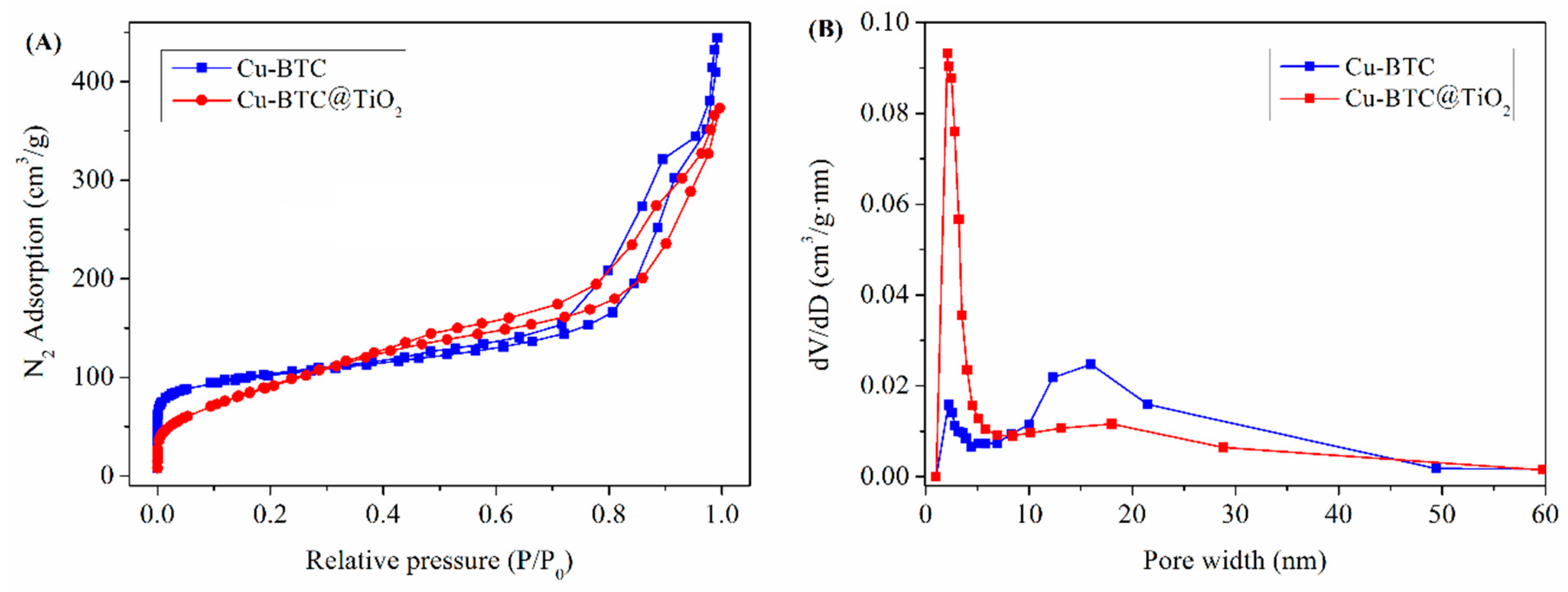
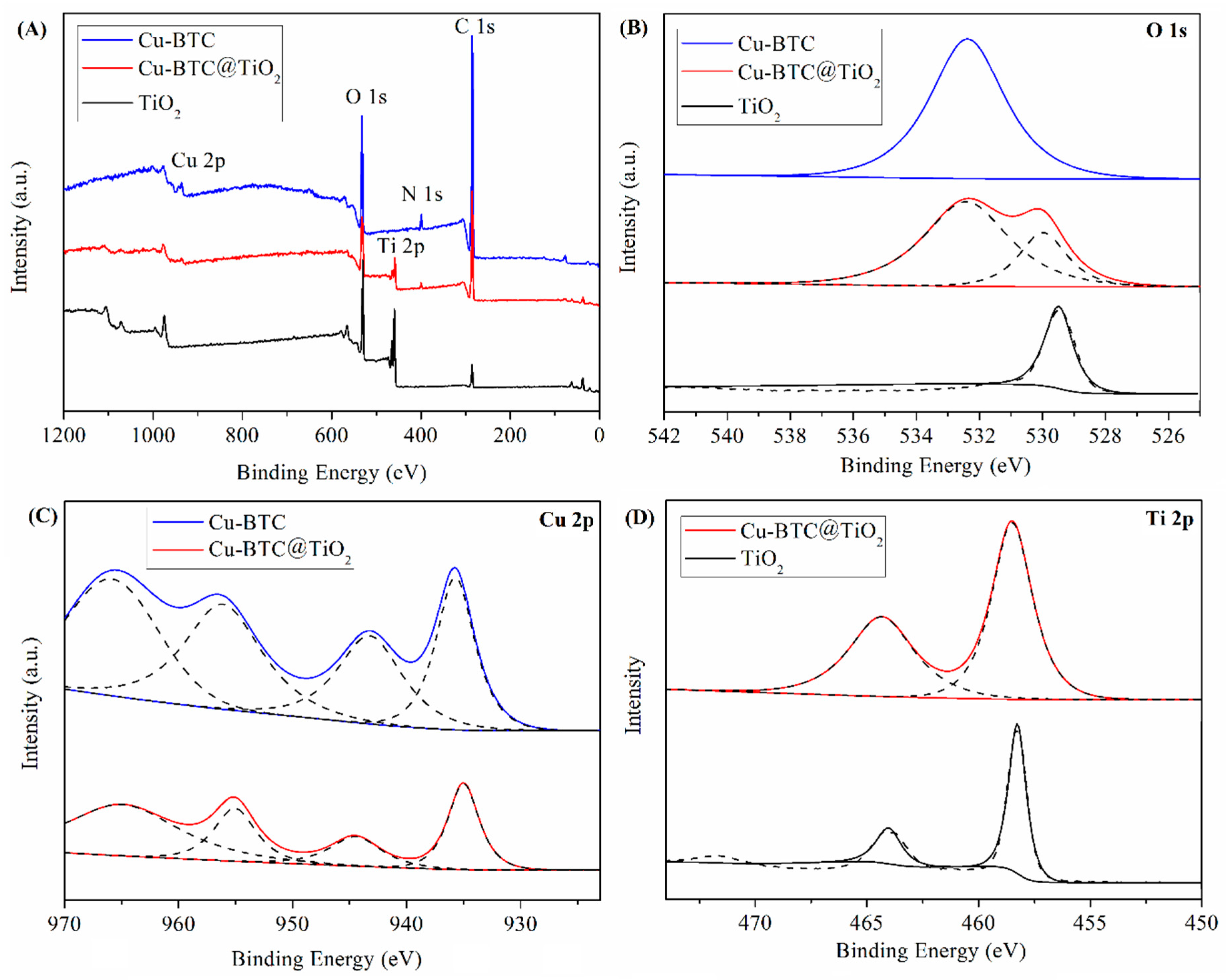
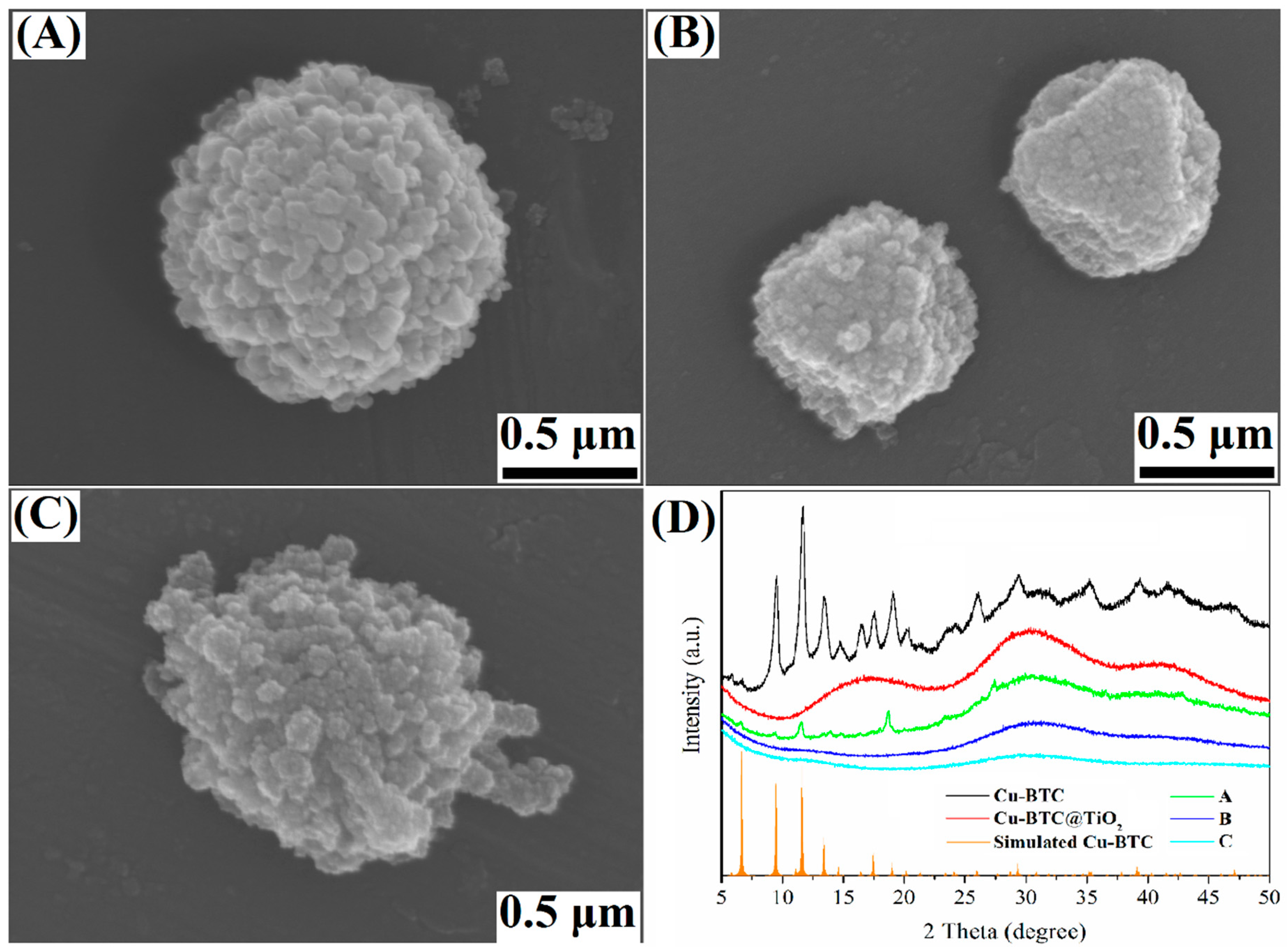
3.1. Characterization of the Cu-BTC@TiO2 Microsphere
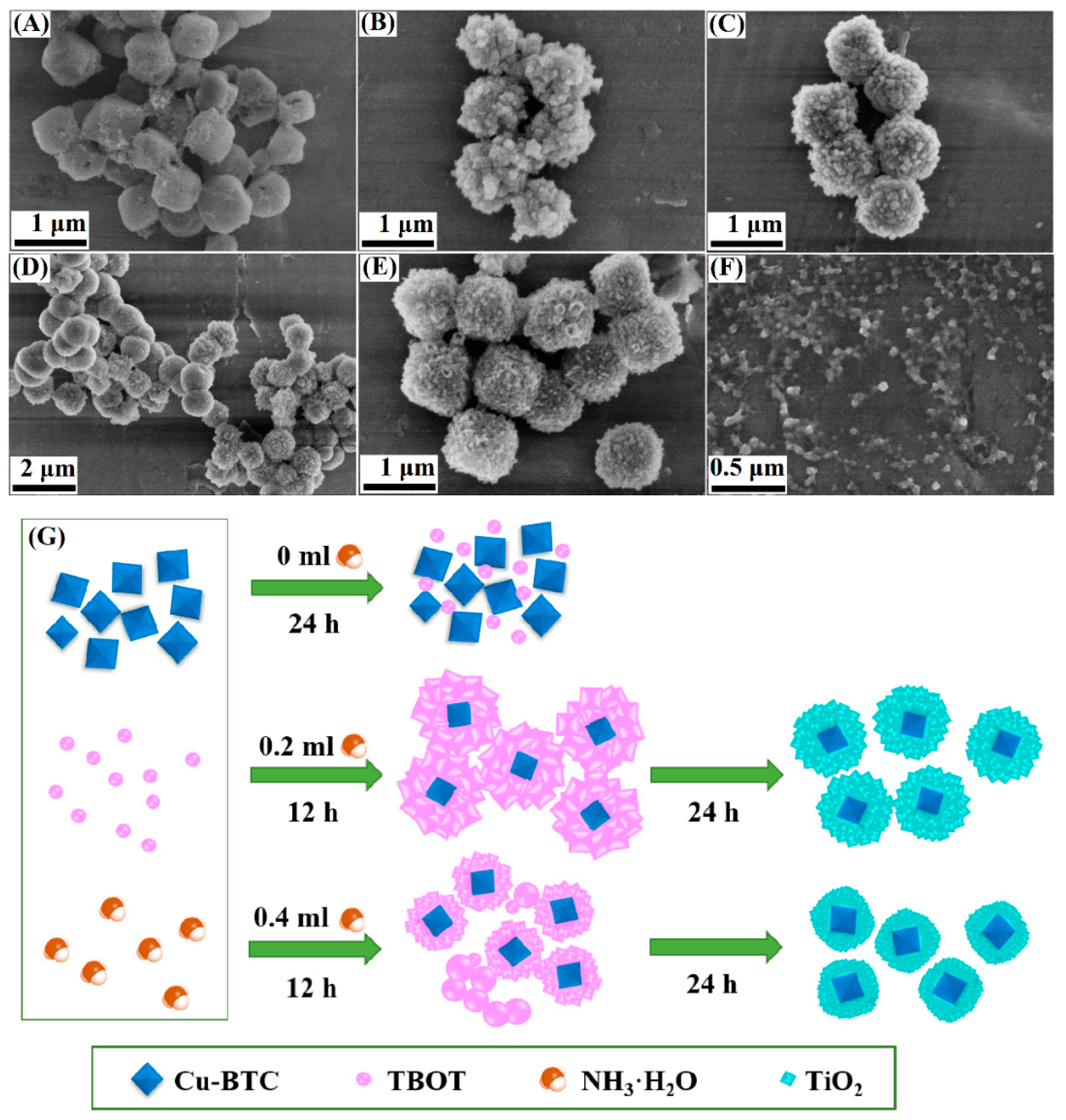
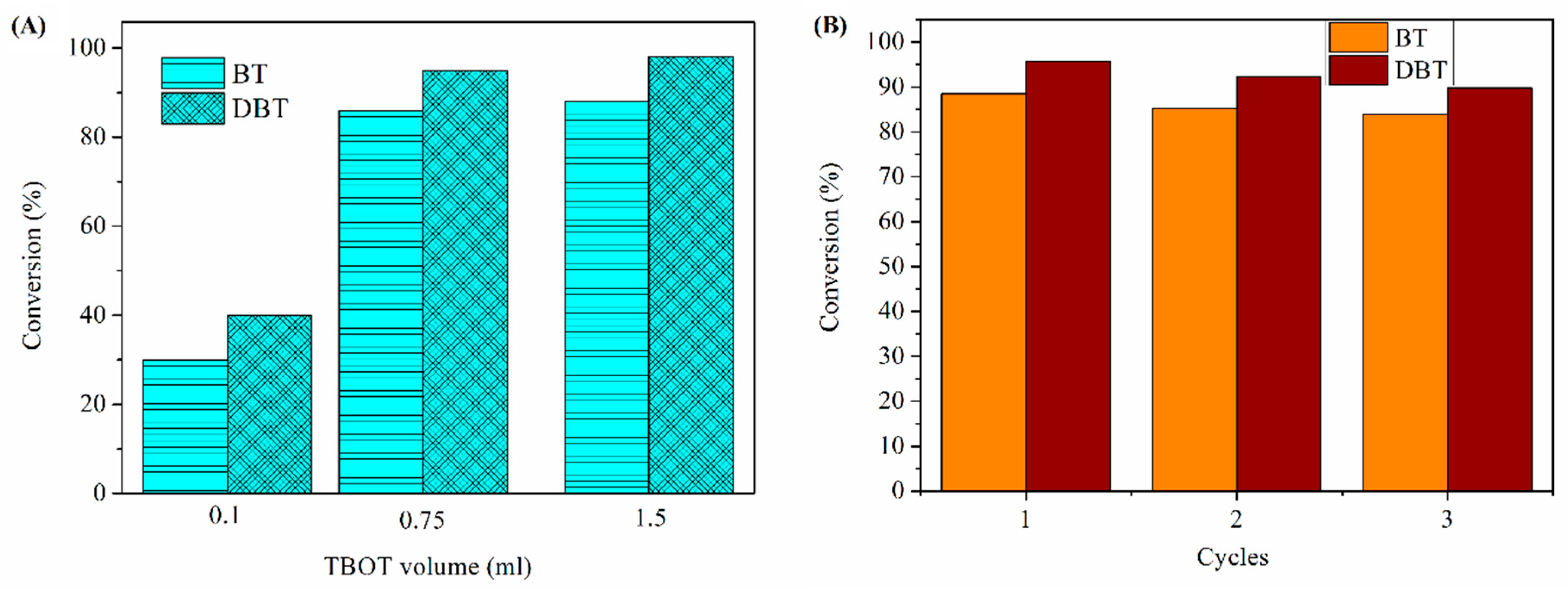
3.2. General Analysis of Controlling Factors
3.3. Formation Mechanism
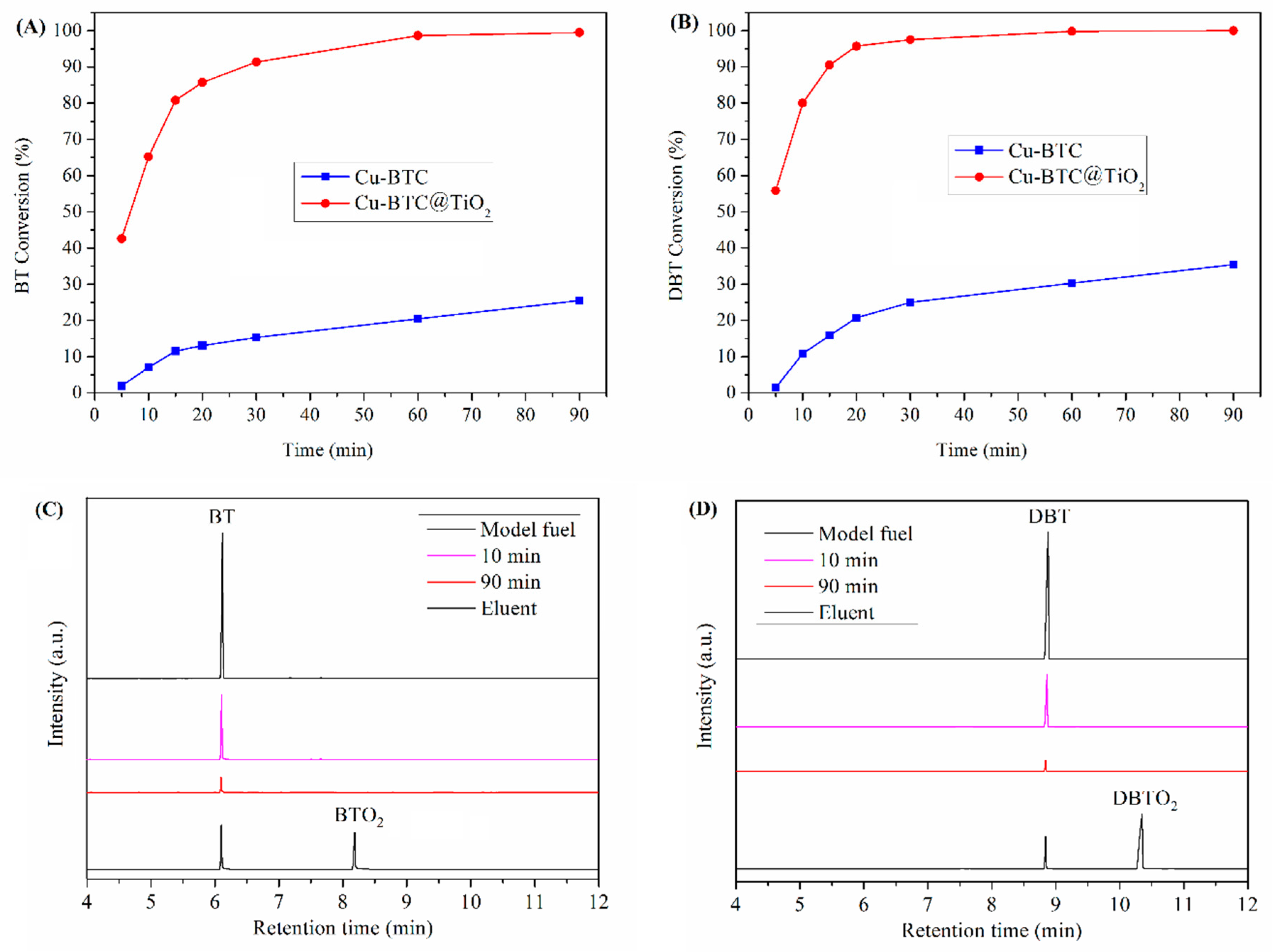
3.4. Desulfurization Performance
3.5. The Proposed Desulfurization Mechanism
3.6. Interaction between Thiophenic Sulfur Compounds and Cu-BTC@TiO2 Microspheres
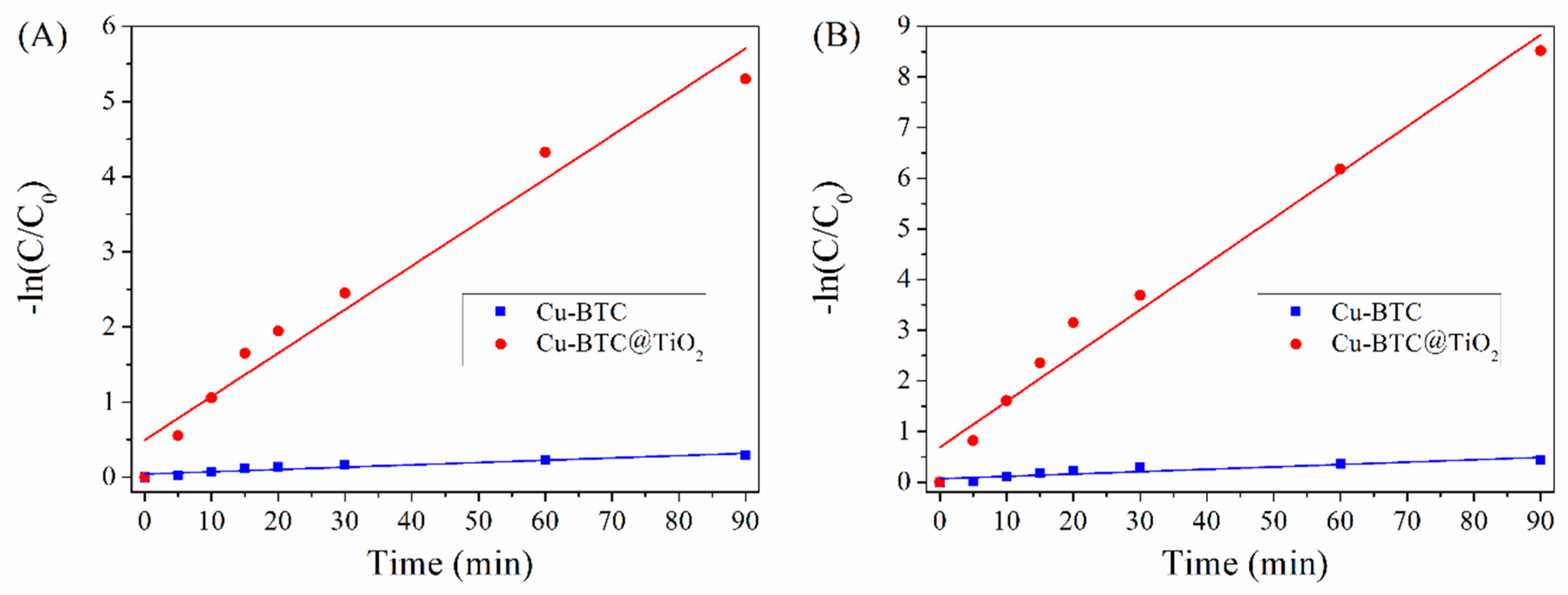
3.7. Kinetic Calculations
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wei, S.; He, H.; Cheng, Y.; Yang, C.; Zeng, G.; Qiu, L. Performances, kinetics and mechanisms of catalytic oxidative desulfurization from oils. RSC Adv. 2016, 6, 103253–103269. [Google Scholar] [CrossRef]
- Ma, W.; Xu, Y.; Ma, K.; Zhang, H. Electrospinning synthesis of H3PW12O40/TiO2 nanofiber catalytic materials and their application in ultra-deep desulfurization. Appl. Catal. A Gen. 2016, 526, 147–154. [Google Scholar] [CrossRef]
- Kulkarni, P.S.; Afonso, C.A.M. Deep Desulfurization of Diesel Fuel Using Ionic Liquids: Current Status and Future Challenges. Green Chem. 2010, 12, 1139–1149. [Google Scholar] [CrossRef]
- Baeza, P.; Aguila, G.; Gracia, F.; Araya, P. Desulfurization by adsorption with copper supported on zirconia. Catal. Commun. 2008, 9, 751–755. [Google Scholar] [CrossRef]
- Rang, H.; Kann, J.; Oja, V. Advances in desulfurization research of liquid fuel. Oil Shale 2006, 23, 164–176. [Google Scholar]
- Shi, C.; Wang, W.; Liu, N.; Xu, X.; Wang, D.; Zhang, M.; Sun, P.; Chen, T. Low temperature oxidative desulfurization with hierarchically mesoporous titaniumsilicate Ti-SBA-2 single crystals. Chem. Commun. 2015, 51, 11500–11503. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Miao, G.; Xiao, Z.; Ye, F.; Li, Z.; Wang, H.; Xiao, J. Catalytic adsorptive desulfurization of model diesel fuel using TiO2/SBA-15 under mild conditions. Fuel 2016, 174, 118–125. [Google Scholar] [CrossRef]
- Luna, M.D.G.D.; Samaniego, M.L.; Ong, D.C.; Wan, M.W.; Lu, M.C. Kinetics of sulfur removal in high shear mixing-assisted oxidative-adsorptive desulfurization of diesel. J. Clean. Prod. 2018, 178, 468–475. [Google Scholar] [CrossRef]
- Yang, R.T.; Hernández-Maldonado, A.J.; Yang, F.H. Desulfurization of transportation fuels with zeolites under ambient conditions. Science 2003, 301, 79–81. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Wang, Z.; Mominou, N.; Liu, L.; Li, S. Ultra-deep Desulfurization of Gasoline through Aqueous Phase in-situ Hydrogenation and Photocatalytic Oxidation. Appl. Catal. B Environ. 2016, 193, 180–188. [Google Scholar]
- Mansouri, A.; Khodadadi, A.A.; Mortazavi, Y. Ultra-deep adsorptive desulfurization of a model diesel fuel on regenerable Ni-Cu/γ-Al2O3 at low temperatures in absence of hydrogen. J. Hazard. Mater. 2014, 271, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Li, X.; Qian, J.; Miao, N.; Yao, C.; Chen, Z. Synthesis and characterization of CeO2/TiO2 nanotube arrays and enhanced photocatalytic oxidative desulfurization performance. J. Alloys Compd. 2016, 661, 363–371. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Y.; Han, H.; Yang, M.; Wang, L.; Zhang, Y.; Jiang, Z.; Li, C. Ultra-deep desulfurization via reactive adsorption on Ni/ZnO: The effect of ZnO particle size on the adsorption performance. Appl. Catal. B Environ. 2012, 119–120, 13–19. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Zhao, J.; Liu, Y.; Liu, C. Ultra-deep desulfurization by reactive adsorption desulfurization on copper-based catalysts. J. Energy Chem. 2018. [Google Scholar] [CrossRef]
- Al-Degs, Y.S.; El-Sheikh, A.H.; Al Bakain, R.Z.; Newman, A.P.; Al-Ghouti, M.A. Conventional and Upcoming Sulfur-Cleaning Technologies for Petroleum Fuel: A Review. Energy Technol. 2016, 4, 679–699. [Google Scholar] [CrossRef]
- Ma, X.; Sun, L.; Song, C. A new approach to deep desulfurization of gasoline, diesel fuel and jet fuel by selective adsorption for ultra-clean fuels and for fuel cell applications. Catal. Today 2002, 77, 107–116. [Google Scholar] [CrossRef]
- Wang, T.; Fang, Y.; Dai, W.; Hu, L.; Ma, N.; Yu, L. The remarkable adsorption capacity of zinc/nickel/copper-based metal-organic frameworks for thiophenic sulfurs. RSC Adv. 2016, 6, 105827–105832. [Google Scholar] [CrossRef]
- Wang, T.; Li, X.; Dai, W.; Fang, Y.; Huang, H. Enhanced adsorption of dibenzothiophene with zinc/copper-based metal-organic frameworks. J. Mater. Chem. A 2015, 3, 21044–21050. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, H.; Li, C.; Meng, H.; Lu, Y.; Zhong, C.; Liu, D.; Yang, Q. Adsorption Behavior of Metal–Organic Frameworks for Thiophenic Sulfur from Diesel Oil. Ind. Eng. Chem. Res. 2012, 51, 12449–12455. [Google Scholar] [CrossRef]
- Qin, Y.; Huang, L.; Zhang, D.; Sun, L. Mixed-node A-Cu-BTC and porous carbon based oxides derived from A-Cu-BTC as low temperature NO–CO catalyst. Inorg. Chem. Commun. 2016, 66, 64–68. [Google Scholar] [CrossRef]
- Tian, F.; Fu, Z.; Zhang, H.; Zhang, J.; Chen, Y.; Jia, C. Thiophene adsorption onto metal-organic framework HKUST-1 in the presence of toluene and cyclohexene. Fuel 2015, 158, 200–206. [Google Scholar] [CrossRef]
- Miao, G.; Ye, F.Y.; Wu, L.M.; Ren, X.L.; Xiao, J.; Li, Z.; Wang, H.H. Selective adsorption of thiophenic compounds from fuel over TiO2/SiO2 under UV-irradiation. J. Hazard. Mater. 2015, 300, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cai, H.; Li, S.; Mominou, N. Ultra-deep removal of thiophene compounds in diesel oil over catalyst TiO2/Ni-ZSM-5 assisted by ultraviolet irradiating. Fuel 2013, 105, 752–756. [Google Scholar] [CrossRef]
- Lin, F.; Jiang, Z.; Tang, N.; Zhang, C.; Chen, Z.; Liu, T.; Dong, B. Photocatalytic oxidation of thiophene on RuO2/SO42−-TiO2: Insights for cocatalyst and solid-acid. Appl. Catal. B: Environ. 2016, 188, 253–258. [Google Scholar] [CrossRef]
- Hitam, C.N.C.; Jalil, A.A.; Triwahyono, S.; Ahmad, A.; Jaafar, N.F.; Salamun, N.; Fatah, N.A.A.; Teh, L.P.; Khusnun, N.F.; Ghazali, Z. Synergistic interactions of Cu and N on surface altered amorphous TiO2 nanoparticles for enhanced photocatalytic oxidative desulfurization of dibenzothiophene. RSC Adv. 2016, 6, 76259–76268. [Google Scholar] [CrossRef]
- Sun, X.; Tatarchuk, B.J. Photo-assisted adsorptive desulfurization of hydrocarbon fuels over TiO2 and Ag/TiO2. Fuel 2016, 183, 550–556. [Google Scholar] [CrossRef]
- Sun, X.; Hussain, A.H.M.S.; Chi, M.; Cheng, X.; Tatarchuk, B.J. Persistent adsorptive desulfurization enhancement of TiO2 after one-time ex-situ UV-treatment. Fuel 2017, 193, 95–100. [Google Scholar] [CrossRef]
- Shen, L.; Xu, C.; Qi, X.; Cao, Y.; Tang, J.; Zheng, Y.; Jiang, L. Highly efficient CuxO/TiO2 catalysts: Controllable dispersion and isolation of metal active species. Dalton Trans. 2016, 45, 4491–4495. [Google Scholar] [CrossRef] [PubMed]
- Ökte, A.N.; Karamanis, D.; Chalkia, E.; Tuncel, D. The effect of ZnO or TiO2 loaded nanoparticles on the adsorption and photocatalytic performance of Cu-BTC and ZIF-8 MOFs. Mater. Chem. Phys. 2017, 187, 5–10. [Google Scholar] [CrossRef]
- Ramasubbu, V.; Alwin, S.; Mothi, E.M.; Sahaya Shajan, X. TiO2 aerogel–Cu-BTC metal-organic framework composites for enhanced photon absorption. Mater. Lett. 2017, 197, 236–240. [Google Scholar] [CrossRef]
- Abedi, S.; Morsali, A. Ordered Mesoporous Metal Organic Frameworks Incorporated with Amorphous TiO2 As Photocatalyst for Selective Aerobic Oxidation in Sunlight Irradiation. ACS Catal. 2014, 4, 1398–1403. [Google Scholar] [CrossRef]
- Tuncel, D.; Ökte, A.N. Efficient photoactivity of TiO2-hybrid-porous nanocomposite: Effect of humidity. Appl. Surf. Sci. 2018, 458, 546–554. [Google Scholar] [CrossRef]
- Wang, H.M.; Yu, T.; Tan, X.; Zhang, H.B.; Li, P.; Liu, H.M.; Shi, L.; Li, X.L.; Ye, J.H. Enhanced Photocatalytic Oxidation of Isopropanol by HKUST-1@TiO2 Core-Shell Structure with Ultrathin Anatase Porous Shell: Toxic Intermediate Control. Ind. Eng. Chem. Res. 2016, 55, 8096–8103. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, W.; Wu, L.; Ye, F.; Xiao, J.; Li, Z. One-pot photocatalysis-assisted adsorptive desulfurization of diesel over doped-TiO2 under ambient conditions. RSC Adv. 2014, 4, 56567–56570. [Google Scholar] [CrossRef]
- Zhang, R.; Hu, L.; Bao, S.; Li, R.; Gao, L.; Li, R.; Chen, Q. Surface polarization enhancement: High catalytic performance of Cu/CuOx/C nanocomposites derived from Cu-BTC for CO oxidation. J. Mater. Chem. A 2016, 4, 8412–8420. [Google Scholar] [CrossRef]
- Li, W.; Yang, J.; Wu, Z.; Wang, J.; Li, B.; Feng, S.; Deng, Y.; Zhang, F.; Zhao, D. A Versatile Kinetics-Controlled Coating Method to Construct Uniform Porous TiO2 Shells for Multifunctional Core–Shell Structures. J. Am. Chem. Soc. 2012, 134, 11864–11867. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Kondo, T.; Mine, E.; Nagao, D.; Kobayashi, Y.; Konno, M. Fabrication of sub-micrometer-sized jingle bell-shaped hollow spheres from multilayered core–shell particles. J. Colloid Interface Sci. 2004, 279, 281–283. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Miao, Y.; Li, S.; Gao, L.; Xiao, G. Metal-organic frameworks derived bimetallic Cu-Co catalyst for efficient and selective hydrogenation of biomass-derived furfural to furfuryl alcohol. Mol. Catal. 2017, 436, 128–137. [Google Scholar] [CrossRef]
- Xiong, Y.; Ye, F.; Zhang, C.; Shen, S.; Su, L.; Zhao, S. Synthesis of magnetic porous γ-Fe2O3/C@HKUST-1 composites for efficient removal of dyes and heavy metal ions from aqueous solution. RSC Adv. 2015, 5, 5164–5172. [Google Scholar] [CrossRef]
- Jabbari, V.; Veleta, J.M.; Zarei-Chaleshtori, M.; Gardea-Torresdey, J.; Villagrán, D. Green synthesis of magnetic MOF@GO and MOF@CNT hybrid nanocomposites with high adsorption capacity towards organic pollutants. Chem. Eng. J. 2016, 304, 774–783. [Google Scholar] [CrossRef] [Green Version]
- Kalantari, K.; Kalbasi, M.; Sohrabi, M.; Royaee, S.J. Synthesis and characterization of N-doped TiO2 nanoparticles and their application in photocatalytic oxidation of dibenzothiophene under visible light. Ceram. Int. 2016, 42, 14834–14842. [Google Scholar] [CrossRef]
- Kalantari, K.; Kalbasi, M.; Sohrabi, M.; Royaee, S.J. Enhancing the photocatalytic oxidation of dibenzothiophene using visible light responsive Fe and N co-doped TiO2 nanoparticles. Ceram. Int. 2017, 43, 973–981. [Google Scholar] [CrossRef]
- Senthil Kumar, R.; Senthil Kumar, S.; Anbu Kulandainathan, M. Efficient electrosynthesis of highly active Cu3(BTC)2-MOF and its catalytic application to chemical reduction. Microporous Mesoporous Mater. 2013, 168, 57–64. [Google Scholar] [CrossRef]
- Chen, H.; Wang, L.; Yang, J.; Yang, R.T. Investigation on Hydrogenation of Metal–Organic Frameworks HKUST-1, MIL-53, and ZIF-8 by Hydrogen Spillover. J. Phys. Chem. C 2013, 117, 7565–7576. [Google Scholar] [CrossRef]
- Pan, L.; Ji, Z.; Yi, X.; Zhu, X.; Chen, X.; Shang, J.; Liu, G.; Li, R.W. Metal-Organic Framework Nanofilm for Mechanically Flexible Information Storage Applications. Adv. Funct. Mater. 2015, 25, 2677–2685. [Google Scholar] [CrossRef]
- Duke, A.S.; Dolgopolova, E.A.; Galhenage, R.P.; Ammal, S.C.; Heyden, A.; Smith, M.D.; Chen, D.A.; Shustova, N.B. Active Sites in Copper-Based Metal–Organic Frameworks: Understanding Substrate Dynamics, Redox Processes, and Valence-Band Structure. J. Phys. Chem. C 2015, 119, 27457–27466. [Google Scholar] [CrossRef]
- Wu, Y.P.; Zhou, W.; Dong, W.W.; Zhao, J.; Qiao, X.Q.; Hou, D.F.; Li, D.; Zhang, Q.; Feng, P. Temperature-controlled synthesis of porous CuO particles with different morphologies for highly sensitive detection of triethylamine. Cryst. Growth Des. 2017, 17, 2158–2165. [Google Scholar] [CrossRef]
- Wang, A.; Zhou, Y.; Wang, Z.; Chen, M.; Sun, L.; Liu, X. Titanium incorporated with UiO-66(Zr)-type Metal-Organic Framework (MOF) for photocatalytic application. RSC Adv. 2016, 6, 3671–3679. [Google Scholar] [CrossRef]
- Li, X.; Pi, Y.; Xia, Q.; Li, Z.; Xiao, J. TiO2 encapsulated in Salicylaldehyde-NH2-MIL-101(Cr) for enhanced visible light-driven photodegradation of MB. Appl. Catal. B: Environ. 2016, 191, 192–201. [Google Scholar] [CrossRef]
- Chui, S.S.Y.; Lo, S.M.F.; Charmant, J.P.H.; Orpen, A.G.; Williams, I.D. A Chemically Functionalizable Nanoporous Material [Cu3(TMA)2(H2O)3]n. Science 1999, 283, 1148–1150. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Q.; Qiu, L.G.; Xu, T.; Wu, Y.; Wang, W.; Wu, Z.Y.; Jiang, X. Ultrasonic synthesis of the microporous metal–organic framework Cu3(BTC)2 at ambient temperature and pressure: An efficient and environmentally friendly method. Mater. Lett. 2009, 63, 78–80. [Google Scholar] [CrossRef]
- Chen, B.; Zhao, N.; Wei, C.; Zhou, J.; He, F.; Shi, C.; He, C.; Liu, E. Multi-functional integration of pore P25@C@MoS2 core-double shell nanostructures as robust ternary anodes with enhanced lithium storage properties. Appl. Surf. Sci. 2017, 401, 232–240. [Google Scholar] [CrossRef]
- Li, W.; Wang, F.; Feng, S.; Wang, J.; Sun, Z.; Li, B.; Li, Y.; Yang, J.; Elzatahry, A.A.; Xia, Y.; et al. Sol–Gel Design Strategy for Ultradispersed TiO2 Nanoparticles on Graphene for High-Performance Lithium Ion Batteries. J. Am. Chem. Soc. 2013, 135, 18300–18303. [Google Scholar] [CrossRef] [PubMed]
- Otsuki, S.; Nonaka, T.; Takashima, N.; Qian, W.; Ishihara, A.; Imai, T.; Kabe, T. Oxidative desulfurization of light gas oil and vacuum gas oil by oxidation and solvent extraction. Energy Fuels 2000, 14, 1232–1239. [Google Scholar] [CrossRef]
- Wang, F.; Wang, G.; Sun, W.; Wang, T.; Chen, X. Metallophthalocyanine functionalized magnetic mesoporous silica nanoparticles and its application in ultrasound-assisted oxidation of benzothiophene. Microporous Mesoporous Mater. 2015, 217, 203–209. [Google Scholar] [CrossRef]
- Zhang, X.; Luo, G.; Zhu, M.; Kang, L.; Yu, F.; Dai, B. Application of a H4SiMo12O40@SiO2 catalyst with a hollow core–shell structure to oxidative desulfurization. RSC Adv. 2015, 5, 76182–76189. [Google Scholar] [CrossRef]
- Yang, J.; Hu, D.; Li, W.; Yi, S. Highly efficient microreactors with simultaneous separation of catalysts and products in deep desulfurization. Chem. Eng. J. 2015, 267, 93–101. [Google Scholar] [CrossRef]
- Rivoira, L.P.; Vallés, V.A.; Ledesma, B.C.; Ponte, M.V.; Martínez, M.L.; Anunziata, O.A.; Beltramone, A.R. Sulfur elimination by oxidative desulfurization with titanium-modified SBA-16. Catal. Today 2016, 271, 102–113. [Google Scholar] [CrossRef]
- Ahmed, I.; Jhung, S.H. Adsorptive desulfurization and denitrogenation using metal-organic frameworks. J. Hazard. Mater. 2016, 301, 259–276. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.H.; Wu, J.; Yu, M.X.; Zhu, L.G. Ti (Phen)(OC2H5)2Cl2: A highly efficient pre-catalyst for selective oxidation of organic sulfides to sulfoxides by hydrogen peroxide. RSC Adv. 2017, 7, 44259–44264. [Google Scholar] [CrossRef]
- Khan, N.A.; Jhung, S.H. Adsorptive removal and separation of chemicals with metal-organic frameworks: Contribution of π-complexation. J. Hazard. Mater. 2017, 325, 198–213. [Google Scholar] [CrossRef] [PubMed]
- Salmasi, M.; Fatemi, S.; Mortazavi, Y. Fabrication of promoted TiO2 nanotubes with superior catalytic activity against TiO2 nanoparticles as the catalyst of oxi-desulfurization process. J. Ind. Eng. Chem. 2016, 39, 66–76. [Google Scholar] [CrossRef]
- Bhadra, B.N.; Ji, Y.S.; Khan, N.A.; Jhung, S.H. TiO2-Containing Carbon Derived from a Metal–Organic Framework Composite: A Highly Active Catalyst for Oxidative Desulfurization. ACS Appl. Mater. Interfaces 2017, 9, 31192–31202. [Google Scholar] [CrossRef] [PubMed]

| Catalysts | BT | DBT | ||
|---|---|---|---|---|
| k (min−1) | R2 | k (min−1) | R2 | |
| Cu-BTC | 0.0424 | 0.9230 | 0.0680 | 0.9558 |
| Cu-BTC@TiO2 | 0.4953 | 0.9598 | 0.6867 | 0.9735 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Li, X.-M.; He, J.; Wang, L.-Y.; Lei, J.-D. Combining the Photocatalysis and Absorption Properties of Core-Shell Cu-BTC@TiO2 Microspheres: Highly Efficient Desulfurization of Thiophenic Compounds from Fuel. Materials 2018, 11, 2209. https://doi.org/10.3390/ma11112209
Liu J, Li X-M, He J, Wang L-Y, Lei J-D. Combining the Photocatalysis and Absorption Properties of Core-Shell Cu-BTC@TiO2 Microspheres: Highly Efficient Desulfurization of Thiophenic Compounds from Fuel. Materials. 2018; 11(11):2209. https://doi.org/10.3390/ma11112209
Chicago/Turabian StyleLiu, Jing, Xiao-Min Li, Jing He, Lu-Ying Wang, and Jian-Du Lei. 2018. "Combining the Photocatalysis and Absorption Properties of Core-Shell Cu-BTC@TiO2 Microspheres: Highly Efficient Desulfurization of Thiophenic Compounds from Fuel" Materials 11, no. 11: 2209. https://doi.org/10.3390/ma11112209
APA StyleLiu, J., Li, X.-M., He, J., Wang, L.-Y., & Lei, J.-D. (2018). Combining the Photocatalysis and Absorption Properties of Core-Shell Cu-BTC@TiO2 Microspheres: Highly Efficient Desulfurization of Thiophenic Compounds from Fuel. Materials, 11(11), 2209. https://doi.org/10.3390/ma11112209






