Thermal and Mechanical Properties of Expanded Graphite/Paraffin Gypsum-Based Composite Material Reinforced by Carbon Fiber
Abstract
:1. Introduction
2. Experimental
2.1. Materials
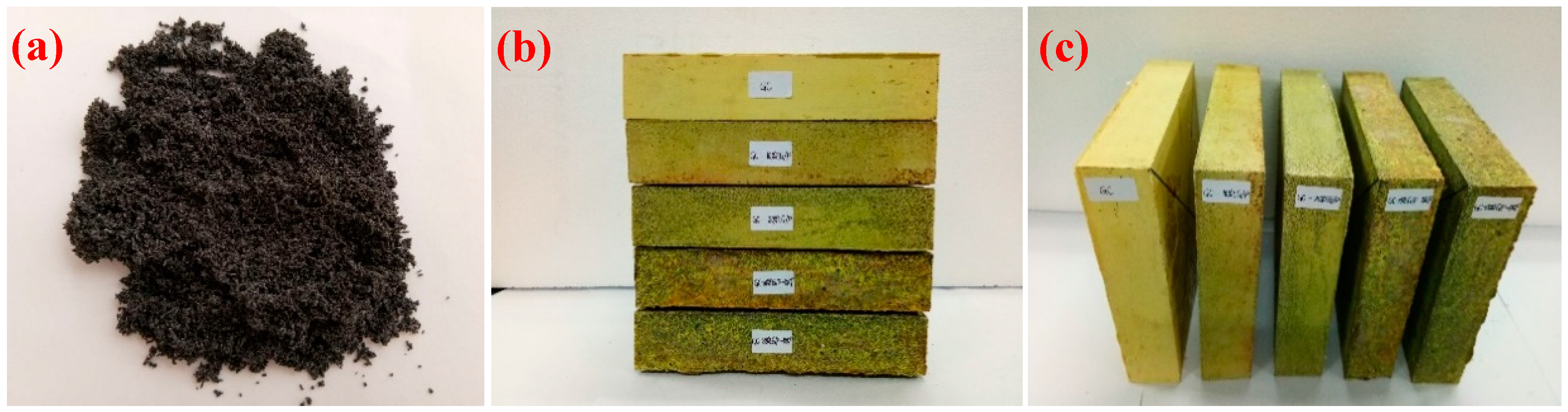
2.2. The Preparation and Characterization of EG/P
2.3. The Preparation and Characterization of EGPG
2.3.1. The Sample Preparation of EGPG
2.3.2. Thermal Conductivity Test
2.3.3. Thermo-Regulated Performance Test
2.3.4. Temperature Cycling Test
2.3.5. Strength Tests
2.3.6. Chemical Structure Tests
3. Results and Discussion
3.1 Characterization of EG/P
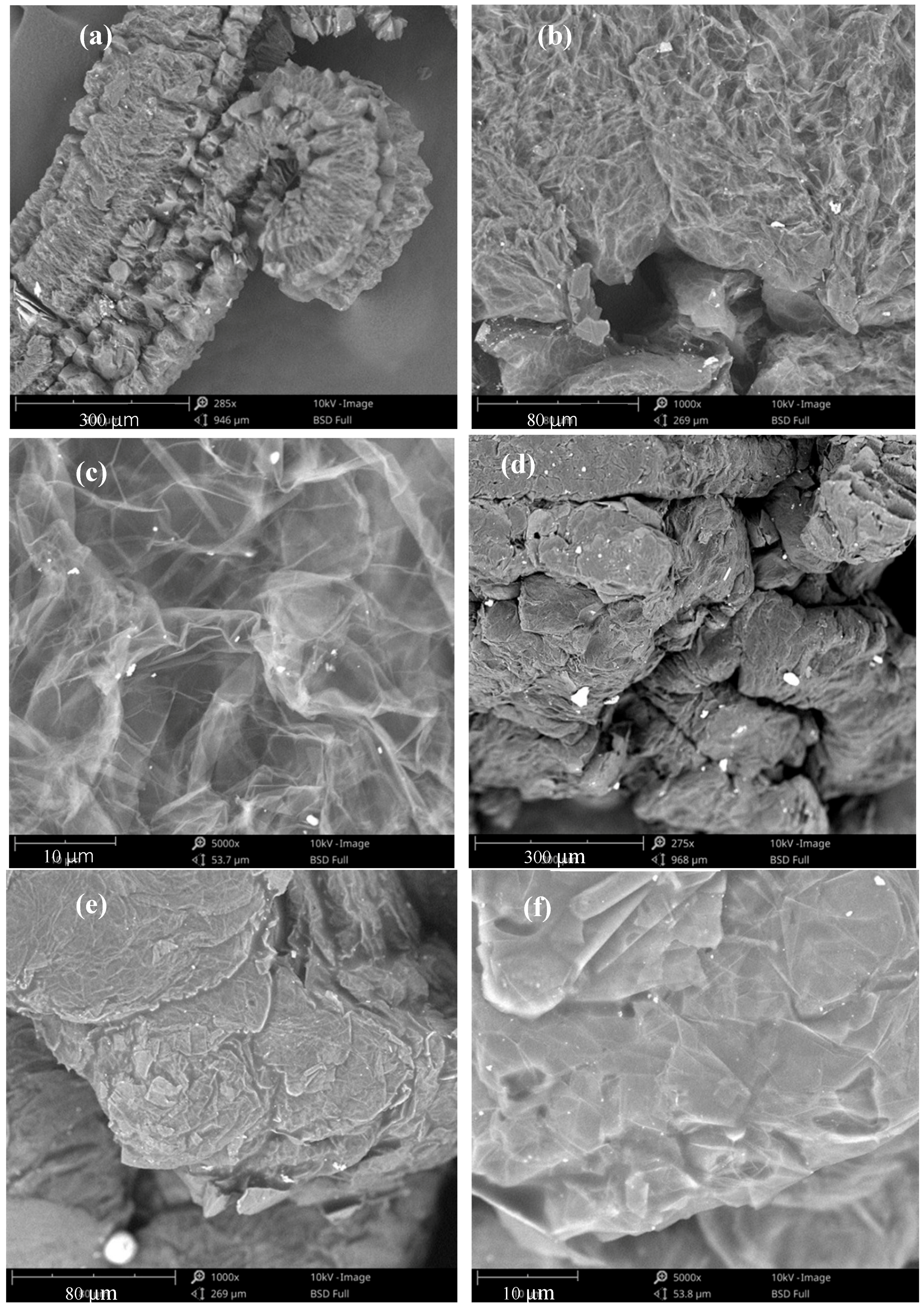
3.1.1. The Microstructure of EG and EG/P
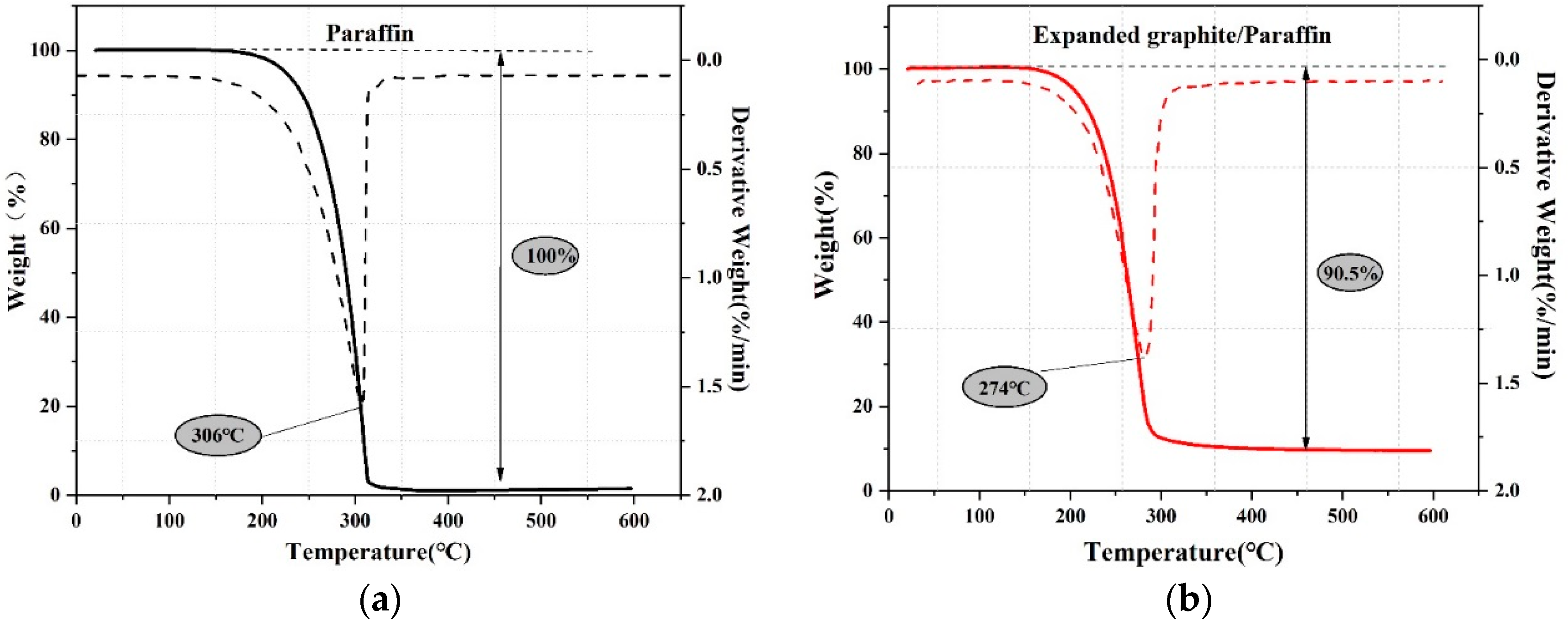
3.1.2. The Thermal Properties of EG/P
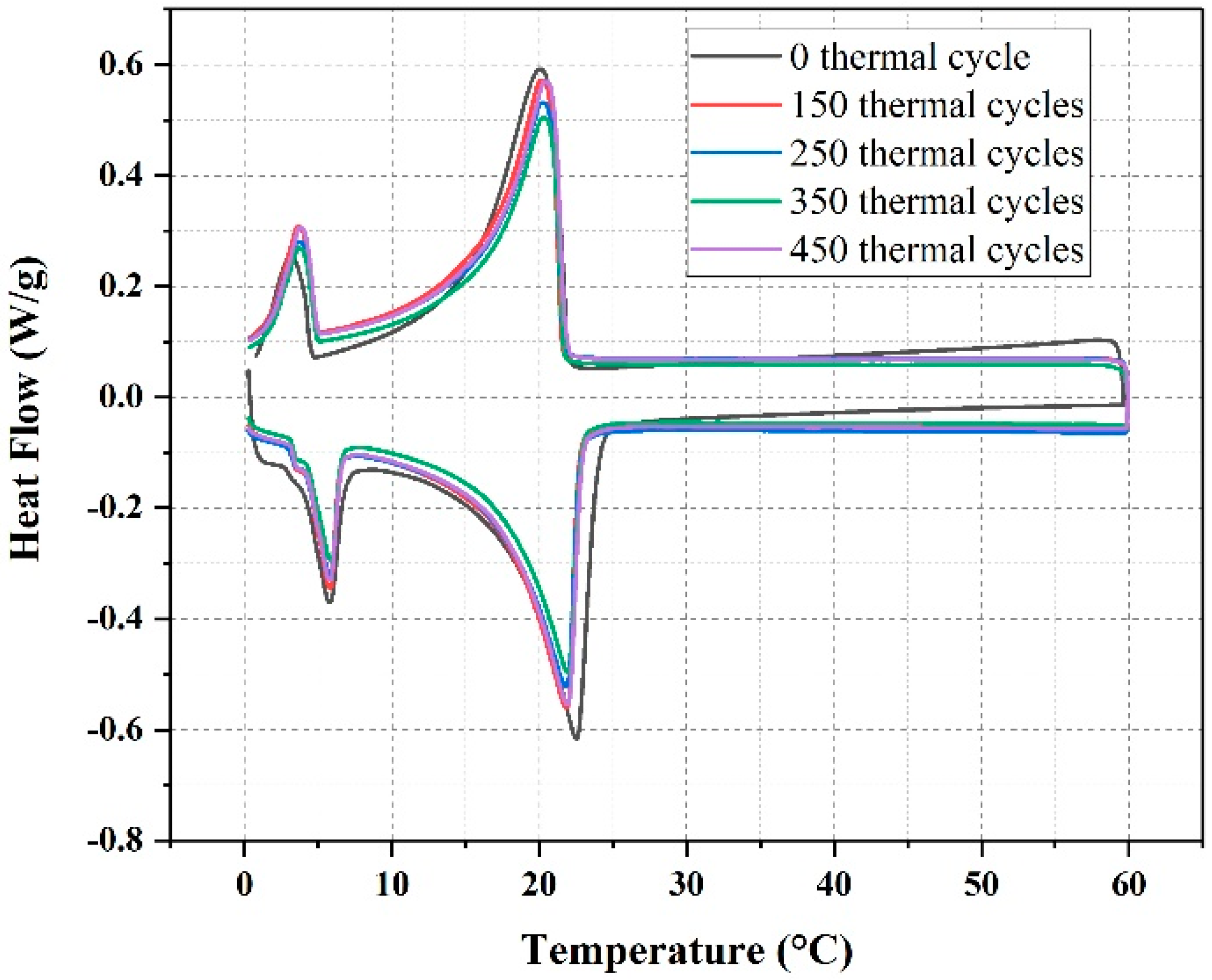
3.1.3. The Thermal Reliability of EG/P
3.1.4. The Chemical Structure of EG/P
3.2. Thermal Conductivity of EGPG
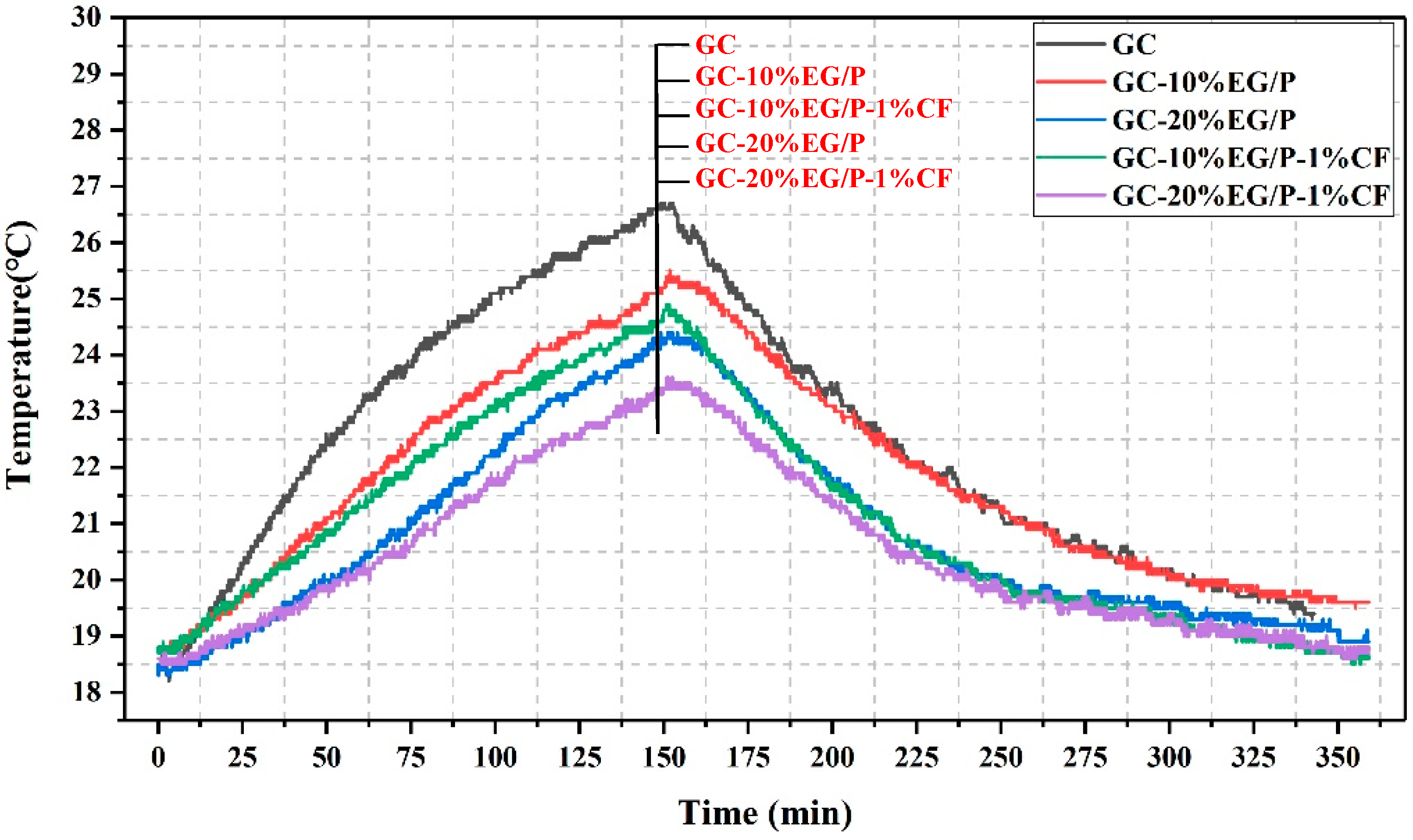
3.3. The Thermo-Regulated Performance of EGPG
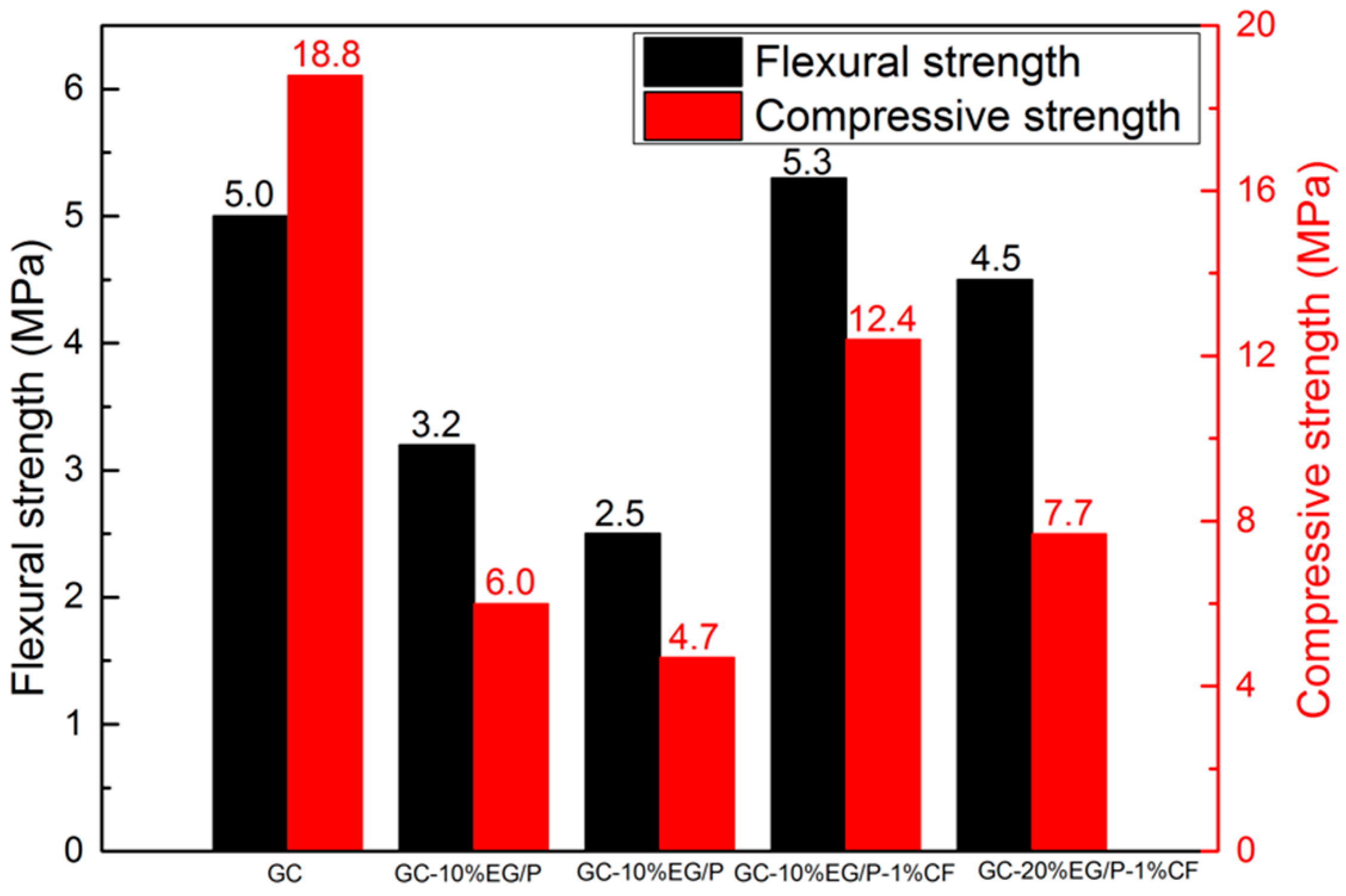
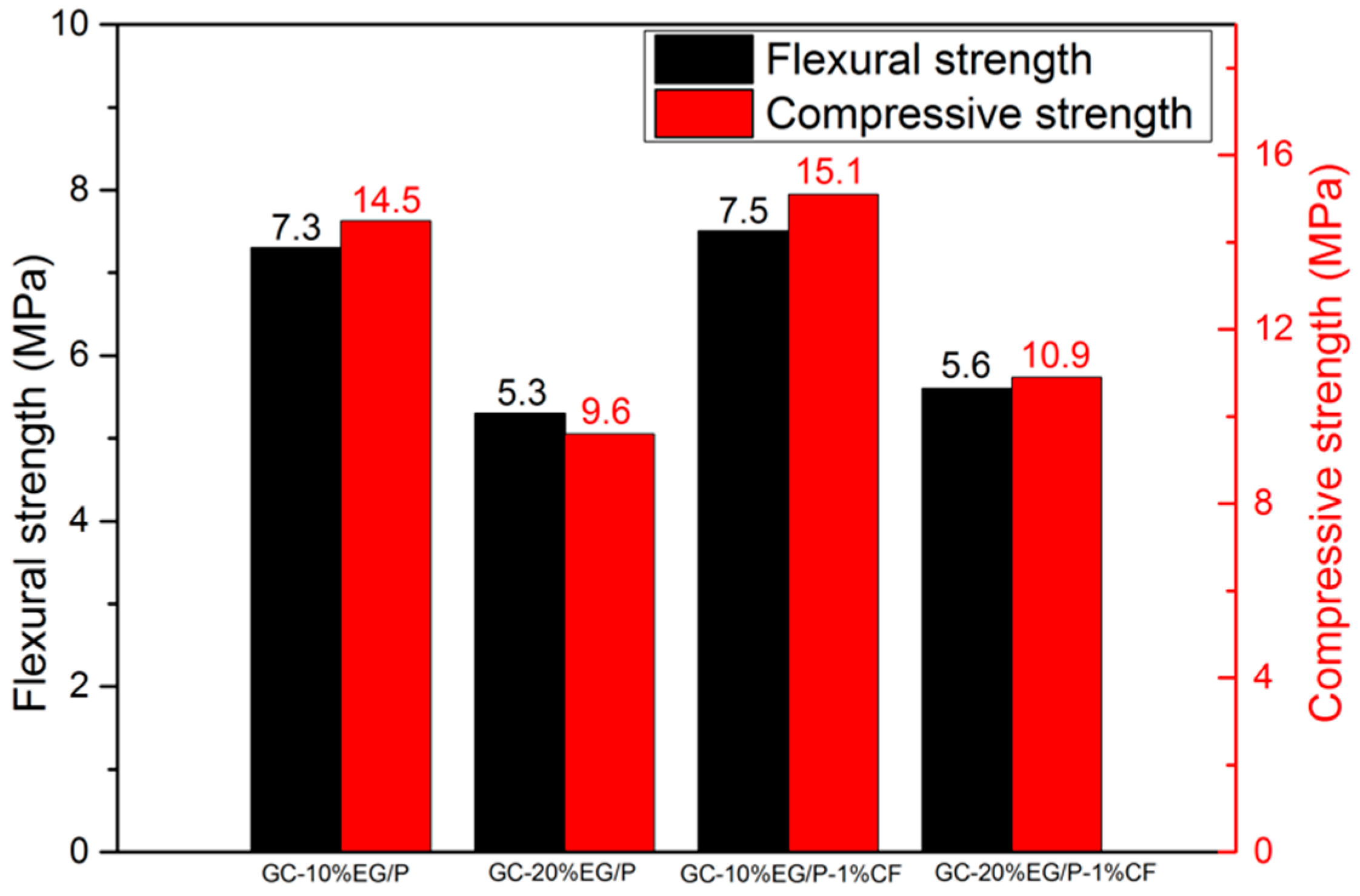
3.4. The Mechanical Properties of EGPG
4. Conclusions
- EG/P was prepared successfully by the vacuum impregnation method, which produces material with a latent heat storage capacity of 105.3 J/g and phase change temperature of 22.28 °C. The results of FT-IR reveal that the components within EG/P are chemically compatible, and TGA proves that EG/P can meet the requirements for thermal stability. As a result, EG/P is demonstrated as a potential candidate for buildings as part of overall efficient energy management scheme.
- Both EG/P and CF improved the thermal conductivity of gypsum (GC). Compared with GC, the thermal conductivity of the GC-10%EG/P and GC-20%EG/P was improved by 33.7% and 53.2%, respectively. When 1 wt % CF was added, the thermal conductivity of GC-10%EG/P and GC-20%EG/P further increases by 36.0% and 28.6%, respectively.
- EG/P can significantly reduce temperature fluctuations. The maximum temperature of indoor center position decreased by 2.4 °C with the addition of 20 wt % EG/P into GC. CF also has a role in increasing the rate of heat transfer to EG/P within gypsum, and thus the greatest improvement in temperature was 3.2 °C, observed between GC-20%EG/P-1%CF and GC.
- With the increase of EG/P content, the flexural and compressive strengths of EGPG demonstrate a dramatically decreasing trend. However, the presence of CF can improve the flexural strength of GC-20%EG/P by 51.6%, with no change in compressive strength.
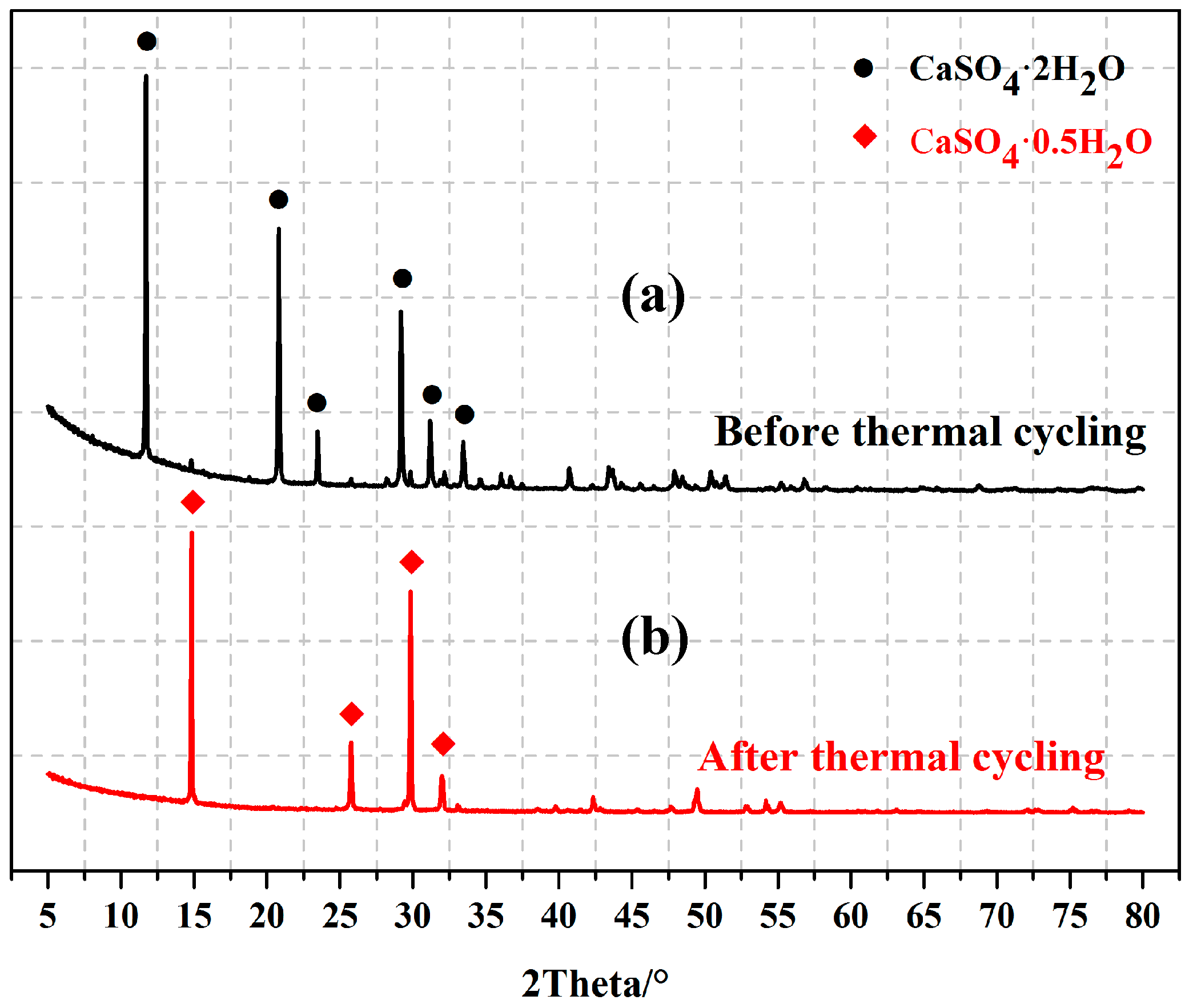
- After 500 heating/cooling cycles, the mechanical properties of EGPG increased by about 100%, which is attributed to the dehydration of calcium sulfate dihydrate.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PCMs | Phase Change Materials |
| EG | Expanded graphite |
| EG/P | Expanded graphite/Paraffin composites |
| SSPCM | Stabilized-shape Phase Change Material |
| EGPG | Expanded graphite/paraffin gypsum-based composite material |
| CF | Carbon Fiber |
References
- Rao, V.V.; Parameshwaran, R.; Ram, V.V. PCM-mortar based construction materials for energy efficient buildings: A review on research trends. Energy Build. 2018, 158, 95–122. [Google Scholar] [CrossRef]
- Stritih, U.; Tyagi, V.V.; Stropnik, R.; Paksoy, H.; Haghighat, F.; Joybari, M.M. Integration of passive PCM technologies for net-zero energy buildings. Sustain. Cities Soc. 2018, 41, 286–295. [Google Scholar] [CrossRef]
- Fernandes, D.; Pitié, F.; Cáceres, G.; Baeyens, J. Thermal energy storage: “How previous findings determine current research priorities”. Energy 2012, 39, 246–257. [Google Scholar] [CrossRef]
- Bhale, P.V.; Rathod, M.K.; Sahoo, L. Thermal Analysis of a Solar Concentrating System Integrated with Sensible and Latent Heat Storage. Energy Procedia 2015, 75, 2157–2162. [Google Scholar] [CrossRef]
- Memon, S.A.; Lo, T.Y.; Cui, H.; Barbhuiya, S. Preparation, characterization and thermal properties of dodecanol/cement as novel form-stable composite phase change material. Energy Build. 2013, 66, 697–705. [Google Scholar] [CrossRef]
- Şahan, N.; Paksoy, H. Novel shapeable phase change material (PCM) composites for thermal energy storage (TES) applications. Sol. Energy Mater. Sol. Cells 2018, 174, 380–387. [Google Scholar] [CrossRef]
- Xu, X.; Cui, H.; Memon, S.A.; Yang, H.; Tang, W. Development of novel composite PCM for thermal energy storage using CaCl2·6H2O with graphene oxide and SrCl2·6H2O. Energy Build. 2017, 156, 163–172. [Google Scholar] [CrossRef]
- Liu, L.; Su, D.; Tang, Y.; Fang, G. Thermal conductivity enhancement of phase change materials for thermal energy storage: A review. Renew. Sustain. Energy Rev. 2016, 62, 305–317. [Google Scholar] [CrossRef]
- Yang, Y.; Pang, Y.; Liu, Y.; Guo, H. Preparation and thermal properties of polyethylene glycol/expanded graphite as novel form-stable phase change material for indoor energy saving. Mater. Lett. 2018, 216, 220–223. [Google Scholar] [CrossRef]
- Li, L.; Wang, G.; Guo, C. Influence of intumescent flame retardant on thermal and flame retardancy of eutectic mixed paraffin/polypropylene form-stable phase change materials. Appl. Energy 2016, 162, 428–434. [Google Scholar] [CrossRef]
- Chang, T.-C.; Lee, S.; Fuh, Y.-K.; Peng, Y.-C.; Lin, Z.-Y. PCM based heat sinks of paraffin/nanoplatelet graphite composite for thermal management of IGBT. Appl. Therm. Eng. 2017, 112, 1129–1136. [Google Scholar] [CrossRef]
- Zhang, Z.; Fang, X. Study on paraffin/expanded graphite composite phase change thermal energy storage material. Energy Convers. Manag. 2006, 47, 303–310. [Google Scholar] [CrossRef]
- Karaipekli, A.; Biçer, A.; Sarı, A.; Tyagi, V.V. Thermal characteristics of expanded perlite/paraffin composite phase change material with enhanced thermal conductivity using carbon nanotubes. Energy Convers. Manag. 2017, 134, 373–381. [Google Scholar] [CrossRef]
- Yang, H.; Memon, S.; Bao, X.; Cui, H.; Li, D. Design and Preparation of Carbon Based Composite Phase Change Material for Energy Piles. Materials 2017, 10, 391. [Google Scholar] [CrossRef] [PubMed]
- Chuang, W.; Geng-Sheng, J.; Bing-Liang, L.; Lei, P.; Ying, F.; Ni, G.; Ke-Zhi, L. Dispersion of carbon fibers and conductivity of carbon fiber-reinforced cement-based composites. Ceram. Int. 2017, 43, 15122–15132. [Google Scholar] [CrossRef]
- Hou, L.G.; Wu, R.Z.; Wang, X.D.; Zhang, J.H.; Zhang, M.L.; Dong, A.P.; Sun, B.D. Microstructure, mechanical properties and thermal conductivity of the short carbon fiber reinforced magnesium matrix composites. J. Alloys Compd. 2017, 695, 2820–2826. [Google Scholar] [CrossRef]
- Han, B.; Zhang, L.; Zhang, C.; Wang, Y.; Yu, X.; Ou, J. Reinforcement effect and mechanism of carbon fibers to mechanical and electrically conductive properties of cement-based materials. Constr. Build. Mater. 2016, 125, 479–489. [Google Scholar] [CrossRef]
- Cui, H.; Feng, T.; Yang, H.; Bao, X.; Tang, W.; Fu, J. Experimental study of carbon fiber reinforced alkali-activated slag composites with micro-encapsulated PCM for energy storage. Constr. Build. Mater. 2018, 161, 442–451. [Google Scholar] [CrossRef]
- Jiang, Z.; Ouyang, T.; Yang, Y.; Chen, L.; Fan, X.; Chen, Y.; Li, W.; Fei, Y. Thermal conductivity enhancement of phase change materials with form-stable carbon bonded carbon fiber network. Mater. Des. 2018, 143, 177–184. [Google Scholar] [CrossRef]
- Oliver, A. Thermal characterization of gypsum boards with PCM included: Thermal energy storage in buildings through latent heat. Energy Build. 2012, 48, 1–7. [Google Scholar] [CrossRef]
- Karaipekli, A.; Sarı, A. Development and thermal performance of pumice/organic PCM/gypsum composite plasters for thermal energy storage in buildings. Sol. Energy Mater. Sol. Cells 2016, 149, 19–28. [Google Scholar] [CrossRef]
- Ali Memon, S.; Yiu Lo, T.; Shi, X.; Barbhuiya, S.; Cui, H. Preparation, characterization and thermal properties of Lauryl alcohol/Kaolin as novel form-stable composite phase change material for thermal energy storage in buildings. Appl. Therm. Eng. 2013, 59, 336–347. [Google Scholar] [CrossRef]
- Zhang, X.; Wen, R.; Huang, Z.; Tang, C.; Huang, Y.; Liu, Y.; Fang, M.; Wu, X.; Min, X.; Xu, Y. Enhancement of thermal conductivity by the introduction of carbon nanotubes as a filler in paraffin/expanded perlite form-stable phase-change materials. Energy Build. 2017, 149, 463–470. [Google Scholar] [CrossRef]
- Rao, Z.; Zhang, G.; Xu, T.; Hong, K. Experimental study on a novel form-stable phase change materials based on diatomite for solar energy storage. Sol. Energy Mater. Sol. Cells 2018, 182, 52–60. [Google Scholar] [CrossRef]
- Cui, H.; Tang, W.; Qin, Q.; Xing, F.; Liao, W.; Wen, H. Development of structural-functional integrated energy storage concrete with innovative macro-encapsulated PCM by hollow steel ball. Appl. Energy 2017, 185, 107–118. [Google Scholar] [CrossRef]
- Aydın, A.A.; Okutan, H. Polyurethane rigid foam composites incorporated with fatty acid ester-based phase change material. Energy Convers. Manag. 2013, 68, 74–81. [Google Scholar] [CrossRef]
- Yuan, Y.; Yuan, Y.; Zhang, N.; Du, Y.; Cao, X. Preparation and thermal characterization of capric–myristic–palmitic acid/expanded graphite composite as phase change material for energy storage. Mater. Lett. 2014, 125, 154–157. [Google Scholar] [CrossRef]
- Ren, Y.; Xu, C.; Yuan, M.; Ye, F.; Ju, X.; Du, X. Ca(NO3)2-NaNO3/expanded graphite composite as a novel shape-stable phase change material for mid- to high-temperature thermal energy storage. Energy Convers. Manag. 2018, 163, 50–58. [Google Scholar] [CrossRef]
- Xia, L.; Zhang, P.; Wang, R.Z. Preparation and thermal characterization of expanded graphite/paraffin composite phase change material. Carbon 2010, 48, 2538–2548. [Google Scholar] [CrossRef]
- Zhang, D.; Zhou, J.; Wu, K.; Li, Z. Granular phase changing composites for thermal energy storage. Sol. Energy 2005, 78, 471–480. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, M.; Wu, S.; Liu, Q.; Wan, J. Preparation of expanded graphite/polyethylene glycol composite phase change material for thermoregulation of asphalt binder. Constr. Build. Mater. 2018, 169, 513–521. [Google Scholar] [CrossRef]
- Bao, X.; Memon, S.; Yang, H.; Dong, Z.; Cui, H. Thermal Properties of Cement-Based Composites for Geothermal Energy Applications. Materials 2017, 10, 462. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, S.; Wang, X.; Sanjayan, J.; Wilson, J. Assessing the feasibility of integrating form-stable phase change material composites with cementitious composites and prevention of PCM leakage. Mater. Lett. 2017, 192, 88–91. [Google Scholar] [CrossRef]
- Wen, R.; Zhang, X.; Huang, Z.; Fang, M.; Liu, Y.; Wu, X.; Min, X.; Gao, W.; Huang, S. Preparation and thermal properties of fatty acid/diatomite form-stable composite phase change material for thermal energy storage. Sol. Energy Mater. Sol. Cells 2018, 178, 273–279. [Google Scholar] [CrossRef]
- Xu, T.; Li, Y.; Chen, J.; Wu, H.; Zhou, X.; Zhang, Z. Improving thermal management of electronic apparatus with paraffin (PA)/expanded graphite (EG)/graphene (GN) composite material. Appl. Therm. Eng. 2018, 140, 13–22. [Google Scholar] [CrossRef]
- Xu, B.; Li, Z. Paraffin/diatomite/multi-wall carbon nanotubes composite phase change material tailor-made for thermal energy storage cement-based composites. Energy 2014, 72, 371–380. [Google Scholar] [CrossRef]
- Han, X.; Zhao, T.; Gao, X.; Li, H. Preparation and characterization of high-temperature non-flowing SiO2/EG/paraffin composites by high-temperature refining. Colloids Surf. A Physicochem. Eng. Asp. 2018, 542, 1–7. [Google Scholar] [CrossRef]
- Mourid, A.; El Alami, M.; Kuznik, F. Experimental investigation on thermal behavior and reduction of energy consumption in a real scale building by using phase change materials on its envelope. Sustain. Cities Soc. 2018, 41, 35–43. [Google Scholar] [CrossRef]
- Cui, H.; Memon, S.A.; Liu, R. Development, mechanical properties and numerical simulation of macro encapsulated thermal energy storage concrete. Energy Build. 2015, 96, 162–174. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, M.; Gao, X.; Yang, Y.; Sang, Y. Experimental exploration of incorporating form-stable hydrate salt phase change materials into cement mortar for thermal energy storage. Appl. Therm. Eng. 2018, 140, 112–119. [Google Scholar] [CrossRef]
- Pilehvar, S.; Cao, V.D.; Szczotok, A.M.; Valentini, L.; Salvioni, D.; Magistri, M.; Pamies, R.; Kjøniksen, A.-L. Mechanical properties and microscale changes of geopolymer concrete and Portland cement concrete containing micro-encapsulated phase change materials. Cem. Concr. Res. 2017, 100, 341–349. [Google Scholar] [CrossRef]
- Zhu, C.; Zhang, J.; Peng, J.; Cao, W.; Liu, J. Physical and mechanical properties of gypsum-based composites reinforced with PVA and PP fibers. Constr. Build. Mater. 2018, 163, 695–705. [Google Scholar] [CrossRef]
- Tan, H.; Dong, F.; Liu, J. Morphology control of calcium sulfate hemihydrates and application in size screening iron/sulfur of jarosite sediment. J. Phys. Chem. Solids 2018, 112, 239–245. [Google Scholar] [CrossRef]






| No. | Gypsum (g) | Water (g) | EG/P (g) | CF (g) |
|---|---|---|---|---|
| GC | 3600 | 1300 | -- | -- |
| GC-10%EG/P | 3600 | 1300 | 360 | -- |
| GC-20%EG/P | 3600 | 1300 | 720 | -- |
| GC-10%EG/P-1%CF | 3600 | 1300 | 360 | 36 |
| GC-20%EG/P-1%CF | 3600 | 1300 | 720 | 36 |
| Cycles | Melting Temperature (°C) | Melting Enthalpy (J/g) | Freezing Temperature (°C) | Freezing Enthalpy (J/g) |
|---|---|---|---|---|
| 0 | 22.3 | 105.0 | 19.9 | 105.5 |
| 150 | 22.0 | 104.9 | 20.2 | 105.2 |
| 250 | 22.0 | 103.3 | 20.2 | 104.5 |
| 350 | 22.1 | 102.7 | 20.3 | 103.8 |
| 450 | 22.1 | 101.8 | 20.3 | 104.1 |
| No. | Thermal Conductivity (W/m·K) | Energy Storage Capacity (J/g) |
|---|---|---|
| GC | 0.742 | 0 |
| GC-10%EG/P | 0.992 | 7.25 |
| GC-20%EG/P | 1.137 | 13.65 |
| GC-10%EG/P-1%CF | 1.350 | 7.20 |
| GC-20%EG/P-1%CF | 1.462 | 13.57 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Tian, Y.; Jin, X.; Lo, T.Y.; Cui, H. Thermal and Mechanical Properties of Expanded Graphite/Paraffin Gypsum-Based Composite Material Reinforced by Carbon Fiber. Materials 2018, 11, 2205. https://doi.org/10.3390/ma11112205
Zhang B, Tian Y, Jin X, Lo TY, Cui H. Thermal and Mechanical Properties of Expanded Graphite/Paraffin Gypsum-Based Composite Material Reinforced by Carbon Fiber. Materials. 2018; 11(11):2205. https://doi.org/10.3390/ma11112205
Chicago/Turabian StyleZhang, Bo, Yuanyuan Tian, Xiaoyan Jin, Tommy Y. Lo, and Hongzhi Cui. 2018. "Thermal and Mechanical Properties of Expanded Graphite/Paraffin Gypsum-Based Composite Material Reinforced by Carbon Fiber" Materials 11, no. 11: 2205. https://doi.org/10.3390/ma11112205
APA StyleZhang, B., Tian, Y., Jin, X., Lo, T. Y., & Cui, H. (2018). Thermal and Mechanical Properties of Expanded Graphite/Paraffin Gypsum-Based Composite Material Reinforced by Carbon Fiber. Materials, 11(11), 2205. https://doi.org/10.3390/ma11112205





