Bone Healing in Rabbit Calvaria Defects Using a Synthetic Bone Substitute: A Histological and Micro-CT Comparative Study
Abstract
1. Introduction
2. Experimental Section
2.1. Animals
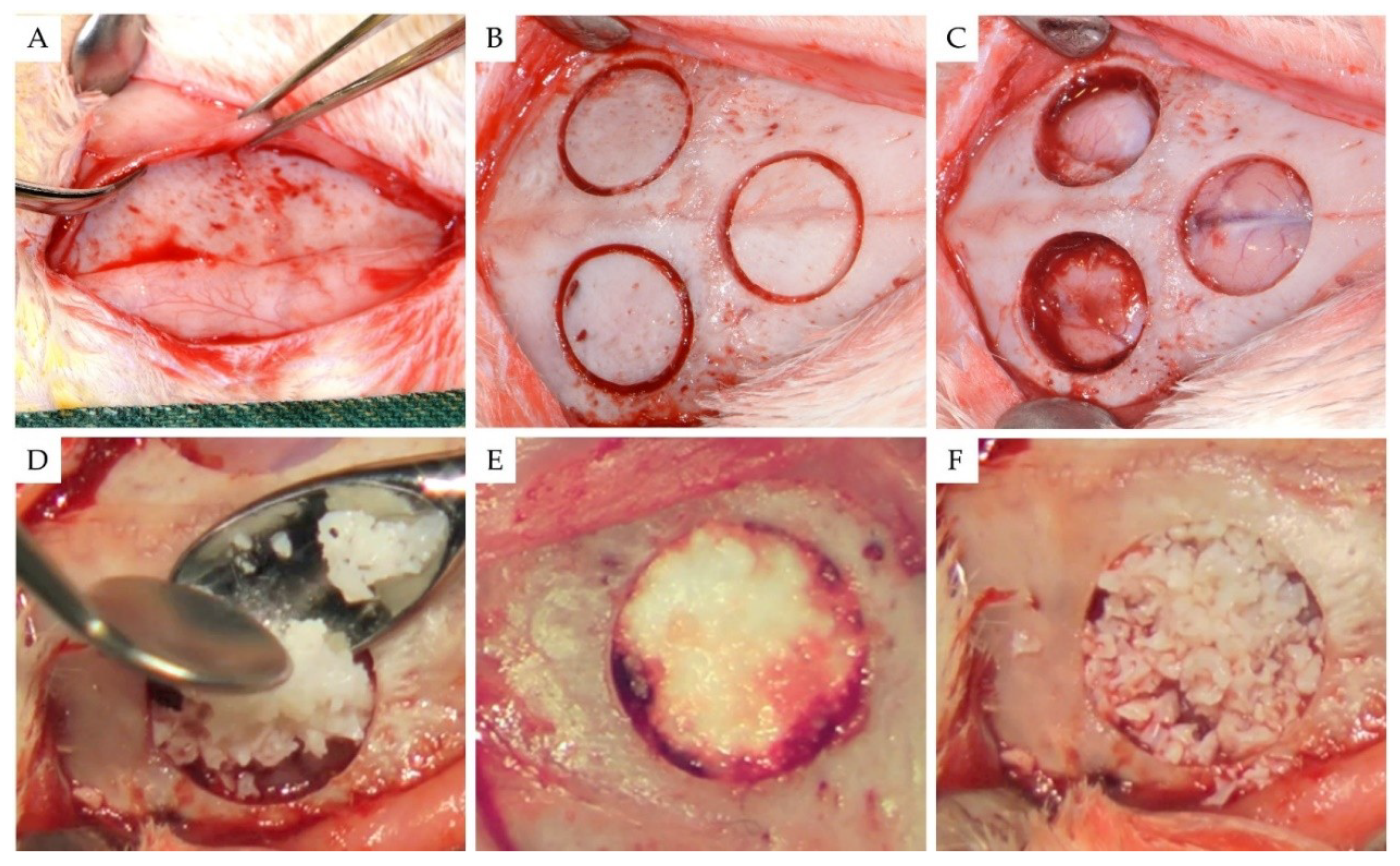
2.2. Surgical Procedures
2.3. Micro-CT Evaluation
2.4. Histology
2.5. Statistics
3. Results
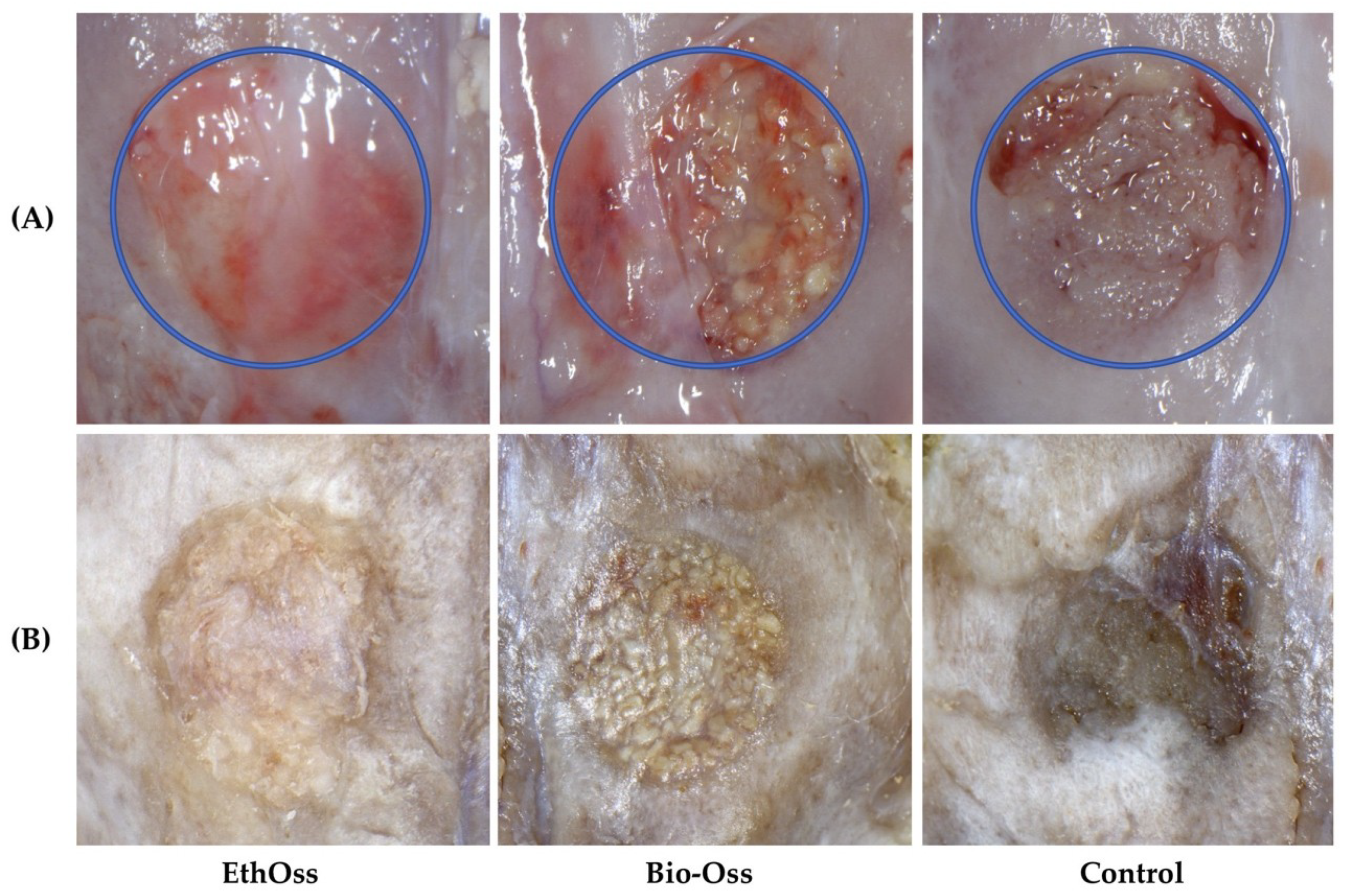
3.1. Overall
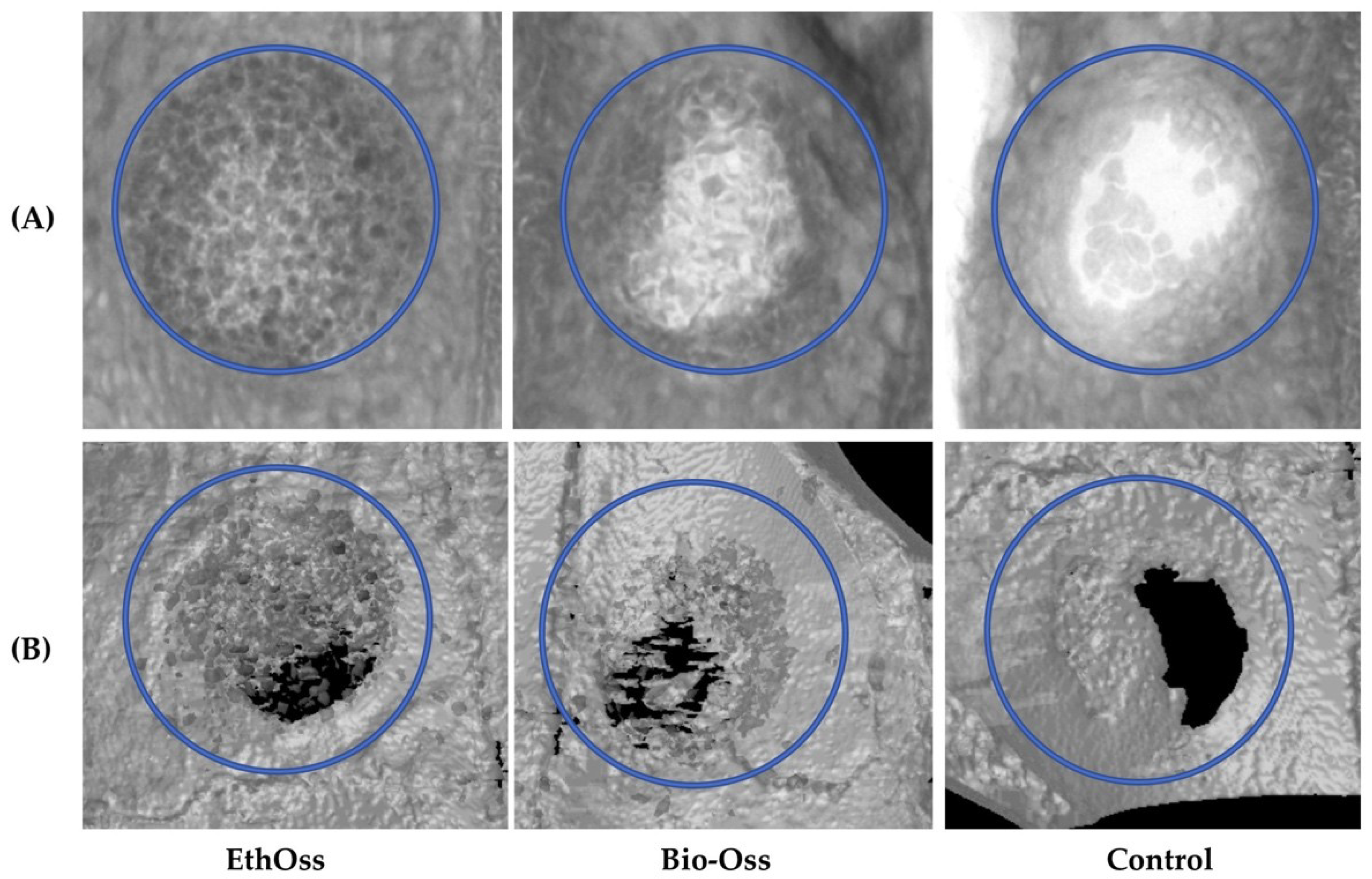
3.2. Micro-CT Evaluation
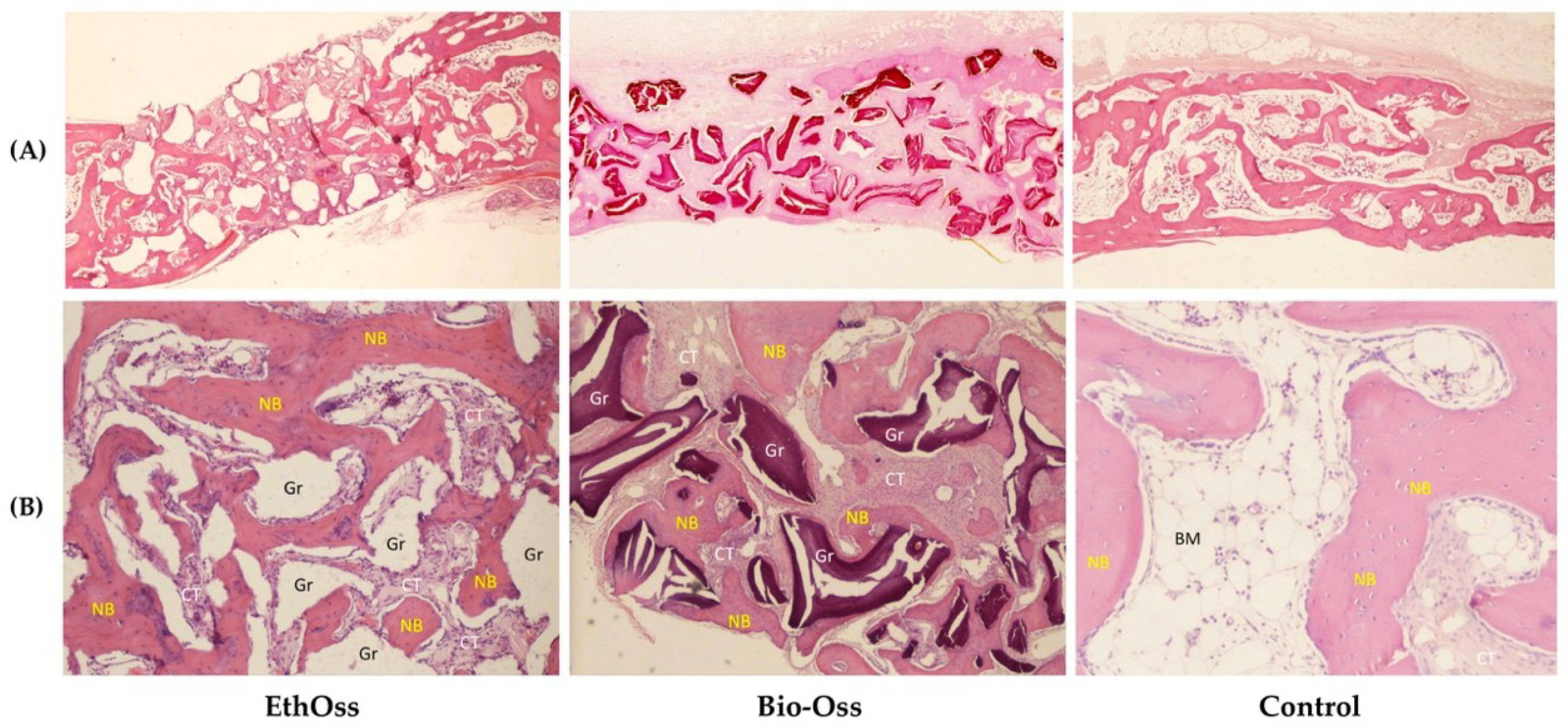
3.3. Histology
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Yip, I.; Ma, L.; Mattheos, N.; Dard, M.; Lang, N.P. Defect healing with various bone substitutes. Clin. Oral Implants Res. 2015, 26, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.E.; Lang, N.P. Ridge preservation after tooth extraction. Clin. Oral Implants Res. 2012, 23 (Suppl. 6), 147–156. [Google Scholar] [CrossRef]
- Misch, C.M. Autogenous bone: Is it still the gold standard? Implant Dent. 2010, 19, 361. [Google Scholar] [CrossRef] [PubMed]
- Le, B.Q.; Nurcombe, V.; Cool, S.M.; van Blitterswijk, C.A.; de Boer, J.; La Pointe, V.L.S. The Components of Bone and What They Can Teach Us about Regeneration. Materials 2017, 11, E14. [Google Scholar] [CrossRef] [PubMed]
- Henkel, J.; Woodruff, M.A.; Epari, D.R.; Steck, R.; Glatt, V.; Dickinson, I.C.; Choong, P.F.; Schuetz, M.A.; Hutmacher, D.W. Bone regeneration based on tissue engineering conceptions—A 21st century perspective. Bone Res. 2013, 1, 216–248. [Google Scholar] [CrossRef] [PubMed]
- Schlickewei, W.; Schlickewei, C. The Use of Bone Substitutes in the Treatment of Bone Defects-the Clinical View and History. Macromol. Symp. 2007, 253, 10–23. [Google Scholar] [CrossRef]
- Chan, H.L.; Lin, G.H.; Fu, J.H.; Wang, H.L. Alterations in bone quality after socket preservation with grafting materials: A systematic review. Int. J. Oral Maxillofac. Implants 2013, 28, 710–720. [Google Scholar] [CrossRef] [PubMed]
- Iocca, O.; Farcomeni, A.; Pardiñas Lopez, S.; Talib, H.S. Alveolar ridge preservation after tooth extraction: A Bayesian Network meta-analysis of grafting materials efficacy on prevention of bone height and width reduction. J. Clin. Periodontol. 2017, 44, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Danesh-Sani, S.A.; Engebretson, S.P.; Janal, M.N. Histomorphometric results of different grafting materials and effect of healing time on bone maturation after sinus floor augmentation: A systematic review and meta-analysis. J. Periodontal Res. 2017, 52, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Jambhekar, S.; Kernen, F.; Bidra, A.S. Clinical and histologic outcomes of socket grafting after flapless tooth extraction: A systematic review of randomized controlled clinical trials. J. Prosthet. Dent. 2015, 113, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, R.A.; Leventis, M.D.; Rohrer, M.D.; Prasad, H.S. Bone grafting: History, rationale, and selection of materials and techniques. Compend. Contin. Educ. Dent. 2014, 35 (Suppl. 4), 1–6. [Google Scholar]
- Pryor, L.S.; Gage, E.; Langevin, C.J.; Herrera, F.; Breithaupt, A.D.; Gordon, C.R.; Afifi, A.M.; Zins, J.E.; Meltzer, H.; Gosman, A.; et al. Review of bone substitutes. Craniomaxillofac. Trauma Reconstr. 2009, 2, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Peng, S.; Feng, P.; Shuai, C. Bone biomaterials and interactions with stem cells. Bone Res. 2017, 5, 17059. [Google Scholar] [CrossRef] [PubMed]
- Glenske, K.; Donkiewicz, P.; Köwitsch, A.; Milosevic-Oljaca, N.; Rider, P.; Rofall, S.; Franke, J.; Jung, O.; Smeets, R.; Schnettler, R.; et al. Applications of Metals for Bone Regeneration. Int. J. Mol. Sci. 2018, 19, E826. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V. Calcium orthophosphates in nature, biology and medicine. Materials 2009, 2, 399–498. [Google Scholar] [CrossRef]
- Araújo, M.G.; Liljenberg, B.; Lindhe, J. β-tricalcium phosphate in the early phase of socket healing: An experimental study in the dog. Clin. Oral Implants Res. 2010, 21, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Trisi, P.; Rao, W.; Rebaudi, A.; Fiore, P. Histologic effect of pure-phase beta-tricalcium phosphate on bone regeneration in human artificial jawbone defects. Int. J. Periodontics Restorative Dent. 2003, 23, 69–78. [Google Scholar] [PubMed]
- Roh, J.; Kim, J.Y.; Choi, Y.M.; Ha, S.M.; Kim, K.N.; Kim, K.M. Bone Regeneration Using a Mixture of Silicon-Substituted Coral HA and β-TCP in a Rat Calvarial Bone Defect Model. Materials 2016, 9, E97. [Google Scholar] [CrossRef] [PubMed]
- Gregori, G.; Kleebe, H.J.; Mayr, H.; Ziegler, G. EELS characterisation of β-tricalcium phosphate and hydroxyapatite. J. Eur. Ceram. Soc. 2006, 26, 1473–1479. [Google Scholar] [CrossRef]
- Daculsi, G.; Laboux, O.; Malard, O.; Weiss, P. Current state of the art of biphasic calcium phosphate bioceramics. J. Mater. Sci. Mater. Med. 2003, 14, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Harel, N.; Moses, O.; Palti, A.; Ormianer, Z. Long-term results of implants immediately placed into extraction sockets grafted with β-tricalcium phosphate: A retrospective study. J. Oral Maxillofac. Surg. 2013, 71, E63–E68. [Google Scholar] [CrossRef] [PubMed]
- Kucera, T.; Sponer, P.; Urban, K.; Kohout, A. Histological assessment of tissue from large human bone defects repaired with β-tricalcium phosphate. Eur. J. Orthop. Surg. Traumatol. 2014, 24, 1357–1365. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Fernandes, H.; Habibovic, P.; de Boer, J.; Barradas, A.M.; de Ruiter, A.; Walsh, W.R.; van Blitterswijk, C.A.; de Bruijn, J.D. Osteoinductive ceramics as a synthetic alternative to autologous bone grafting. Proc. Natl. Acad. Sci. USA 2010, 107, 13614–13619. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Li, X.; Tan, Y.; Fan, H.; Zhang, X. The material and biological characteristics of osteoinductive calcium phosphate ceramics. Regen. Biomater. 2018, 5, 43–59. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Zhang, Q.; Sculean, A.; Buser, D.; Pippenger, B.E.; Dard, M.; Shirakata, Y.; Chandad, F.; Zhang, Y. Osteoinductive potential of 4 commonly employed bone grafts. Clin. Oral Investig. 2016, 20, 2259–2265. [Google Scholar] [CrossRef] [PubMed]
- Barradas, A.M.; Yuan, H.; van Blitterswijk, C.; Habibovic, P. Osteoinductive biomaterials: Current knowledge of properties, experimental models and biological mechanisms. Eur. Cell. Mater. 2010, 21, 407–429. [Google Scholar] [CrossRef]
- Cheng, L.; Shi, Y.; Ye, F.; Bu, H. Osteoinduction of calcium phosphate biomaterials in small animals. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 1254–1260. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, A.; Habibovic, P. Calcium phosphates and angiogenesis: Implications and advances for bone regeneration. Trends Biotechnol. 2016, 34, 983–992. [Google Scholar] [CrossRef] [PubMed]
- Ruga, E.; Gallesio, C.; Chiusa, L.; Boffano, P. Clinical and histologic outcomes of calcium sulfate in the treatment of postextraction sockets. J. Craniofac. Surg. 2011, 22, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Anson, D. Using calcium sulfate in guided tissue regeneration: A recipe for success. Compend. Contin. Educ. Dent. 2000, 21, 365–370. [Google Scholar] [PubMed]
- Eleftheriadis, E.; Leventis, M.D.; Tosios, K.I.; Faratzis, G.; Titsinidis, S.; Eleftheriadi, I.; Dontas, I. Osteogenic activity of β-tricalcium phosphate in a hydroxyl sulphate matrix and demineralized bone matrix: A histological study in rabbit mandible. J. Oral Sci. 2010, 52, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Podaropoulos, L.; Veis, A.A.; Papadimitriou, S.; Alexandridis, C.; Kalyvas, D. Bone regeneration using b-tricalcium phosphate in a calcium sulfate matrix. J. Oral Implantol. 2009, 35, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Leventis, M.D.; Fairbairn, P.; Dontas, I.; Faratzis, G.; Valavanis, K.D.; Khaldi, L.; Kostakis, G.; Eleftheriadis, E. Biological response to β-tricalcium phosphate/calcium sulfate synthetic graft material: An experimental study. Implant Dent. 2014, 23, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Fairbairn, P.; Leventis, M. Protocol for Bone Augmentation with Simultaneous Early Implant Placement: A Retrospective Multicenter Clinical Study. Int. J. Dent. 2015, 2015, 589135. [Google Scholar] [CrossRef] [PubMed]
- Fairbairn, P.; Leventis, M.; Mangham, C.; Horowitz, R. Alveolar Ridge Preservation Using a Novel Synthetic Grafting Material: A Case with Two-Year Follow-Up. Case Rep. Dent. 2018, 2018, 6412806. [Google Scholar] [CrossRef] [PubMed]
- Giannoudis, P.V.; Einhorn, T.A.; Marsh, D. Fracture healing: The diamond concept. Injury 2007, 38, S3–S6. [Google Scholar] [CrossRef]
- Troedhan, A.; Schlichting, I.; Kurrek, A.; Wainwright, M. Primary implant stability in augmented sinuslift-sites after completed bone regeneration: A randomized controlled clinical study comparing four subantrally inserted biomaterials. Sci. Rep. 2014, 4, 5877. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, R.; Mataliotakis, G.I.; Calori, G.M.; Giannoudis, P.V. The role of barrier membranes for guided bone regeneration and restoration of large bone defects: Current experimental and clinical evidence. BMC Med. 2012, 10, 81. [Google Scholar] [CrossRef] [PubMed]
- Pecora, G.; Andreana, S.; Margarone, J.E.; Covani, U.; Sottosanti, J.S. Bone regeneration with a calcium sulfate barrier. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1997, 84, 424–429. [Google Scholar] [CrossRef]
- Mazor, Z.; Mamidwar, S.; Ricci, J.L.; Tovar, N.M. Bone repair in periodontal defect using a composite of allograft and calcium sulfate (DentoGen) and a calcium sulfate barrier. J. Oral Implantol. 2011, 37, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Strocchi, R.; Orsini, G.; Iezzi, G.; Scarano, A.; Rubini, C.; Pecora, G.; Piattelli, A. Bone regeneration with calcium sulfate: Evidence for increased angiogenesis in rabbits. J. Oral Implantol. 2002, 28, 273–278. [Google Scholar] [CrossRef]
- Artzi, Z.; Weinreb, M.; Givol, N.; Rohrer, M.D.; Nemcovsky, C.E.; Prasad, H.S.; Tal, H. Biomaterial Resorption Rate and Healing Site Morphology of Inorganic Bovine Bone and β-Tricalcium Phosphate in the Canine: A 24-month Longitudinal Histologic Study and Morphometric Analysis. Int. J. Oral Maxillofac. Implants 2004, 19, 357–368. [Google Scholar] [PubMed]
- Palti, A.; Hoch, T. A concept for the treatment of various dental bone defects. Implant Dent. 2002, 11, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Artzi, Z.; Tal, H.; Dayan, D. Porous bovine bone mineral in healing of human extraction sockets. Part 1: Histomorphometric evaluations at 9 months. J. Periodontol. 2000, 71, 1015–1023. [Google Scholar] [CrossRef] [PubMed]
- Artzi, Z.; Tal, H.; Dayan, D. Porous bovine bone mineral in healing of human extraction sockets: 2. Histochemical observations at 9 months. J. Periodontol. 2001, 72, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Pang, C.; Ding, Y.; Zhou, H.; Qin, R.; Hou, R.; Zhang, G.; Hu, K. Alveolar ridge preservation with deproteinized bovine bone graft and collagen membrane and delayed implants. J. Craniofac. Surg. 2014, 25, 1698–1702. [Google Scholar] [CrossRef] [PubMed]
- Aludden, H.C.; Mordenfeld, A.; Hallman, M.; Dahlin, C.; Jensen, T. Lateral ridge augmentation with Bio-Oss alone or Bio-Oss mixed with particulate autogenous bone graft: A systematic review. Int. J. Oral Maxillofac. Surg. 2017, 46, 1030–1038. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.; Schou, S.; Stavropoulos, A.; Terheyden, H.; Holmstrup, P. Maxillary sinus floor augmentation with Bio-Oss or Bio-Oss mixed with autogenous bone as graft: A systematic review. Clin. Oral Implants Res. 2012, 23, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Scheyer, E.T.; Velasquez-Plata, D.; Brunsvold, M.A.; Lasho, D.J.; Mellonig, J.T. A clinical comparison of a bovine-derived xenograft used alone and in combination with enamel matrix derivative for the treatment of periodontal osseous defects in humans. J. Periodontοl. 2002, 73, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Chappuis, V.; Rahman, L.; Buser, R.; Janner, S.F.M.; Belser, U.C.; Buser, D. Effectiveness of contour augmentation with guided bone regeneration: 10-year results. J. Dent. Res. 2018, 97, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Mordenfeld, A.; Hallman, M.; Johansson, C.B.; Albrektsson, T. Histological and histomorphometrical analyses of biopsies harvested 11 years after maxillary sinus floor augmentation with deproteinized bovine and autogenous bone. Clin. Oral Implants Res. 2010, 21, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Heberer, S.; Al-Chawaf, B.; Hildebrand, D.; Nelson, J.J.; Nelson, K. Histomorphometric analysis of extraction sockets augmented with Bio-Oss Collagen after a 6-week healing period: A prospective study. Clin. Oral Implants Res. 2008, 19, 1219–1225. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Nowzari, H.; Rich, S.K. Risk of Prion Disease Transmission through Bovine-Derived Bone Substitutes: A Systematic Review. Clin. Implant Dent. Relat. Res. 2013, 15, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Rodriguez, A.E.; Nowzari, H. The risk of prion infection through bovine grafting materials. Clin. Implant Dent. Relat. Res. 2016, 18, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.L.; Zhu, X.S.; Chen, L.; Chen, C.M.; Mangham, D.C.; Coulton, L.A.; Aiken, S.S. Bone healing response to a synthetic calcium sulfate/β-tricalcium phosphate graft material in a sheep vertebral body defect model. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 1911–1921. [Google Scholar] [CrossRef] [PubMed]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Müller, R. Guidelines for assessment of bone microstructure in rodents using micro–computed tomography. J. Bone Miner. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef] [PubMed]
- Bouxsein, M.L. Determinants of skeletal fragility. Best Pract. Res. Clin. Rheumatol. 2005, 19, 897–911. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Abbreviation | Description | Standard Unit |
|---|---|---|---|
| Bone volume fraction | BV/TV | Ratio of the segmented newly-formed bone volume to the total volume of the region of interest | % |
| Residual material volume fraction | RMVF | Ratio of the residual grafting material volume to the total volume of the region of interest | % |
| Trabecular number | Tb.N | Measure of the average number of trabeculae per unit length | 1/mm |
| Trabecular thickness | Tb.Th | Mean thickness of trabeculae, assessed using direct 3D methods | mm |
| Trabecular separation | Tb.Sp | Mean distance between trabeculae, assessed using direct 3D methods | mm |
| Parameter | Site | N | Mean | SD | p-Value |
|---|---|---|---|---|---|
| BV/TV | EthOss | 6 | 33.70 | 8.94 | 0.525 |
| Bio-Oss | 6 | 24.07 | 9.69 | ||
| Control | 6 | 27.36 | 10.95 | ||
| RMVF | EthOss | 6 | 13.41 | 6.43 | 0.070 |
| Bio-Oss | 6 | 21.36 | 10.05 | ||
| Control | 6 | - | - | - |
| Parameter | Site | N | Mean | SD | p-Value |
|---|---|---|---|---|---|
| Tb.N | EthOss | 6 | 1.511 a | 0.255 a | <0.001 |
| Bio-Oss | 6 | 1.213 a | 0.198 a | ||
| Control | 6 | 0.541 | 0.239 | ||
| Tb.Th | EthOss | 6 | 0.219 b | 0.018 b | <0.001 |
| Bio-Oss | 6 | 0.291 | 0.029 | ||
| Control | 6 | 0.210 b | 0.028 b | ||
| Tb.S | EthOss | 6 | 0.486 a | 0.136 a | <0.001 |
| Bio-Oss | 6 | 0.713 a | 0.238 a | ||
| Control | 6 | 1.686 | 0.455 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leventis, M.; Fairbairn, P.; Mangham, C.; Galanos, A.; Vasiliadis, O.; Papavasileiou, D.; Horowitz, R. Bone Healing in Rabbit Calvaria Defects Using a Synthetic Bone Substitute: A Histological and Micro-CT Comparative Study. Materials 2018, 11, 2004. https://doi.org/10.3390/ma11102004
Leventis M, Fairbairn P, Mangham C, Galanos A, Vasiliadis O, Papavasileiou D, Horowitz R. Bone Healing in Rabbit Calvaria Defects Using a Synthetic Bone Substitute: A Histological and Micro-CT Comparative Study. Materials. 2018; 11(10):2004. https://doi.org/10.3390/ma11102004
Chicago/Turabian StyleLeventis, Minas, Peter Fairbairn, Chas Mangham, Antonios Galanos, Orestis Vasiliadis, Danai Papavasileiou, and Robert Horowitz. 2018. "Bone Healing in Rabbit Calvaria Defects Using a Synthetic Bone Substitute: A Histological and Micro-CT Comparative Study" Materials 11, no. 10: 2004. https://doi.org/10.3390/ma11102004
APA StyleLeventis, M., Fairbairn, P., Mangham, C., Galanos, A., Vasiliadis, O., Papavasileiou, D., & Horowitz, R. (2018). Bone Healing in Rabbit Calvaria Defects Using a Synthetic Bone Substitute: A Histological and Micro-CT Comparative Study. Materials, 11(10), 2004. https://doi.org/10.3390/ma11102004





