In Situ Vapor Polymerization of Poly(3,4-ethylenedioxythiophene) Coated SnO2-Fe2O3 Continuous Electrospun Nanotubes for Rapid Detection of Iodide Ions
Abstract
:1. Introduction
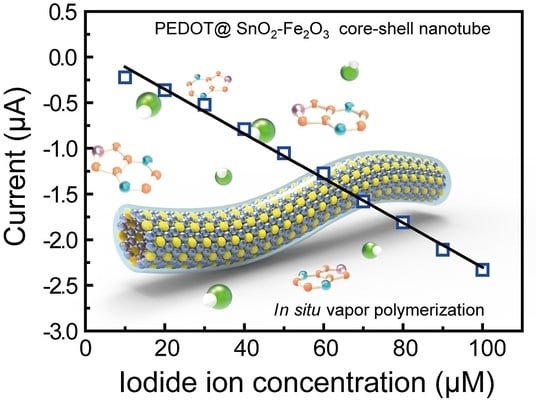
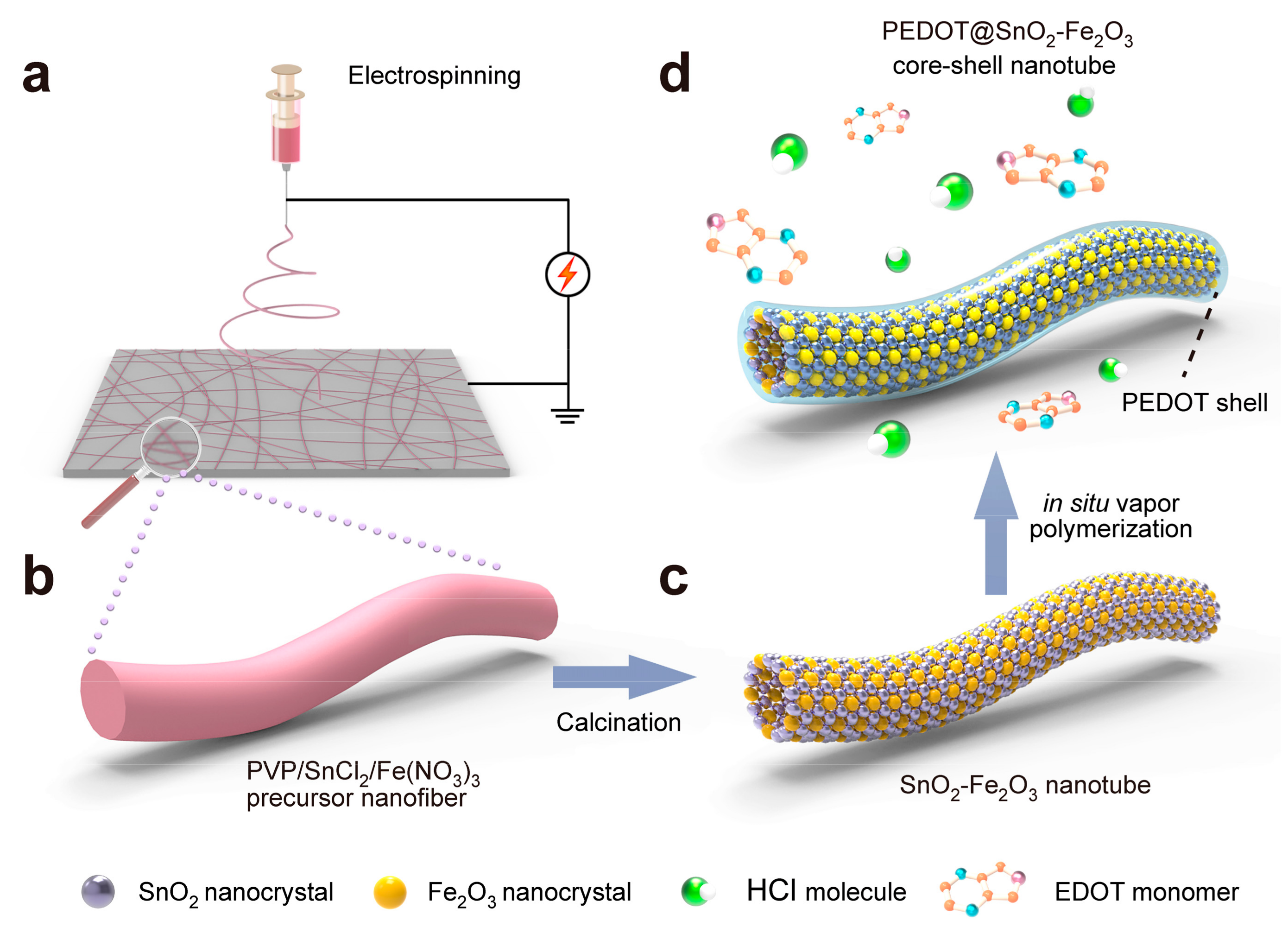
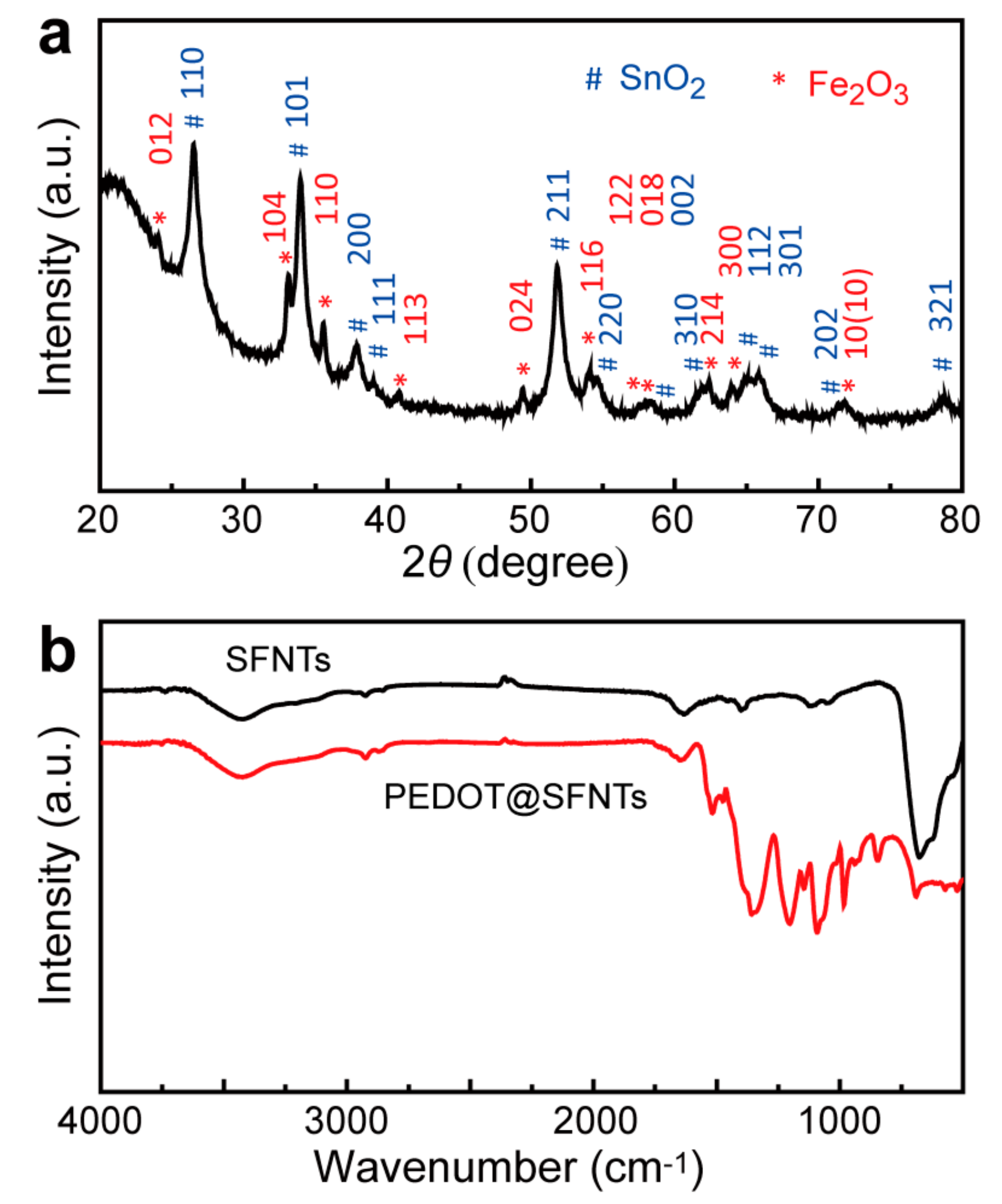
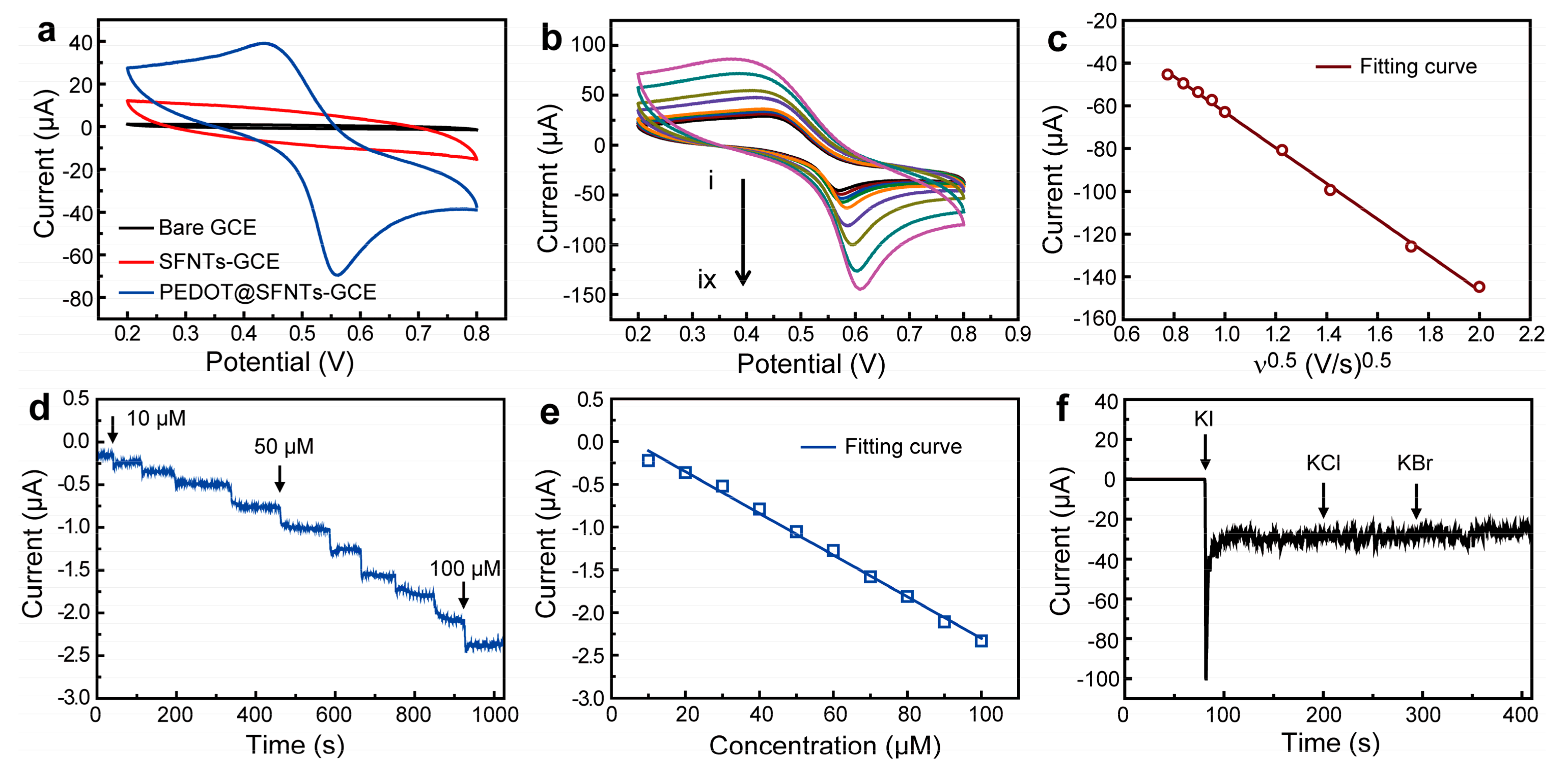
2. Results and Discussion
3. Materials and Methods
3.1. Preparation of PVP/SnCl2/Fe(NO3)3 Precursor Electrospun Nanofibers
3.2. Preparation of PEDOT@SnO2-Fe2O3 Core-Shell Nanotubes
3.3. Fabrication of PEDOT@SnO2-Fe2O3 Core-Shell Nanotube Modified GCE
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dissanayake, C. Of stones and health: Medical geology in Sri Lanka. Science 2005, 309, 883–885. [Google Scholar] [CrossRef] [PubMed]
- Jacob, P.; Goulko, G.; Heidenreich, W.F.; Likhtarev, I.; Kairo, I.; Tronko, N.D.; Bogdanova, T.I.; Kenigsberg, J.; Buglova, E.; Drozdovitch, V.; et al. Thyroid cancer risk to children calculated. Nature 1998, 392, 31–32. [Google Scholar] [CrossRef] [PubMed]
- Haldimann, M.; Zimmerli, B.; Als, C.; Gerber, H. Direct determination of urinary iodine by inductively coupled plasma mass spectrometry using isotope dilution with iodine-129. Clin. Chem. 1998, 44, 817–824. [Google Scholar] [PubMed]
- Yebra, M.C.; Bollain, M.H. A simple indirect automatic method to determine total iodine in milk products by flame atomic absorption spectrometry. Talanta 2010, 82, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Puchnin, K.; Andrianova, M.; Kuznetsov, A.; Kovalev, V. Field-effect transition sensor for KI detection based on self-assembled calixtube monolayers. Biosen. Bioelectron. 2017, 98, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Nacapricha, D.; Sangkarn, P.; Karuwan, C.; Mantim, T.; Waiyawat, W.; Wilairat, P.; Cardwell, I.; McKelvie, I.D.; Ratanawimarnwong, N. Pervaporation-flow injection with chemiluminescence detection for determination of iodide in multivitamin tablets. Talanta 2007, 72, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Biallozor, S.; Kupniewska, A. Study on poly(3,4-ethylenedioxythiophene) behaviour in the I−/I2 solution. Electrochem. Commun. 2000, 2, 480–486. [Google Scholar] [CrossRef]
- Dielacher, B.; Tiefenauer, R.F.; Junesch, J.; Voeroes, J. Iodide sensing via electrochemical etching of ultrathin gold films. Nanotechnology 2015, 26, 025202. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Lu, X.; Wang, C.; Zhang, W. Investigation on PEDOT/β-Fe3+O(OH,Cl) nanospindles as a new steady electrode material for detecting iodic compounds. Electrochem. Commun. 2009, 11, 603–607. [Google Scholar] [CrossRef]
- Huang, J.; Wang, D.; Hou, H.; You, T. Electrospun palladium nanoparticle-loaded carbon nanofibers and their electrocatalytic activities towards hydrogen peroxide and NADH. Adv. Funct. Mater. 2008, 18, 441–448. [Google Scholar] [CrossRef]
- Ju, Y.-W.; Choi, G.-R.; Jung, H.-R.; Lee, W.-J. Electrochemical properties of electrospun PAN/MWCNT carbon nanofibers electrodes coated with polypyrrole. Electrochim. Acta 2008, 53, 5796–5803. [Google Scholar] [CrossRef]
- Chi, M.; Nie, G.; Jiang, Y.; Yang, Z.; Zhang, Z.; Wang, C.; Lu, X. Self-assembly fabrication of coaxial Te@poly(3,4-ethylenedioxythiophene) nanocables and their conversion to Pd@poly(3,4-ethylenedioxythiophene) nanocables with a high peroxidase-like activity. ACS Appl. Mater. Interfaces 2016, 8, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Wang, C.; Favier, F.; Pinna, N. Electrospun nanomaterials for supercapacitor electrodes: Designed architectures and electrochemical performance. Adv. Energy Mater. 2017, 7, 1601301. [Google Scholar] [CrossRef]
- Trevisan, R.; Dobbelin, M.; Boix, P.P.; Braea, E.M.; Tena-Zaera, R.; Mora-Sero, I.; Bisquert, J. PEDOT nanotube arrays as high performing counter electrodes for dye sensitized solar cells. Study of the interactions among electrolytes and counter electrodes. Adv. Energy Mater. 2011, 1, 781–784. [Google Scholar] [CrossRef]
- Greene, L.E.; Law, M.; Goldberger, J.; Kim, F.; Johnson, J.C.; Zhang, Y.F.; Saykally, R.J.; Yang, P.D. Low-temperature wafer-scale production of ZnO nanowire arrays. Angew. Chem. Int. Ed. 2003, 42, 3031–3034. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.D.; Yan, H.Q.; Mao, S.; Russo, R.; Johnson, J.; Saykally, R.; Morris, N.; Pham, J.; He, R.R.; Choi, H.J. Controlled growth of ZnO nanowires and their optical properties. Adv. Funct. Mater. 2002, 12, 323–331. [Google Scholar] [CrossRef]
- Back, J.-W.; Lee, S.; Hwang, C.-R.; Chi, C.-S.; Kim, J.-Y. Fabrication of conducting PEDOT nanotubes using vapor deposition polymerization. Macromol. Res. 2011, 19, 33–37. [Google Scholar] [CrossRef]
- Cho, S.I.; Choi, D.H.; Kim, S.-H.; Lee, S.B. Electrochemical synthesis and fast electrochromics of poly(3,4-ethylenedioxythiophene) nanotubes in flexible substrate. Chem. Mater. 2005, 17, 4564–4566. [Google Scholar] [CrossRef]
- Wang, W.; Lu, X.; Li, Z.; Lei, J.; Liu, X.; Wang, Z.; Zhang, H.; Wang, C. One-dimensional polyelectrolyte/polymeric semiconductor core/shell structure: Sulfonated poly(arylene ether ketone)/polyaniline nanofibers for organic field-effect transistors. Adv. Mater. 2011, 23, 5109–5112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Jiang, Y.; Chi, M.; Yang, Z.; Nie, G.; Lu, X.; Wang, C. Fabrication of Au nanoparticles supported on CoFe2O4 nanotubes by polyaniline assisted self-assembly strategy and their magnetically recoverable catalytic properties. Appl. Surf. Sci. 2016, 363, 578–585. [Google Scholar] [CrossRef]
- Winther-Jensen, B.; West, K. Vapor-phase polymerization of 3,4-ethylenedioxythiophene: A route to highly conducting polymer surface layers. Macromolecules 2004, 37, 4538–4543. [Google Scholar] [CrossRef]
- Winther-Jensen, B.; Winther-Jensen, O.; Forsyth, M.; MacFarlane, D.R. High rates of oxygen reduction over a vapor phase-polymerized PEDOT electrode. Science 2008, 321, 671–674. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lee, J.Y.; Zeng, H.C. Polycrystalline SnO2 nanotubes prepared via infiltration casting of nanocrystallites and their electrochemical application. Chem. Mater. 2005, 17, 3899–3903. [Google Scholar] [CrossRef]
- Cao, K.; Jiao, L.; Liu, H.; Liu, Y.; Wang, Y.; Guo, Z.; Yuan, H. 3D hierarchical porous alpha-Fe2O3 nanosheets for high-performance lithium-ion batteries. Adv. Energy Mater. 2015, 5, 1401421. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, X.; Yang, Y.; Wang, L.; Zhu, M.; Hsiao, B.S.; Chu, B. Aligned and molecularly oriented semihollow ultrafine polymer fiber yarns by a facile method. J. Polym. Sci. B Polym. Phys. 2010, 48, 1118–1125. [Google Scholar] [CrossRef]
- Sun, W.; Lu, X.; Xue, Y.; Tong, Y.; Wang, C. One-step preparation of CoFe2O4/polypyrrole/Pd ternary nanofibers and their catalytic activity toward p-nitrophenol hydrogenation reaction. Macromol. Mater. Eng. 2014, 299, 361–367. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, Z.; Jiang, Y.; Chi, M.; Nie, G.; Lu, X.; Wang, C. Palladium nanoparticles modified electrospun CoFe2O4 nanotubes with enhanced peroxidase-like activity for colorimetric detection of hydrogen peroxide. RSC Adv. 2016, 6, 33636–33642. [Google Scholar] [CrossRef]
- Xu, X.; Yin, M.; Li, N.; Wang, W.; Sun, B.; Liu, M.; Zhang, D.; Li, Z.; Wang, C. Vanadium-doped tin oxide porous nanofibers: Enhanced responsivity for hydrogen detection. Talanta 2017, 167, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Ghosh, D.; Chen, S. Large-scale electrochemical synthesis of SnO2 nanoparticles. J. Mater. Sci. 2008, 43, 5291–5299. [Google Scholar] [CrossRef]
- Qin, W.; Yang, C.; Yi, R.; Gao, G. Hydrothermal synthesis and characterization of single-crystalline α-Fe2O3 nanocubes. J. Nanomater. 2011, 2011, 159259. [Google Scholar] [CrossRef]
- Funda, S.; Ohki, T.; Liu, Q.; Hossain, J.; Ishimaru, Y.; Ueno, K.; Shirai, H. Correlation between the fine structure of spin-coated PEDOT:PSS and the photovoltaic performance of organic/crystalline-silicon heterojunction solar cells. J. Appl. Phys. 2016, 120, 033103. [Google Scholar] [CrossRef]
- Lin, W.-J.; Liao, C.-S.; Jhang, J.-H.; Tsai, Y.-C. Graphene modified basal and edge plane pyrolytic graphite electrodes for electrocatalytic oxidation of hydrogen peroxide and β-nicotinamide adenine dinucleotide. Electrochem. Commun. 2009, 11, 2153–2156. [Google Scholar] [CrossRef]
- Tong, Y.; Li, Z.; Lu, X.; Yang, L.; Sun, W.; Nie, G. Wang, Z.; Wang, C. Electrochemical determination of dopamine based on electrospun CeO2/Au composite nanofibers. Electrochim. Acta 2013, 95, 12–17. [Google Scholar] [CrossRef]
- Tang, H.; Kitani, A.; Shiotani, M. Cyclic voltammetry of KI at polyaniline-filmed Pt electrodes part I: Formation of polyaniline-iodine charge transfer complexes. J. Appl. Electrochem. 1996, 26, 36–44. [Google Scholar] [CrossRef]
- Tang, H.; Kitani, A.; Shiotani, M. Cyclic voltammetry of KI at polyaniline-filmed Pt electrodes part II: Effects of pH. J. Appl. Electrochem. 1996, 26, 45–50. [Google Scholar] [CrossRef]

| Detecting Method | LOD | Linear Range | Linear R2 | Response Time |
|---|---|---|---|---|
| ICP-MS [6] a | 2.5 μg/L | 25–355 μg/L | 5 min | |
| AAS [7] b | 2.75 μg/L | 11–350 μg/L | 0.998 | ~3 min |
| FET [8] c | 0.03 μM | 0.1 μM–10 mM | >100 s | |
| FIA [9] d | 500 μg/L | 1–10 mg/L | 0.999 | 2 min |
| Current work | 1.5 μM | 10–100 μM | 0.9935 | ~4 s |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Wang, W.; Sun, B.; Zhang, X.; Zhao, R.; Wang, C. In Situ Vapor Polymerization of Poly(3,4-ethylenedioxythiophene) Coated SnO2-Fe2O3 Continuous Electrospun Nanotubes for Rapid Detection of Iodide Ions. Materials 2018, 11, 2084. https://doi.org/10.3390/ma11112084
Xu X, Wang W, Sun B, Zhang X, Zhao R, Wang C. In Situ Vapor Polymerization of Poly(3,4-ethylenedioxythiophene) Coated SnO2-Fe2O3 Continuous Electrospun Nanotubes for Rapid Detection of Iodide Ions. Materials. 2018; 11(11):2084. https://doi.org/10.3390/ma11112084
Chicago/Turabian StyleXu, Xiuru, Wei Wang, Bolun Sun, Xue Zhang, Rui Zhao, and Ce Wang. 2018. "In Situ Vapor Polymerization of Poly(3,4-ethylenedioxythiophene) Coated SnO2-Fe2O3 Continuous Electrospun Nanotubes for Rapid Detection of Iodide Ions" Materials 11, no. 11: 2084. https://doi.org/10.3390/ma11112084
APA StyleXu, X., Wang, W., Sun, B., Zhang, X., Zhao, R., & Wang, C. (2018). In Situ Vapor Polymerization of Poly(3,4-ethylenedioxythiophene) Coated SnO2-Fe2O3 Continuous Electrospun Nanotubes for Rapid Detection of Iodide Ions. Materials, 11(11), 2084. https://doi.org/10.3390/ma11112084