Role of Reversed Austenite Behavior in Determining Microstructure and Toughness of Advanced Medium Mn Steel by Welding Thermal Cycle
Abstract
:1. Introduction
- (1)
- The reversed austenite nucleation characterization on heating.
- (2)
- The reversed austenite growth kinetic.
- (3)
- The effect of different austenite grain size on continuous cooling transformation temperature, and thus martensite transformation and reversed austenite transformation.
- (4)
- The role of microstructure in influencing the cryogenic toughness of heat-affected zone.
2. Experimental
3. Results and Discussion
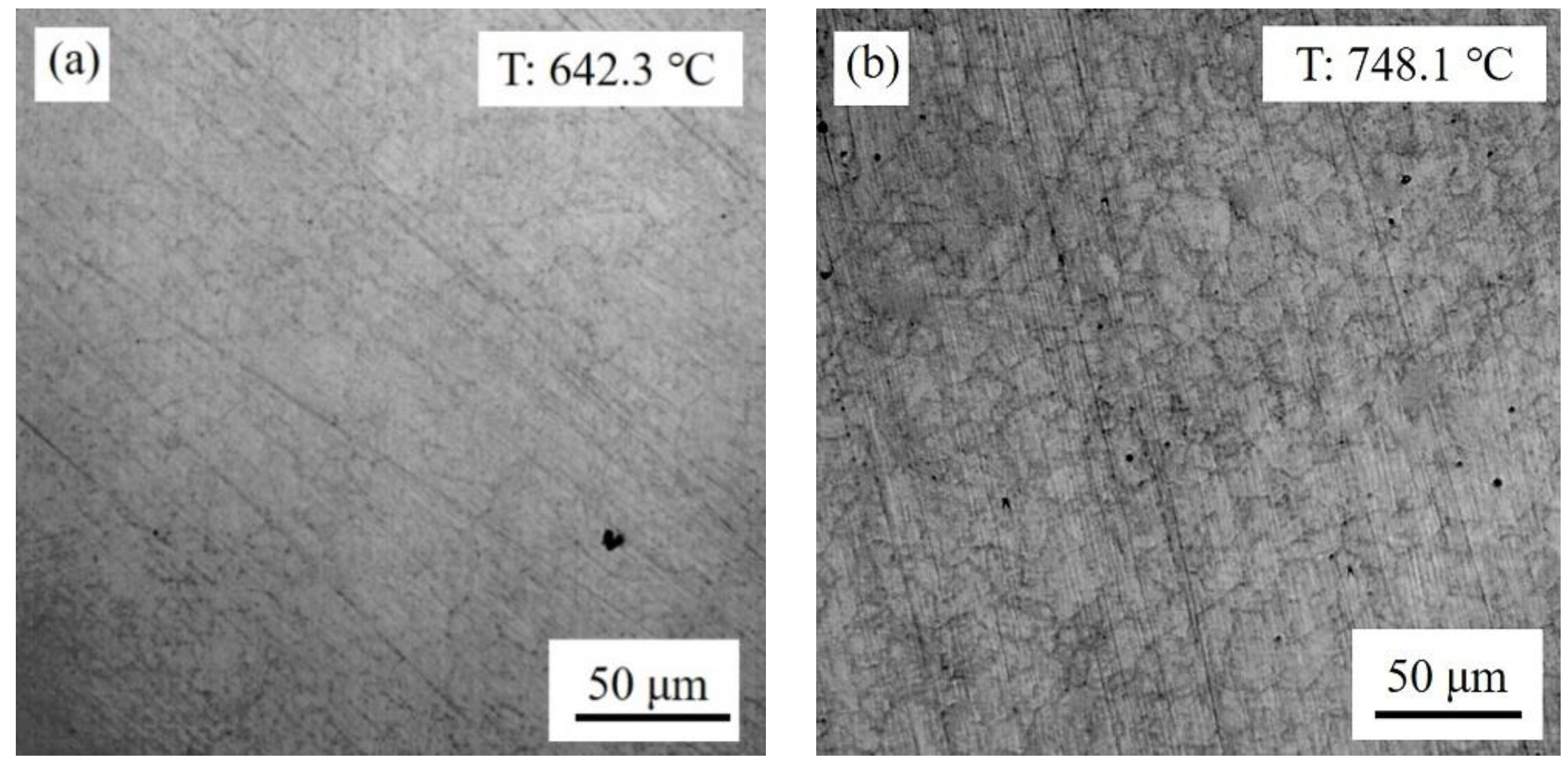
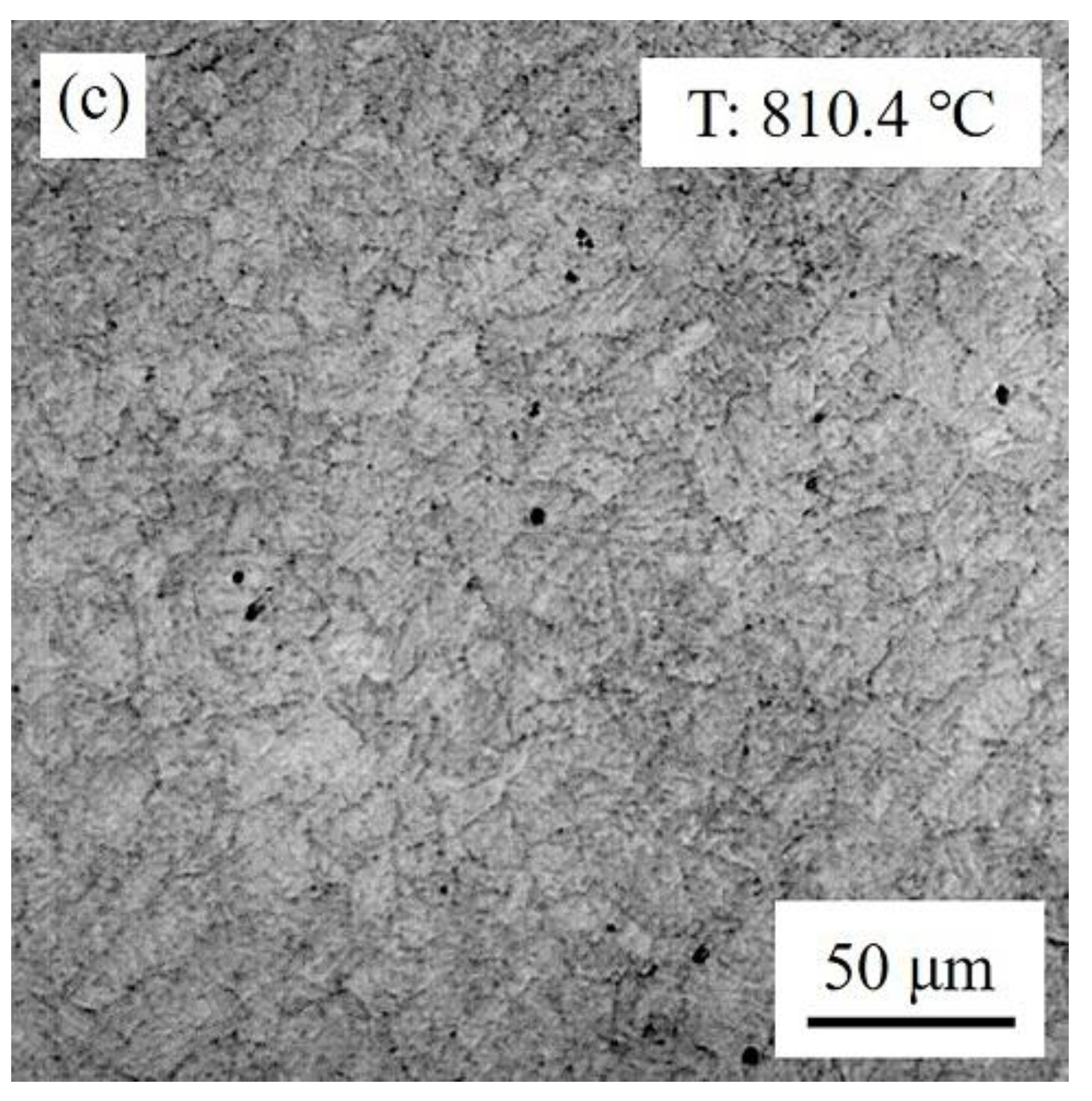
3.1. Nucleation and Growth of Reversed Austenite
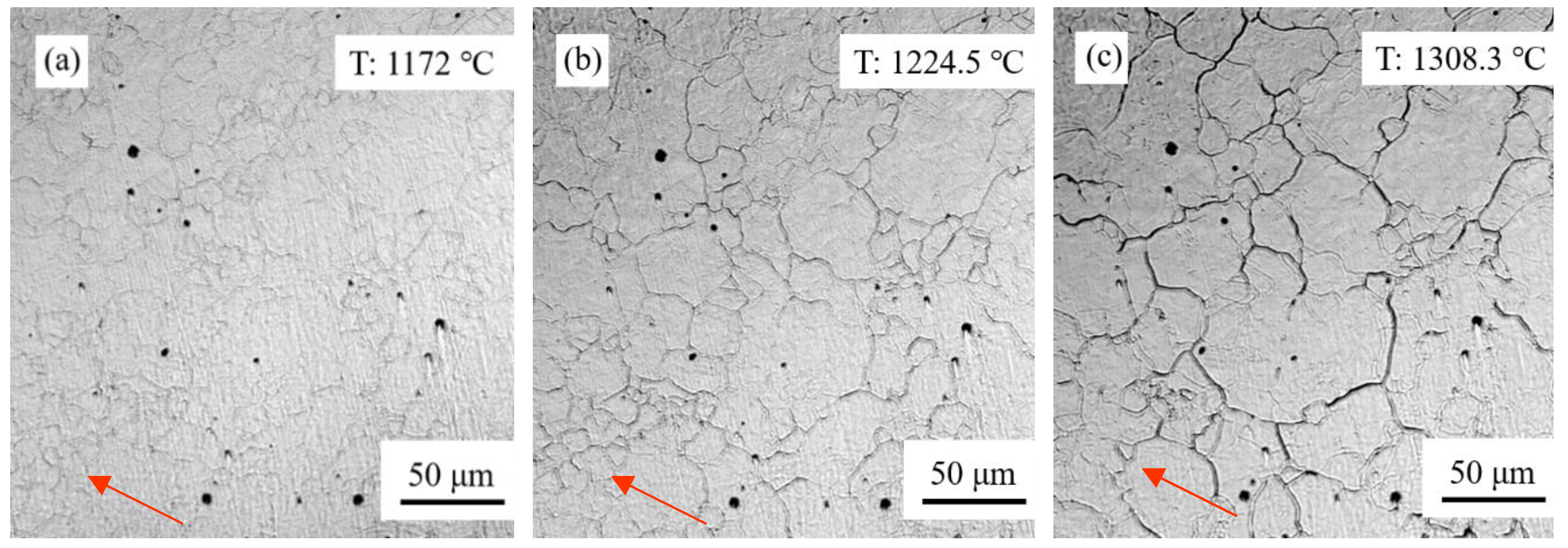
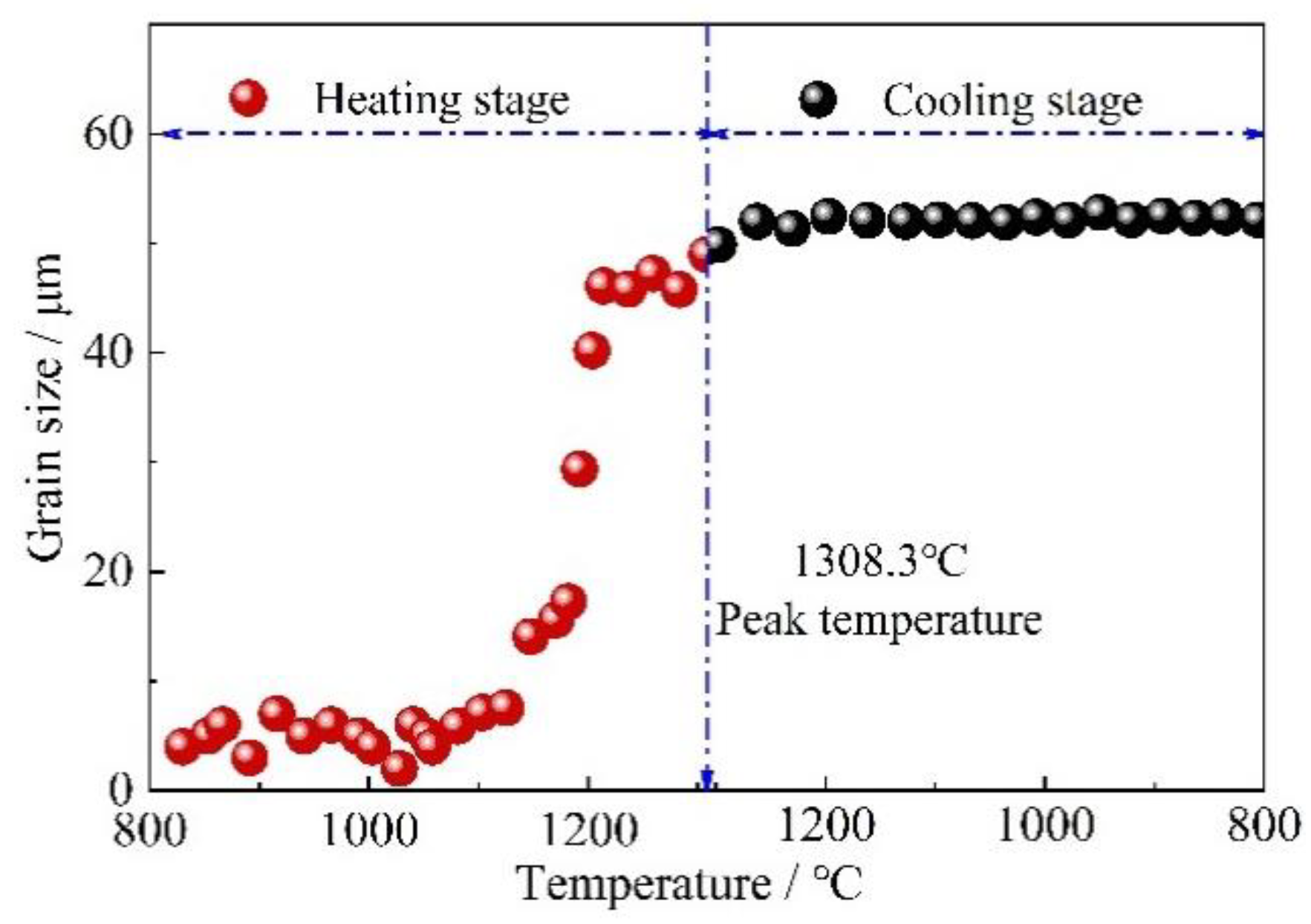
3.2. Effect of Prior Austenite Grain Size on the Continuous Cooling Transformation Temperature and Transformation Rate
3.3. Martensite Transformation and Retained Austenite
3.4. Hardness
3.5. Impact Toughness and the Microstructure Effect
4. Conclusions
- (1)
- The pre-existing retained austenite in the investigated 5Mn steel enhanced the reversed austenite nucleation number during heating of the welding thermal cycle and led to the relatively small grain size in simulated CGHAZ and FGHAZ.
- (2)
- Austenite growth dominantly took place in the heating process and there was almost no austenite growth in the cooling process above peak temperature during the simulated welding thermal cycle. Therefore, the relatively small size was found in CGHAZ.
- (3)
- The higher transformation rate was measured to be a maximum of 0.98 in the simulated FGHAZ and a maximum of 0.60 in the simulated CGHAZ. Thus, a 10~11% austenite was retained in FGHAZ.
- (4)
- Compared to CGHAZ with the larger grain size, the impact toughness in fine grain FGHAZ was not significantly improved. This was due to the transformation of relatively harder martensite and the TRIP effect during the impact test in simulated FGHAZ. Therefore, how to retain austenite will be the future work of how to improve impact toughness of HAZ.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guthrie, R.I.L.; Jonas, J.J. Properties and selection: Irons, steels and high performance alloys. In ASM Handbook, 10th ed.; ASM International: Materials Park, OH, USA, 1990; Volume 1, pp. 115–116. [Google Scholar]
- Militzer, M.; Hawbolt, E.B.; Meadowcroft, T.R.; Giumelli, A. Austenite grain growth kinetics in Al-killed plain carbon steel. Metall. Mater. Trans. A 1996, 27, 3399–3409. [Google Scholar] [CrossRef]
- Easterling, K. Introduction to the Physical Metallurgy of Welding; Elsevier Science & Technology: Oxford, UK, 1992; pp. 117–125. ISBN 978-0750603942. [Google Scholar]
- Zhang, L.P.; Davis, C.L.; Strangwood, M. Effect of TiN particles and microstructure on fracture toughness in simulated heat affected zones of a structural steel. Metall. Mater. Trans. A 1999, 30A, 2089–2096. [Google Scholar] [CrossRef]
- Eroglu, M.; Aksoy, M. Effect of initial grain size on microstructure and toughness of intercritical heat-affected zone of a low carbon steel. Mater. Sci. Eng. 2000, 286, 289–297. [Google Scholar] [CrossRef]
- Enomoto, M.; Li, S.; Yang, Z.N.; Zhang, C.; Yang, Z.G. Partition and non-partition transition of austenite growth from a ferrite and cementite mixture in hypo- and hypereutectoid Fe-C-Mn alloys. Calphad 2018, 61, 116–125. [Google Scholar] [CrossRef]
- Annan, K.A.; Siyasiya, C.W.; Stumpf, W.E. Austenite grain growth kinetics after isothermal deformation in microalloyed steels with varying Nb concentrations. ISIJ Int. 2018, 58, 333–339. [Google Scholar] [CrossRef]
- Zhang, S.S.; Li, M.Q.; Liu, Y.G.; Luo, J.; Liu, T.Q. The growth behavior of austenite grain in the heating process of 300M steel. Mater. Sci. Eng. A-Struct. 2011, 528, 4967–4972. [Google Scholar] [CrossRef]
- Gladman, T. The Physical Metallurgy of Microalloyed Steels; CRC Press: London, UK, 2002; pp. 236–254. ISBN 978-1902653815. [Google Scholar]
- Manohar, P.A.; Ferry, M.; Chandra, T. Five decades of the Zener equation. ISIJ Int. 1998, 38, 913–924. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H.; Honerycombe, R. Steels Microstructure and Properties, 2nd ed.; Butterworth-Heinemann: Oxford, UK, 2006; p. 176. ISBN 978-0750680844. [Google Scholar]
- Gladman, T.; Pickering, F.B. Grain-coarsening of austenite. Iron Steel Inst. J. 1967, 205, 653–664. [Google Scholar]
- Li, Y.M.; Sun, X.J.; Li, Z.D.; Yong, Q.L.; Wang, X.J.; Zhang, K. Stability of reversed austenite in Mn-Ni steel. Heat Treat. Met. 2016, 41, 111–116. [Google Scholar]
- Himeno, M.; Shibata, K.; Fujita, T. Effects of the Widmanstatten-like Reversed Austenite on Mechanical Properties in Fe-Ni Alloys. ISIJ Int. 1979, 65, 235–244. [Google Scholar]
- Li, J.H.; Wang, H.H.; Luo, Q.; Li, L.; Sun, C.; Misra, R.D.K. Correlation between microstructure and impact toughness of weld heat affected zone in 5 wt.% manganese steel. J. Iron Steel Res. Int. 2018, in press. [Google Scholar]
- Law, N.C.; Edmonds, D.V. The formaiton of austenite in a low-alloy steel. Metall. Mater. Trans. A 1980, 11, 33–46. [Google Scholar] [CrossRef]
- Zhang, W.Y. Welding Metallurgy; China Machine Press: Beijing, China, 2014; p. 183. ISBN 978-7111044123. [Google Scholar]
- Majka, T.F.; Matlock, D.K.; Krauss, G. Development of microstructural banding in low-alloy steel with simulated Mn segregation. Metall. Mater. Trans. A 2002, 33, 1627–1637. [Google Scholar] [CrossRef]
- Senuma, T. Physical Metallurgy of Modern High Strength Steel Sheets. ISIJ Int. 2001, 41, 520–532. [Google Scholar] [CrossRef]
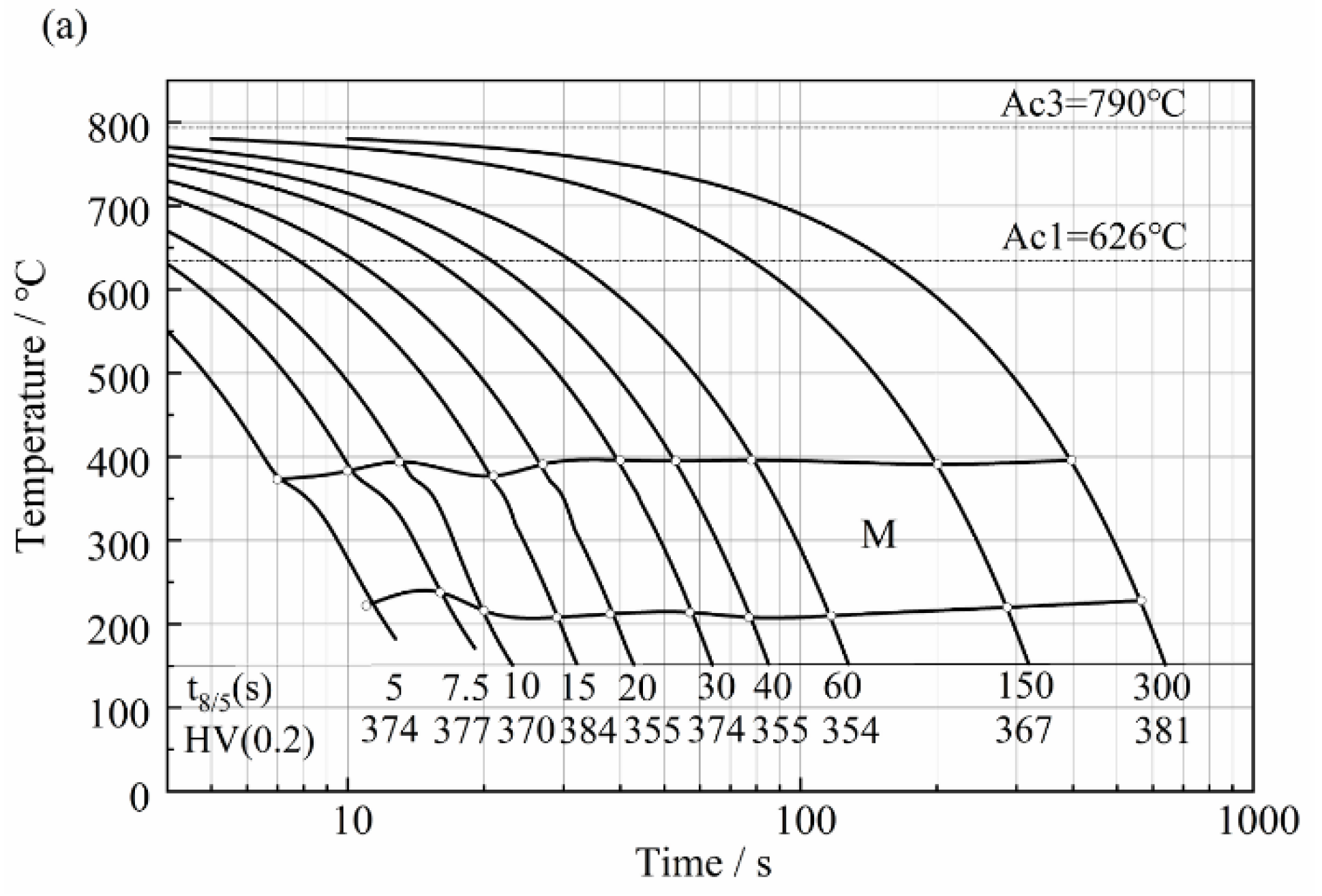

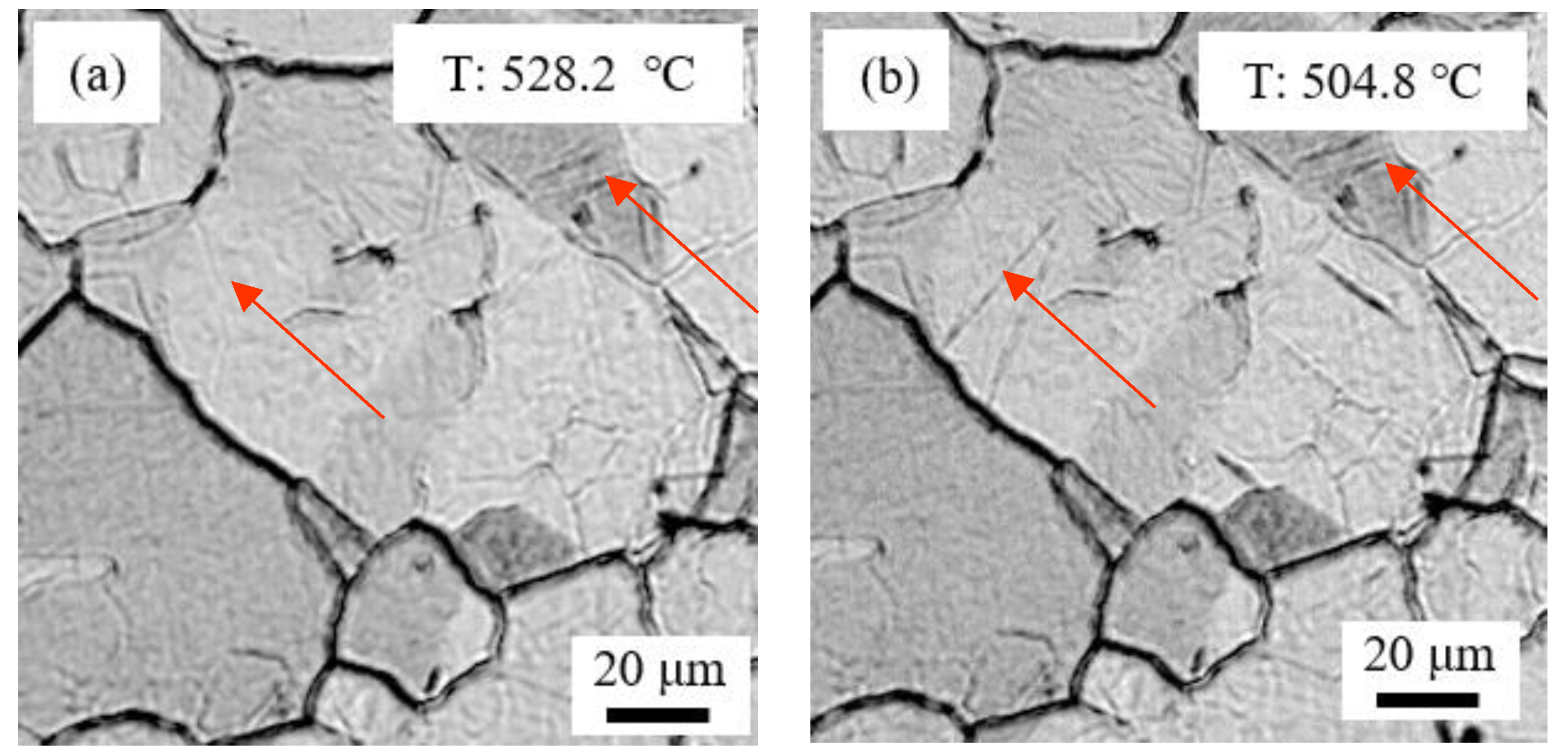



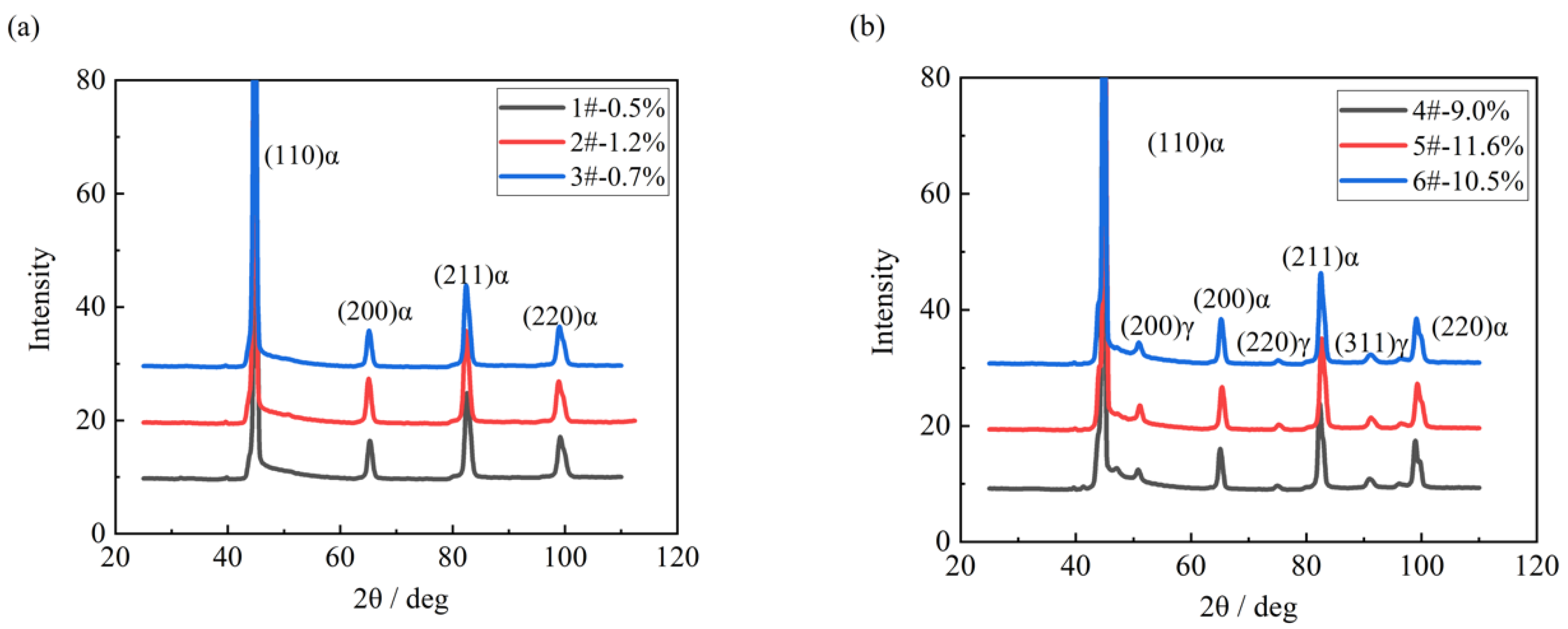

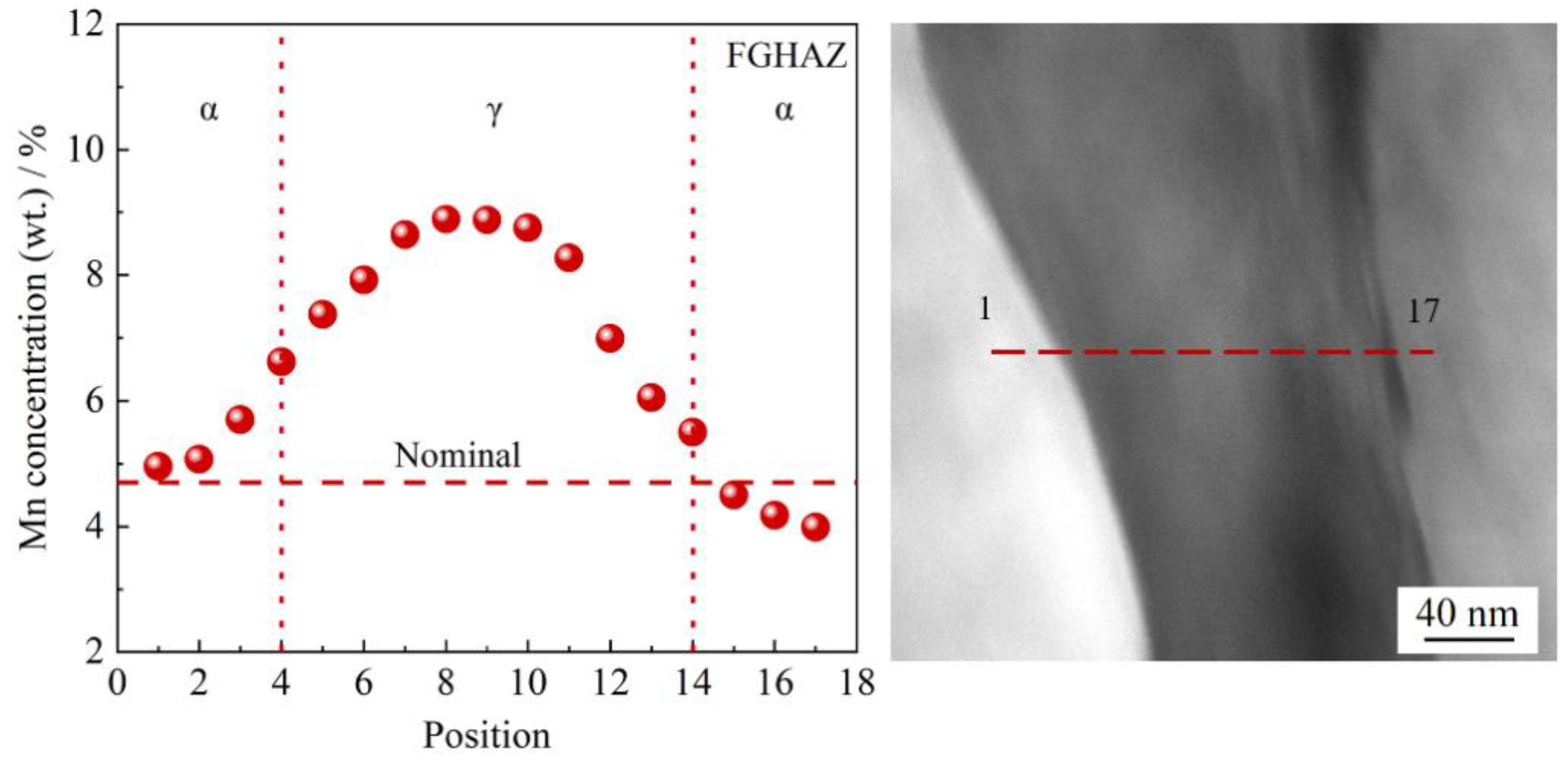

| C | Si | Mn | S | P | Ni | Cr + Cu + Mo | Alt |
|---|---|---|---|---|---|---|---|
| 0.04~0.06 | 0.2~0.25 | 4.9~5.2 | ≤0.0012 | ≤0.009 | 0.27~0.30 | 0.2~0.5 | ≤0.023 |
| Rel/MPa | Rm/MPa | A/% | KV2 (−60 °C)/J |
|---|---|---|---|
| 645~650 | 770~775 | 25~27 | 200~205 |
| t8/5/s | 5 | 7.5 | 20 | 30 | 60 | Peak Temperature |
|---|---|---|---|---|---|---|
| Start transformation temperature/°C | 371 | 380 | 383 | 388 | 394 | Tp = 1320 °C |
| 397 | 403 | 414 | 405 | 423 | Tp = 850 °C | |
| Finish transformation temperature/°C | 220 | 235 | 212 | 213 | 200 | Tp = 1320 °C |
| 251 | 214 | 256 | 261 | 258 | Tp = 850 °C | |
| Transformation temperature range/°C | 151 | 145 | 171 | 175 | 194 | Tp = 1320 °C |
| 146 | 189 | 158 | 144 | 165 | Tp = 850 °C |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Wang, H.; Cai, H.; Li, J.; Chen, Y. Role of Reversed Austenite Behavior in Determining Microstructure and Toughness of Advanced Medium Mn Steel by Welding Thermal Cycle. Materials 2018, 11, 2127. https://doi.org/10.3390/ma11112127
Chen Y, Wang H, Cai H, Li J, Chen Y. Role of Reversed Austenite Behavior in Determining Microstructure and Toughness of Advanced Medium Mn Steel by Welding Thermal Cycle. Materials. 2018; 11(11):2127. https://doi.org/10.3390/ma11112127
Chicago/Turabian StyleChen, Yunxia, Honghong Wang, Huan Cai, Junhui Li, and Yongqing Chen. 2018. "Role of Reversed Austenite Behavior in Determining Microstructure and Toughness of Advanced Medium Mn Steel by Welding Thermal Cycle" Materials 11, no. 11: 2127. https://doi.org/10.3390/ma11112127
APA StyleChen, Y., Wang, H., Cai, H., Li, J., & Chen, Y. (2018). Role of Reversed Austenite Behavior in Determining Microstructure and Toughness of Advanced Medium Mn Steel by Welding Thermal Cycle. Materials, 11(11), 2127. https://doi.org/10.3390/ma11112127




