Solid Oxide Electrochemical Systems: Material Degradation Processes and Novel Mitigation Approaches
Abstract
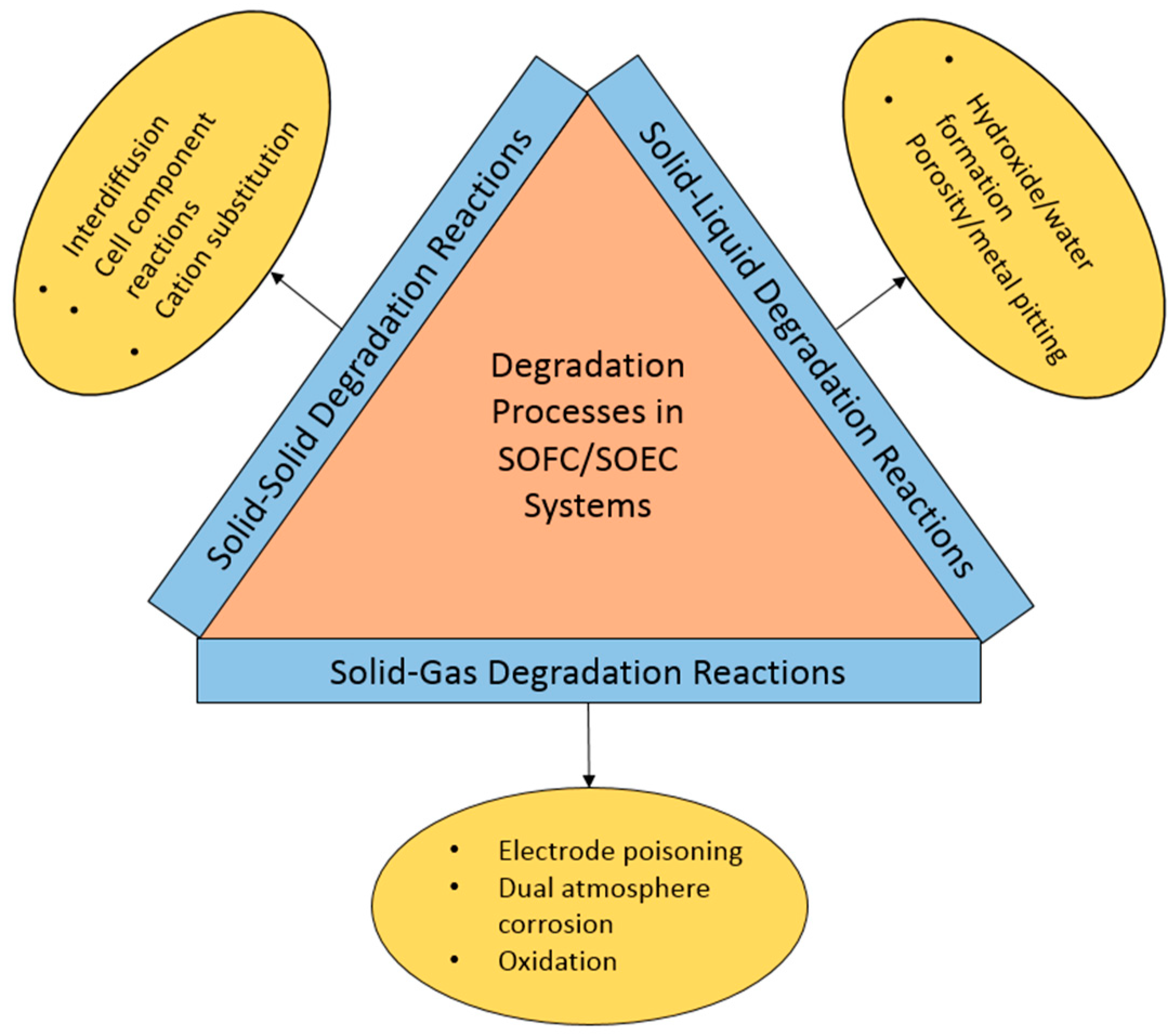
:1. Introduction
1.1. Solid Oxide Electrochemical Systems
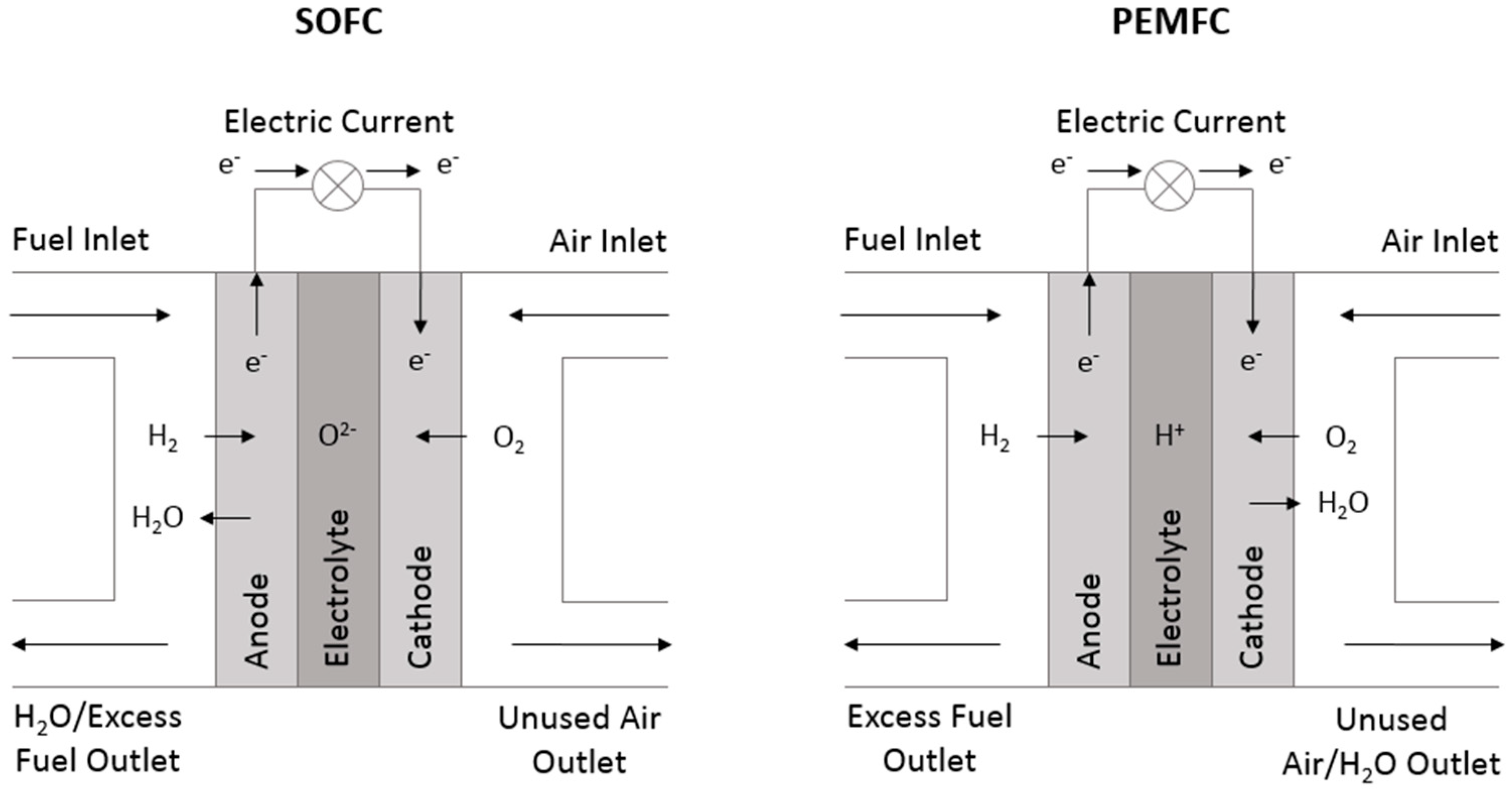
1.1.1. Solid Oxide Fuel Cells
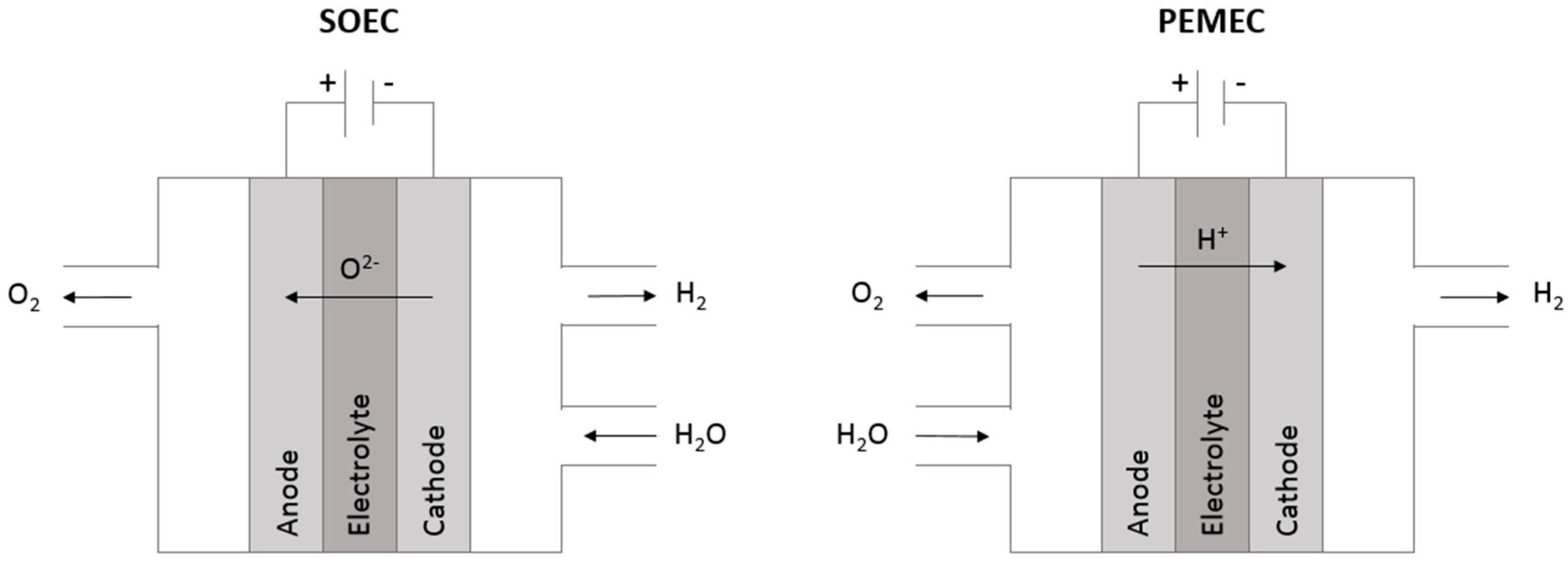
1.1.2. Solid Oxide Electrolysis Cells
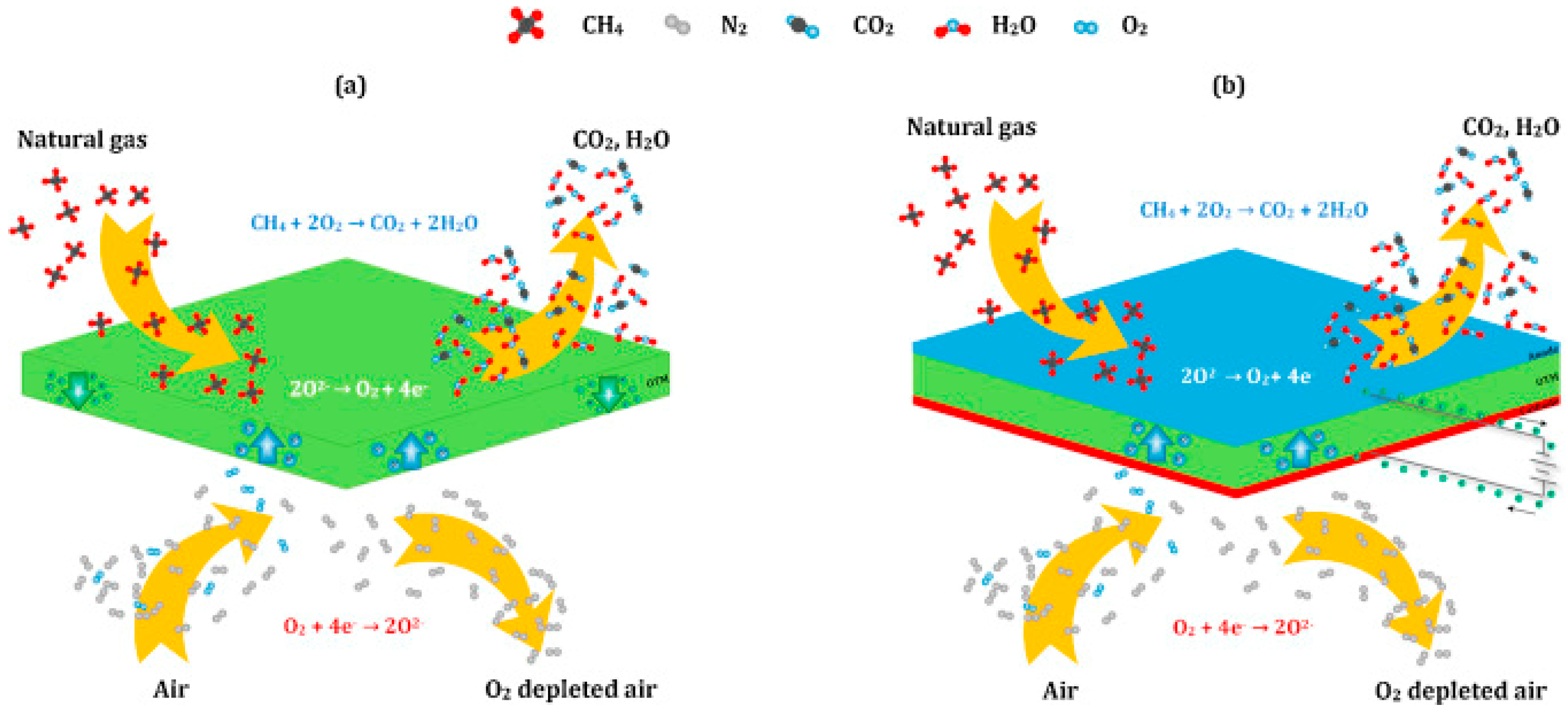
1.1.3. Oxygen Transport Membranes
1.2. Device and System Materials
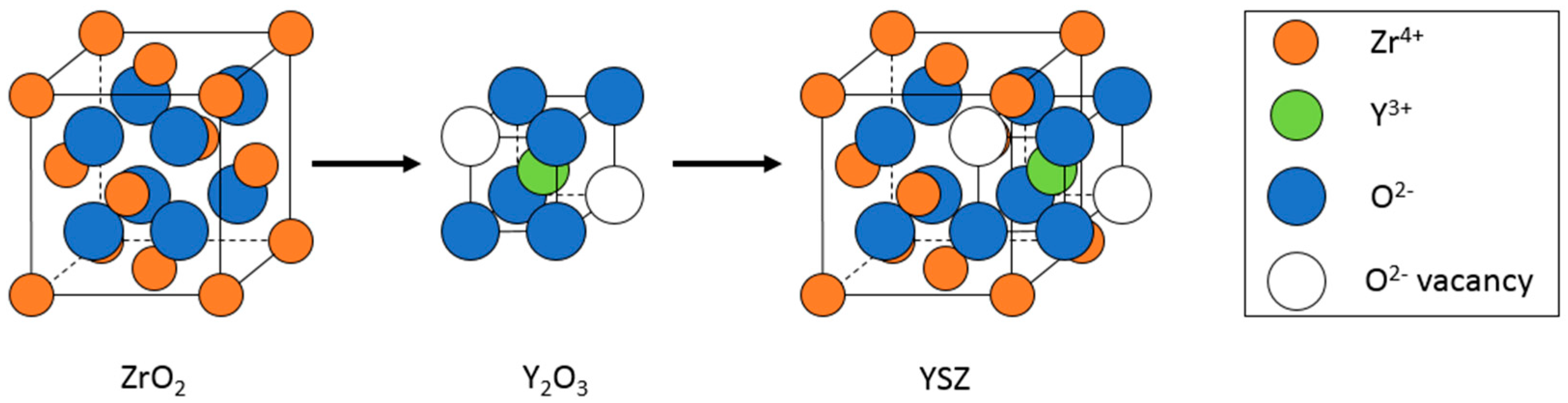
1.2.1. Electrolytes
1.2.2. Electrodes
1.2.3. Interconnects/Sealants/Balance-of-Plant
2. Material Corrosion/Degradation Phenomena
2.1. Electrode Poisoning and Degradation
2.1.1. Sulfur
2.1.2. Chromium
2.1.3. Silicon
2.2. Corrosion of Metallic Components
3. Mitigation Approaches
3.1. Electrode Poisoning Mitigation
3.2. Metallic Component Corrosion Suppression
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods Fundamentals and Applications, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2001; ISBN 978-0-471-04372-0. [Google Scholar]
- Mahato, N.; Banerjee, A.; Gupta, A.; Omar, S.; Balani, K. Progress in Material Selection for Solid Oxide Fuel Cell Technology: A Review. Prog. Mater. Sci. 2015, 72, 141–337. [Google Scholar] [CrossRef]
- Minh, N.Q.; Mizusaki, J.; Singhal, S.C. Advances in Solid Oxide Fuel Cells: Review of Progress through Three Decades of the International Symposia on Solid Oxide Fuel Cells. ECS Trans. 2017, 78, 63–73. [Google Scholar] [CrossRef]
- Stambouli, A.B.; Traversa, E. Solid Oxide Fuel Cells (SOFCs): A Review of an Environmentally Clean and Efficient Source of Energy. Renew. Sustain. Energy Rev. 2002, 6, 433–455. [Google Scholar] [CrossRef]
- Yildiz, B. Reversible Solid Oxide Electrolytic Cells for Large-Scale Energy Storage: Challenges and Opportunities. In Woodhead Publishing Series in Energy; Kilner, J.A., Skinner, S.J., Irvine, S.J.C., Edwards, P., Eds.; Woodhead Publishing: Cambridge, UK, 2012; pp. 149–179. [Google Scholar]
- Yokokawa, H.; Tu, H.; Iwanschitz, B.; Mai, A. Fundamental Mechanisms Limiting Solid Oxide Fuel Cell Durability. J. Power Sources 2008, 182, 400–412. [Google Scholar] [CrossRef]
- Adams, T.A.; Nease, J.; Tucker, D.; Barton, P.I. Energy Conversion with Solid Oxide Fuel Cell Systems: A Review of Concepts and Outlooks for the Short- and Long-Term. Ind. Eng. Chem. Res. 2013, 52, 3089–3111. [Google Scholar] [CrossRef]
- Kirubakaran, A.; Jain, S.; Nema, R.K. A Review on Fuel Cell Technologies and Power Electronic Interface. Renew. Sustain. Energy Rev. 2009, 13, 2430–2440. [Google Scholar] [CrossRef]
- Ji, M.; Wei, Z. A Review of Water Management in Polymer Electrolyte Membrane Fuel Cells. Energies 2009, 2, 1057–1106. [Google Scholar] [CrossRef] [Green Version]
- Bi, L.; Da’as, E.H.; Shafi, S.P. Proton-Conducting Solid Oxide Fuel Cell (SOFC) with Y-Doped BaZrO3 Electrolyte. Electrochem. Commun. 2017, 80, 20–23. [Google Scholar] [CrossRef]
- Bi, L.; Fabbri, E.; Traversa, E. Solid Oxide Fuel Cells with Proton-Conducting La0.99Ca0.01NbO4 Electrolyte. Electrochim. Acta 2018, 260, 748–754. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, Y.; Hu, X.; Sun, L.; Ling, Y. High Performance Proton-Conducting Solid Oxide Fuel Cells with a Layered Perovskite GdBaCuCoO5+xCathode. Electron. Mater. Lett. 2018, 14, 147–153. [Google Scholar] [CrossRef]
- Lefebvre-Joud, F.; Gauthier, G.; Mougin, J. Current Status of Proton-Conducting Solid Oxide Fuel Cells Development. J. Appl. Electrochem. 2009, 39, 535–543. [Google Scholar] [CrossRef]
- Chen, K.; Jiang, S.P. Review—Materials Degradation of Solid Oxide Electrolysis Cells. J. Electrochem. Soc. 2016, 163, F3070–F3083. [Google Scholar] [CrossRef]
- Schmidt, O.; Gambhir, A.; Staffell, I.; Hawkes, A.; Nelson, J.; Few, S. Future Cost and Performance of Water Electrolysis: An Expert Elicitation Study. Int. J. Hydrogen Energy 2017, 42, 30470–30492. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, J.; Yu, B.; Zhang, W.; Chen, J.; Qiao, J.; Zhang, J. A Review of High Temperature Co-Electrolysis of H2O and CO2 to Produce Sustainable Fuels Using Solid Oxide Electrolysis Cells (SOECs): Advanced Materials and Technology. Chem. Soc. Rev. 2017, 46, 1427–1463. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Mahapatra, M.; Singh, P. Lanthanum Chromite Based Perovskites for Oxygen Transport Membrane. Mater. Sci. Eng. R Rep. 2015, 90, 1–36. [Google Scholar] [CrossRef]
- Eichler, A. Tetragonal Y-Doped Zirconia: Structure and Ion Conductivity. Phys. Rev. B 2001, 64, 174103. [Google Scholar] [CrossRef]
- Ormerod, R.M. Solid Oxide Fuel Cells. Chem. Soc. Rev. 2003, 32, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, E.; D’Epifanio, A.; Bartolomeo, E.; Licoccia, S.; Traversa, E. BaCe1-x-YZrxYyO3-d Protonic Conductor for Intermediate Temperature Solid Oxide Fuel Cells (IT-SOFCs). ECS Trans. 2008, 6, 23–28. [Google Scholar]
- Sun, C.; Hui, R.; Roller, J. Cathode Materials for Solid Oxide Fuel Cells: A Review. J. Solid State Electrochem. 2010, 14, 1125–1144. [Google Scholar] [CrossRef]
- Sun, C.; Stimming, U. Recent Anode Advances in Solid Oxide Fuel Cells. J. Power Sources 2007, 171, 247–260. [Google Scholar] [CrossRef]
- Skinner, S.J.; Kilner, J.A. Oxygen Ion Conductors. Mater. Today 2003, 6, 30–37. [Google Scholar] [CrossRef]
- Shaikh, S.P.S.; Muchtar, A.; Somalu, M.R. A Review on the Selection of Anode Materials for Solid-Oxide Fuel Cells. Renew. Sustain. Energy Rev. 2015, 51, 1–8. [Google Scholar] [CrossRef]
- Sakai, N.; Yokokawa, H.; Horita, T.; Yamaji, K. Lanthanum Chromite-Based Interconnects as Key Materials for SOFC Stack Development. Int. J. Appl. Ceram. Technol. 2004, 1, 23–30. [Google Scholar] [CrossRef]
- Yang, Z. Recent Advances in Metallic Interconnects for Solid Oxide Fuel Cells. Int. Mater. Rev. 2008, 53, 39–54. [Google Scholar] [CrossRef]
- Yang, Z.; Xia, G.G.; Walker, M.S.; Wang, C.M.; Stevenson, J.W.; Singh, P. High Temperature Oxidation/Corrosion Behavior of Metals and Alloys under a Hydrogen Gradient. Int. J. Hydrogen Energy 2007, 32, 3770–3777. [Google Scholar] [CrossRef]
- Quadakkers, W.J.; Piron-Abellan, J.; Shemet, V.; Singheiser, L. Metallic Interconnectors for Solid Oxide Fuel Cells—A Review. Mater. High Temp. 2003, 20, 115–127. [Google Scholar]
- Mahapatra, M.K.; Lu, K. Glass-Based Seals for Solid Oxide Fuel and Electrolyzer Cells—A Review. Mater. Sci. Eng. R Rep. 2010, 67, 65–85. [Google Scholar] [CrossRef]
- Atkinson, A.; Aguiar, P.; Brandon, N.P.; Brett, D.J.L. Fuel Cells for Autonomous Vehicles, SEAS DTC Annual Technical Conference; SEAS DTC: Edinburgh, UK, 2006; p. C5. [Google Scholar]
- Grgicak, C.M.; Green, R.G.; Giorgi, J.B. SOFC Anodes for Direct O00000xidation of Hydrogen and Methane Fuels Containing H2S. J. Power Sources 2008, 179, 317–328. [Google Scholar] [CrossRef]
- Sun, S.; Cheng, Z. H2S Poisoning of Proton Conducting Solid Oxide Fuel Cell and Comparison with Conventional Oxide-Ion Conducting Solid Oxide Fuel Cell. J. Electrochem. Soc. 2018, 165, F836–F844. [Google Scholar] [CrossRef]
- Sasaki, K.; Susuki, K.; Iyoshi, A.; Uchimura, M.; Imamura, N.; Kusaba, H.; Teraoka, Y.; Fuchino, H.; Tsujimoto, K.; Uchida, Y.; et al. H2S Poisoning of Solid Oxide Fuel Cells. J. Electrochem. Soc. 2006, 153, A2023. [Google Scholar] [CrossRef]
- Xiong, Y.; Yamaji, K.; Horita, T.; Yokokawa, H.; Akikusa, J.; Eto, H.; Inagaki, T. Sulfur Poisoning of SOFC Cathodes. J. Electrochem. Soc. 2009, 156, B588. [Google Scholar] [CrossRef]
- Aphale, A.; Liang, C.; Hu, B.; Singh, P. Chapter 6—Cathode Degradation from Airborne Contaminants in Solid Oxide Fuel Cells—A Review. Solid Oxide Fuel Cell Lifetime Reliab. Crit. Chall. Fuel Cells 2017, 102–114. [Google Scholar] [CrossRef]
- Falk-Windisch, H.; Svensson, J.E.; Froitzheim, J. The Effect of Temperature on Chromium Vaporization and Oxide Scale Growth on Interconnect Steels for Solid Oxide Fuel Cells. J. Power Sources 2015, 287, 25–35. [Google Scholar] [CrossRef]
- Falk-Windisch, H.; Claquesin, J.; Sattari, M.; Svensson, J.-E.; Froitzheim, J. Co- and Ce/Co-Coated Ferritic Stainless Steel as Interconnect Material for Intermediate Temperature Solid Oxide Fuel Cells. J. Power Sources 2017, 343, 1–10. [Google Scholar] [CrossRef]
- Badwal, S.P.S.; Deller, R.; Foger, K.; Ramprakash, Y.; Zhang, J.P. Interaction between Chromia Forming Alloy Interconnects and Air Electrode of Solid Oxide Fuel Cells. Solid State Ion. 1997, 99, 297–310. [Google Scholar] [CrossRef]
- Jiang, S.P.; Zhang, J.P.; Zheng, X.G. A Comparative Investigation of Chromium Deposition at Air Electrodes of Solid Oxide Fuel Cells. J. Eur. Ceram. Soc. 2002, 22, 361–373. [Google Scholar] [CrossRef]
- Kurokawa, H.; Jacobson, C.P.; DeJonghe, L.C.; Visco, S.J. Chromium Vaporization of Bare and of Coated Iron–chromium Alloys at 1073 K. Solid State Ion. 2007, 178, 287–296. [Google Scholar] [CrossRef]
- Haga, K.; Adachi, S.; Shiratori, Y.; Itoh, K.; Sasaki, K. Poisoning of SOFC Anodes by Various Fuel Impurities. Solid State Ion. 2008, 179, 1427–1431. [Google Scholar] [CrossRef]
- Hauch, A.; Jensen, S.H.; Bilde-Sorensen, J.B.; Mogensen, M. Silica Segregation in the Ni/YSZ Electrode. J. Electrochem. Soc. 2007, 154, A619–A626. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Xia, G.; Singh, P.; Stevenson, J.W. Effects of Water Vapor on Oxidation Behavior of Ferritic Stainless Steels under Solid Oxide Fuel Cell Interconnect Exposure Conditions. Solid State Ion. 2005, 176, 1495–1503. [Google Scholar] [CrossRef]
- Alnegren, P.; Sattari, M.; Svensson, J.E.; Froitzheim, J. Severe Dual Atmosphere Effect at 600 °C for Stainless Steel 441. J. Power Sources 2016, 301, 170–178. [Google Scholar] [CrossRef]
- Wongpromrat, W.; Berthomé, G.; Parry, V.; Chandra-ambhorn, S.; Chandra-ambhorn, W.; Pascal, C.; Galerie, A.; Wouters, Y. Reduction of Chromium Volatilisation from Stainless Steel Interconnector of Solid Oxide Electrochemical Devices by Controlled Preoxidation. Corros. Sci. 2016, 106, 172–178. [Google Scholar] [CrossRef]
- Promdirek, P.; Lothongkum, G.; Chandra-Ambhorn, S.; Wouters, Y.; Galerie, A. Oxidation Kinetics of AISI441 Ferritic Stainless Steel at High Temperatures in CO2 Atmosphere. Oxid. Met. 2014, 81, 315–329. [Google Scholar] [CrossRef]
- Shaigan, N.; Qu, W.; Ivey, D.G.; Chen, W. A Review of Recent Progress in Coatings, Surface Modifications and Alloy Developments for Solid Oxide Fuel Cell Ferritic Stainless Steel Interconnects. J. Power Sources 2010, 195, 1529–1542. [Google Scholar] [CrossRef]
- Mah, J.C.W.; Muchtar, A.; Somalu, M.R.; Ghazali, M.J. Metallic Interconnects for Solid Oxide Fuel Cell: A Review on Protective Coating and Deposition Techniques. Int. J. Hydrogen Energy 2017, 42, 9219–9229. [Google Scholar] [CrossRef]
- Yang, Z.; Xia, G.-G.; Li, X.-H.; Stevenson, J.W. (Mn,Co)3O4 Spinel Coatings on Ferritic Stainless Steels for SOFC Interconnect Applications. Int. J. Hydrogen Energy 2007, 32, 3648–3654. [Google Scholar] [CrossRef]
- Marina, O.A.; Coyle, C.A.; Engelhard, M.H.; Pederson, L.R. Mitigation of Sulfur Poisoning of Ni/Zirconia SOFC Anodes by Antimony and Tin. J. Electrochem. Soc. 2011, 158, B424. [Google Scholar] [CrossRef]
- Aphale, A.; Uddin, M.A.; Hu, B.; Heo, S.J.; Hong, J.; Singh, P. Synthesis and Stability of SrxNiyOz Chromium Getter for Solid Oxide Fuel Cells. J. Electrochem. Soc. 2018, 165, F635–F640. [Google Scholar] [CrossRef]
- Uddin, M.A.; Banas, C.J.; Liang, C.; Pasaogullari, U.; Recknagle, K.P.; Koeppel, B.J.; Stevenson, J.W.; Singh, P. Design and Optimization of Chromium Getter for SOFC Systems through Computational Modeling. ECS Trans. 2017, 78, 1063–1072. [Google Scholar] [CrossRef]
- Rufner, J.; Gannon, P.; White, P.; Deibert, M.; Teintze, S.; Smith, R.; Chen, H. Oxidation Behavior of Stainless Steel 430 and 441 at 800 °C in Single (Air/Air) and Dual Atmosphere (Air/Hydrogen) Exposures. Int. J. Hydrogen Energy 2008, 33, 1392–1398. [Google Scholar] [CrossRef]
- Faust, R. Dual Atmosphere Corrosion of Ferritic Stainless Steel Used for Solid Oxide Fuel Cell Applications; Chalmers University of Technology: Göteborg, Sweden, 2017. [Google Scholar]
- Sabioni, A.C.S.; Huntz, A.M.; Silva, F.; Jomard, F. Diffusion of Iron in Cr2O3: Polycrystals and Thin Films. Mater. Sci. Eng. A 2005, 392, 254–261. [Google Scholar] [CrossRef]
- Talic, B.; Molin, S.; Wiik, K.; Hendriksen, P.V.; Lein, H.L. Comparison of Iron and Copper Doped Manganese Cobalt Spinel Oxides as Protective Coatings for Solid Oxide Fuel Cell Interconnects. J. Power Sources 2017, 372, 145–156. [Google Scholar] [CrossRef]
- Bateni, R.; Wei, P.; Deng, X.; Petric, A. Spinel Coatings for UNS 430 Stainless Steel Interconnects. Surf. Coat. Technol. 2007, 201, 4677–4684. [Google Scholar] [CrossRef]
- Grolig, J.G.; Froitzheim, J.; Svensson, J.-E. Coated Stainless Steel 441 as Interconnect Material for Solid Oxide Fuel Cells: Evolution of Electrical Properties. J. Power Sources 2015, 284, 321–327. [Google Scholar] [CrossRef]
- Fontana, S.; Amendola, R.; Chevalier, S.; Piccardo, P.; Caboche, G.; Viviani, M.; Molins, R.; Sennour, M. Metallic Interconnects for SOFC: Characterisation of Corrosion Resistance and Conductivity Evaluation at Operating Temperature of Differently Coated Alloys. J. Power Sources 2007, 171, 652–662. [Google Scholar] [CrossRef]
- Pint, B.A. Progress in Understanding the Reactive Element Effect since the Whittle and Stringer Literature Review. In John Stringer Symposium on High Temperature Corrosion; Asm Intl: Materials Park, OH, USA, 2003; pp. 9–19. [Google Scholar]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reisert, M.; Aphale, A.; Singh, P. Solid Oxide Electrochemical Systems: Material Degradation Processes and Novel Mitigation Approaches. Materials 2018, 11, 2169. https://doi.org/10.3390/ma11112169
Reisert M, Aphale A, Singh P. Solid Oxide Electrochemical Systems: Material Degradation Processes and Novel Mitigation Approaches. Materials. 2018; 11(11):2169. https://doi.org/10.3390/ma11112169
Chicago/Turabian StyleReisert, Michael, Ashish Aphale, and Prabhakar Singh. 2018. "Solid Oxide Electrochemical Systems: Material Degradation Processes and Novel Mitigation Approaches" Materials 11, no. 11: 2169. https://doi.org/10.3390/ma11112169




