ZnO Nanorods with High Photocatalytic and Antibacterial Activity under Solar Light Irradiation
Abstract
1. Introduction
2. Materials and Methods
2.1. Photocatalytic Degradation of Orange II
2.2. Photocatalytic Inactivation of E. coli
2.3. Fluorescence Spectroscopy Measurements
2.4. Estimation of Cell Membrane Integrity by Fluorescence Microscopy
2.5. Assessing Cell Alteration by Flow Cytometry
2.6. Scanning Electron Microscope (SEM) Observations of E. coli Cells
2.7. E. coli Cell Morphology Analysis by Atomic Force Microscopy (AFM)
3. Results
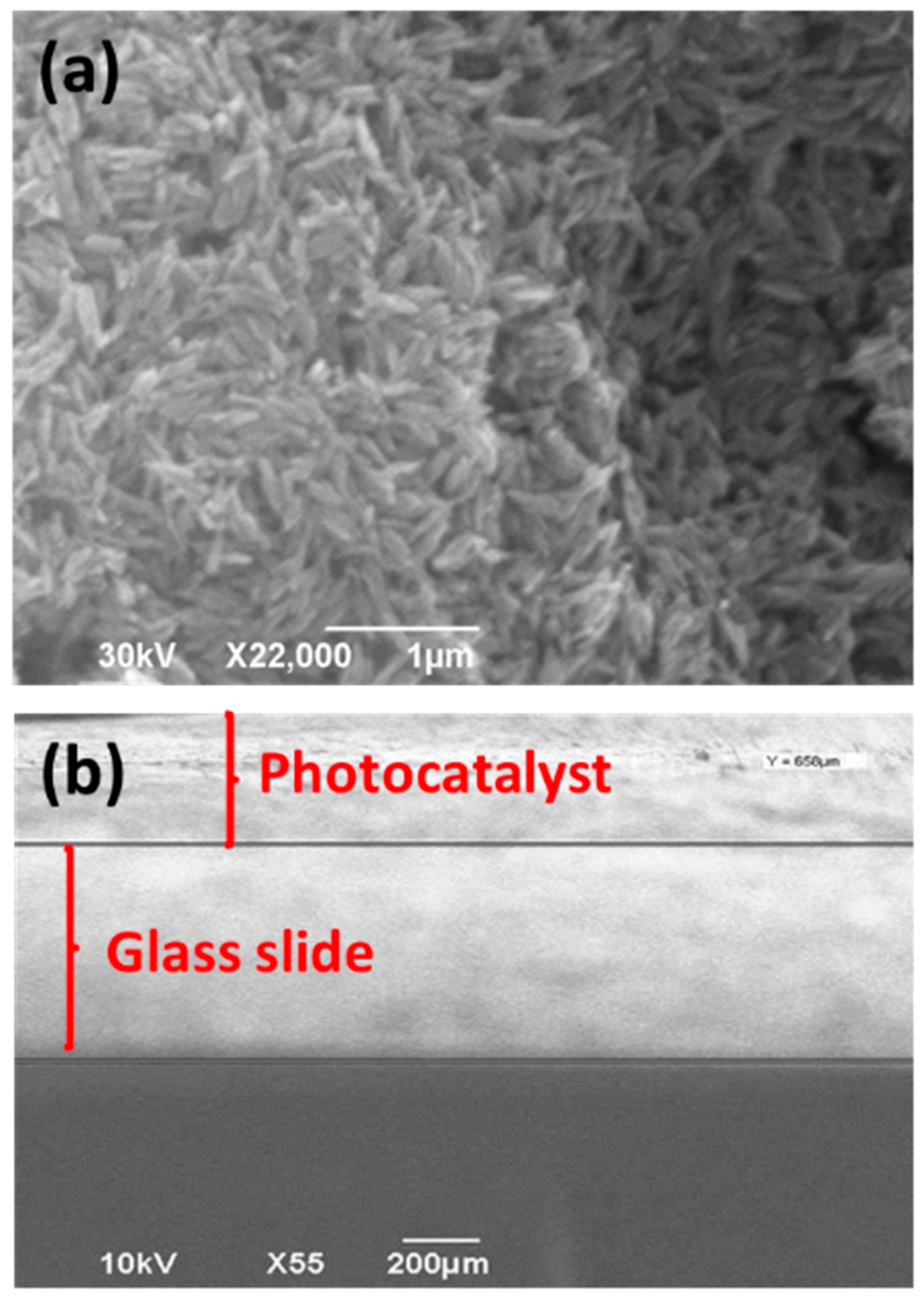
3.1. Synthesis and Characterization of ZnO NRs
3.2. Photocatalytic Degradation of Orange II
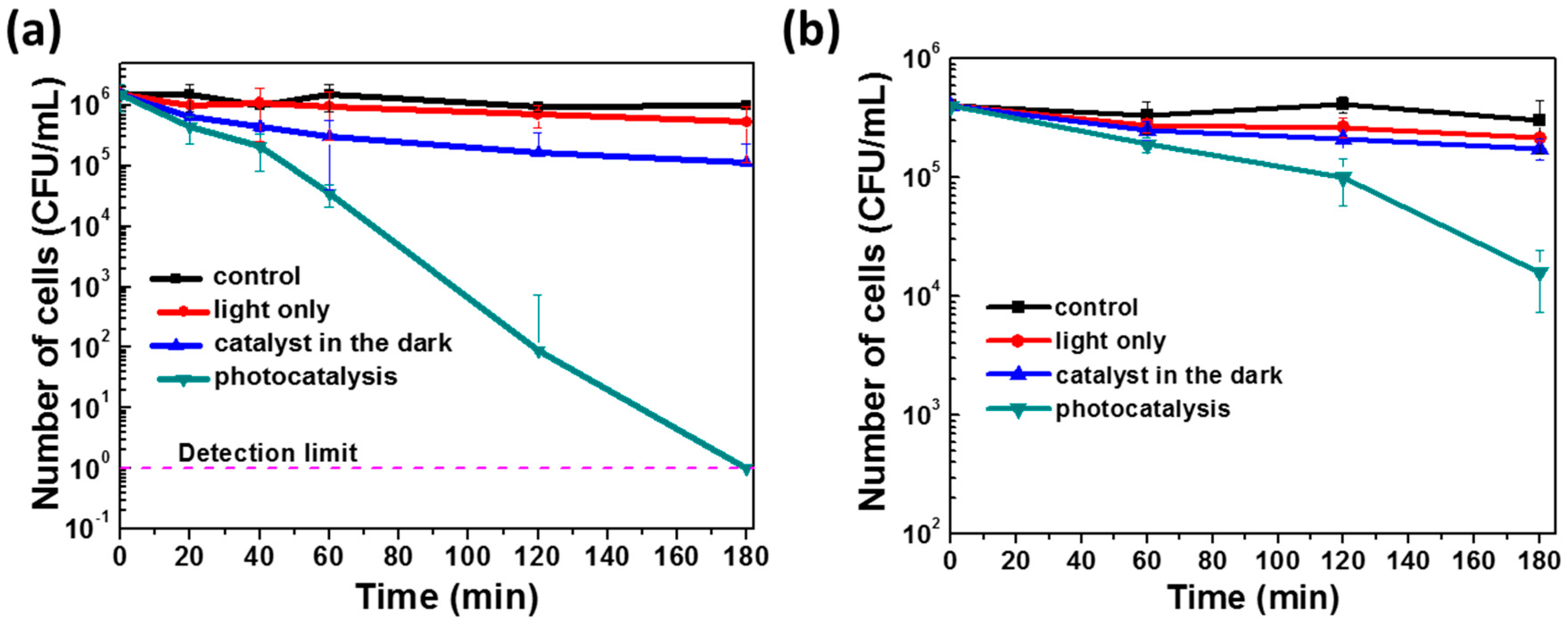
3.3. Photocatalytic Inactivation of E. coli MG1655
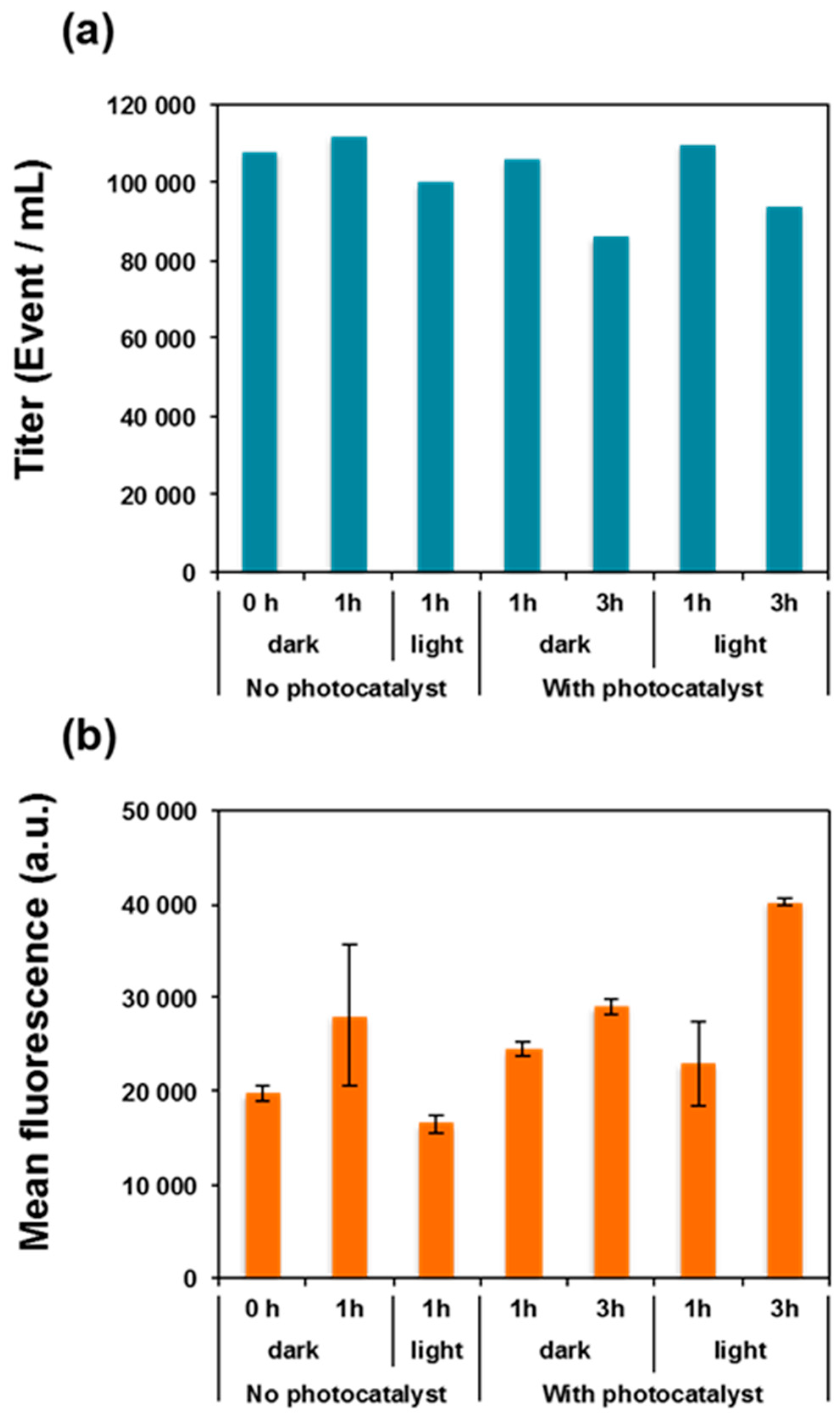
3.4. Bacterial Protein Interaction with ZnO Nanorods
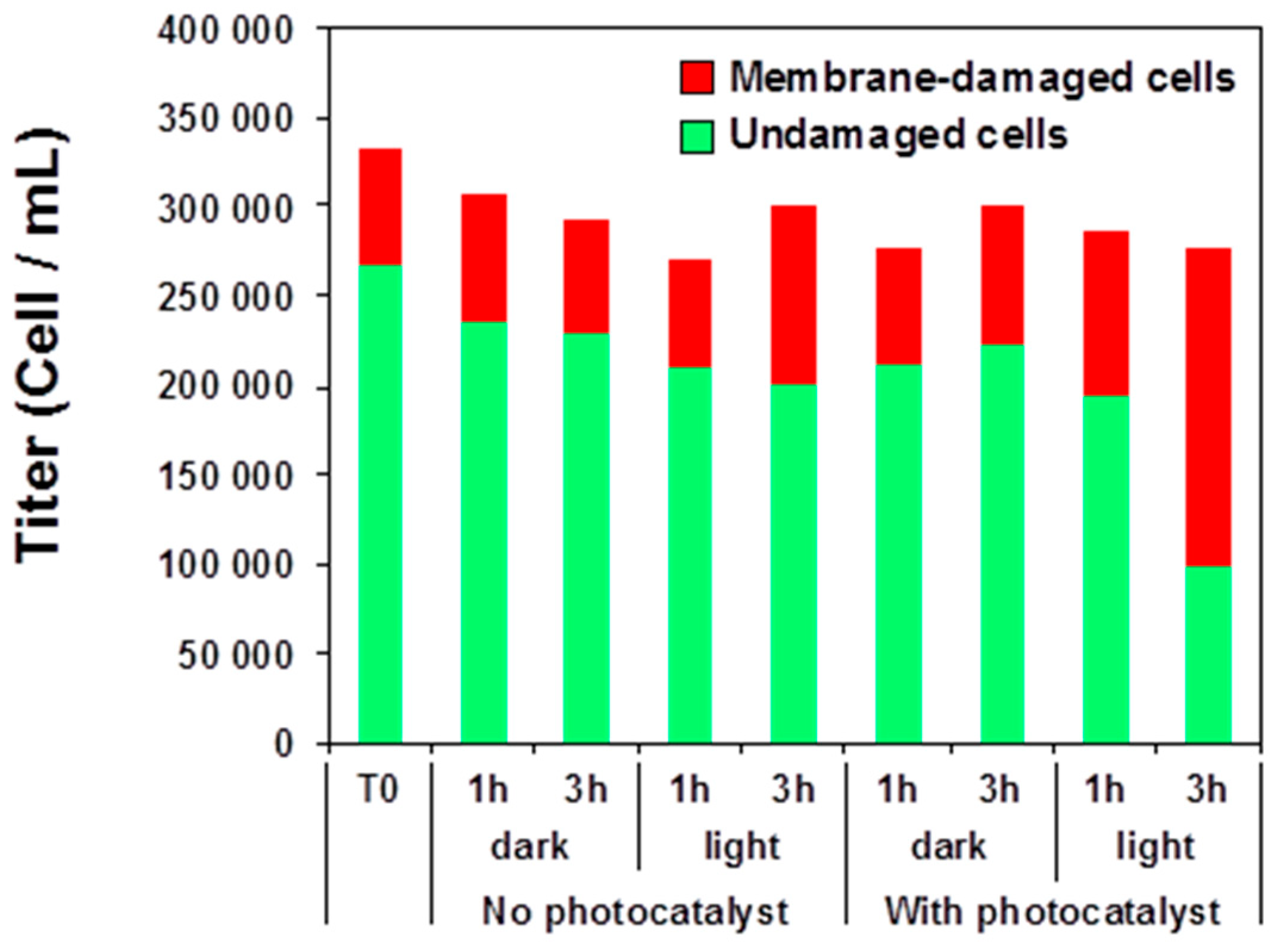
3.5. Photocatalysis-Induced Cell Surface Alterations
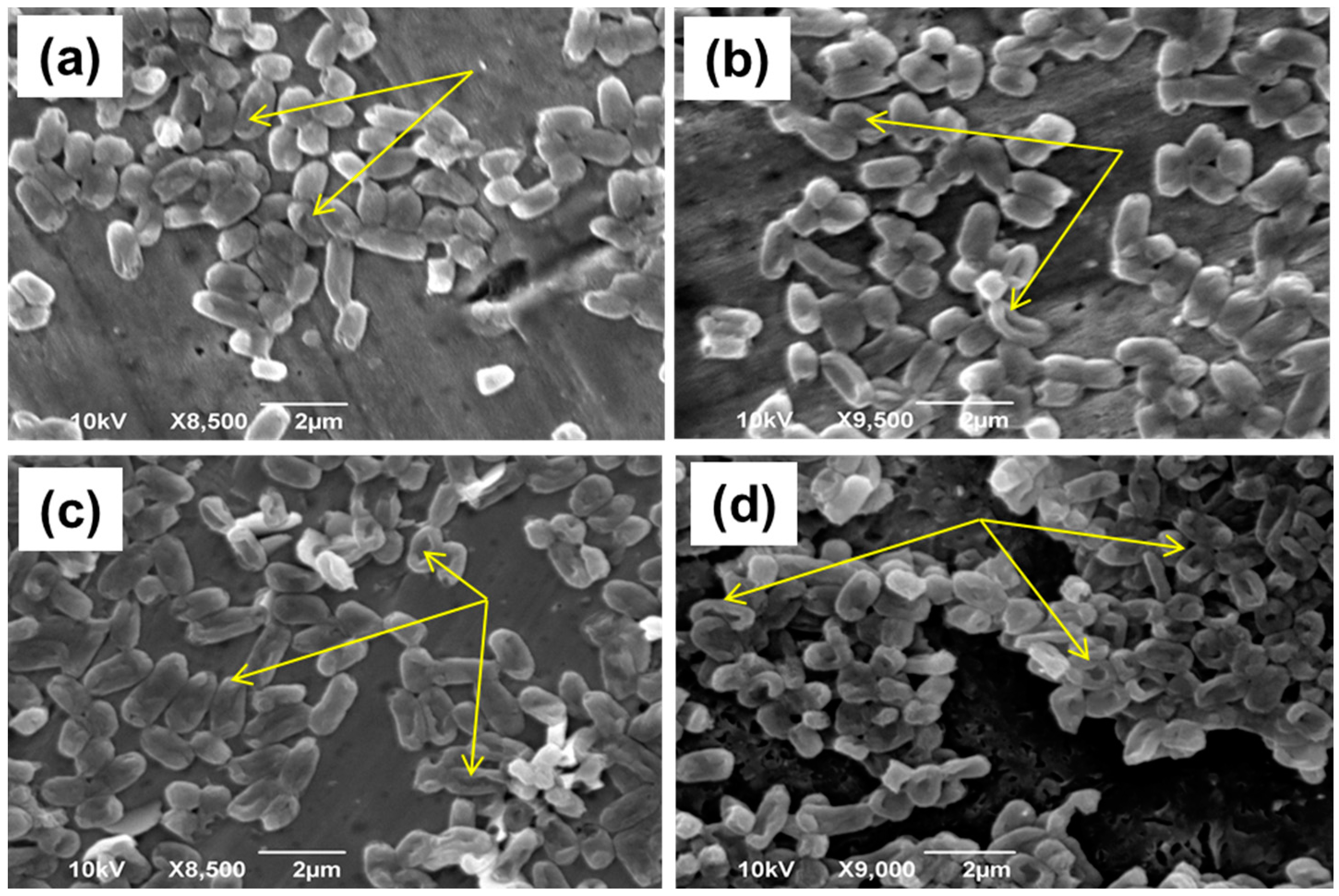
3.6. Photocatalysis-Induced Cell Structure Damages
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Matsunaga, T.; Tomoda, R.; Nakajima, T.; Wake, H. Photoelectrochemical sterilization of microbial cells by semiconductor powders. FEMS Microbiol. Lett. 1985, 29, 211–214. [Google Scholar] [CrossRef]
- Karaolia, P.; Michael-Kordatou, I.; Hapeshi, E.; Drosou, C.; Bertakis, Y.; Christofilos, D.; Armatas, G.S.; Sygellou, L.; Schwartz, T.; Xekoukoulotakis, N.P.; et al. Removal of antibiotics, antibiotic-resistant bacteria and their associated genes by graphene-based TiO2 composite photocatalysts under solar radiation in urban wastewaters. Appl. Catal. B Environ. 2018, 224, 810–824. [Google Scholar] [CrossRef]
- Regmi, C.; Joshi, B.; Ray, S.K.; Gyawali, G.; Pandey, R.P. Understanding mechanism of photocatalytic microbial decontamination of environmental wastewater. Front. Chem. 2018, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Leyland, N.S.; Podporska-Carroll, J.P.; Browne, J.; Hinder, S.J.; Quilty, B.; Pillai, S.C. Highly efficient F, Cu doped TiO2 anti-bacterial visible light active photocatalytic coatings to combat hospital-acquired infections. Sci. Rep. 2016, 6, 24770. [Google Scholar] [CrossRef] [PubMed]
- Helali, S.; Polo-Lopez, M.I.; Fernandez-Ibanez, P.; Ohtani, B.; Amano, F.; Malato, S.; Guillard, C. Solar photocatalysis: A green technology for E. coli contaminated water disinfection. Effect of concentration and different types of suspended catalyst. J. Photochem. Photobiol. A Chem. 2014, 276, 31–40. [Google Scholar] [CrossRef]
- Nakano, R.; Hara, M.; Ishiguro, H.; Yao, Y.; Ochiai, T.; Nakata, K.; Murakami, T.; Kajioka, J.; Sunada, K.; Hashimoto, K.; et al. Broad spectrum microbicidal activity of photocatalysis by TiO2. Catalysts 2013, 3, 310–323. [Google Scholar] [CrossRef]
- Wang, W.; Huang, G.; Yu, J.C.; Wong, P.K. Advances in photocatalytic disinfection of bacteria: Development of photocatalysts and mechanisms. J. Environ. Sci. 2015, 34, 232–247. [Google Scholar] [CrossRef] [PubMed]
- Ebert, D.; Bhushan, B. Transparent, superhydrophobic, and wear-resistant coatings on glass and polymer substrates using SiO2, ZnO, and ITO nanoparticles. Langmuir 2012, 28, 11391–11399. [Google Scholar] [CrossRef] [PubMed]
- Mokari, T.; Sztrum, C.G.; Salant, A.; Rabani, E.; Banin, U. Formation of asymmetric one-sided metal-tipped semiconductor nanocrystal dots and rods. Nat. Mater. 2005, 4, 855–863. [Google Scholar] [CrossRef]
- Phillips, J.; Bowen, W.; Cagin, E.; Wang, W. Electronic and optoelectronic devices based on semiconducting zinc oxide. Semicond. Sci. Technol. 2011, 6, 101–127. [Google Scholar] [CrossRef]
- Wang, Z.L. ZnO nanowire and nanobelt platform for nanotechnology. Mater. Sci. Eng. R 2009, 64, 33–71. [Google Scholar] [CrossRef]
- Xie, W.; Li, Y.; Sun, W.; Huang, J.; Xie, H.; Zhao, X. Surface modification of ZnO with Ag improves its photocatalytic efficiency and photostability. J. Photochem. Photobiol. A Chem. 2010, 216, 149–155. [Google Scholar] [CrossRef]
- Quintana, M.; Ricra, E.; Rodriguez, J.; Estrada, W. Spray pyrolysis deposited zinc oxide films for photo-electrocatalytic degradation of methyl orange: Influence of the pH. Catal. Today 2002, 76, 141–148. [Google Scholar] [CrossRef]
- Singh, S.; Barick, K.C.; Bahadur, D. Shape-controlled hierarchical ZnO architectures: Photocatalytic and antibacterial activities. CrystEngComm 2013, 15, 4631–4639. [Google Scholar] [CrossRef]
- Das, J.; Khushalani, D. Nonhydrolytic route for synthesis of ZnO and its use as a recyclable photocatalyst. J. Phys. Chem. C 2010, 114, 2544–2550. [Google Scholar] [CrossRef]
- Lee, K.M.; Lai, C.W.; Ngai, K.S.; Juan, J.C. Recent developments of zinc oxide based photocatalyst in water treatment technology: A review. Water Res. 2016, 88, 428–448. [Google Scholar] [CrossRef] [PubMed]
- Girish Kumar, S.; Koteswara Rao, K.S.R. Zinc oxide based photocatalysis: Tailoring surface-bulk structure and related interfacial charge carrier dynamics for better environmental applications. RSC Adv. 2015, 5, 3306–3351. [Google Scholar] [CrossRef]
- Sun, T.; Qiu, J.; Liang, C. Controllable fabrication and photocatalytic activity of ZnO nanobelt arrays. J. Phys. Chem. C 2008, 11, 715–721. [Google Scholar] [CrossRef]
- Li, B.; Wang, Y.J. Facile synthesis and enhanced photocatalytic performance of flower-like ZnO hierarchical microstructures. Phys. Chem. C 2010, 114, 890–896. [Google Scholar] [CrossRef]
- Kong, X.Y.; Wang, Z.L. Single-crystal nanorings formed by epitaxial self-coiling of polar nanobelts. Science 2004, 303, 1348–1351. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.B.; Zhuang, H.Z.; Wang, D.X.; Xue, C.S.; Liu, H. Growth and characterization of ZnO nanoporous belts. Cryst. Growth Des. 2009, 9, 2187–2190. [Google Scholar] [CrossRef]
- Moussa, H.; Girot, E.; Mozet, K.; Alem, H.; Medjahdi, G.; Schneider, R. ZnO rods/reduced graphene oxide composites prepared via a solvothermal reaction for efficient sunlight-driven photocatalysis. Appl. Catal. B Environ. 2016, 185, 11–21. [Google Scholar] [CrossRef]
- Chouchene, B.; Ben Chaabane, T.; Balan, L.; Girot, E.; Mozet, K.; Medjahdi, G.; Schneider, R. High performance Ce-doped ZnO nanorods for sunlight-driven photocatalysis. Beilstein J. Nanotechnol. 2016, 7, 1338–1349. [Google Scholar] [CrossRef] [PubMed]
- Baruah, S.; Joisai, M.; Dutta, J. Development of a visible light active photocatalytic portable water purification unit using ZnO. J. Catal. Sci. Technol. 2012, 2, 918–921. [Google Scholar] [CrossRef]
- Zhang, X.; Qin, J.; Xue, Y.; Yu, P.; Zhang, B.; Liu, R. Effect of aspect ratio and surface defects on the photocatalytic activity of ZnO nanorods. Sci. Rep. 2014, 4, 4596. [Google Scholar] [CrossRef] [PubMed]
- Meenakshi, G.; Sivasamy, A.; Suganya Josephine, G.A.; Kavithae, S. Preparation, characterization and enhanced photocatalytic activities of zinc oxide nano rods/silicon carbide composite under UV and visible light irradiations. J. Mol. Catal. A Chem. 2016, 411, 167–178. [Google Scholar] [CrossRef]
- Achouri, F.; Corbel, S.; Balan, L.; Mozet, K.; Girot, E.; Medjahdi, G.; Ben Said, M.; Ghrabi, A.; Schneider, R. Porous Mn-doped ZnO nanoparticles for enhanced solar and visible light photocatalysis. Mater. Des. 2016, 101, 309–316. [Google Scholar] [CrossRef]
- Rachel, A.; Subrahmanyan, M.; Boule, P. Comparison of photocatalytic efficiencies of TiO2 in suspended and immobilised form for the photocatalytic degradation of nitrobenzenesulfonic acids. Appl. Catal. B Environ. 2002, 37, 301–308. [Google Scholar] [CrossRef]
- Arabatzis, I.M.; Antonarakis, S.; Stergiopoulos, T.; Hiskia, A.; Papaconstantinou, E.; Bernard, M.C.; Falaras, P. Preparation, characterization and photocatalytic activity of nanocrystalline thin film TiO2 catalysts towards 3,5-dichlorophenol degradation. J. Photochem. Photobiol. A 2002, 149, 237–245. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, H.; He, C.; Qiu, L.; Cao, X. Uniform carbon-coated ZnO nanorods: Microwave-assisted preparation, cytotoxicity, and photocatalytic activity. Langmuir 2009, 25, 4678–4684. [Google Scholar] [CrossRef] [PubMed]
- Blattner, F.R.; Plunkett, G.; Bloch, C.A.; Perna, N.T.; Burland, V.; Riley, M.; Collado-Vides, J.; Glasner, J.D.; Rode, C.K.; Mayhew, G.F.; et al. The complete genome sequence of Escherichia coli K-12. Science 1997, 277, 1453–1462. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, M.F.J.; Buwalda, H.; de Jong, A.W.F.; Michorius, A.; Winkelman, J.G.M.; Beenackers, A.A.C.M. Experimental comparison of three reactor designs for photocatalytic water purification. Chem. Eng. Sci. 2001, 56, 547–555. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef] [PubMed]
- Daskalaki, V.M.; Frontistis, Z.; Mantzavino, D.; Katsaounis, A. Solar light-induced degradation of bisphenol-A with TiO2 immobilized on Ti. Catal. Today 2011, 161, 110–114. [Google Scholar] [CrossRef]
- Heinlaan, M.; Ivask, A.; Blinova, I.; Dubourguier, H.C.; Kahru, A. Toxicity of nanosized and bulk ZnO, CuO and TiO2 to bacteria Vibrio fischeri and crustaceans Daphnia magna and Thamnocephalus platyurus. Chemosphere 2008, 71, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Mashayekhi, H.; Xing, B. Bacterial toxicity comparison between nano-and micro-scaled oxide particles. Environ. Pollut. 2009, 15, 1619–1625. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Sasidharan, A.; Divya Rani, V.V.; Menon, D.; Nair, S.; Manzoor, K.; Raina, S. Role of size scale of ZnO nanoparticles and microparticles on toxicity toward bacteria and osteoblast cancer cells. J. Mater. Sci. Mater. Med. 2009, 20, 235. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; He, Y.; Irwin, P.L.; Jin, T.; Shi, X. Antibacterial activity and mechanism of action of zinc oxide nanoparticles against Campylobacter jejuni. Appl. Environ. Microbiol. 2011, 77, 2325–2331. [Google Scholar] [CrossRef] [PubMed]
- Premanathan, M.; Karthikeyan, K.; Jeyasubramanian, K.; Manivannan, G. Selective toxicity of ZnO nanoparticles toward Gram-positive bacteria and cancer cells by apoptosis through lipid peroxidation. Nanomed. Nanotech. Biol. Med. 2011, 7, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhu, L.; Lin, D. Toxicity of ZnO nanoparticles to Escherichia coli: Mechanism and the influence of medium components. Environ. Sci. Technol. 2011, 45, 1977–1983. [Google Scholar] [CrossRef] [PubMed]
- Franklin, N.M.; Rogers, N.J.; Apte, S.C.; Batley, G.E.; Gadd, G.E.; Casey, P.S. Comparative toxicity of nanoparticulate ZnO, bulk ZnO, and ZnCl2 to a freshwater microalga (Pseudokirchneriella subcapitata): The importance of particle solubility. Environ. Sci. Technol. 2007, 41, 8484–8490. [Google Scholar] [CrossRef] [PubMed]
- Lele, B.S.; Russell, A.J. Rational protein modification leading to resistance of enzymes to TiO2-UV irradiation-induced inactivation. Biomacromolecules 2004, 5, 1947–1955. [Google Scholar] [CrossRef] [PubMed]
- Akbar, S.; Sreeramulu, K.; Sharma, H.C. Tryptophan fluorescence quenching as a binding assay to monitor protein conformation changes in the membrane of intact mitochondria. J. Bioenergy Biomembr. 2016, 48, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, T.; Chakraborti, S.; Joshi, P.; Singh, S.P.; Gupta, V.; Chakrabarti, P. The effect of zinc oxide nanoparticles on the structure of the periplasmic domain of the Vibrio cholerae ToxR protein. FEBS J. 2010, 277, 4184–4194. [Google Scholar] [CrossRef] [PubMed]
- Berney, M.; Weilenmann, H.U.; Egli, T. Flow-cytometric study of vital cellular functions in Escherichia coli during solar disinfection (SODIS). Microbiol. SGM 2006, 152, 1719–1729. [Google Scholar] [CrossRef] [PubMed]
- Prütz, W.A. Hypochlorous acid interactions with thiols, nucleotides, DNA, and other biological substrates. Biochem. Biophys. 1996, 332, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Prütz, W.A. Arch. Interactions of hypochlorous acid with pyrimidine nucleotides, and secondary reactions of chlorinated pyrimidines with GSH, NADH, and other substrates. Biochem. Biophys. 1998, 349, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Phe, M.H.; Dossot, M.; Guilloteau, H.; Block, J.C. Nucleic acid fluorochromes and flow cytometry prove useful in assessing the effect of chlorination on drinking water bacteria. Water Res. 2005, 39, 3618–3628. [Google Scholar] [CrossRef] [PubMed]
- Phe, M.H.; Dossot, M.; Block, J.C. Chlorination effect on the fluorescence of nucleic acid staining dyes. Water. Res. 2004, 38, 3729–3737. [Google Scholar] [CrossRef] [PubMed]
- Phe, M.H.; Hajj Chehade, M.; Guilloteau, H.; Merlin, C.; Block, J.C. Assessment of damage to nucleic acids and repair machinery in Salmonella typhimurium exposed to chlorine. Int. J. Microbiol. 2009, 2009, 201868. [Google Scholar] [CrossRef] [PubMed]
- Grégori, G.; Citterio, S.; Ghiani, A.; Labra, M.; Sgorbati, S.; Brown, S.; Denis, M. Resolution of viable and membrane-compromised bacteria in freshwater and marine waters based on analytical flow cytometry and nucleic acid double staining. Appl. Environ. Microbiol. 2001, 67, 4662–4670. [Google Scholar] [CrossRef] [PubMed]
- Hammes, F.; Berney, M.; Wang, Y.; Vital, M.; Köster, O.; Egli, T. Flow-cytometric total bacterial counts as a descriptive microbiological parameter for drinking water treatment processes. Water Res. 2008, 42, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Kiwi, J.; Nadtochenko, V. Evidence for the mechanism of photocatalytic degradation of the bacterial wall membrane at the TiO2 interface by ATR-FTIR and laser kinetic spectroscopy. Langmuir 2005, 21, 4631–4641. [Google Scholar] [CrossRef] [PubMed]
- Gogniat, G.; Thyssen, M.; Denis, M.; Pulgarin, C.; Dukan, S. The bactericidal effect of TiO2 photocatalysis involves adsorption onto catalyst and the loss of membrane integrity. FEMS Microbiol. Lett. 2006, 258, 18–24. [Google Scholar] [CrossRef] [PubMed]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Achouri, F.; Merlin, C.; Corbel, S.; Alem, H.; Mathieu, L.; Balan, L.; Medjahdi, G.; Ben Said, M.; Ghrabi, A.; Schneider, R. ZnO Nanorods with High Photocatalytic and Antibacterial Activity under Solar Light Irradiation. Materials 2018, 11, 2158. https://doi.org/10.3390/ma11112158
Achouri F, Merlin C, Corbel S, Alem H, Mathieu L, Balan L, Medjahdi G, Ben Said M, Ghrabi A, Schneider R. ZnO Nanorods with High Photocatalytic and Antibacterial Activity under Solar Light Irradiation. Materials. 2018; 11(11):2158. https://doi.org/10.3390/ma11112158
Chicago/Turabian StyleAchouri, Faouzi, Christophe Merlin, Serge Corbel, Halima Alem, Laurence Mathieu, Lavinia Balan, Ghouti Medjahdi, Myriam Ben Said, Ahmed Ghrabi, and Raphaël Schneider. 2018. "ZnO Nanorods with High Photocatalytic and Antibacterial Activity under Solar Light Irradiation" Materials 11, no. 11: 2158. https://doi.org/10.3390/ma11112158
APA StyleAchouri, F., Merlin, C., Corbel, S., Alem, H., Mathieu, L., Balan, L., Medjahdi, G., Ben Said, M., Ghrabi, A., & Schneider, R. (2018). ZnO Nanorods with High Photocatalytic and Antibacterial Activity under Solar Light Irradiation. Materials, 11(11), 2158. https://doi.org/10.3390/ma11112158







