Materials and Devices for Biodegradable and Soft Biomedical Electronics
Abstract
:1. Introduction
2. Materials
2.1. Inorganic Functional Materials
2.2. Organic Functional Materials
2.3. Substrate Materials
2.4. Encapsulation Materials
3. Fabrication Schemes
4. Representative Soft and Biodegradable Devices for Biomedical Applications
4.1. Diagnostic Platforms
4.2. Therapeutic Devices
4.3. Power Supply
5. Summary and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dubal, D.P.; Chodankar, N.R.; Kim, D.H.; Gomez-Romero, P. Towards flexible solid-state supercapacitors for smart and wearable electronics. Chem. Soc. Rev. 2018, 47, 2065–2129. [Google Scholar] [CrossRef] [PubMed]
- Annapureddy, V.; Na, S.M.; Hwang, G.T.; Kang, M.G.; Sriramdas, R.; Palneedi, H.; Yoon, W.H.; Hahn, B.D.; Kim, J.W.; Ahn, C.W.; et al. Exceeding milli-watt powering magneto-mechano-electric generator for standalone-powered electronics. Energy Environ. Sci. 2018, 11, 818–829. [Google Scholar] [CrossRef]
- Salauddin, M.; Toyabur, R.M.; Maharjan, P.; Park, J.Y. High performance human-induced vibration driven hybrid energy harvester for powering portable electronics. Nano Energy 2018, 45, 236–246. [Google Scholar] [CrossRef]
- Wang, S.H.; Xu, J.; Wang, W.C.; Wang, G.J.N.; Rastak, R.; Molina-Lopez, F.; Chung, J.W.; Niu, S.M.; Feig, V.R.; Lopez, J.; et al. Skin electronics from scalable fabrication of an intrinsically stretchable transistor array. Nature 2018, 555, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Falco, A.; Rivadeneyra, A.; Loghin, F.C.; Salmeron, J.F.; Lugli, P.; Abdelhalim, A. Towards low-power electronics: Self-recovering and flexible gas sensors. J. Mater. Chem. A 2018, 6, 7107–7113. [Google Scholar] [CrossRef]
- Li, R.F.; Wang, L.; Kong, D.Y.; Yin, L. Recent progress on biodegradable materials and transient electronics. Bioact. Mater. 2018, 3, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, Y.; Wang, X.; Sim, K.; Liu, J.S.; Chen, J.; Feng, X.; Xu, H.X.; Yu, C.J. Moisture-triggered physically transient electronics. Sci. Adv. 2017, 3, e1701222. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Lee, J.; Choi, B.; Lee, D.; Kim, D.H.; Kim, D.M.; Moon, D.I.; Lim, M.; Kim, S.; Choi, S.J. Flammable carbon nanotube transistors on a nitrocellulose paper substrate for transient electronics. Nano Res. 2017, 10, 87–96. [Google Scholar] [CrossRef]
- Lee, G.; Kang, S.K.; Won, S.M.; Gutruf, P.; Jeong, Y.R.; Koo, J.; Lee, S.S.; Rogers, J.A.; Ha, J.S. Fully Biodegradable Microsupercapacitor for Power Storage in Transient Electronics. Adv. Energy Mater. 2017, 7, 1700157. [Google Scholar] [CrossRef]
- Kang, S.-K.; Hwang, S.-W.; Cheng, H.; Yu, S.; Kim, B.H.; Kim, J.-H.; Huang, Y.; Rogers, J.A. Dissolution Behaviors and Applications of Silicon Oxides and Nitrides in Transient Electronics. Adv. Funct. Mater. 2014, 24, 4427–4434. [Google Scholar] [CrossRef]
- Khanra, S.; Cipriano, T.; Lam, T.; White, T.A.; Fileti, E.E.; Alves, W.A.; Guha, S. Self-Assembled Peptide-Polyfluorene Nanocomposites for Biodegradable Organic Electronics. Adv. Mater. Interfaces 2015, 2, 1500265. [Google Scholar] [CrossRef]
- Feig, V.R.; Tran, H.; Bao, Z.N. Biodegradable Polymeric Materials in Degradable Electronic Devices. ACS Cent. Sci. 2018, 4, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.K.; Koo, J.; Lee, Y.K.; Rogers, J.A. Advanced Materials and Devices for Bioresorbable Electronics. Acc. Chem. Res. 2018, 51, 988–998. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.W.; Tao, H.; Kim, D.H.; Cheng, H.Y.; Song, J.K.; Rill, E.; Brenckle, M.A.; Panilaitis, B.; Won, S.M.; Kim, Y.S.; et al. A Physically Transient Form of Silicon Electronics. Science 2012, 337, 1640–1644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, S.K.; Murphy, R.K.J.; Hwang, S.W.; Lee, S.M.; Harburg, D.V.; Krueger, N.A.; Shin, J.H.; Gamble, P.; Cheng, H.Y.; Yu, S.; et al. Bioresorbable silicon electronic sensors for the brain. Nature 2016, 530, 71. [Google Scholar] [CrossRef] [PubMed]
- Irimia-Vladu, M.; Glowacki, E.D.; Voss, G.; Bauer, S.; Sariciftci, N.S. Green and biodegradable electronics. Mater. Today 2012, 15, 340–346. [Google Scholar] [CrossRef]
- Park, C.W.; Kang, S.K.; Hernandez, H.L.; Kaitz, J.A.; Wie, D.S.; Shin, J.; Lee, O.P.; Sottos, N.R.; Moore, J.S.; Rogers, J.A.; et al. Thermally Triggered Degradation of Transient Electronic Devices. Adv. Mater. 2015, 27, 3783–3788. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, H.L.; Kang, S.K.; Lee, O.P.; Hwang, S.W.; Kaitz, J.A.; Inci, B.; Park, C.W.; Chung, S.J.; Sottos, N.R.; Moore, J.S.; et al. Triggered Transience of Metastable Poly(phthalaldehyde) for Transient Electronics. Adv. Mater. 2014, 26, 7637–7642. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Lu, N.S.; Ma, R.; Kim, Y.S.; Kim, R.H.; Wang, S.D.; Wu, J.; Won, S.M.; Tao, H.; Islam, A.; et al. Epidermal Electronics. Science 2011, 333, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.W.; Kim, D.H.; Tao, H.; Kim, T.I.; Kim, S.; Yu, K.J.; Panilaitis, B.; Jeong, J.W.; Song, J.K.; Omenetto, F.G.; et al. Materials and Fabrication Processes for Transient and Bioresorbable High-Performance Electronics. Adv. Funct. Mater. 2013, 23, 4087–4093. [Google Scholar] [CrossRef]
- Chang, J.K.; Chang, H.P.; Guo, Q.; Koo, J.; Wu, C.I.; Rogers, J.A. Biodegradable Electronic Systems in 3D, Heterogeneously Integrated Formats. Adv. Mater. 2018, 30, 1704955. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.-W.; Park, G.; Edwards, C.; Corbin, E.A.; Kang, S.-K.; Cheng, H.; Song, J.-K.; Kim, J.-H.; Yu, S.; Ng, J.; et al. Dissolution Chemistry and Biocompatibility of Single-Crystalline Silicon Nanomembranes and Associated Materials for Transient Electronics. ACS Nano 2014, 8, 5843–5851. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.W.; Park, G.; Cheng, H.; Song, J.K.; Kang, S.K.; Yin, L.; Kim, J.H.; Omenetto, F.G.; Huang, Y.G.; Lee, K.M.; et al. 25th Anniversary Article: Materials for High-Performance Biodegradable Semiconductor Devices. Adv. Mater. 2014, 26, 1992–2000. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Farimani, A.B.; Min, K.; Vishal, N.; Lam, J.; Lee, Y.K.; Aluru, N.R.; Rogers, J.A. Mechanisms for hydrolysis of silicon nanomembranes as used in bioresorbable electronics. Adv. Mater. 2015, 27, 1857–1864. [Google Scholar] [CrossRef] [PubMed]
- Dagdeviren, C.; Hwang, S.W.; Su, Y.W.; Kim, S.; Cheng, H.Y.; Gur, O.; Haney, R.; Omenetto, F.G.; Huang, Y.G.; Rogers, J.A. Transient, Biocompatible Electronics and Energy Harvesters Based on ZnO. Small 2013, 9, 3398–3404. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.K.; Park, G.; Kim, K.; Hwang, S.W.; Cheng, H.Y.; Shin, J.H.; Chung, S.J.; Kim, M.; Yin, L.; Lee, J.C.; et al. Dissolution Chemistry and Biocompatibility of Silicon- and Germanium-Based Semiconductors for Transient Electronics. ACS Appl. Mater. Interfaces 2015, 7, 9297–9305. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.H.; Kang, S.K.; Cho, I.T.; Han, S.Y.; Chung, H.U.; Lee, D.J.; Shin, J.; Baek, G.W.; Kim, T.I.; Lee, J.H.; et al. Water-Soluble Thin Film Transistors and Circuits Based on Amorphous Indium-Gallium-Zinc Oxide. ACS Appl. Mater. Interfaces 2015, 7, 8268–8274. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liu, Y.H.; Hwang, S.W.; Kang, S.K.; Patnaik, D.; Cortes, J.F.; Rogers, J.A. Biodegradable Materials for Multilayer Transient Printed Circuit Boards. Adv. Mater. 2014, 26, 7371–7377. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Cheng, H.; Mao, S.; Haasch, R.; Liu, Y.; Xie, X.; Hwang, S.-W.; Jain, H.; Kang, S.-K.; Su, Y.; et al. Dissolvable Metals for Transient Electronics. Adv. Funct. Mater. 2014, 24, 645–658. [Google Scholar] [CrossRef]
- Yu, K.J.; Kuzum, D.; Hwang, S.W.; Kim, B.H.; Juul, H.; Kim, N.H.; Won, S.M.; Chiang, K.; Trumpis, M.; Richardson, A.G.; et al. Bioresorbable silicon electronics for transient spatiotemporal mapping of electrical activity from the cerebral cortex. Nat. Mater. 2016, 15, 782. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US) Panel on Micronutrients. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; A Report of the Panel on Micronutrients, Subcommittees on Upper Reference Levels of Nutrients and of Interpretation and Uses of Dietary Reference Intakes, and the Standing Committee on the Scientific Evaluation of Dietary Reference Intakes; National Academy Press: Washington, DC, USA, 2001. [Google Scholar]
- Ysart, G.; Miller, P.; Crews, H.; Robb, P.; Baxter, M.; De L’Argy, C.; Lofthouse, S.; Sargent, C.; Harrison, N. Dietary exposure estimates of 30 elements from the UK Total Diet Study. Food Addit. Contam. 1999, 16, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Millour, S.; Noel, L.; Chekri, R.; Vastel, C.; Kadar, A.; Sirot, V.; Leblanc, J.C.; Guerin, T. Strontium, silver, tin, iron, tellurium, gallium, germanium, barium and vanadium levels in foodstuffs from the Second French Total Diet Study. J. Food Compos. Anal. 2012, 25, 108–129. [Google Scholar] [CrossRef]
- Tao, S.H.; Bolger, P.M. Hazard assessment of germanium supplements. Regul. Toxicol. Pharm. 1997, 25, 211–219. [Google Scholar] [CrossRef] [PubMed]
- James, B.; Zhang, W.L.; Sun, P.; Wu, M.Y.; Li, H.H.; Khaliq, M.A.; Jayasuriya, P.; James, S.; Wang, G. Tungsten (W) bioavailability in paddy rice soils and its accumulation in rice (Oryza sativa). Int. J. Environ. Health Res. 2017, 27, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride; National Academy Press: Washington, DC, USA, 1997. [Google Scholar]
- Rogers, J.A.; Lagally, M.G.; Nuzzo, R.G. Synthesis, assembly and applications of semiconductor nanomembranes. Nature 2011, 477, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, S.; Ducati, C.; Neill, R.J.; Piscanec, S.; Ferrari, A.C.; Geng, J.; Dunin-Borkowski, R.E.; Robertson, J. Gold catalyzed growth of silicon nanowires by plasma enhanced chemical vapor deposition. J. Appl. Phys. 2003, 94, 6005–6012. [Google Scholar] [CrossRef]
- Guo, X.L.; Tabata, H.; Kawai, T. Pulsed laser reactive deposition of p-type ZnO film enhanced by an electron cyclotron resonance source. J. Cryst. Growth 2001, 223, 135–139. [Google Scholar] [CrossRef] [Green Version]
- Schropp, R.E.I.; Feenstra, K.E.; Molenbroek, E.C.; Meiling, H.; Rath, J.K. Device-quality polycrystalline and amorphous silicon films by hot-wire chemical vapour deposition. Philos. Mag. B 1997, 76, 309–321. [Google Scholar] [CrossRef]
- Lee, W.J.; Park, W.T.; Park, S.; Sung, S.; Noh, Y.Y.; Yoon, M.H. Large-Scale Precise Printing of Ultrathin Sol-Gel Oxide Dielectrics for Directly Patterned Solution-Processed Metal Oxide Transistor Arrays. Adv. Mater. 2015, 27, 5043–5048. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Ghaffari, R.; Lu, N.S.; Rogers, J.A. Flexible and Stretchable Electronics for Biointegrated Devices. Annu. Rev. Biomed. Eng. 2012, 14, 113–128. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.H.; Kim, H.S.; Lee, K.J.; Jeon, S.; Kang, S.J.; Sun, Y.G.; Nuzzo, R.G.; Rogers, J.A. Heterogeneous three-dimensional electronics by use of printed semiconductor nanomaterials. Science 2006, 314, 1754–1757. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Yu, K.J.; Song, E.M.; Farimani, A.B.; Vitale, F.; Xie, Z.Q.; Yoon, Y.; Kim, Y.; Richardson, A.; Luan, H.W.; et al. Dissolution of Monocrystalline Silicon Nanomembranes and Their Use as Encapsulation Layers and Electrical Interfaces in Water-Soluble Electronics. ACS Nano 2017, 11, 12562–12572. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Park, Y.J.; Kang, M.; Kang, S.K.; Koo, J.; Shinde, S.M.; Shin, J.; Jeon, S.; Park, G.; Yan, Y.; et al. CVD-grown monolayer MoS2 in bioabsorbable electronics and biosensors. Nat. Commun. 2018, 9, 1690. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, B.K.; Yu, X.W.; Shou, W.; Pan, H.; Huang, X. Mechanically Milled Irregular Zinc Nanoparticles for Printable Bioresorbable Electronics. Small 2017, 13, 1700065. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-K.; Hwang, S.-W.; Yu, S.; Seo, J.-H.; Corbin, E.A.; Shin, J.; Wie, D.S.; Bashir, R.; Ma, Z.; Rogers, J.A. Biodegradable Thin Metal Foils and Spin-On Glass Materials for Transient Electronics. Adv. Funct. Mater. 2015, 25, 1789–1797. [Google Scholar] [CrossRef]
- Kirkland, N.T.; Birbilis, N.; Staiger, M.P. Assessing the corrosion of biodegradable magnesium implants: A critical review of current methodologies and their limitations. Acta Biomater. 2012, 8, 925–936. [Google Scholar] [CrossRef] [PubMed]
- Bowen, P.K.; Drelich, J.; Goldman, J. Zinc Exhibits Ideal Physiological Corrosion Behavior for Bioabsorbable Stents. Adv. Mater. 2013, 25, 2577–2582. [Google Scholar] [CrossRef] [PubMed]
- Badawy, W.A.; Al-Kharafi, F.M. Corrosion and passivation behaviors of molybdenum in aqueous solutions of different pH. Electrochim. Acta 1998, 44, 693–702. [Google Scholar] [CrossRef]
- Patrick, E.; Orazem, M.E.; Sanchez, J.C.; Nishida, T. Corrosion of tungsten microelectrodes used in neural recording applications. J. Neurosci. Meth. 2011, 198, 158–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hemstreet, J.M. Dielectric constant of cotton. J. Electrost. 1982, 13, 345–353. [Google Scholar] [CrossRef]
- Jayamani, E.; Hamdan, S.; Rahman, M.R.; Bin Bakri, M.K. Comparative Study of Dielectric Properties of Hybrid Natural Fiber Composites. Procedia Eng. 2014, 97, 536–544. [Google Scholar] [CrossRef]
- Irimia-Vladu, M.; Troshin, P.A.; Reisinger, M.; Shmygleva, L.; Kanbur, Y.; Schwabegger, G.; Bodea, M.; Schwodiauer, R.; Mumyatov, A.; Fergus, J.W.; et al. Biocompatible and Biodegradable Materials for Organic Field-Effect Transistors. Adv. Funct. Mater. 2010, 20, 4069–4076. [Google Scholar] [CrossRef]
- Singh, T.B.; Sariciftci, N.S.; Grote, J.G. Bio-Organic Optoelectronic Devices Using DNA. Org. Electron. 2010, 223, 189–212. [Google Scholar]
- Yumusak, C.; Singh, T.B.; Sariciftci, N.S.; Grote, J.G. Bioorganic field effect transistors based on crosslinked deoxyribonucleic acid (DNA) gate dielectric. Appl. Phys. Lett. 2009, 95, 263304. [Google Scholar] [CrossRef]
- Singh, B.; Sariciftci, N.S.; Grote, J.G.; Hopkins, F.K. Bioorganic-semiconductor-field-effect-transistor based on deoxyribonucleic acid gate dielectric. J. Appl. Phys. 2006, 100, 024514. [Google Scholar] [CrossRef]
- Wang, L.; Yoshida, J.; Ogata, N.; Sasaki, S.; Kajiyama, T. Self-Assembled Supramolecular Films Derived from Marine Deoxyribonucleic Acid (DNA)−Cationic Surfactant Complexes: Large-Scale Preparation and Optical and Thermal Properties. Chem. Mater. 2001, 13, 1273–1281. [Google Scholar] [CrossRef]
- Worfolk, B.J.; Andrews, S.C.; Park, S.; Reinspach, J.; Liu, N.; Toney, M.F.; Mannsfeld, S.C.; Bao, Z.N. Ultrahigh electrical conductivity in solutionsheared polymeric transparent films. Proc. Natl. Acad. Sci. USA 2015, 112, 14138–14143. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.L.; Guo, Y.L.; Yu, C.M.; Zhang, F.J.; Xu, W.; Liu, Y.Q.; Zhu, D.B. Diketopyrrolopyrrole-Containing Quinoidal Small Molecules for High-Performance, Air-Stable, and Solution-Processable n-Channel Organic Field-Effect Transistors. J. Am. Chem. Soc. 2012, 134, 4084–4087. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.J.; Guo, Y.L.; Yu, G.; Zhao, Y.; Zhang, J.; Gao, D.; Liu, H.T.; Liu, Y.Q. Highly p-Extended Copolymers with Diketopyrrolopyrrole Moieties for High-Performance Field-Effect Transistors. Adv. Mater. 2012, 24, 4618–4622. [Google Scholar] [CrossRef] [PubMed]
- Madrigal, M.M.P.; Giannotti, M.I.; Oncins, G.; Franco, L.; Armelin, E.; Puiggali, J.; Sanz, F.; del Valle, L.J.; Aleman, C. Bioactive nanomembranes of semiconductor polythiophene and thermoplastic polyurethane: Thermal, nanostructural and nanomechanical properties. Polym. Chem. 2013, 4, 568–583. [Google Scholar] [CrossRef]
- Lei, T.; Guan, M.; Liu, J.; Lin, H.C.; Pfattner, R.; Shaw, L.; McGuire, A.F.; Huang, T.C.; Shao, L.L.; Cheng, K.T.; et al. Biocompatible and totally disintegrable semiconducting polymer for ultrathin and ultralightweight transient electronics. Proc. Natl. Acad. Sci. USA 2017, 114, 5107–5112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irimia-Vladu, M.; Glowacki, E.D.; Troshin, P.A.; Schwabegger, G.; Leonat, L.; Susarova, D.K.; Krystal, O.; Ullah, M.; Kanbur, Y.; Bodea, M.A.; et al. Indigo—A Natural Pigment for High Performance Ambipolar Organic Field Effect Transistors and Circuits. Adv. Mater. 2012, 24, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Mostert, A.B.; Powell, B.J.; Pratt, F.L.; Hanson, G.R.; Sarna, T.; Gentle, I.R.; Meredith, P. Role of semiconductivity and ion transport in the electrical conduction of melanin. Proc. Natl. Acad. Sci. USA 2012, 109, 8943–8947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bettinger, C.J.; Bruggeman, P.P.; Misra, A.; Borenstein, J.T.; Langer, R. Biocompatibility of biodegradable semiconducting melanin films for nerve tissue engineering. Biomaterials 2009, 30, 3050–3057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramachandran, G.K.; Tomfohr, J.K.; Li, J.; Sankey, O.F.; Zarate, X.; Primak, A.; Terazono, Y.; Moore, T.A.; Moore, A.L.; Gust, D.; et al. Electron transport properties of a carotene molecule in a metal-(single molecule)-metal junction. J. Phys. Chem. B 2003, 107, 6162–6169. [Google Scholar] [CrossRef]
- Koh, L.D.; Yeo, J.; Lee, Y.Y.; Ong, Q.; Han, M.Y.; Tee, B.C.K. Advancing the frontiers of silk fibroin protein-based materials for futuristic electronics and clinical wound-healing. Mater. Sci. Eng. C Mater. 2018, 86, 151–172. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Li, Y.; Chen, G.Q.; He, J.H.; Han, Y.F.; Wang, X.Q.; Kaplan, D.L. Silk-Based Biomaterials in Biomedical Textiles and Fiber-Based Implants. Adv. Healthc. Mater. 2015, 4, 1134–1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, M.B.; Li, Y.; Li, J.S.; Chen, A.Z.; Zhao, Z.; Li, G. Biomedical Applications of Silk Fibroin. In Textile Bioengineering and Informatics Symposium Proceedings; Textile Bioengineering & Informatics Society Ltd.: Hong Kong, China, 2014; Volumes 1 and 2, pp. 207–218. [Google Scholar]
- Taddei, P.; Chiono, V.; Anghileri, A.; Vozzi, G.; Freddi, G.; Ciardelli, G. Silk Fibroin/Gelatin Blend Films Crosslinked with Enzymes for Biomedical Applications. Macromol. Biosci. 2013, 13, 1492–1510. [Google Scholar] [CrossRef] [PubMed]
- Pal, R.K.; Farghaly, A.A.; Wang, C.Z.; Collinson, M.M.; Kundu, S.C.; Yadavalli, V.K. Conducting polymer-silk biocomposites for flexible and biodegradable electrochemical sensors. Biosens. Bioelectron. 2016, 81, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Edupuganti, V.; Solanki, R. Fabrication, characterization, and modeling of a biodegradable battery for transient electronics. J. Power Sources 2016, 336, 447–454. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, J. Biodegradable and biobased polymers. In Applied Plastics Engineering Handbook, 2nd ed.; William Andrew Publishing: Oxford, UK, 2017; pp. 127–143. [Google Scholar]
- Zhu, H.L.; Fang, Z.Q.; Preston, C.; Li, Y.Y.; Hu, L.B. Transparent paper: Fabrications, properties, and device applications. Energy Environ. Sci. 2014, 7, 269–287. [Google Scholar] [CrossRef]
- Zhu, H.L.; Xiao, Z.G.; Liu, D.T.; Li, Y.Y.; Weadock, N.J.; Fang, Z.Q.; Huang, J.S.; Hu, L.B. Biodegradable transparent substrates for flexible organic-light-emitting diodes. Energy Environ. Sci. 2013, 6, 2105–2111. [Google Scholar] [CrossRef]
- Jung, Y.H.; Chang, T.H.; Zhang, H.L.; Yao, C.H.; Zheng, Q.F.; Yang, V.W.; Mi, H.Y.; Kim, M.; Cho, S.J.; Park, D.W.; et al. High-performance green flexible electronics based on biodegradable cellulose nanofibril paper. Nat. Commun. 2015, 6, 7170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.Q.; Wang, H.C.; Lv, S.Z.; Liu, L.; Guo, W.Y.; Yuan, M.; Yan, H.B.; Zhao, H.J.; Lang, S.P. Clinical Comparative Study on Efficacy and Safety for Treatment of Coronary Heart Disease with Cobalt-Base Alloy Bio Absorbable Polymer Sirolimus-Eluting Stent and Partner Stent. Heart 2011, 97, A149. [Google Scholar] [CrossRef]
- Wu, Y.T.; Gao, Y.C. Five Years Follow Up Result after Application of Biodegradable Polymer Sirolimus-Eluting Stent in Patients with Coronary Heart Disease and Diabetes Mellitus. Heart 2013, 99, E175. [Google Scholar]
- Inigo-Garcia, L.A.; Martinez-Garcia, F.J.; Milan-Pinilla, A.; Valle-Alberca, A.; Fernandez-Lopez, L.; Traverso-Castilla, V.V.; Delgado-Aguilar, A.; Bravo-Marques, R.; Ramirez-Moreno, A.; Siles-Rubio, J.R. Biodegradable Polymer Drug Eluting Stent: Efficacy and Safety with Short Regimen of Antiplatelet Therapy. Cardiology 2016, 134, 48. [Google Scholar]
- Pilgrim, T.; Heg, D.; Roffi, M.; Tuller, D.; Muller, O.; Vuilliomenet, A.; Cook, S.; Weilenmann, D.; Kaiser, C.; Jamshidi, P.; et al. Ultrathin strut biodegradable polymer sirolimus-eluting stent versus durable polymer everolimus-eluting stent for percutaneous coronary revascularisation (BIOSCIENCE): A randomised, single-blind, non-inferiority trial. Lancet 2014, 384, 2111–2122. [Google Scholar] [CrossRef]
- Waksman, R.; Pakala, R.; Baffour, R.; Seabron, R.; Hellinga, D.; Chan, R.; Su, S.H.; Kolodgie, F.; Virmani, R. In vivo comparison of a polymer-free Biolimus A9-eluting stent with a biodegradable polymer-based Biolimus A9 eluting stent and a bare metal stent in balloon denuded and radiated hypercholesterolemic rabbit iliac arteries. Catheter. Cardiovasc. Interv. 2012, 80, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Balch, O.K.; Collier, M.A.; DeBault, L.E.; Johnson, L.L. Bioabsorbable suture anchor (co-polymer 85/15 D,L lactide/glycolide) implanted in bone: Correlation of physical/mechanical properties, magnetic resonance imaging, and histological response. Arthroscopy 1999, 15, 691–708. [Google Scholar] [CrossRef]
- Im, S.H.; Jung, Y.; Kim, S.H. Current status and future direction of biodegradable metallic and polymeric vascular scaffolds for next-generation stents. Acta Biomater. 2017, 60, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.W.; Song, J.K.; Huang, X.; Cheng, H.Y.; Kang, S.K.; Kim, B.H.; Kim, J.H.; Yu, S.; Huang, Y.G.; Rogers, J.A. High-Performance Biodegradable/Transient Electronics on Biodegradable Polymers. Adv. Mater. 2014, 26, 3905–3911. [Google Scholar] [CrossRef] [PubMed]
- Shou, W.; Mahajan, B.K.; Ludwig, B.; Yu, X.W.; Staggs, J.; Huang, X.; Pan, H. Low-Cost Manufacturing of Bioresorbable Conductors by Evaporation-Condensation-Mediated Laser Printing and Sintering of Zn Nanoparticles. Adv. Mater. 2017, 29, 1700172. [Google Scholar] [CrossRef] [PubMed]
- Najafabadi, A.H.; Tamayol, A.; Annabi, N.; Ochoa, M.; Mostafalu, P.; Akbari, M.; Nikkhah, M.; Rahimi, R.; Dokmeci, M.R.; Sonkusale, S.; et al. Biodegradable Nanofibrous Polymeric Substrates for Generating Elastic and Flexible Electronics. Adv. Mater. 2014, 26, 5823–5830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boutry, C.M.; Kaizawa, Y.; Schroeder, B.C.; Chortos, A.; Legrand, A.; Wang, Z.; Chang, J.; Fox, P.; Bao, Z. A stretchable and biodegradable strain and pressure sensor for orthopaedic application. Nat. Electron. 2018, 1, 314–321. [Google Scholar] [CrossRef]
- Lee, Y.K.; Kim, J.; Kim, Y.; Kwak, J.W.; Yoon, Y.; Rogers, J.A. Room Temperature Electrochemical Sintering of Zn Microparticles and Its Use in Printable Conducting Inks for Bioresorbable Electronics. Adv. Mater. 2017, 29, 1702665. [Google Scholar] [CrossRef] [PubMed]
- Brenckle, M.A.; Cheng, H.Y.; Hwang, S.; Tao, H.; Paquette, M.; Kaplan, D.L.; Rogers, J.A.; Huang, Y.G.; Omenetto, F.G. Modulated Degradation of Transient Electronic Devices through Multilayer Silk Fibroin Pockets. ACS Appl. Mater. Interfaces 2015, 7, 19870–19875. [Google Scholar] [CrossRef] [PubMed]
- Pan, R.Z.; Xuan, W.P.; Chen, J.K.; Dong, S.R.; Jin, H.; Wang, X.Z.; Li, H.L.; Luo, J.K. Fully biodegradable triboelectric nanogenerators based on electrospun polylactic acid and nanostructured gelatin films. Nano Energy 2018, 45, 193–202. [Google Scholar] [CrossRef]
- Lee, S.; Koo, J.; Kang, S.K.; Park, G.; Lee, Y.J.; Chen, Y.Y.; Lim, S.A.; Lee, K.M.; Rogers, J.A. Metal microparticle—Polymer composites as printable, bio/ecoresorbable conductive inks. Mater. Today 2018, 21, 207–215. [Google Scholar] [CrossRef]
- Lee, C.H.; Kim, H.; Harburg, D.V.; Park, G.; Ma, Y.J.; Pan, T.S.; Kim, J.S.; Lee, N.Y.; Kim, B.H.; Jang, K.I.; et al. Biological lipid membranes for on-demand, wireless drug delivery from thin, bioresorbable electronic implants. Npg Asia Mater. 2015, 7, e227. [Google Scholar] [CrossRef] [PubMed]
- White, M.D.; Bosio, C.M.; Duplantis, B.N.; Nano, F.E. Human body temperature and new approaches to constructing temperature-sensitive bacterial vaccines. Cell. Mol. Life Sci. 2011, 68, 3019–3031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duplantis, B.N.; Bosio, C.M.; Nano, F.E. Temperature-sensitive bacterial pathogens generated by the substitution of essential genes from cold-loving bacteria: Potential use as live vaccines. J. Mol. Med. 2011, 89, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Hooke, A.M. Temperature-Sensitive Mutants of Bacterial Pathogens—Isolation and Use to Determine Host Clearance and In-Vivo Replication Rates. Method Enzymol. 1994, 235, 448–457. [Google Scholar]
- Tao, H.; Hwang, S.W.; Marelli, B.; An, B.; Moreau, J.E.; Yang, M.M.; Brenckle, M.A.; Kim, S.; Kaplan, D.L.; Rogers, J.A.; et al. Silk-based resorbable electronic devices for remotely controlled therapy and in vivo infection abatement. Proc. Natl. Acad. Sci. USA 2014, 111, 17385–17389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, L.; Huang, X.; Xu, H.X.; Zhang, Y.F.; Lam, J.; Cheng, J.J.; Rogers, J.A. Materials, Designs, and Operational Characteristics for Fully Biodegradable Primary Batteries. Adv. Mater. 2014, 26, 3879–3884. [Google Scholar] [CrossRef] [PubMed]
- Bouhlala, M.A.; Kameche, M.; Tadji, A.; Benouar, A. Chitosan hydrogel-based electrolyte for clean and biodegradable batteries: Energetic and conductometric studies. Phys. Chem. Liq. 2018, 56, 266–278. [Google Scholar] [CrossRef]
- Jia, X.T.; Wang, C.Y.; Ranganathan, V.; Napier, B.; Yu, C.C.; Chao, Y.F.; Forsyth, M.; Omenetto, F.G.; MacFarlane, D.R.; Wallace, G.G. A Biodegradable Thin-Film Magnesium Primary Battery Using Silk Fibroin-Ionic Liquid Polymer Electrolyte. ACS Energy Lett. 2017, 2, 831–836. [Google Scholar] [CrossRef]
- Jia, X.T.; Wang, C.Y.; Zhao, C.; Ge, Y.; Wallace, G.G. Toward Biodegradable Mg-Air Bioelectric Batteries Composed of Silk Fibroin-Polypyrrole Film. Adv. Funct. Mater. 2016, 26, 1454–1462. [Google Scholar] [CrossRef]
- Huang, X.Y.; Wang, D.; Yuan, Z.Y.; Xie, W.S.; Wu, Y.X.; Li, R.F.; Zhao, Y.; Luo, D.; Cen, L.; Chen, B.B.; et al. A Fully Biodegradable Battery for Self-Powered Transient Implants. Small 2018, 14, e1800994. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.Y.; Yang, Z.J.; Meacham, K.; Cvetkovic, C.; Corbin, E.A.; Vazquez-Guardado, A.; Xue, M.T.; Yin, L.; Boroumand, J.; Pakeltis, G.; et al. Biodegradable Monocrystalline Silicon Photovoltaic Microcells as Power Supplies for Transient Biomedical Implants. Adv. Energy Mater. 2018, 8, 1703035. [Google Scholar] [CrossRef]
- Hwang, S.W.; Huang, X.; Seo, J.H.; Song, J.K.; Kim, S.; Hage-Ali, S.; Chung, H.J.; Tao, H.; Omenetto, F.G.; Ma, Z.Q.; et al. Materials for Bioresorbable Radio Frequency Electronics. Adv. Mater. 2013, 25, 3526–3531. [Google Scholar] [CrossRef] [PubMed]
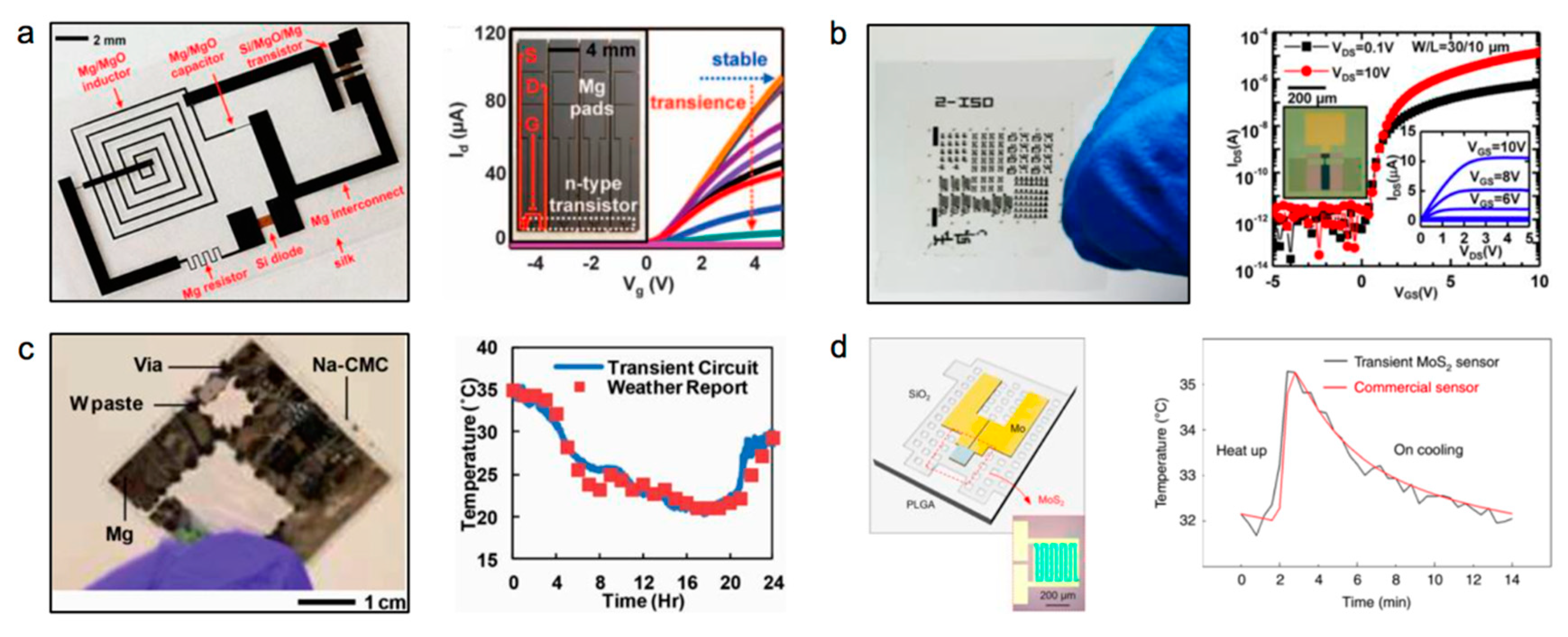
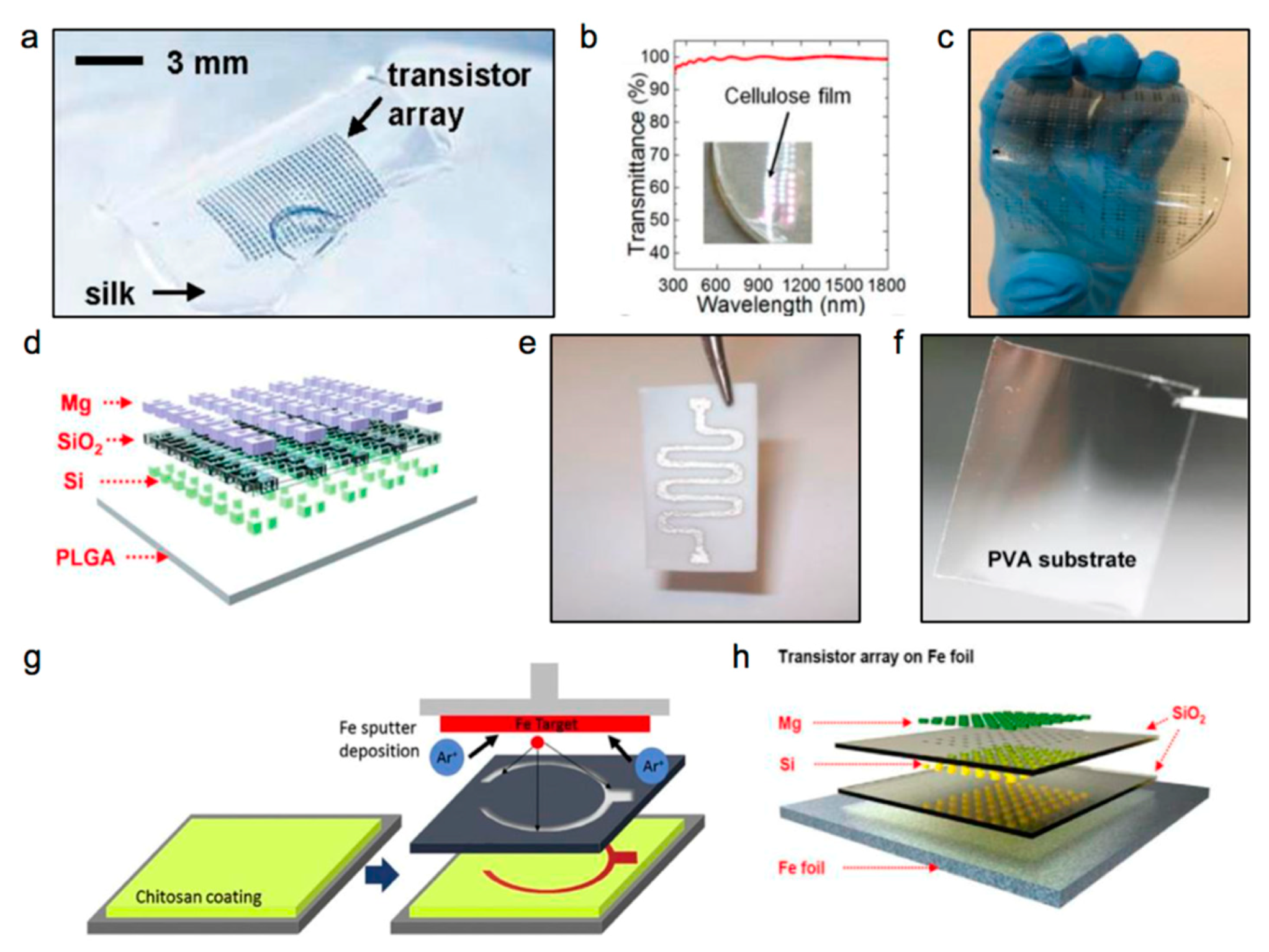
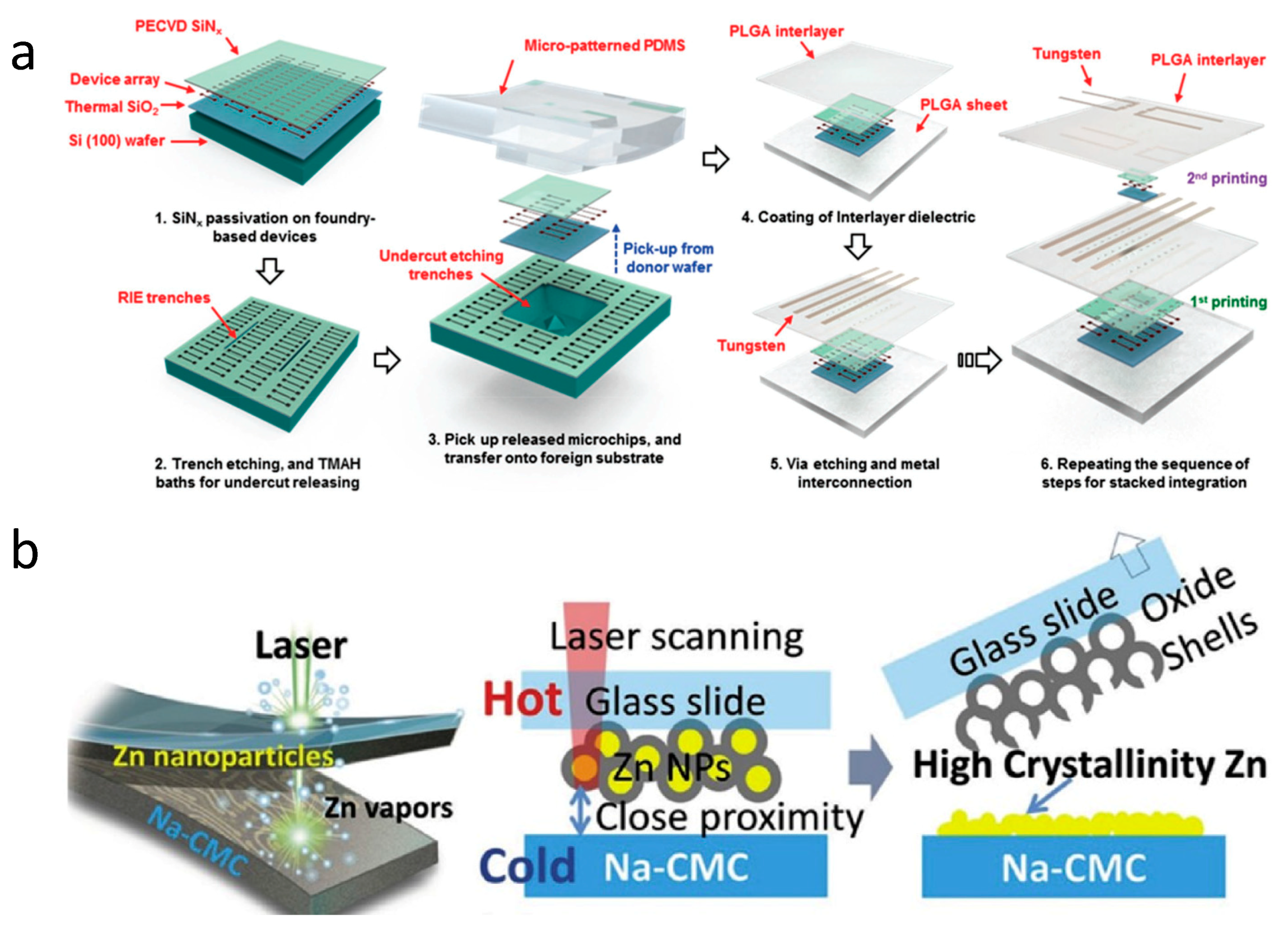
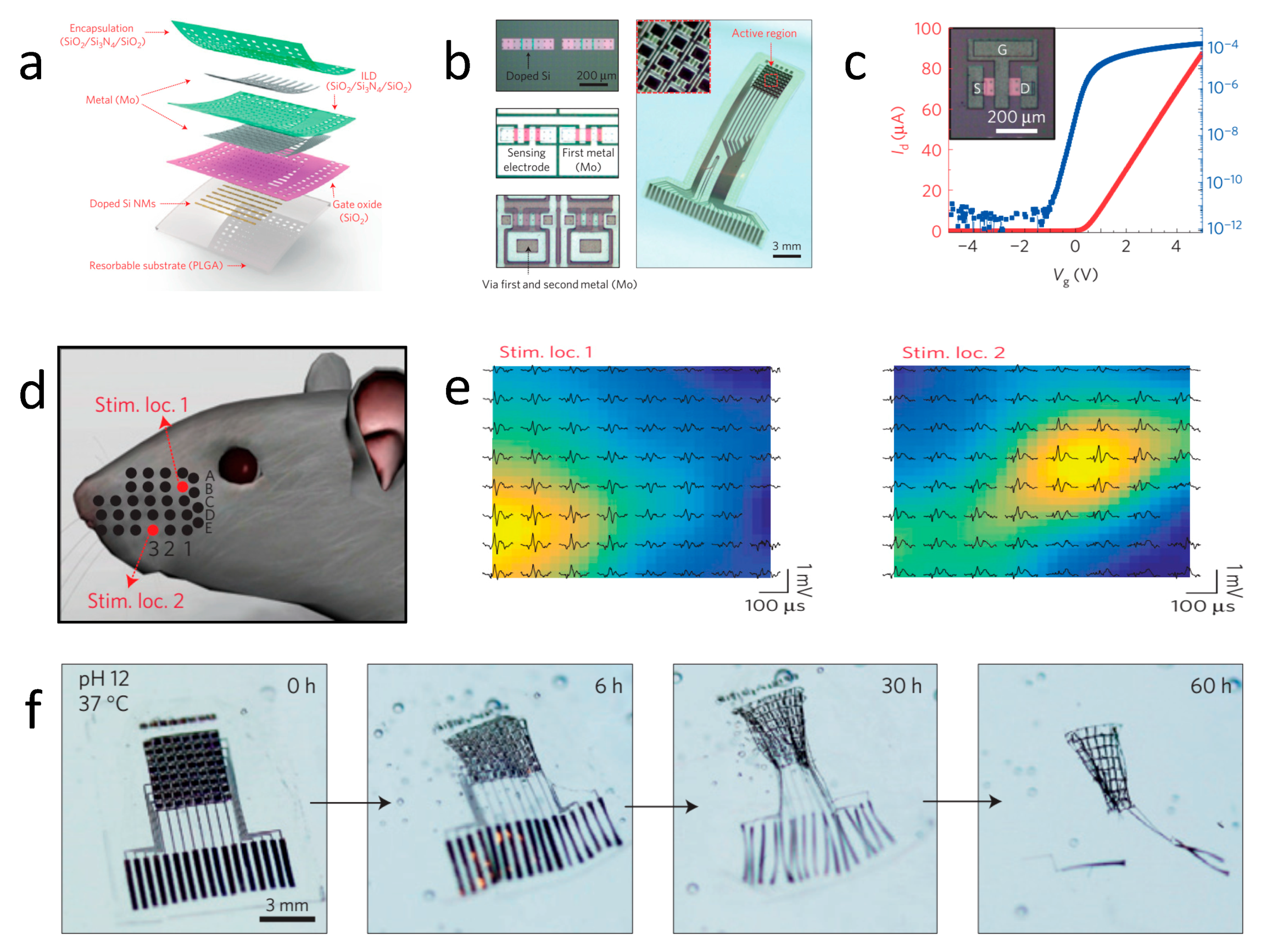
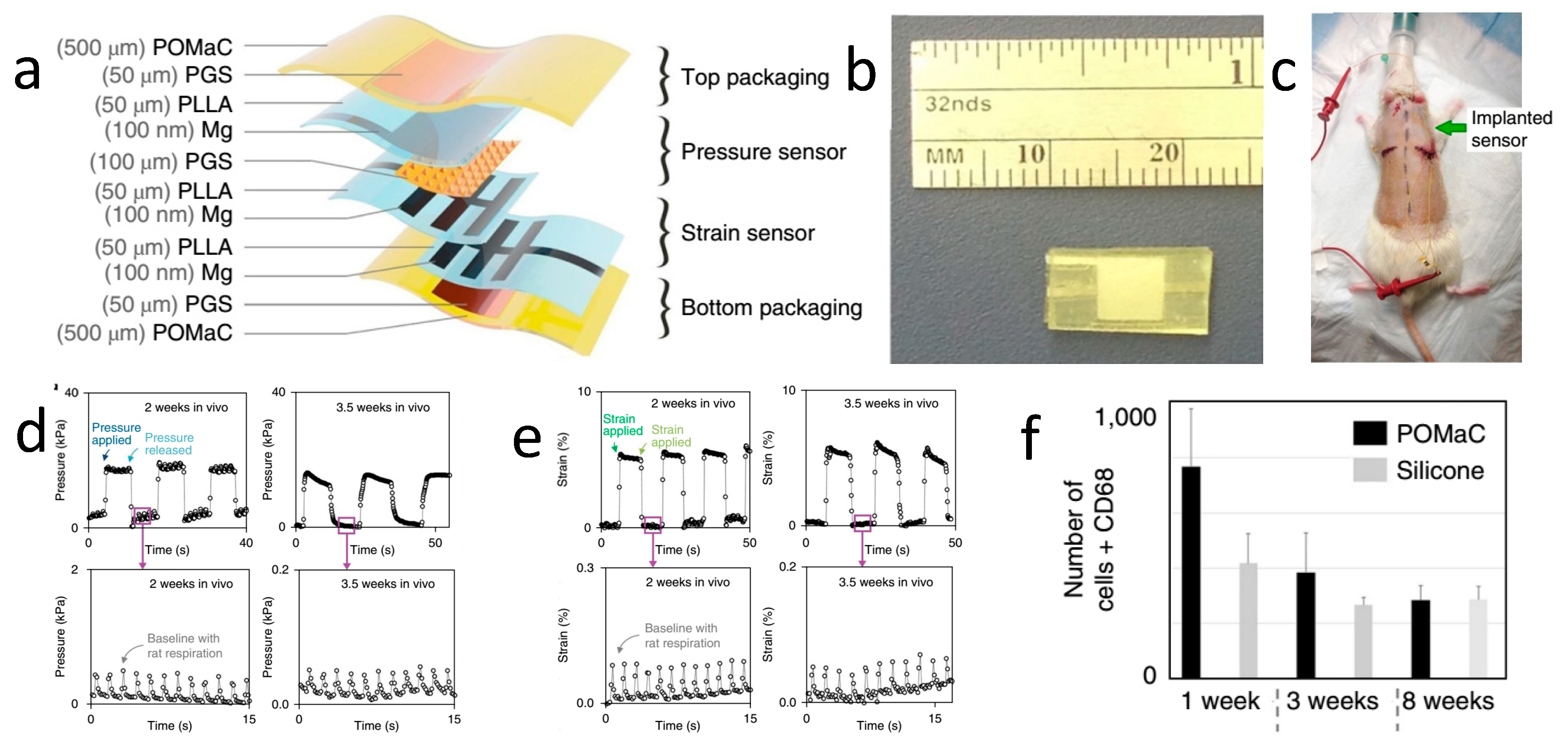
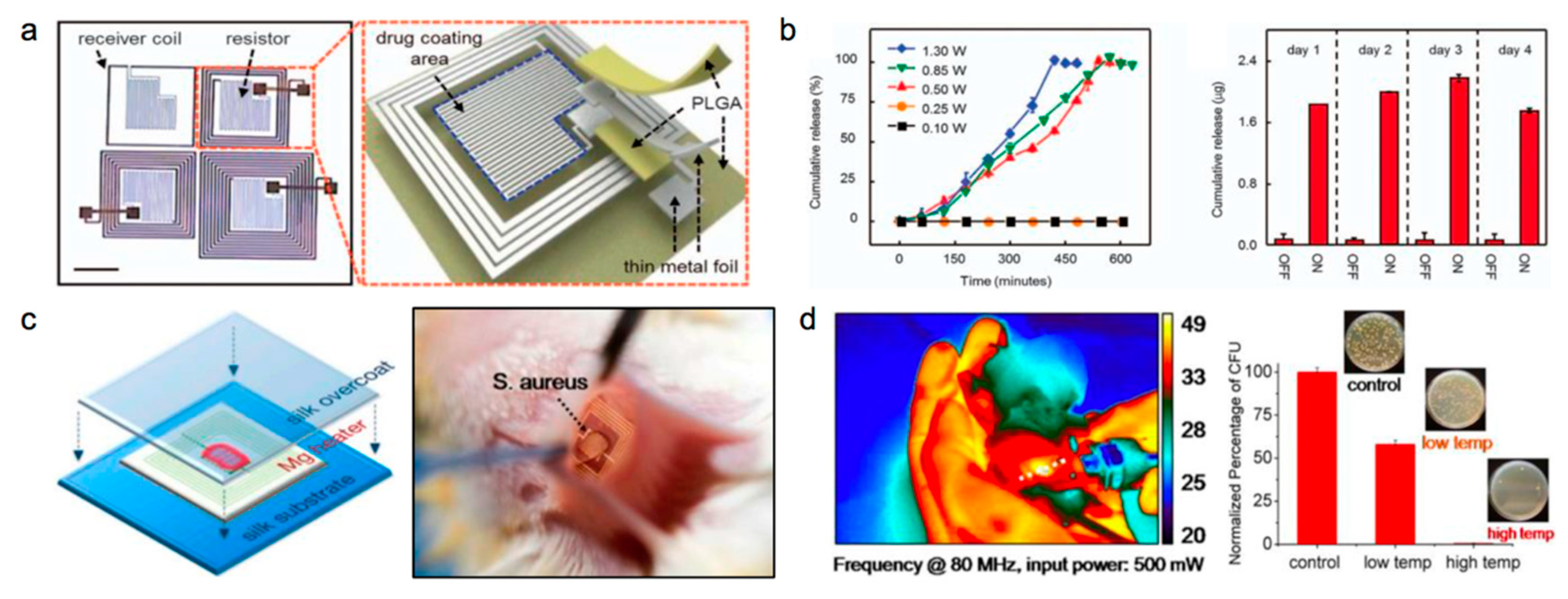
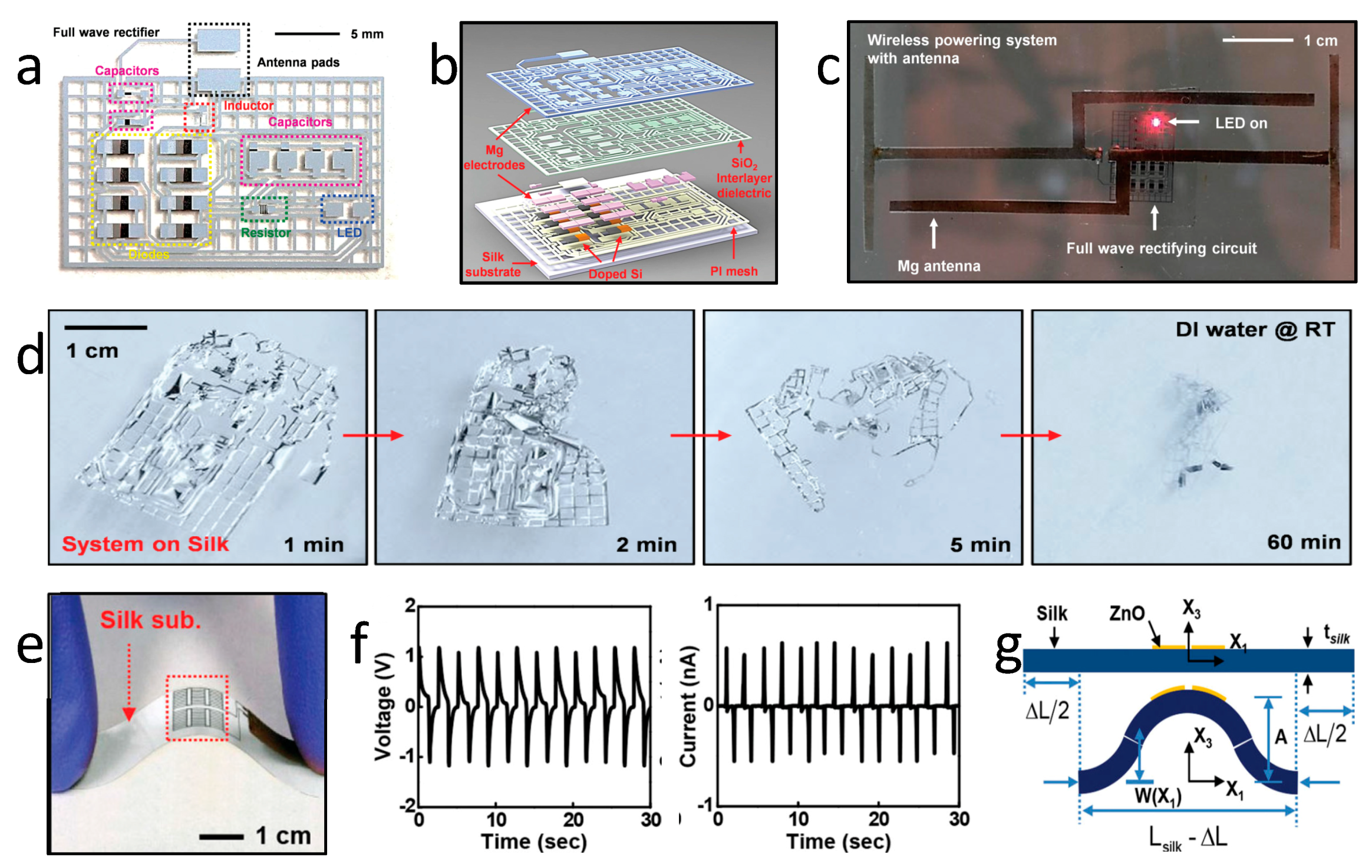
| Life Stage | Category | Element | |||
|---|---|---|---|---|---|
| Mg (mg/day) | Mo (μg/day) | Fe (mg/day) | Zn (mg/day) | ||
| Infants 0–12 months | RDA | 30–75 | 2–3 | 0.27–11 | 2–3 |
| UL | – | – | 40 | 4–5 | |
| Children 1–8 years | RDA | 80–130 | 17–22 | 7–10 | 3–5 |
| UL | 65–110 | 300–600 | 40 | 7–12 | |
| Males ≥9 years | RDA | 240–420 | 34–45 | 8–11 | 8–11 |
| UL | 350 | 1100–2000 | 40–45 | 23–40 | |
| Females ≥9 years | RDA | 240–320 | 34–45 | 8–15 | 8–9 |
| UL | 350 | 1100–2000 | 40–45 | 23–40 | |
| Pregnancy 14–50 years | RDA | 350–400 | 50 | 27 | 11–12 |
| UL | 350 | 1700–2000 | 45 | 34–40 | |
| Lactation 14–50 years | RDA | 310–360 | 50 | 9–10 | 12–13 |
| UL | 350 | 1700–2000 | 45 | 34–40 | |
| Functional Materials | Temperature °C | Aqueous Solution | pH | Doping Type cm−3 | Dissolution Rate nm day−1 |
|---|---|---|---|---|---|
| Mono-Si NMs [24] | 37 | Phosphate 0.05 M | 7.5 | 1017(p) | 2.512 |
| Phosphate 0.1 M | 25.119 | ||||
| Phosphate 0.5 M | 50.119 | ||||
| Phosphate 1 M | 63.096 | ||||
| Chloride 0.05 M | 1.259 | ||||
| Chloride 0.1 M | 6.309 | ||||
| Chloride 0.5 M | 63.096 | ||||
| Chloride 1 M | 63.096 | ||||
| 50 | Phosphate 0.05 M | 7.5 | 1017(p) | 3.162 | |
| Phosphate 0.1 M | 31.623 | ||||
| Phosphate 0.5 M | 125.893 | ||||
| Phosphate 1 M | 251.189 | ||||
| Chloride 0.05 M | 5.012 | ||||
| Chloride 0.1 M | 15.849 | ||||
| Chloride 0.5 M | 199.526 | ||||
| Chloride 1 M | 398.107 | ||||
| Mono-Si NMs [22] | 37 | Buffer solution 0.1 M | 7.4 | 1017(p) | 3.162 |
| 1019(p) | 3.162 | ||||
| 1020(p) | 0.501 | ||||
| 37 | Buffer solution 0.1 M | 7.4 | 1017(b) | 3.162 | |
| 1019(b) | 3.162 | ||||
| 1020(b) | 0.251 | ||||
| Room temperature (RT) | Coke | 2.6 | - | 0.600 | |
| RT | Milk | 6.4 | - | 23.300 | |
| RT | PBS 0.1 M | 7.4 | - | 1.820 | |
| Mono-Si NMs [23] | 37 | Bovine serum | 7.4 | - | 100.800 |
| Sea water | 7.8 | - | 4.115 | ||
| Mono-Si NMs [44] | 37 | Albumin phosphate buffered saline (PBS) Na+ | 7.4 | 1015(b) | 42.100 |
| Albumin PBS Mg2+ | 45.010 | ||||
| Albumin PBS Ca2+ | 51.000 | ||||
| 20 | Purified water | 5.5 | 1015(b) | <0.010 | |
| 1020(b) | <0.010 | ||||
| 20 | Tap water | 7.5 | 1015(b) | 0.710 | |
| 1020(b) | 0.420 | ||||
| 37 | Serum | 7.4 | 1015(b) | 21.020 | |
| 1020(b) | 0.500 | ||||
| 37 | Hank’s balanced salt solution (HBSS) | 7.6 | 1015(b) | 58.010 | |
| 1020(b) | 7.010 | ||||
| 37 | HBSS w/Ca, Mg | 7.6 | 1015(b) | 66.020 | |
| 1020(b) | 8.020 | ||||
| 37 | HBSS | 8.2 | 1015(b) | 129.030 | |
| 1020(b) | 58.000 | ||||
| 37 | HBSS w/Ca, Mg | 8.2 | 1015(b) | 178.000 | |
| 1020(b) | 69.010 | ||||
| 20 | Purified water | 5.5 | 1015(b) | 0.010 | |
| Tap water | 7.5 | 0.720 | |||
| Serum | 7.4 | 3.500 | |||
| 37 | Purified water | 5.5 | 1015(b) | 0.210 | |
| Tap water | 7.5 | 2.620 | |||
| Sweat | 4.5 | 0.530 | |||
| Serum | 7.4 | 21.000 | |||
| HBSS | 7.6 | 58.210 | |||
| HBSS w/Ca, Mg | 7.6 | 66.010 | |||
| HBSS | 8.2 | 129.020 | |||
| HBSS w/Ca, Mg | 8.2 | 178.000 | |||
| poly-Si NMs [26] | RT | Buffer solution | 7 | - | 1.020 |
| 7.4 | 1.585 | ||||
| 8 | 10.010 | ||||
| 10 | 398.107 | ||||
| 37 | Buffer solution | 7 | - | 1.259 | |
| 7.4 | 3.162 | ||||
| 8 | 19.953 | ||||
| 10 | 562.341 | ||||
| a-Si NMs [26] | RT | Buffer solution | 7 | - | 1.778 |
| 7.4 | 3.981 | ||||
| 8 | 15.849 | ||||
| 10 | 501.187 | ||||
| 37 | Buffer solution | 7 | - | 1.585 | |
| 7.4 | 5.011 | ||||
| 8 | 31.623 | ||||
| 10 | 630.957 |
| Functional Materials | Temperature °C | Aqueous Solution | pH | Dissolution Rate nm day−1 |
|---|---|---|---|---|
| Ge [26] | RT | Aqueous Buffer solution | 7 | 0.794 |
| 7.4 | 1.995 | |||
| 8 | 15.849 | |||
| 10 | 501.187 | |||
| 37 | Aqueous Buffer solution | 7 | 1.259 | |
| 7.4 | 3.162 | |||
| 8 | 19.953 | |||
| 10 | 562.341 | |||
| Poly-MoS2 [45] | 40 | 1.0 M PBS | 7.4 | 0.010 |
| 12 | 0.010 | |||
| 60 | 1.0 M PBS | 7.4 | 0.070 | |
| 12 | 0.160 | |||
| 75 | 1.0 M PBS | 7.4 | 0.200 | |
| 12 | 0.270 | |||
| 85 | 1.0 M PBS | 7.4 | 0.270 | |
| 12 | 0.400 |
| Functional Materials | Test Conditions | Dissolution Rate nm day−1 | Dissolution Product |
|---|---|---|---|
| Mg [48] | Deionized water, RT | 1680.000 | Mg(OH)2 |
| Zn [49] | Deionized water, RT | 168.000 | Zn(OH)2 |
| Fe [47] | Simulated body fluids, 37 °C | 5.000–80.000 | Fe(OH)2, Fe(OH)3 |
| Mo [50] | Deionized water, RT | 7.200 | H2MoO4 |
| W [51] | Deionized water, RT | 7.200–3.000–40.800 | H2WO4 |
| Functional Materials | Fabrication Methods | Test Conditions | Dissolution Rate nm day−1 | Dissolution Product |
|---|---|---|---|---|
| SiO2 [10] | E-beam | 37 °C | 10.000 | Si(OH)4 |
| PECVD | 37 °C | 0.100 | ||
| Thermally grown | 37 °C | 0.003 | ||
| Si3N4 [10] | LPCVD | pH 7.4 | 0.158 | Si(OH)4 + NH3 |
| LPCVD | pH 8 | 0.251 | ||
| LPCVD | pH 10 | 0.316 | ||
| LPCVD | pH 12 | 0.631 | ||
| PECVD-LF | pH 7.4 | 0.794 | ||
| PECVD-LF | pH 8 | 1.585 | ||
| PECVD-LF | pH 10 | 1.995 | ||
| PECVD-LF | pH 12 | 3.981 | ||
| PECVD-HF | pH 7.4 | 0.794 | ||
| PECVD-HF | pH 8 | 2.512 | ||
| PECVD-HF | pH 10 | 6.310 | ||
| PECVD-HF | pH 12 | 25.119 | ||
| Spin-on glass (SOG) [47] | cured at 300 °C | PBS, pH 7.4 | 50.000 | Si(OH)4 |
| cured at 800 °C | PBS, pH 7.4 | 6.000 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Wang, L.; Yin, L. Materials and Devices for Biodegradable and Soft Biomedical Electronics. Materials 2018, 11, 2108. https://doi.org/10.3390/ma11112108
Li R, Wang L, Yin L. Materials and Devices for Biodegradable and Soft Biomedical Electronics. Materials. 2018; 11(11):2108. https://doi.org/10.3390/ma11112108
Chicago/Turabian StyleLi, Rongfeng, Liu Wang, and Lan Yin. 2018. "Materials and Devices for Biodegradable and Soft Biomedical Electronics" Materials 11, no. 11: 2108. https://doi.org/10.3390/ma11112108
APA StyleLi, R., Wang, L., & Yin, L. (2018). Materials and Devices for Biodegradable and Soft Biomedical Electronics. Materials, 11(11), 2108. https://doi.org/10.3390/ma11112108





