Teflon-on-Glass Molding Enables High-Throughput Fabrication of Hydrophilic-in-Hydrophobic Microwells for Bead-Based Digital Bioassays
Abstract
1. Introduction
2. Materials and Methods
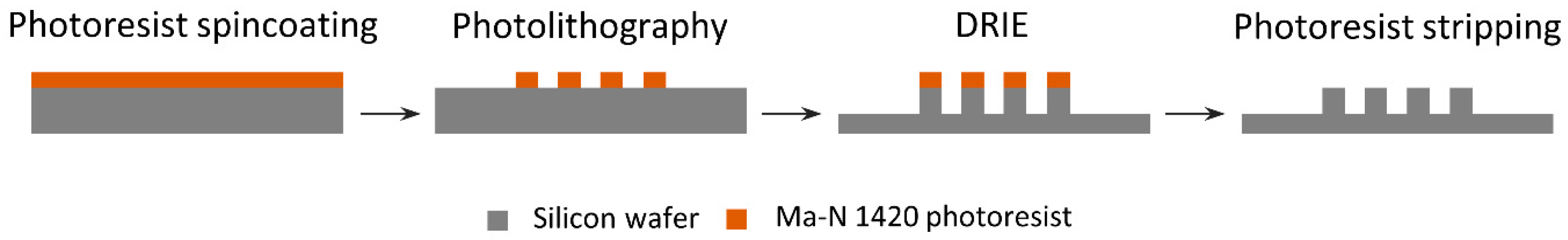
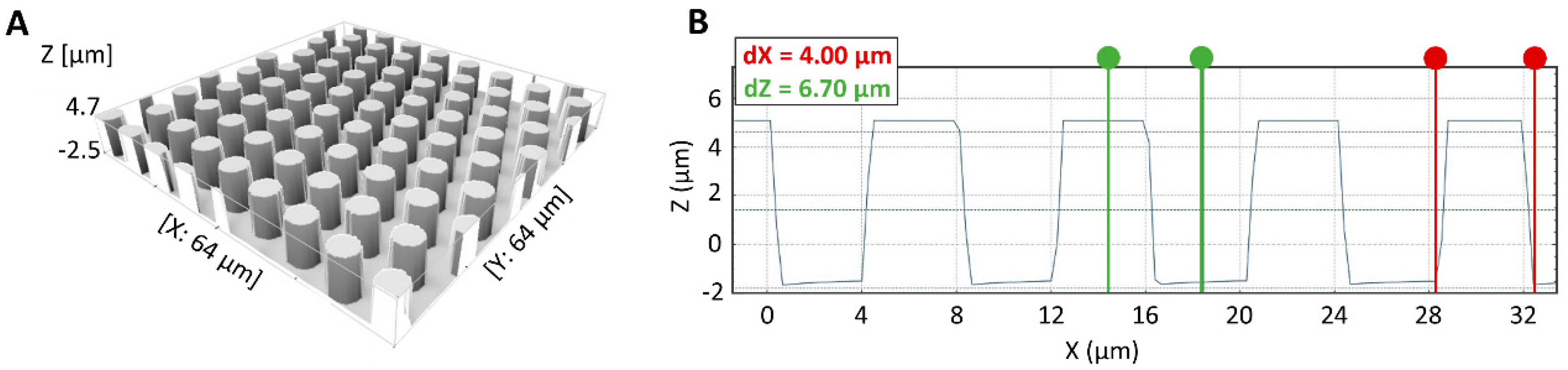
2.1. Silicon Micropillar Mold Fabrication and Characterization
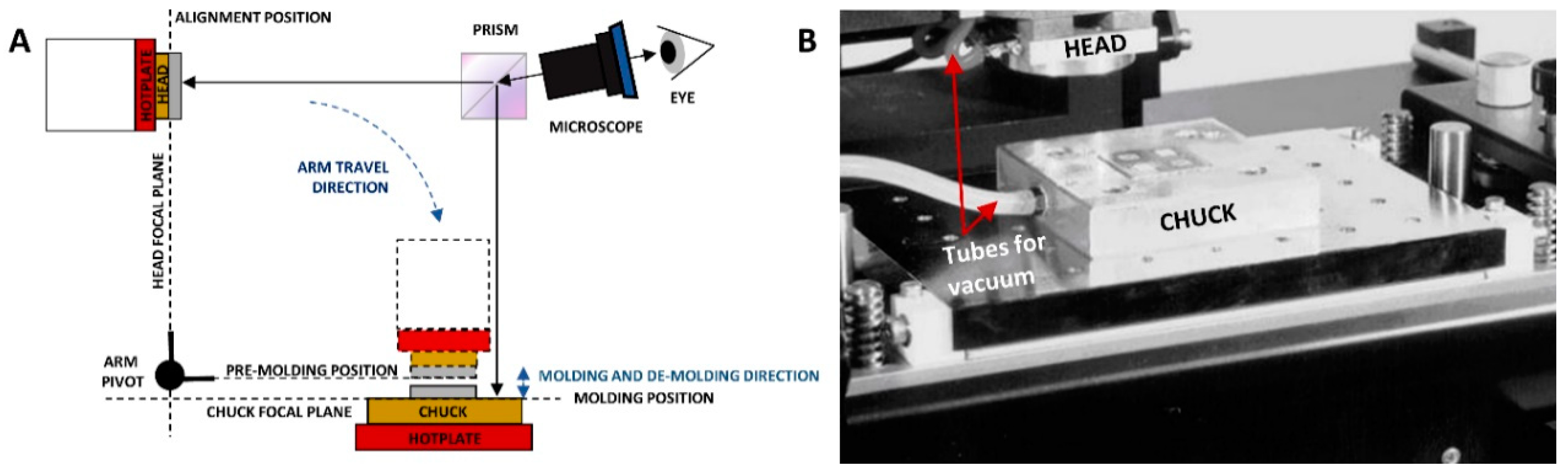
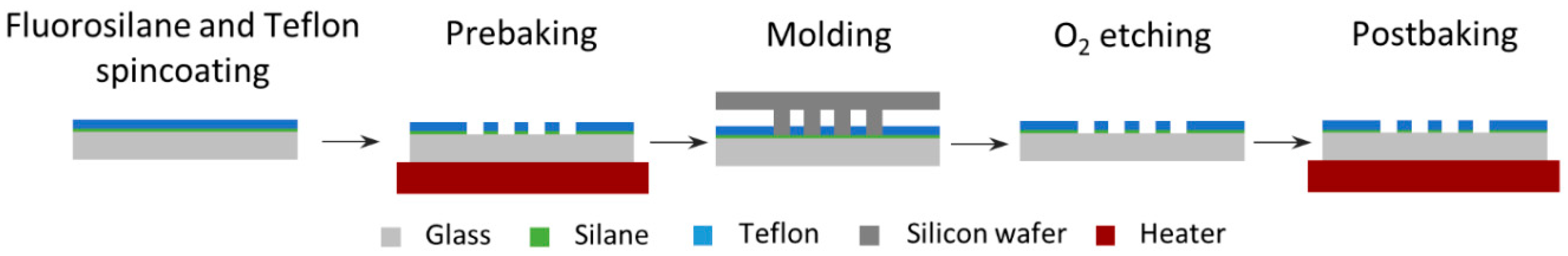
2.2. Microwell Array Fabrication and Characterization
2.3. Bead Seeding and Reagent Sealing
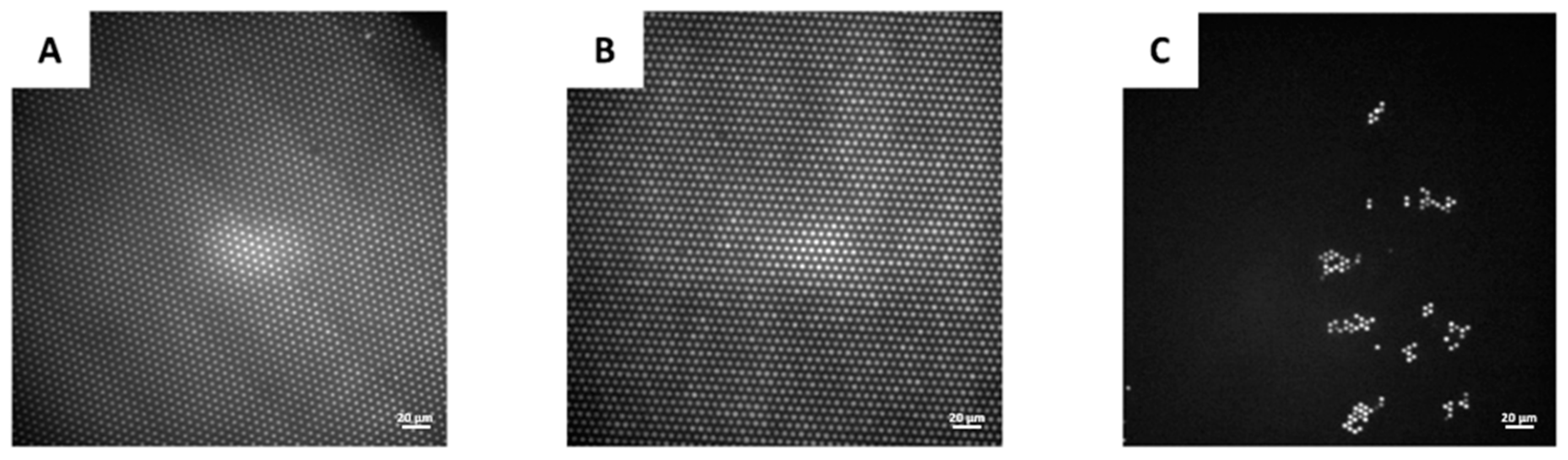
3. Results and Discussion
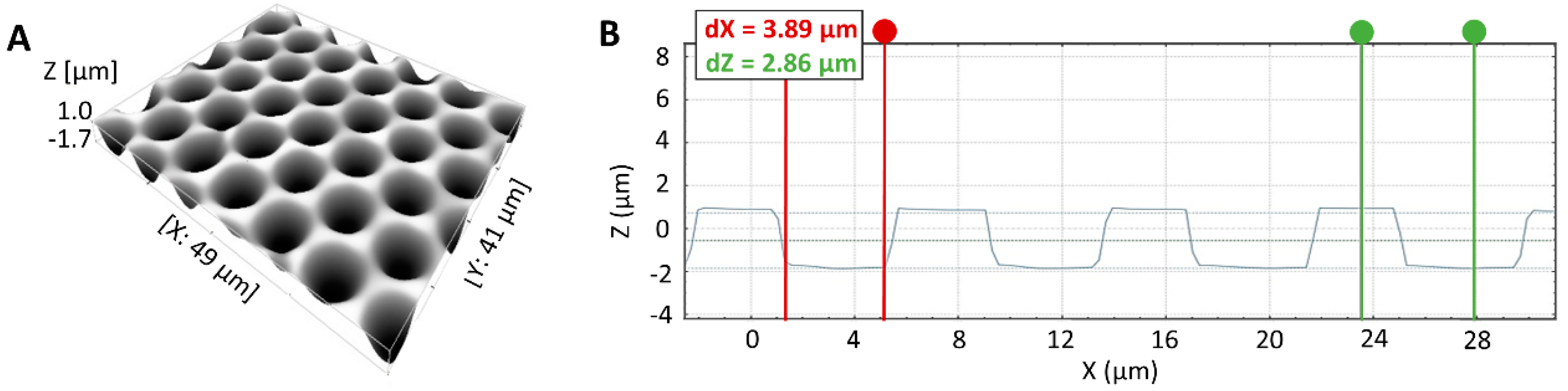
3.1. Silicon Micropillar Mold Fabrication and Characterization
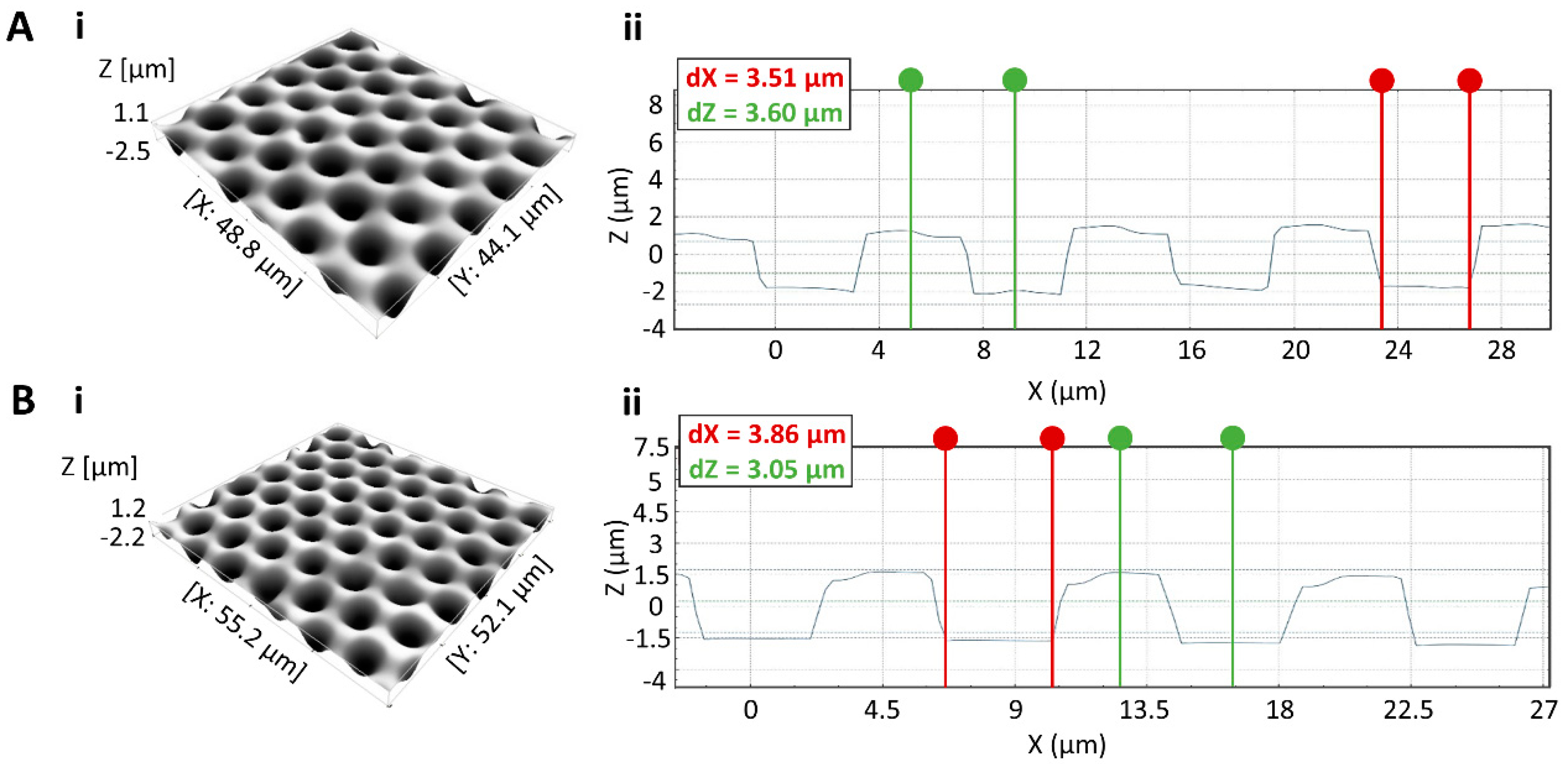
3.2. Microwell Array Fabrication and Characterization
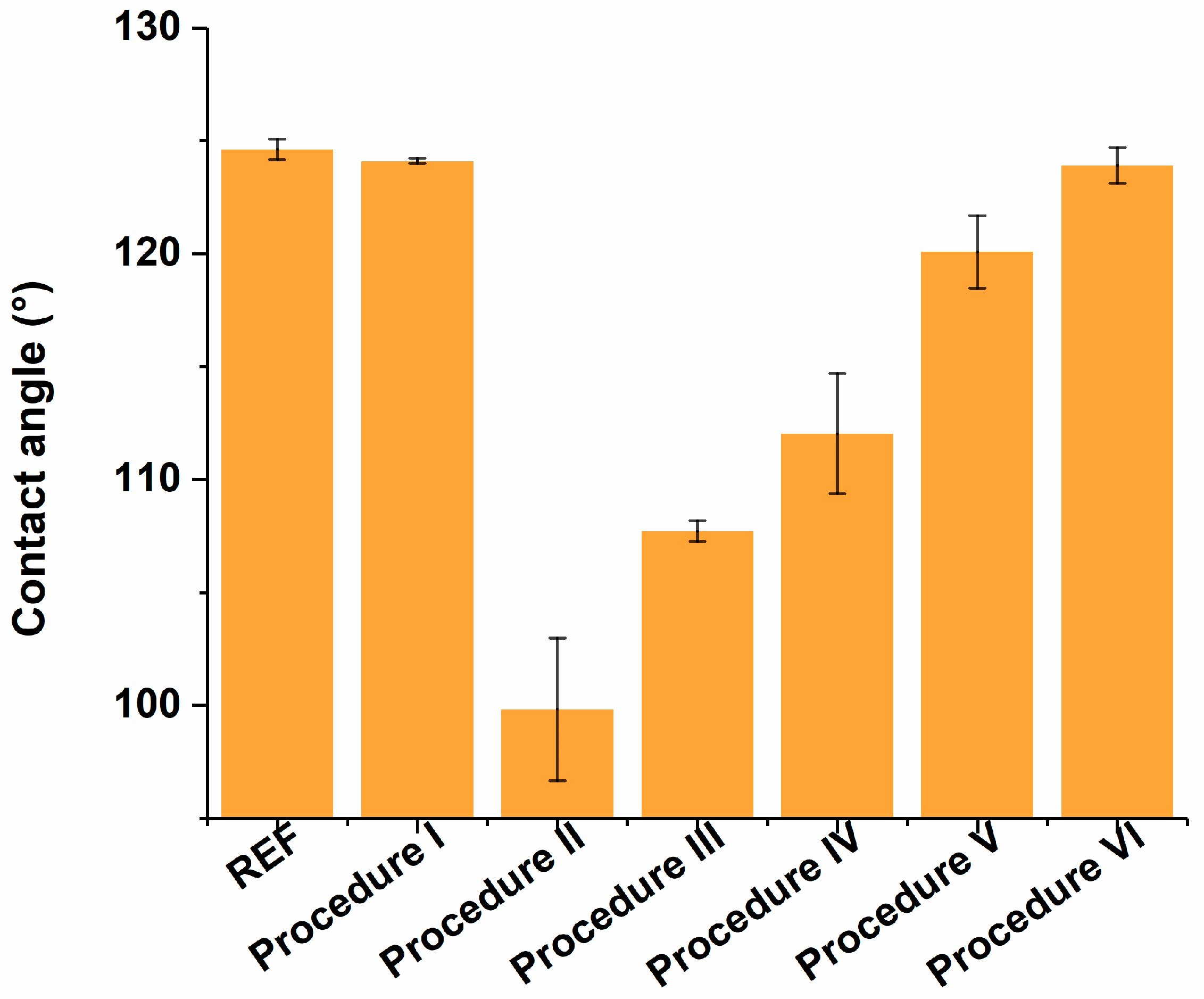
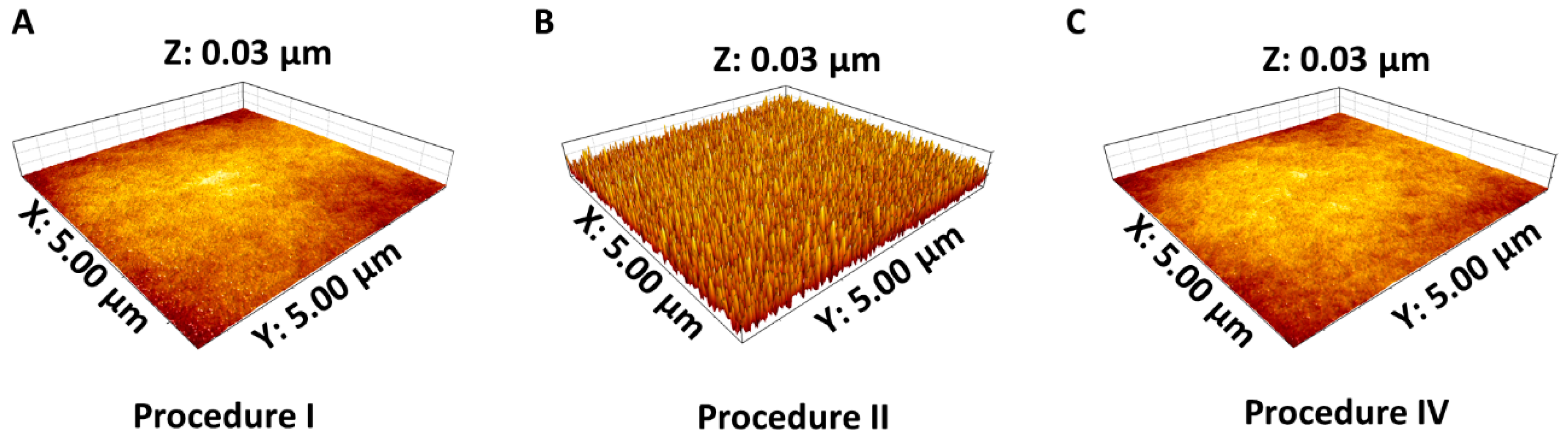
3.3. Contact Angle and AFM Measurements
3.4. Seeding and Sealing Efficiency
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pollack, M.G.; Fair, R.B.; Shenderov, A.D. Electrowetting-based actuation of liquid droplets for microfluidic applications. Appl. Phys. Lett. 2000, 77, 1725–1726. [Google Scholar] [CrossRef]
- Cho, S.K.; Moon, H.; Kim, C.J. Creating, Transporting, Cutting, and Merging Liquid Droplets by Electrowetting-Based Actuation for Digital Microfluidic Circuits. J. Microelectromech. Syst. 2003, 12, 70–80. [Google Scholar]
- Witters, D.; Toffalini, F.; Puers, R.; Lammertyn, J. Digital Microfluidic Femtoliter Droplet Printing: A Versatile Tool for Single-Molecule Detection of Nucleic Acids and Proteins. In Proceedings of the 17th International Conference on Miniaturized Systems for Chemistry and Life Sciences (MicroTAS 2013), Freiburg, Germany, 27–31 October 2013; pp. 296–298. [Google Scholar]
- Pantano, P.; Walt, D.R. Ordered Nanowell Arrays. Chem. Mater. 1996, 8, 2832–2835. [Google Scholar] [CrossRef]
- Chou, J.; Du, N.; Ou, T.; Floriano, P.N.; Christodoulides, N.; McDevitt, J.T. Hot embossed polyethylene through-hole chips for bead-based microfluidic devices. Biosens. Bioelectron. 2013, 42, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Kimerling, T.E.; Liu, W.; Kim, B.H.; Yao, D. Rapid hot embossing of polymer microfeatures. Microsyst. Technol. 2006, 12, 730–735. [Google Scholar] [CrossRef]
- Thompson, J.A.; Du, X.; Grogan, J.M.; Schrlau, M.G.; Bau, H.H. Polymeric microbead arrays for microfluidic applications. J. Micromech. Microeng. 2010, 20, 115017. [Google Scholar] [CrossRef]
- Ren, K.; Dai, W.; Zhou, J.; Su, J.; Wu, H. Whole-Teflon microfluidic chips. Proc. Natl. Acad. Sci. USA 2011, 108, 8162–8166. [Google Scholar] [CrossRef] [PubMed]
- Yeong, Y.H.; Gupta, M.C. Hot embossed micro-textured thin superhydrophobic Teflon FEP sheets for low ice adhesion. Surf. Coat. Technol. 2017, 313, 17–23. [Google Scholar] [CrossRef]
- Jucius, D.; Grigaliunas, V.; Mikolajunas, M.; Guobiene, A.; Kopustinskas, V.; Gudonyte, A.; Narmontas, P. Hot embossing of PTFE: Towards superhydrophobic surfaces. Appl. Surf. Sci. 2011, 257, 2353–2360. [Google Scholar] [CrossRef]
- Jucius, D.; Guobiene, A.; Grigaliunas, V. Surface texturing of polytetrafluoroethylene by hot embossing. Appl. Surf. Sci. 2010, 256, 2164–2169. [Google Scholar] [CrossRef]
- Kim, S.H.; Iwai, S.; Araki, S.; Sakakihara, S.; Iino, R.; Noji, H. Large-scale femtoliter droplet array for digital counting of single biomolecules. Lab Chip 2012, 12, 4986. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Winter, M.; Thierry, B. Quasi-spherical microwells on superhydrophobic substrates for long term culture of multicellular spheroids and high throughput assays. Biomaterials 2014, 35, 6060–6068. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Hu, H.; Wang, Y.; Wang, Y.W.; Wu, Q.; Liu, L.T.; Chen, G.Q. Fabrication of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (PHBHHx) microstructures using soft lithography for scaffold applications. Biomaterials 2006, 27, 2550–2557. [Google Scholar] [CrossRef] [PubMed]
- Kan, C.W.; Rivnak, A.J.; Campbell, T.G.; Piech, T.; Rissin, D.M.; Mosl, M.; Peterca, A.; Niederberger, H.P.; Minnehan, K.A.; Patel, P.P.; et al. Isolation and detection of single molecules on paramagnetic beads using sequential fluid flows in microfabricated polymer array assemblies. Lab Chip 2012, 12, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.H.; Rissin, D.M.; Kan, C.W.; Fournier, D.R.; Piech, T.; Campbell, T.G.; Meyer, R.E.; Fishburn, M.W.; Cabrera, C.; Patel, P.P.; et al. The Simoa HD-1 Analyzer: A Novel Fully Automated Digital Immunoassay Analyzer with Single-Molecule Sensitivity and Multiplexing. J. Lab. Autom. 2016, 21, 533–547. [Google Scholar] [CrossRef] [PubMed]
- Rissin, D.M.; Walt, D.R. Digital Concentration Readout of Single Enzyme Molecules Using Femtoliter Arrays and Poisson Statistics. Nano Lett. 2006, 6, 520–523. [Google Scholar] [CrossRef] [PubMed]
- Rissin, D.M.; Kan, C.W.; Campbell, T.G.; Howes, S.C.; Fournier, D.R.; Song, L.; Piech, T.; Patel, P.P.; Chang, L.; Rivnak, A.J.; et al. Single-molecule enzyme-linked immunosorbent assay detects serum proteins at subfemtomolar concentrations. Nat. Biotechnol. 2010, 28, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Nie, S.; Etson, C.M.; Wang, R.M.; Walt, D.R. Oil-sealed femtoliter fiber-optic arrays for single molecule analysis. Lab Chip 2012, 12, 2229–2239. [Google Scholar] [CrossRef] [PubMed]
- Sakakihara, S.; Araki, S.; Iino, R.; Noji, H. A single-molecule enzymatic assay in a directly accessible femtoliter droplet array. Lab Chip 2010, 10, 3355. [Google Scholar] [CrossRef] [PubMed]
- Witters, D.; Knez, K.; Ceyssens, F.; Puers, R.; Lammertyn, J. Digital microfluidics-enabled single-molecule detection by printing and sealing single magnetic beads in femtoliter droplets. Lab Chip 2013, 13, 2047. [Google Scholar] [CrossRef] [PubMed]
- Tripodi, L.; Witters, D.; Kokalj, T.; Huber, H.J.; Puers, R.; Lammertyn, J.; Spasic, D. Sub-femtomolar detection of DNA and discrimination of mutant strands using microwell-array assisted digital enzyme-linked oligonucleotide assay. Anal. Chim. Acta 2018, 1041, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Leirs, K.; Kumar, P.T.; Decrop, D.; Perez-Ruiz, E.; Leblebici, P.; Van Kelst, B.; Compernolle, G.; Meeuws, H.; Van Wesenbeeck, L.; Lagatie, O.; et al. Bioassay Development for Ultrasensitive Detection of Influenza A Nucleoprotein Using Digital ELISA. Anal. Chem. 2016, 88, 8450–8458. [Google Scholar] [CrossRef] [PubMed]
- Perez-Ruiz, E.; Decrop, D.; Ven, K.; Tripodi, L.; Leirs, K.; Rosseels, J.; de Wouwer, M.V.; Geukens, N.; De Vos, A.; Vanmechelen, E.; et al. Digital ELISA for the quantification of attomolar concentrations of Alzheimer’s disease biomarker protein Tau in biological samples. Anal. Chim. Acta 2018, 1015, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.T.; Vriens, K.; Cornaglia, M.; Gijs, M.; Kokalj, T.; Thevissen, K.; Geeraerd, A.; Cammue, B.P.A.; Puers, R.; Lammertyn, J. Digital microfluidics for time-resolved cytotoxicity studies on single non-adherent yeast cells. Lab Chip 2015, 15, 1852–1860. [Google Scholar] [CrossRef] [PubMed]
- Decrop, D.; Brans, T.; Gijsenbergh, P.; Lu, J.D.; Spasic, D.; Kokalj, T.; Beunis, F.; Goos, P.; Puers, R.; Lammertyn, J. Optical Manipulation of Single Magnetic Beads in a Microwell Array on a Digital Microfluidic Chip. Anal. Chem. 2016, 88, 8596–8603. [Google Scholar] [CrossRef] [PubMed]
- Witters, D.; Vergauwe, N.; Vermeir, S.; Ceyssens, F.; Liekens, S.; Puers, R.; Lammertyn, J. Biofunctionalization of electrowetting-on-dielectric digital microfluidic chips for miniaturized cell-based applications. Lab Chip 2011, 11, 2790. [Google Scholar] [CrossRef] [PubMed]
- Decrop, D.; Pardon, G.; Brancato, L.; Kil, D.; Shafagh, R.Z.; Kokalj, T.; Haraldsson, T.; Puers, R.; van der Wijngaart, W.; Lammertyn, J. Single-Step Imprinting of Femtoliter Microwell Arrays Allows Digital Bioassays with Attomolar Limit of Detection. ACS Appl. Mater. Interfaces 2017, 9, 10418–10426. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.Y. Spatially defined hydrophobic coating of a microwell-patterned hydrophilic polymer substrate for targeted adhesion with high-resolution soft lithography. Colloid Surface B 2013, 111, 313–320. [Google Scholar] [CrossRef] [PubMed]
- FCM User Guide V3 (with Pressure Sensor Head). Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&ved=2ahUKEwjw4JKP5a_eAhXGErwKHXW9BwkQFjAAegQIABAC&url=https%3A%2F%2Fwww.liconic.com%2Fold%2Fsupport%2Fmanuals%2Ffcm-user-guide_v3-2.doc&usg=AOvVaw0N0kerjBkDZsTzHwKrOfQr (accessed on 31 October 2018).
- Chemours_Teflon_AF_Processing_Guide_K16528. Available online: https://www.chemours.com/Teflon_Industrial/zh_CN/assets/downloads/Chemours_Teflon_AF_Processing_Guide_K16528.pdf (accessed on 31 October 2018).
- Uehara, T.; Kubo, S.; Hiroshiba, N.; Nakagawa, M. Anisotropic Oxygen Reactive Ion Etching for Removing Residual Layers from 45 nm-width Imprint Patterns. J. Photopolym. Sci. Technol. 2016, 29, 201–208. [Google Scholar] [CrossRef]
- Zanini, S.; Barni, R.; Della Pergola, R.; Riccardi, C. Modification of the PTFE wettability by oxygen plasma treatments: Influence of the operating parameters and investigation of the ageing behaviour. J. Phys. D Appl. Phys. 2014, 47, 325202. [Google Scholar] [CrossRef]
- Li, R. Time-temperature superposition method for glass transition temperature of plastic materials. Mater. Sci. Eng. A 2000, 278, 36–45. [Google Scholar] [CrossRef]
- Ohkubo, Y.; Ishihara, K.; Shibahara, M.; Nagatani, A.; Honda, K.; Endo, K.; Yamamura, K. Drastic Improvement in Adhesion Property of Polytetrafluoroethylene (PTFE) via Heat-Assisted Plasma Treatment Using a Heater. Sci. Rep. 2017, 7, 9476. [Google Scholar] [CrossRef] [PubMed]

| Procedure | Molding | Plasma | Post-Bake 1 | Post-Bake 2 |
|---|---|---|---|---|
| Procedure I | 110 °C | - | - | |
| Procedure II | 110 °C | Yes | - | - |
| Procedure III | 110 °C | Yes | 10 min at 165 °C | - |
| Procedure IV | 110 °C | Yes | 10 min at 250 °C | - |
| Procedure V | 110 °C | Yes | 15 min at 250 °C | - |
| Procedure VI | 110 °C | Yes | 10 min at 165 °C | 2 min at 330 °C |
| Procedure VII | 110 °C | Yes | 10 min at 250 °C ↕ | - |
| REF | - | - | 5 min at 110 °C | 10 min at 250 °C |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tripodi, L.; Ven, K.; Kil, D.; Rutten, I.; Puers, R.; Lammertyn, J. Teflon-on-Glass Molding Enables High-Throughput Fabrication of Hydrophilic-in-Hydrophobic Microwells for Bead-Based Digital Bioassays. Materials 2018, 11, 2154. https://doi.org/10.3390/ma11112154
Tripodi L, Ven K, Kil D, Rutten I, Puers R, Lammertyn J. Teflon-on-Glass Molding Enables High-Throughput Fabrication of Hydrophilic-in-Hydrophobic Microwells for Bead-Based Digital Bioassays. Materials. 2018; 11(11):2154. https://doi.org/10.3390/ma11112154
Chicago/Turabian StyleTripodi, Lisa, Karen Ven, Dries Kil, Iene Rutten, Robert Puers, and Jeroen Lammertyn. 2018. "Teflon-on-Glass Molding Enables High-Throughput Fabrication of Hydrophilic-in-Hydrophobic Microwells for Bead-Based Digital Bioassays" Materials 11, no. 11: 2154. https://doi.org/10.3390/ma11112154
APA StyleTripodi, L., Ven, K., Kil, D., Rutten, I., Puers, R., & Lammertyn, J. (2018). Teflon-on-Glass Molding Enables High-Throughput Fabrication of Hydrophilic-in-Hydrophobic Microwells for Bead-Based Digital Bioassays. Materials, 11(11), 2154. https://doi.org/10.3390/ma11112154






