1. Introduction
For a long time, coalbed methane (CBM) has been discharged into the atmosphere from coal mines for the safe of coal production. In recent years, in the face of a lack of energy and the pressure of environmental pollution, especially in China, more and more people have gradually realized that CBM should be tapped as an unconventional natural gas and realize the control of atmospheric pollutants at the same time. CBM, which contains significant amounts of nitrogen (N
2 > 70%, CH
4 > 20%), needs to be upgraded in order to meet the quality for civil use (CH
4 ≥ 30%). The mature technology is cryogenic distillation for nitrogen removal from methane, but it is highly energy-intensive and costly. An alternative way of separating the CH
4/N
2 mixture is pressure swing adsorption (PSA) [
1,
2,
3,
4]. The selection of appropriate adsorbent is crucial to the successful separation of methane and nitrogen using PSA.
Up to now, the main adsorbents are activated charcoal, carbon molecular sieve (CMS), and zeolite molecular sieve. The equilibrium adsorption capacity of activated carbon to methane is higher than that of nitrogen, and the separation coefficient is higher. However, in the application process, activated carbon is easily powdered, which often causes blockage of equipment. So, it characterized by large gas circulation, low efficiency, and high costs. The CMS is mainly based on kinetic effect for separation of nitrogen and methane. That is to say, the diffusion rate of nitrogen is higher than that of methane in the micropore of CMS, and a large amount of nitrogen is adsorbed into the pore in a short time, and methane remains outside the pore. The concentrated methane is obtained in the sequence step. However, with the increase of adsorption time, the kinetic effect becomes weaker and the equilibrium effect plays a key role. Then, the equilibrium effect coexists with the kinetic effect, and their separation effect is the opposite. Accordingly, there is difficulty in separating methane and nitrogen. The zeolite molecular sieve is a very promising adsorbent. Zeolites, because of their inherent porosity, crystallinity, and the presence of hydrated aluminosilicates of alkali and alkaline earth cations, can absorb polar molecules and small molecules: they have long been considered as excellent candidate materials for the separation of gases. According to their classification, zeolites occur as one of two types: synthetic zeolites and natural zeolites. Synthetic zeolites are manufactured on a large scale for industrial use. However, compared with synthetic zeolites, natural zeolites are abundant and readily available. Moreover, they are cheap. Therefore, they have been paid attention increasingly by people.
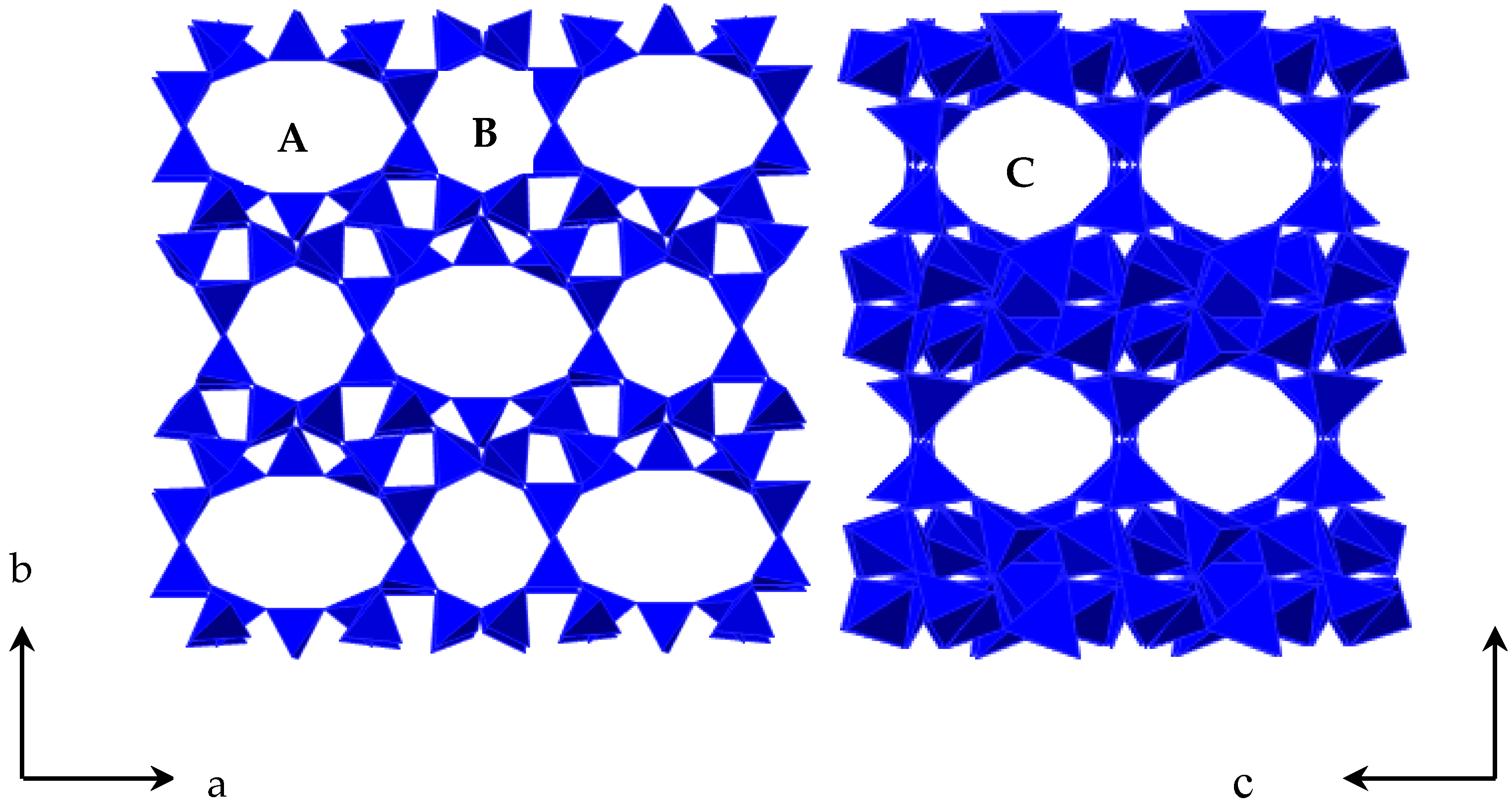
Clinoptilolite (Cp) is a member of heulandite group of natural zeolites and is isostructural with the zeolilte heaulandite. The general formula for natural zeolites is (Na, K)
a(Mg, Ca, Sr, Ba)
d[Al
a+2dSi
n−(a+2d)O
2n]·
mH
2O. The unit cell is monoclinic and is usually characterized on the basis of 72 O atoms (
n = 36) and
m = 24 water molecules. The framework of Cp is formed by two parallel channels of 10-membered rings (channel A, 0.72 × 0.44 nm) and 8-membered rings (channel B, 0.47 × 0.41 nm) that are connected to the other 8-membered rings (channel C, 0.55 × 0.40 nm) [
5]. A schematic diagram of Cp structure is given in
Figure 1. In these channels, there was both zeolite water and metal cations: these (K
+, Na
+, Ca
2+, and Mg
2+) balance the negative charge in the lattice and are readily exchanged with cations in aqueous solution (such as K
+, Sr
2+, Ba
2+, etc.). They do not destroy the crystal structure but can change the electric field within the crystal; thus, the adsorption properties of the Cp can be changed to a significant extent. Cp has been studied with a view to its use in natural gas purification. Aguilar-Armenta et al. measured the adsorption kinetics of pure CO
2, N
2, and CH
4 on a natural clinoptilolite sample from Villa de Reyes [
6]. Jayaraman et al. found that purified and Mg-clinoptilolite showed potential for nitrogen/methane separation [
7]. Faghihian et al. investigated the adsorption of N
2 and CH
4 on natural Cp from the north of Semnan and its cation-exchanged forms (Na-Cp, K-Cp, and H-Cp) at 298 K [
8]. However, it is hard to enrich methane through kinetic PSA, since the methane content has been lower than 20% in the feed gas until now.
In this paper, in view of the fact that large deposits of zeolitic tuffs, primarily of Cp, have been discovered in China, the adsorption properties of modified Cp (using aqueous solutions of salts KCl, NaCl, AgNO3, RbCl, CsCl, LiCl, NH4Cl, CaCl2, MgCl2, BaCl2, SrCl2, CuCl2, ZnCl2, and CeCl3) was investigated to evaluate their possible industrial applications in CBM separation processes. The simulated methane–nitrogen mixture (19.7% CH4, 80.3% N2) was separated using a single-bed unit with the modified Cp in the same conditions of adsorption pressure and temperature in the PSA equipment.
2. Materials and Methods
2.1. Materials
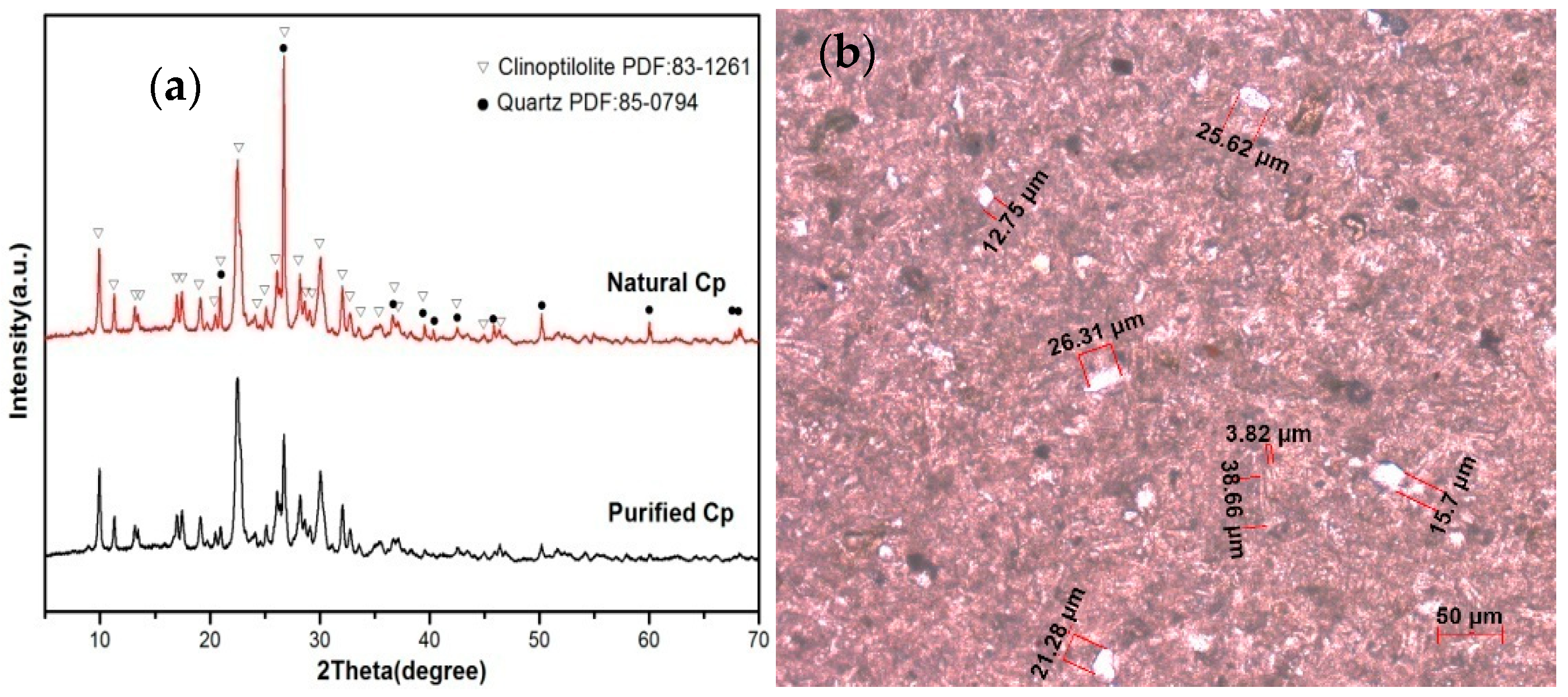
The natural clinoptilolite (Cp) was collected from the south of the Liaoxi metallogenic belt in China, and its structure was shown in
Figure 1. Its chemical composition (wt.%) is SiO
2 68.48%, TiO
2 0.19%, Fe
2O
3 1.35%, Al
2O
3 11.92%, CaO 3.75%, MgO 1.26%, K
2O 1.61%, and Na
2O 0.63%. The natural clinoptilolite was crushed to 200 mesh by wet-grinding, and the milled pulp was poured into a centrifuge to remove any heavy impurities. Then, purified clinoptilolite (p-Cp) was obtained with the following chemical composition (wt.%): SiO
2 66.99%, TiO
2 0.20%, Fe
2O
3 1.37%, Al
2O
3 12.01%, CaO 3.80%, MgO 1.29%, K
2O 1.63%, and Na
2O 0.65%. This process differed from that quoted in the existing literature [
6,
7,
8] in that the purified clinoptilolite was dried at 378 K and stored in a desiccator. It was used as a raw material for the preparation of adsorbents.
Helium (99.999%, pre-purified, Praxair Gases), nitrogen, and methane were used as adsorbates. Helium was used as a purge gas during desorption. All gases were supplied by Praxair (Beijing, China). Other used analytical-grade chemicals were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Deionized water was used for sample washing, and silver nitrate was used to check for the absence of Cl− ions in the washings.
2.2. Preparation of Modified Clinoptilolites
The samples were prepared from p-Cp by ion exchange with 2 M aqueous solutions of respective salt KCl, NaCl, AgNO3, RbCl, CsCl, LiCl, NH4Cl, CaCl2, MgCl2, BaCl2, SrCl2, CuCl2, ZnCl2, and CeCl3 in a three-neck flask in a 90 °C water bath for 2 h on a magnetic stirrer. The above-mentioned all salt solutions were mixed by the weight of the solid to liquid ratio at 1:20. This procedure was repeated three times until the cations contents were no longer changed in the filtrate. After these, the mixture was filtered and washed many times with deionized water until white precipitates disappeared in filtrate by test with silver nitrate. Finally, the samples were through simple processing, drying, and fine grinding, and they were put in a glass dryer for use. All samples were depicted by K-Cp, Na-Cp, Ca-Cp, Mg-Cp, Li-Cp, Rb-Cp, Sr-Cp, Ba-Cp, Ce-Cp, Cu-Cp, Zn-Cp, Cs-Cp, Ag-Cp, H-Cp which were treated by KCl, NaCl, CaCl2, MgCl2, LiCl, RbCl, SrCl2, BaCl2, CeCl3, CuCl2, ZnCl2, CsCl, AgNO3, and NH4Cl solution, respectively. Then, the modified Cppowder was prepared into particles as adsorbents between 0.5 and 1.5 mm.
2.3. Materials Characterization
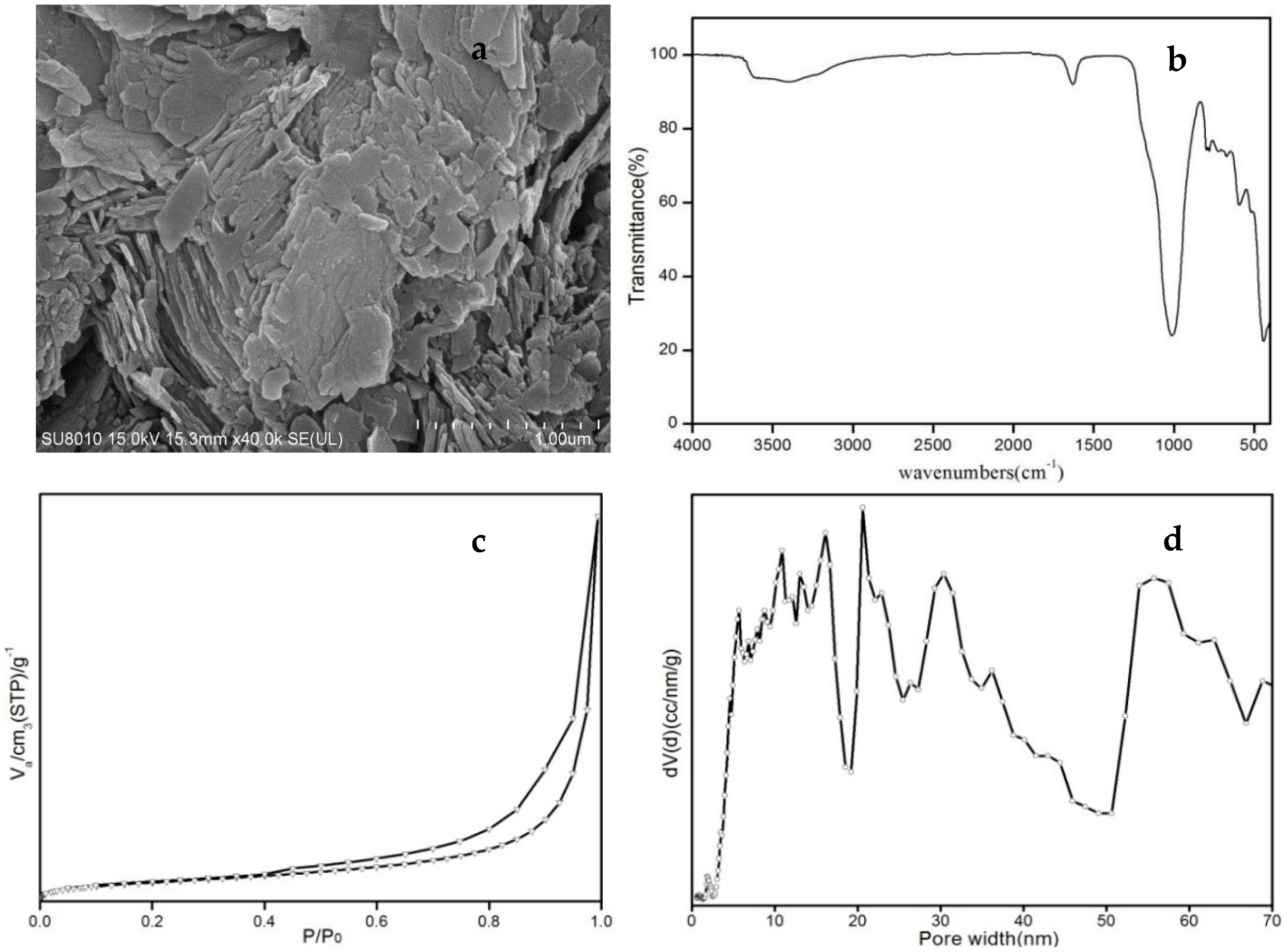
To detect possible structural changes produced by thermal activation, X-ray diffraction patterns of the natural, purified, and cation-exchanged Cp were assessed. X-ray powder diffraction (XRD) patterns of the clinoptilolite samples were obtained from a MiniFlex 600 (Rigaku, Japan) diffractometer using CuKα radiation (λ = 1.5406 Å). The FTIR spectrum of each sample was obtained by using a Nicolet (IS50) spectrophotometer (Thermofisher, Waltham, MA, USA). The silicon content of all samples was analyzed by ultraviolet visible spectrophotometer (SPECORD 200, Analytik Jena, Jena, Germany). The rubidium and silver content of all samples was analyzed by atomic absorption spectrophotometry (SOLAAR M6, Thermofisher, Waltham, MA, USA). Other analysis of chemical components of all samples was analyzed by inductively coupled plasma atomic emission spectrometry (iCAP 7400, Thermofisher, Waltham, MA, USA). The surface morphology and size of sample was observed by SEM (SU8020, Hitachi, Tokyo, Japan). The polarizing microscope (ZEISS Axio Scope A1, Oberkochen, Germany) with plane-polarized light revealed the phase composition.
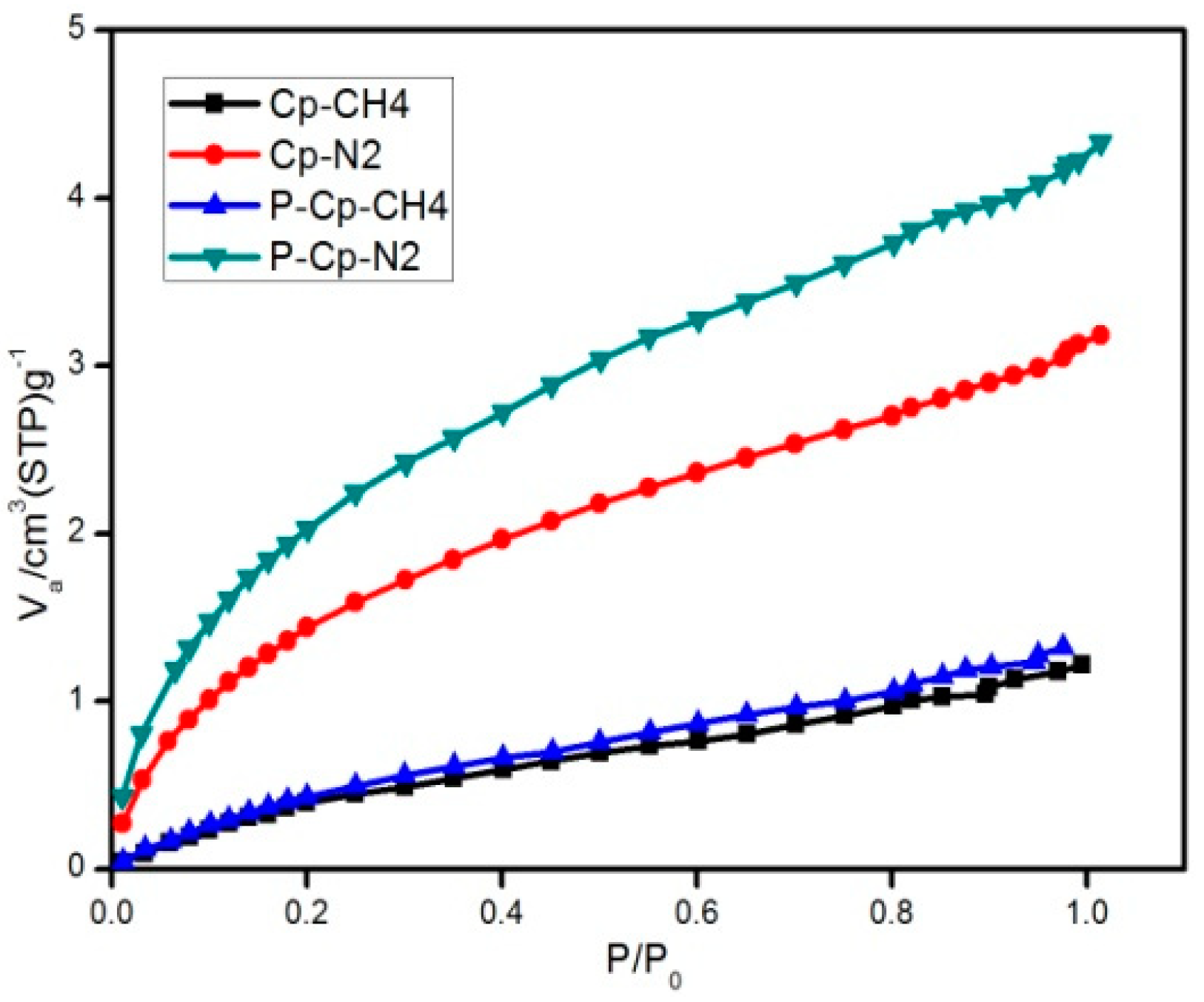
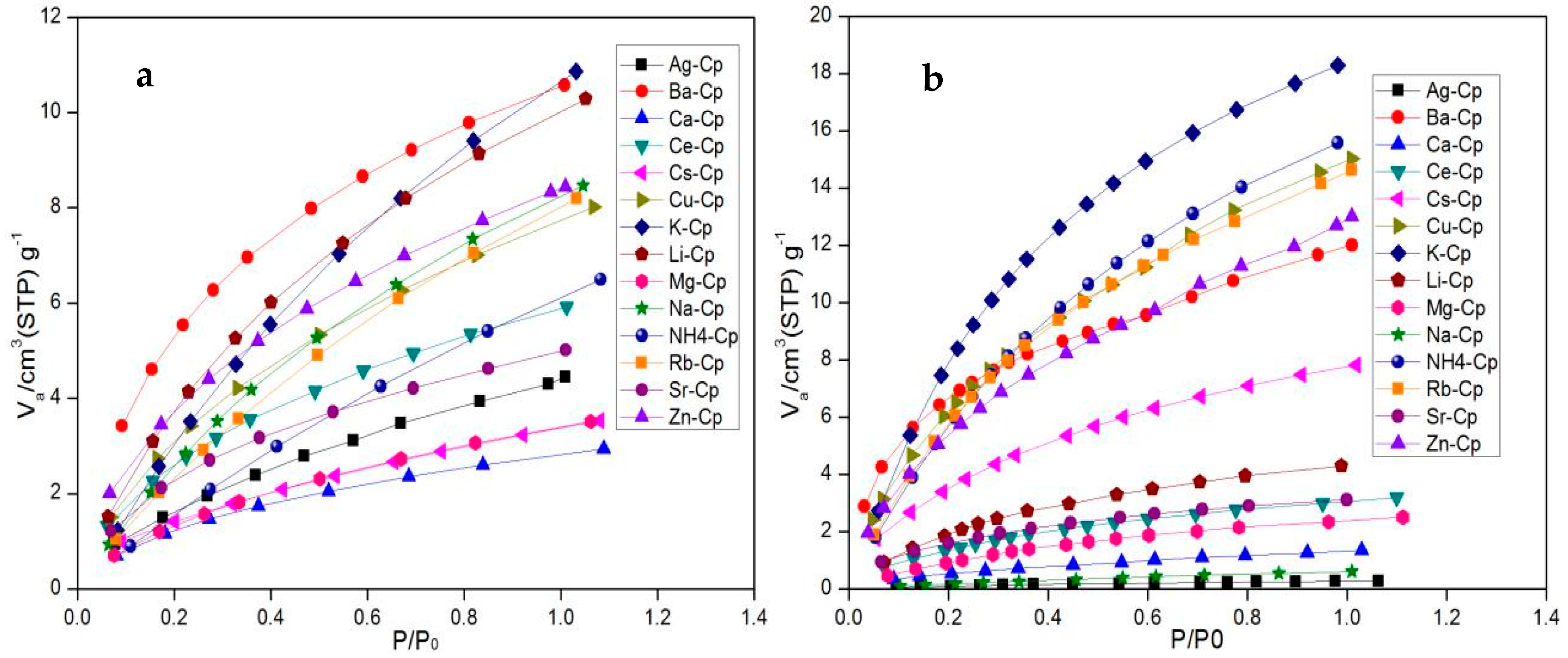
Static adsorption isotherms of samples at low temperatures with argon gas at 87.27 K (Autosorb iQ, Quantachrome, Boynton Beach, FL, USA) were obtained: the static adsorption properties of adsorbents for methane and nitrogen were studied with a static volumetric system (BELSORP-miniII, BEL, Japan). Before measurement, the Cp samples were activated at 573 K in an oven evacuated to a residual pressure of less than 3.2 × 10−2 Pa and maintained in those conditions for 6 h. The static adsorption characteristics of Cp for nitrogen or methane were then tested at 298 K.
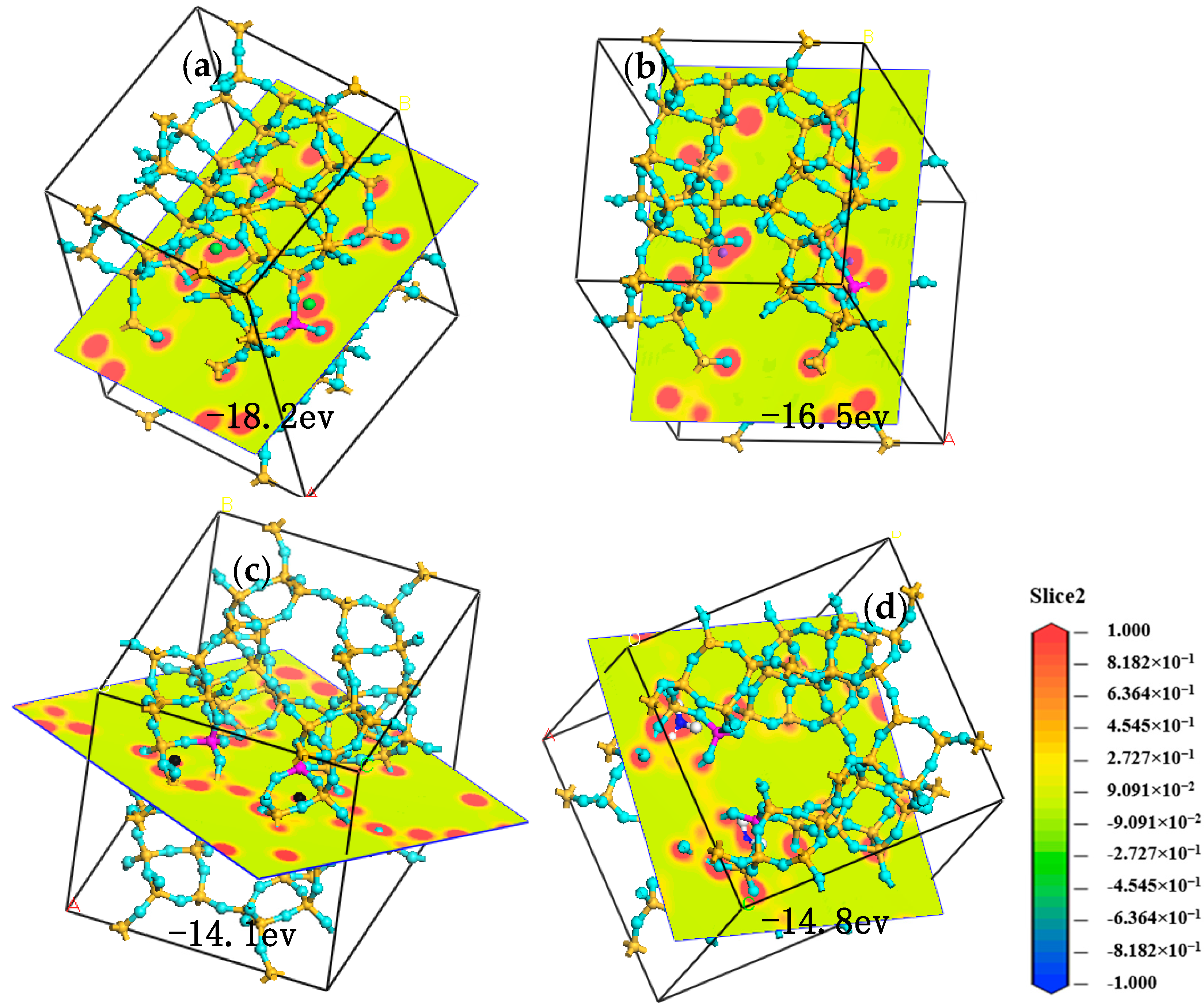
Molecular simulation was performed under the module ‘CASTEP’ of Materials Studio 7.0 software (Accelrys, San Diego, CA, USA) to investigate the sorption sites of cation on Cp. The primitive unit cell of Cp was optimized with the generalized gradient approximation (GGA) for the exchange-correlation potential (PW91). The resulting primitive unit cell was characterized by the parameters a = 17.77 Å, b = 17.95 Å, c = 7.44 Å, and α = γ = 90°, and β = 116°. The number of cycles is 3, and the steps of one cycle are 106, a representative part of the interface devoid of any arbitrary boundary effects.
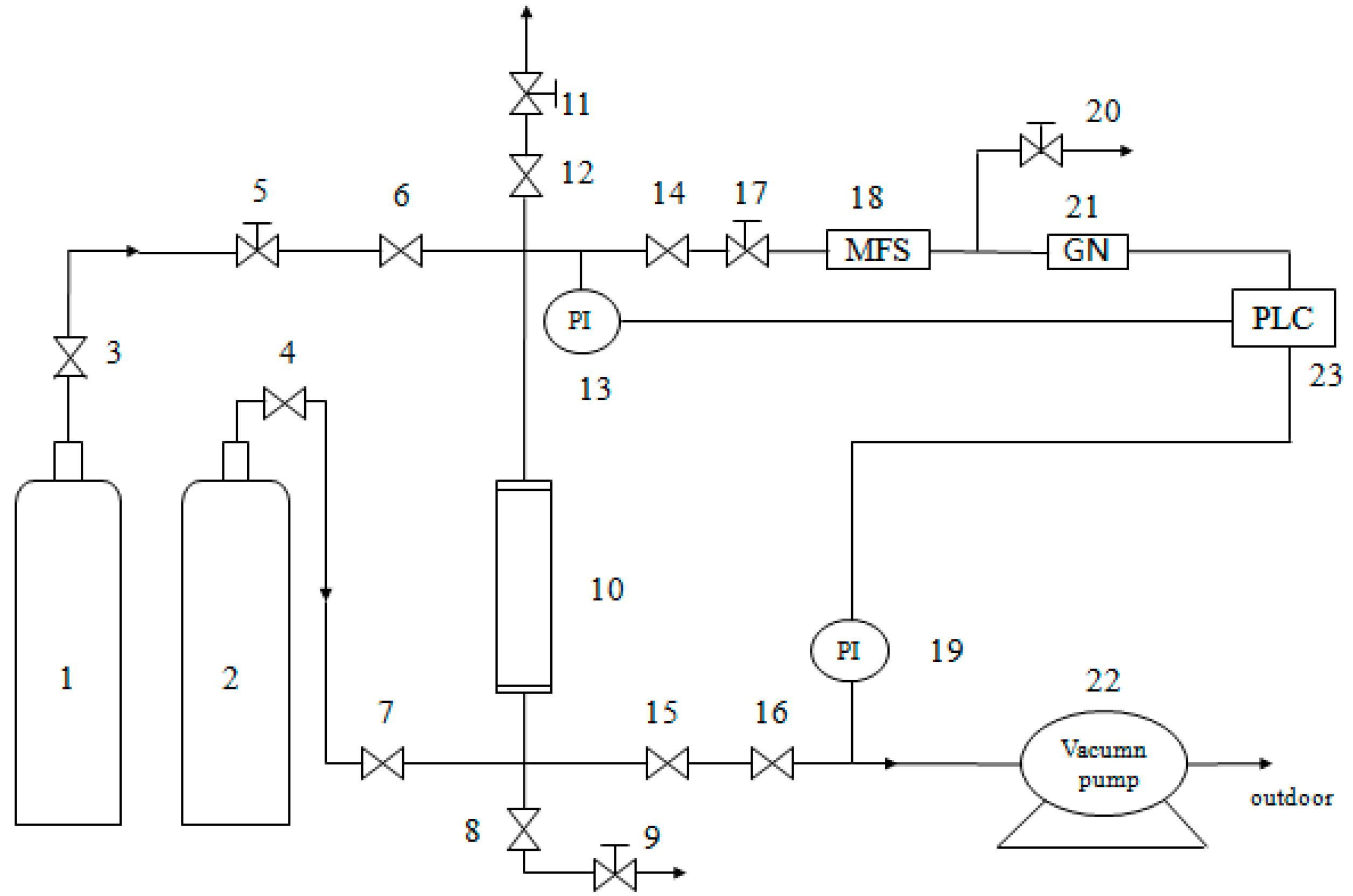
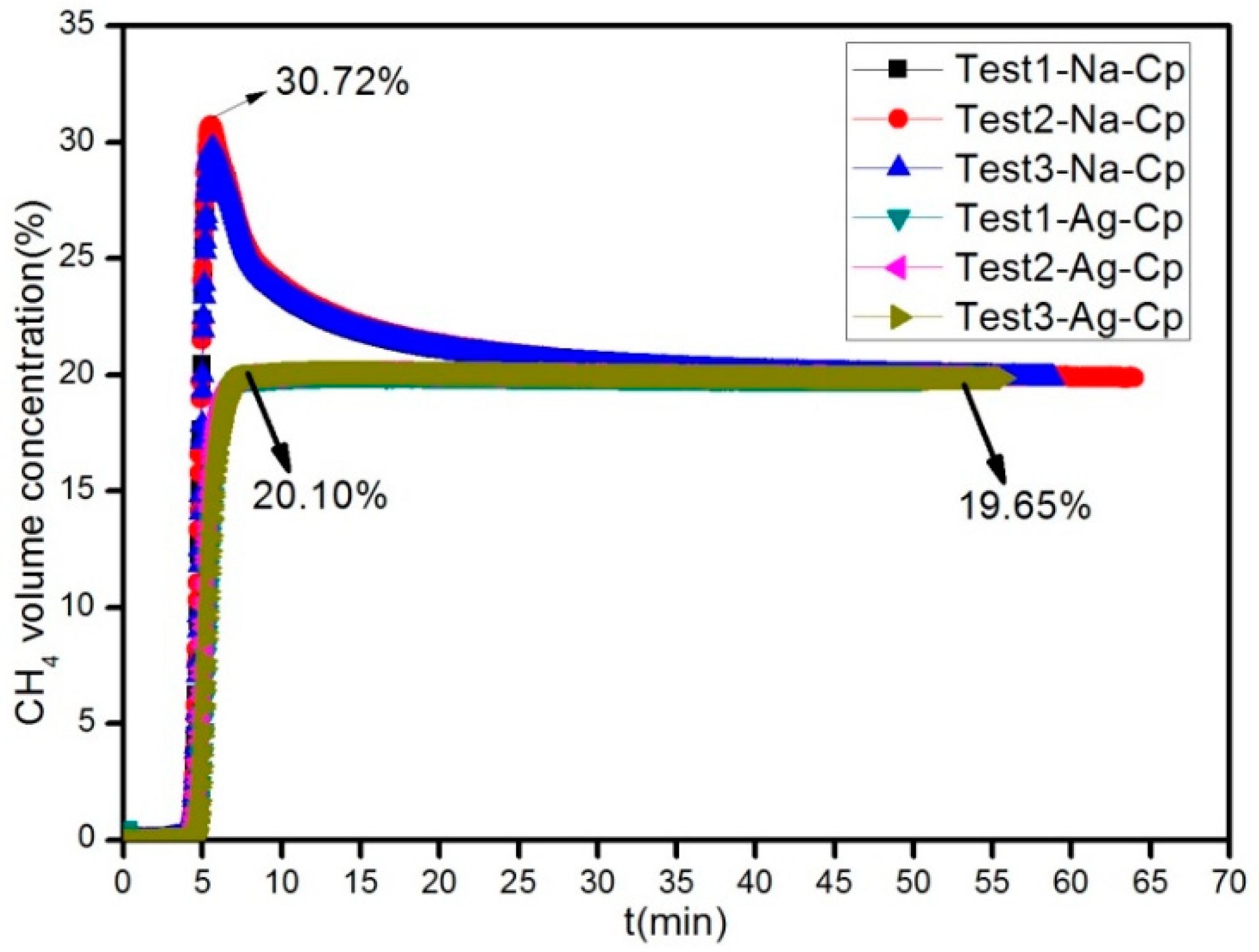
2.4. Dynamic Separation Experiment with CH4/N2
The feed gas is the mixture gas of CH
4 and N
2, and the volume ratio of CH
4/N
2 was 19.7%: 80.3%. The simulated methane-nitrogen mixture (19.7% CH
4, 80.3% N
2) was separated using a single-bed unit (Shown as
Figure 2) with the modified Cp in the same conditions of 0.2 MPa and 298 K in the PSA equipment (self-assembly). The absorbing tower was filled with p-Cp and modified Cp, respectively. At first, the device was pressurized using high-pure standard He until the adsorption pressure was up to the setting pressure at 0.2 MPa. Then, the intake valve of He was closed, and the intake valve of mixture gas was opened (it was the start time of data recording). In order to keep the pressure of absorbing tower reaching the experimental value, it was adjusted by control valves (the flow value of gas was set to 60 mL/min). The outlet discharge was set using mass flow controller before the test. In the process of absorption, the change of concentration of CH
4 was tested and recorded by gas analyzer. The testing was not over until the testing volume concentration of CH
4 had risen to the concentration of CH
4 in the feed gas. Then, all valves were closed, and testing was over. Finally, the activation and regeneration of modified clinoptilolite was not begun until inverse vacuum was pumped for 30 min.
4. Conclusions
In conclusion, natural clinoptilolite with micropores, mesopores, and macropores on its surface was purified using centrifugal concentrator. The clinoptilolite adsorbents prepared via the ion exchange treatment of purified clinoptilolite with K+, NH4+, Rb+, Cu2+, Zn2+, Ba2+, Cs+, Li+, N+, Ce3+, Sr2+, Ag+, Mg2+, and Ca2+ showed different adsorption properties for CH4 and N2. The adsorbents of NH4-Cp, Cu-Cp, and K-Cp showed strong adsorbability for CH4, and the equilibrium separation factors for CH4/N2 were 2.56, 1.95, and 1.82, respectively. Moreover, the adsorbents Cp-Na and Cp-Ag showed good absorbability for N2, and the equilibrium separation factors for N2/CH4 were 7.25 and 6.53, respectively. Lastly, through the dynamic simulation test of CH4 and N2, it was found that best sorbent is Na-Cp, which produced high concentration CH4. The large equilibrium separation factor and smaller investment make the practical application of adsorbent Cp-Na possible.