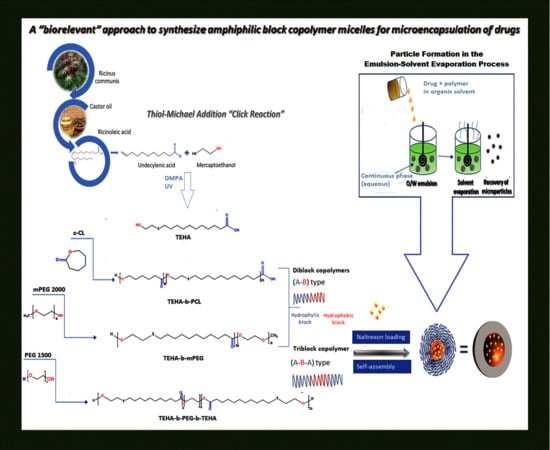
Amphiphilic Block Copolymer Microspheres Derived from Castor Oil, Poly(ε-carpolactone), and Poly(ethylene glycol): Preparation, Characterization and Application in Naltrexone Drug Delivery
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
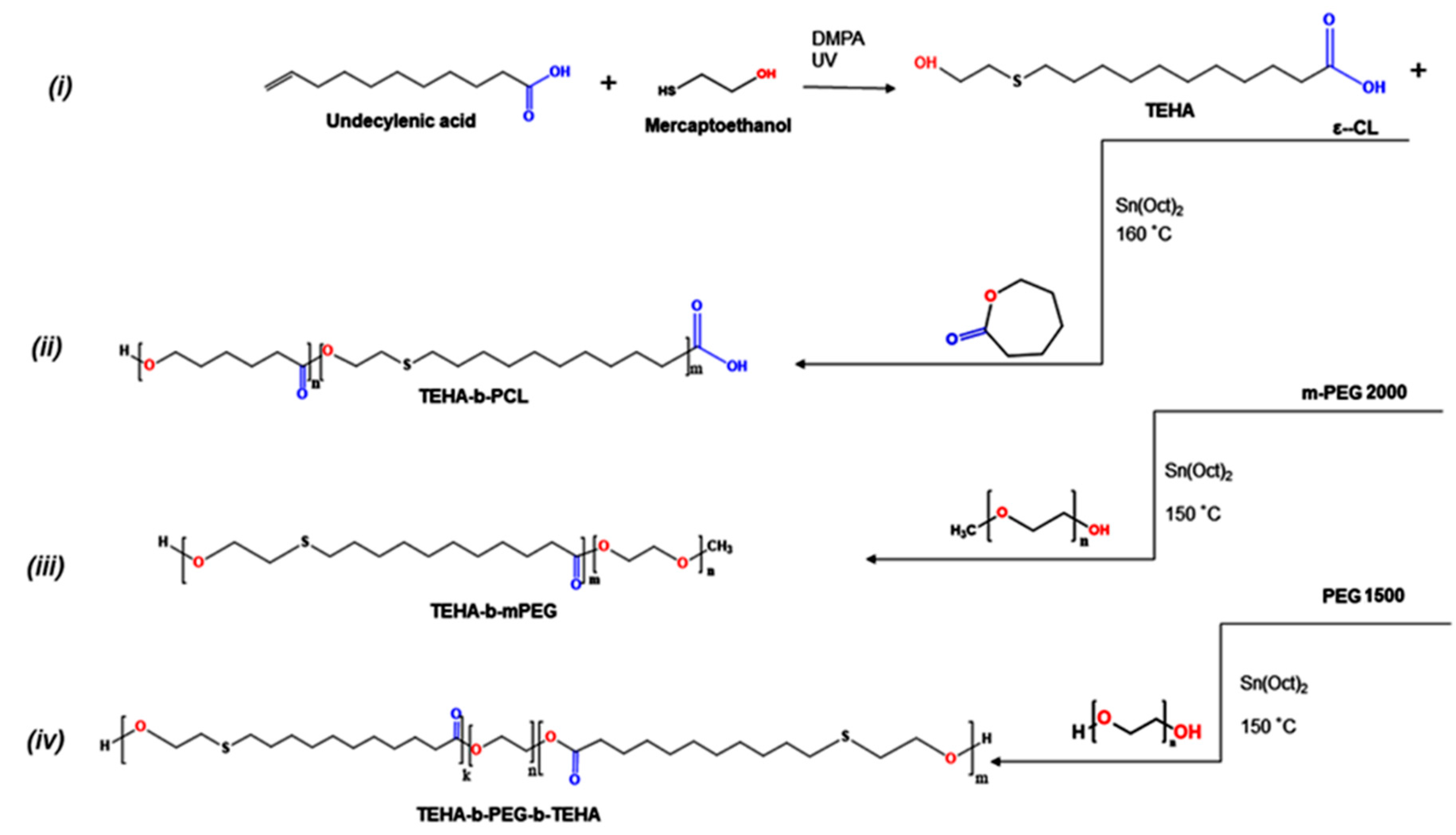
2.2. Synthesis of TEHA, TEHA-b-PCL, TEHA-b-mPEG, and TEHA-b-PEG-b-TEHA
2.3. Preparation of TEHA-b-PCL, TEHA-b-mPEG, and TEHA-b-PEG-b-TEHA Microspheres
2.4. Characterization of Materials
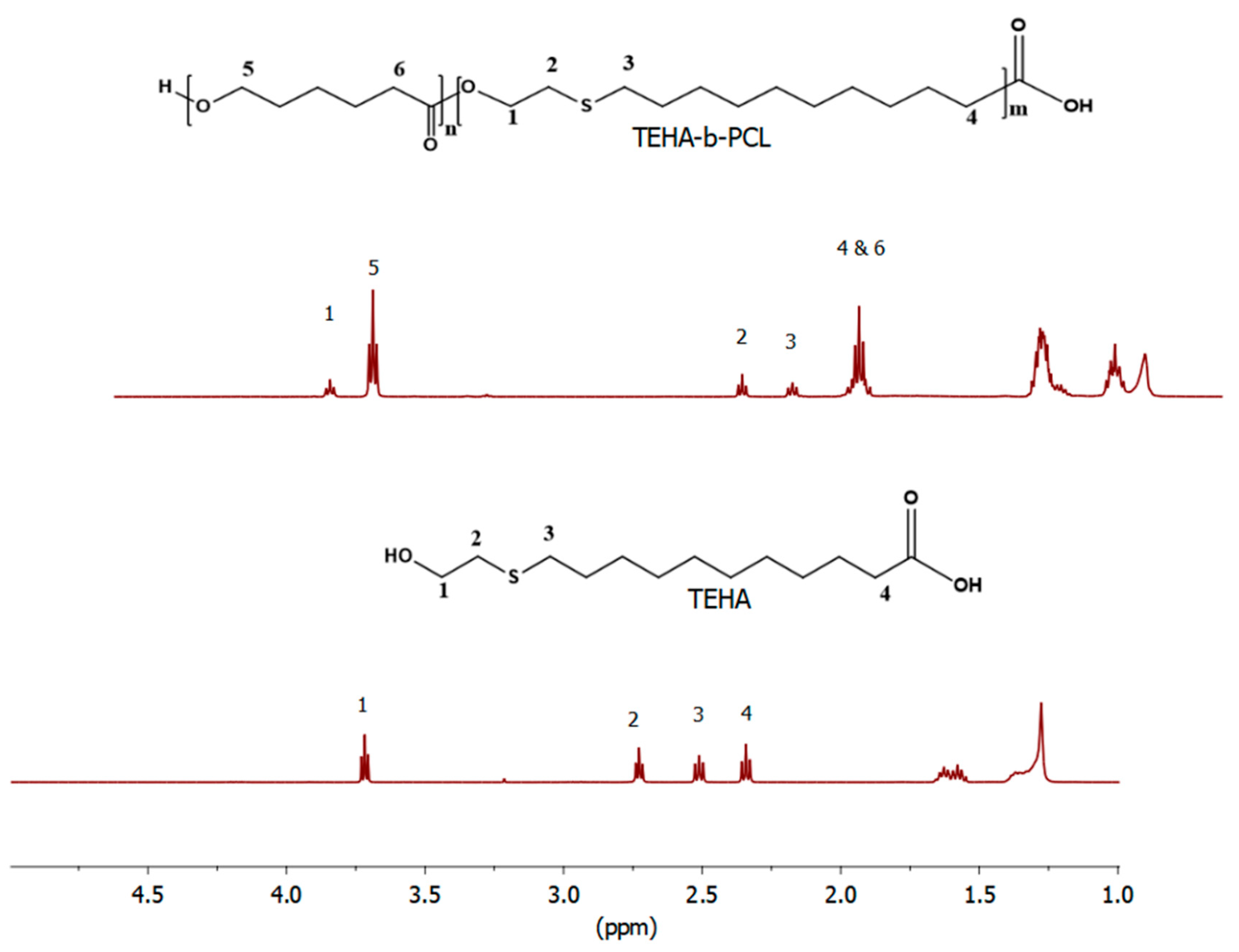
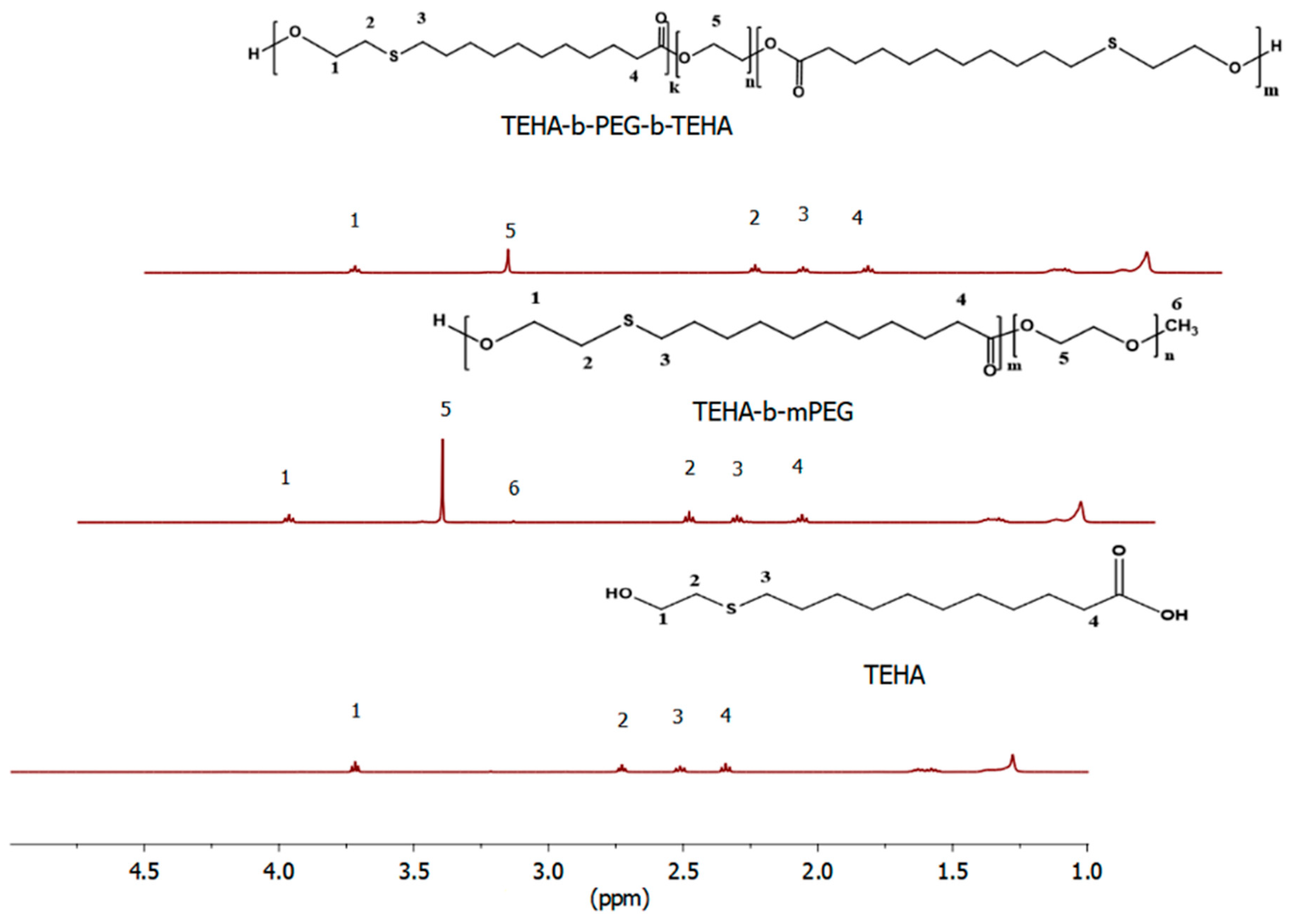
2.4.1. Proton Nuclear Magnetic Resonance (1H-NMR) Spectroscopy
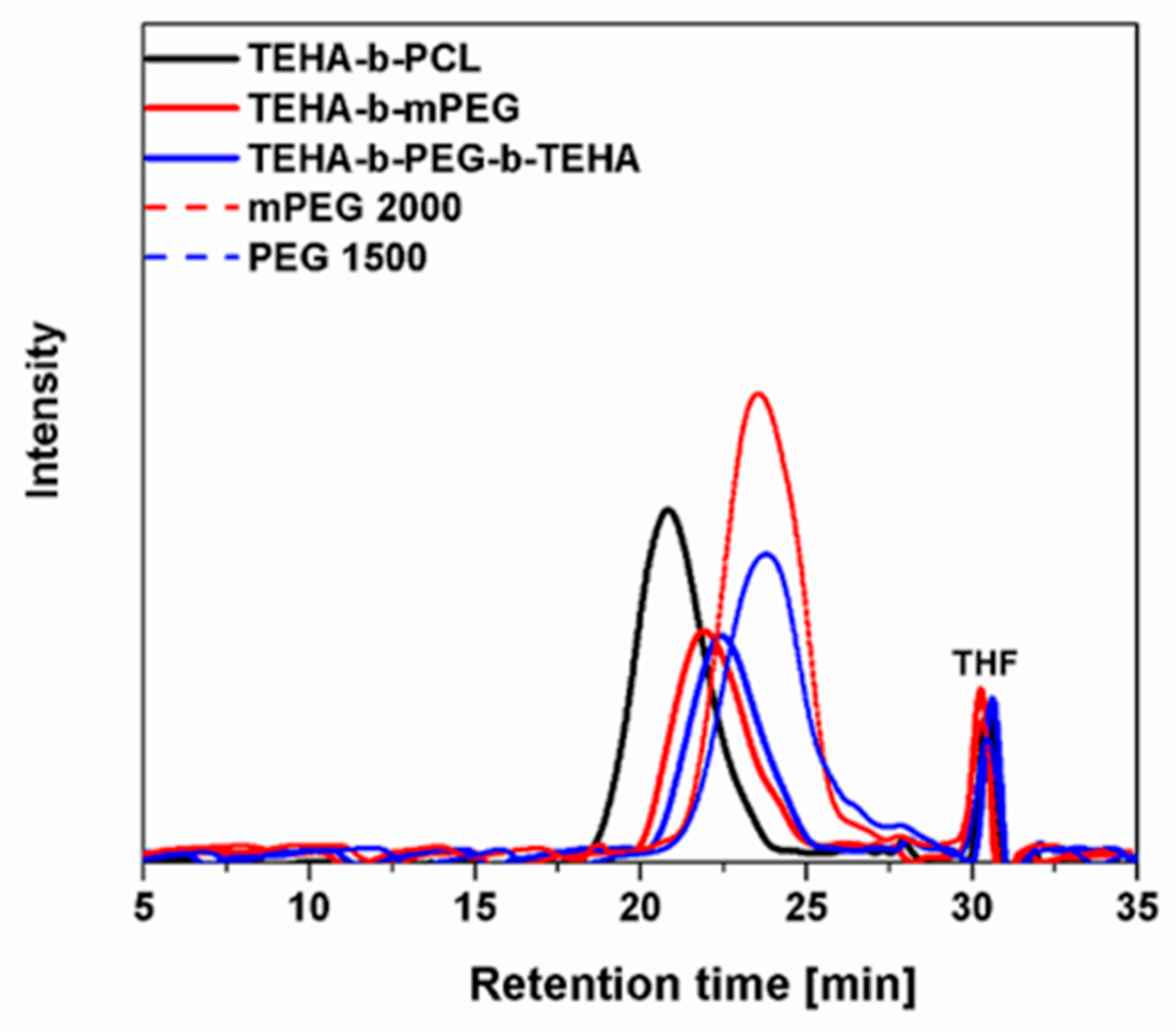
2.4.2. Size Exclusion Chromatography (SEC)
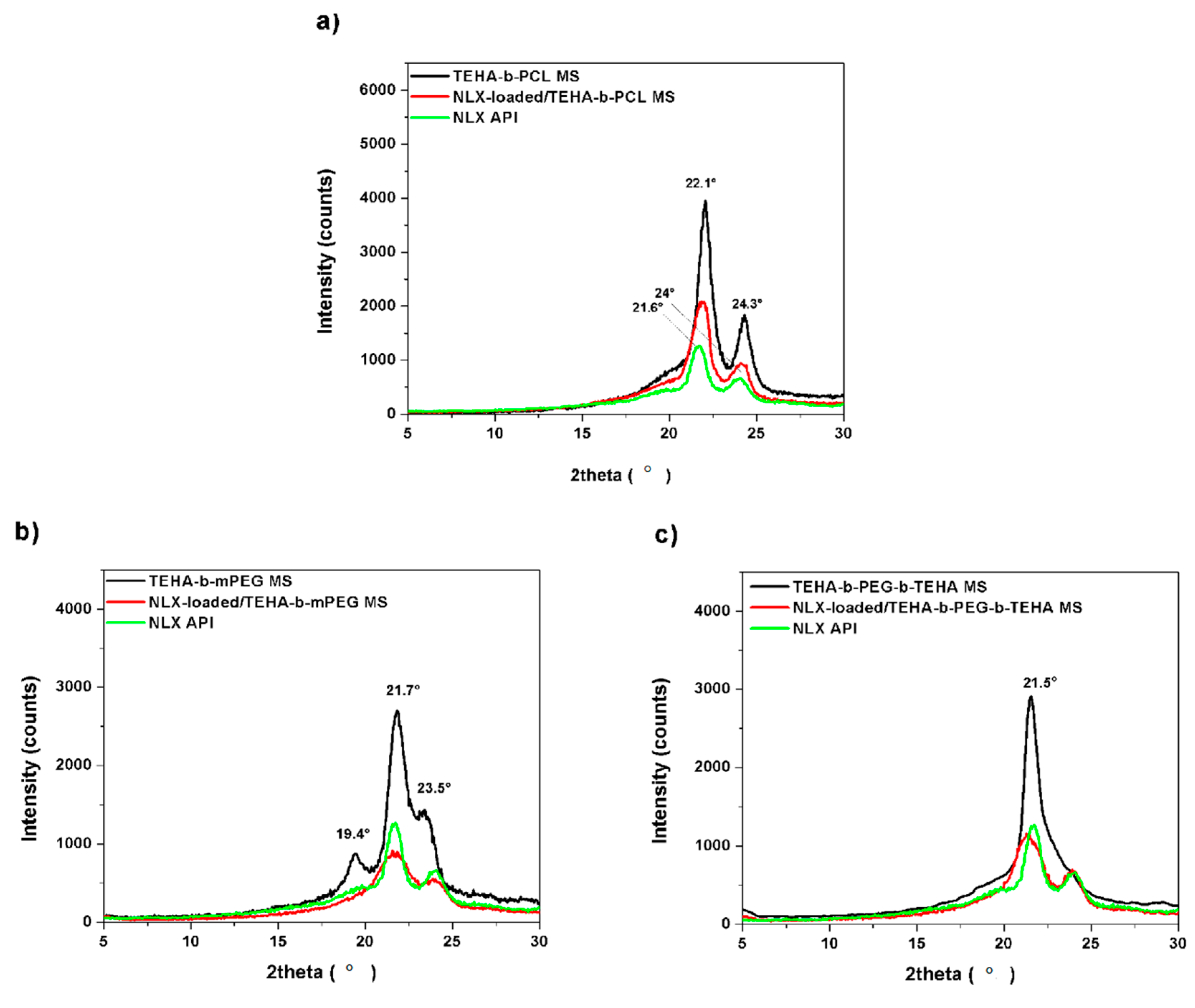
2.4.3. X-ray Diffractometry (XRD)
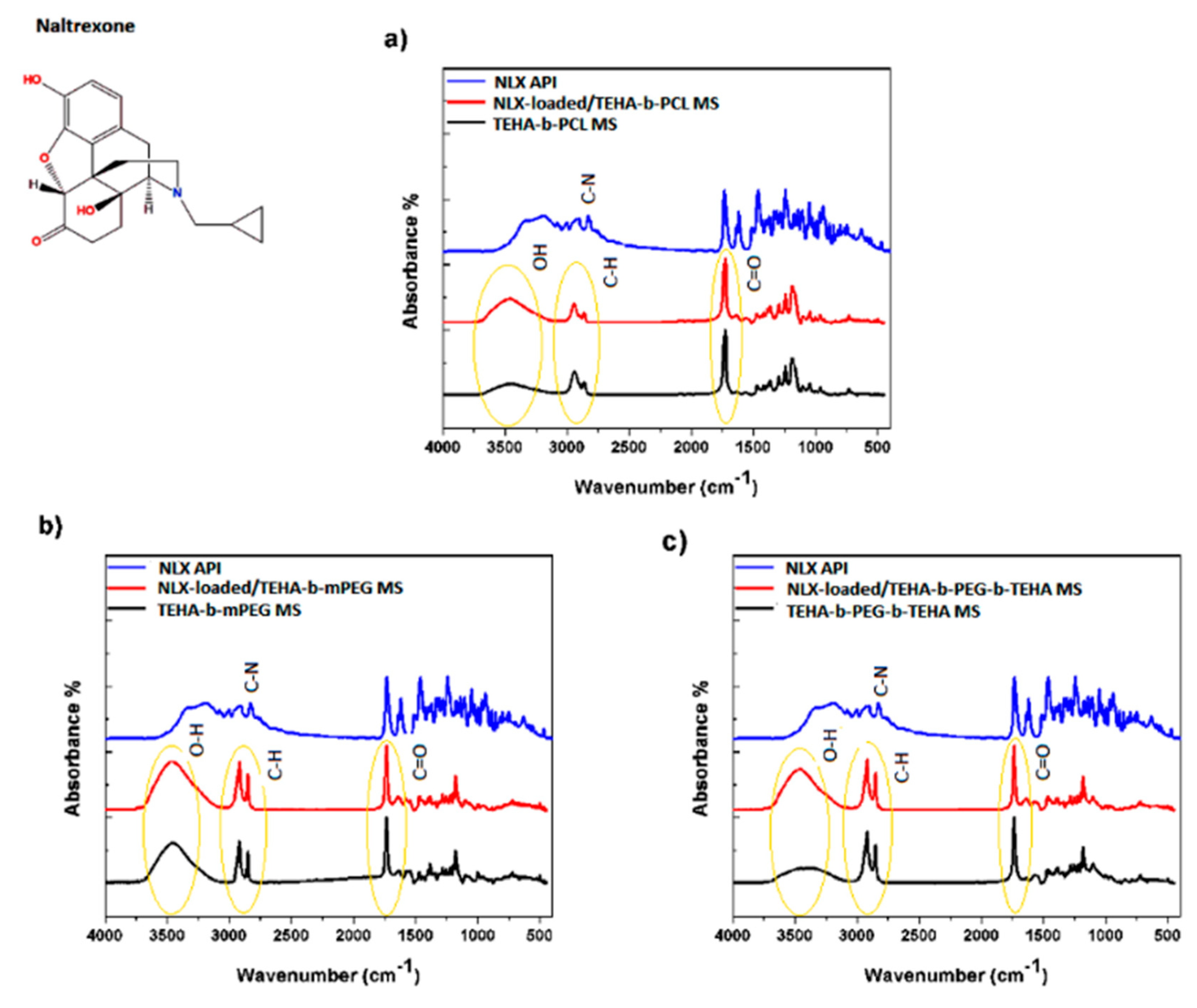
2.4.4. Fourier Transform-Infrared Spectroscopy (FTIR)
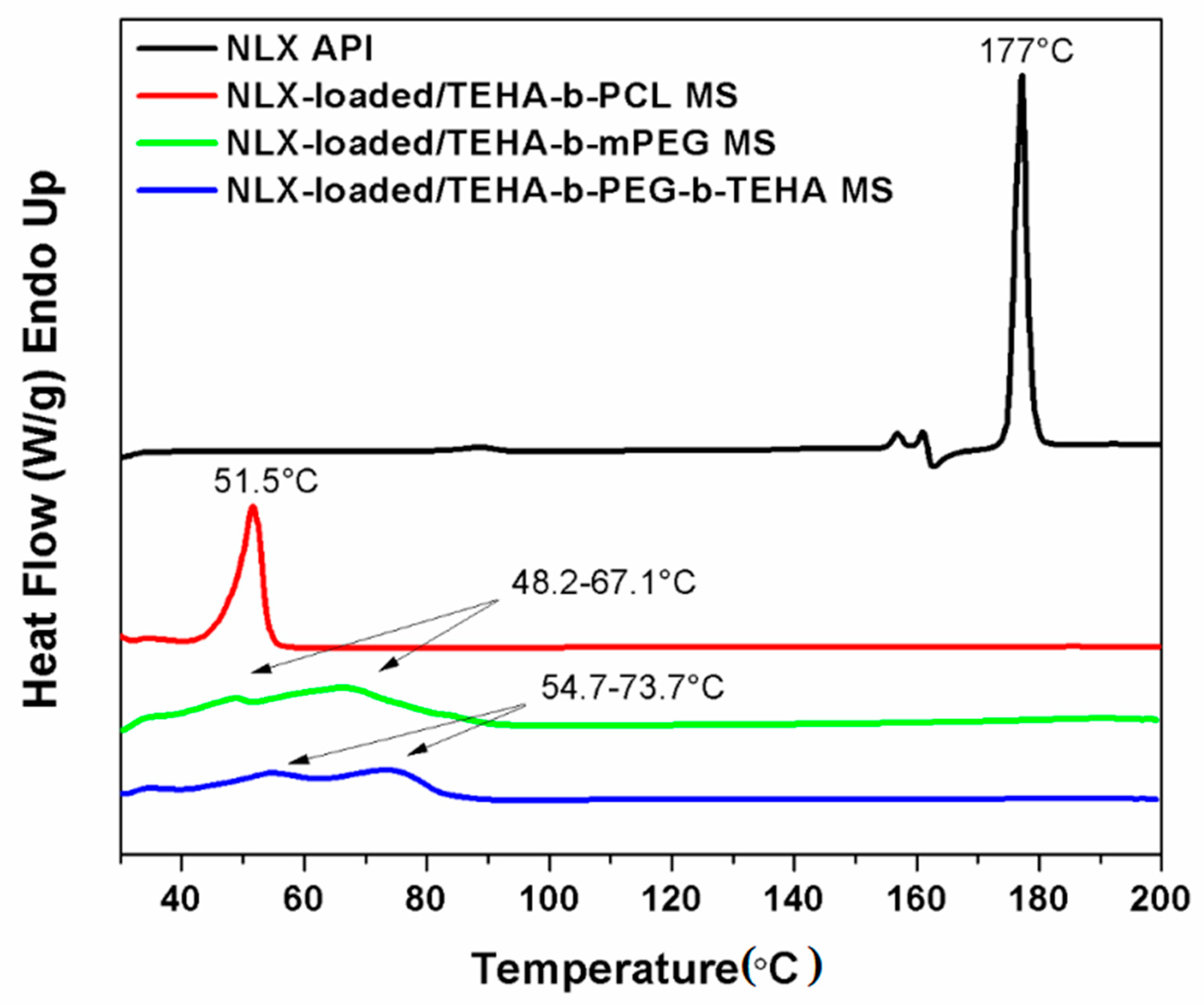
2.4.5. Differential Scanning Calorimetry (DSC)
2.4.6. Scanning Electron Microscopy (SEM)
2.4.7. Evaluation of Drug Encapsulation
2.4.8. In Vitro Release Profile
3. Results and Discussion
3.1. Synthesis and Characterization of Amphiphilic TEHA-Based Block Copolymers
3.2. Evaluation of Drug-Loaded Microspheres Prepared from Amphiphilic TEHA-Based Block Polymers
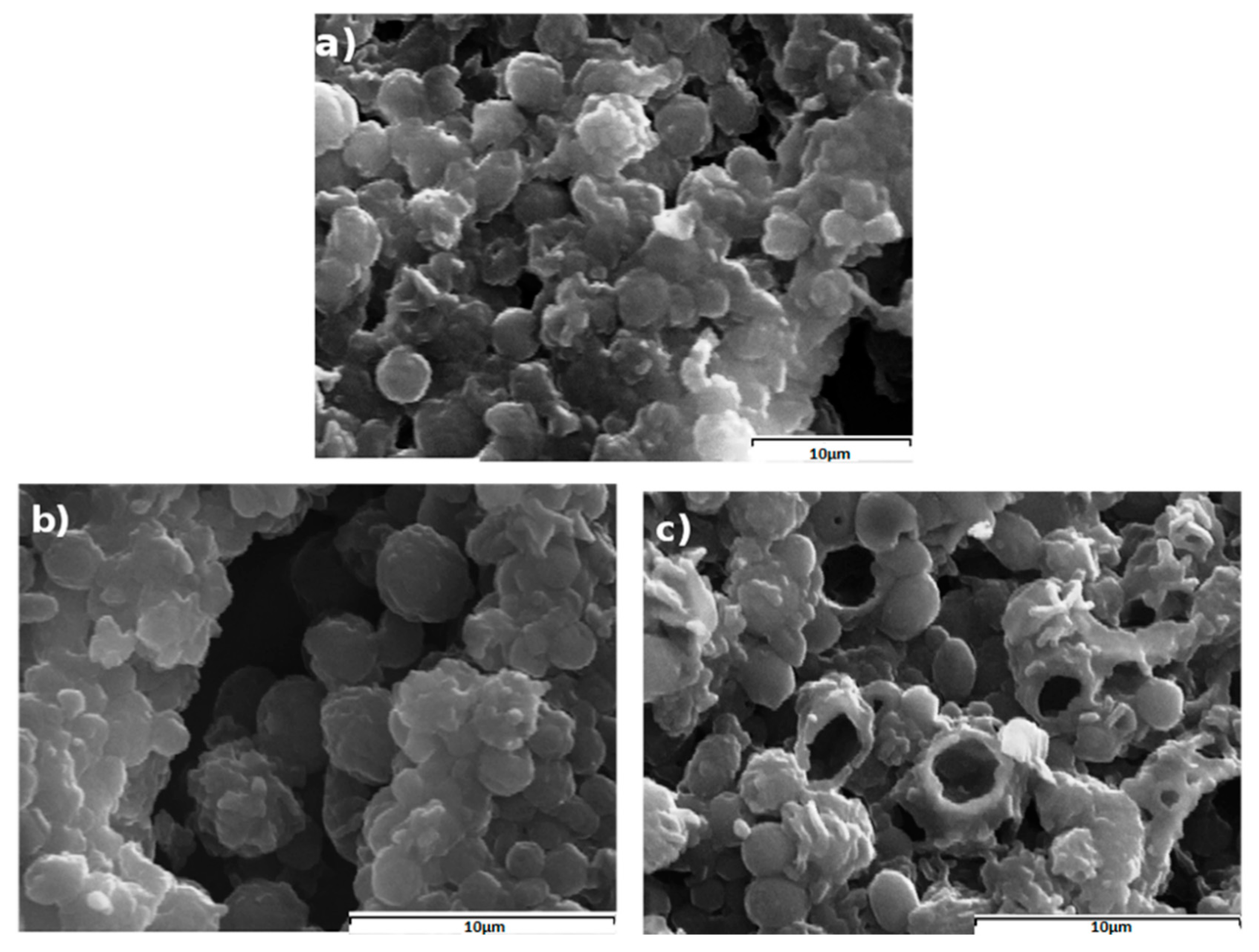
3.2.1. Drug Loading and Morphology of Prepared Microspheres
3.2.2. FTIR, XRD and DSC Analysis of Microparticles
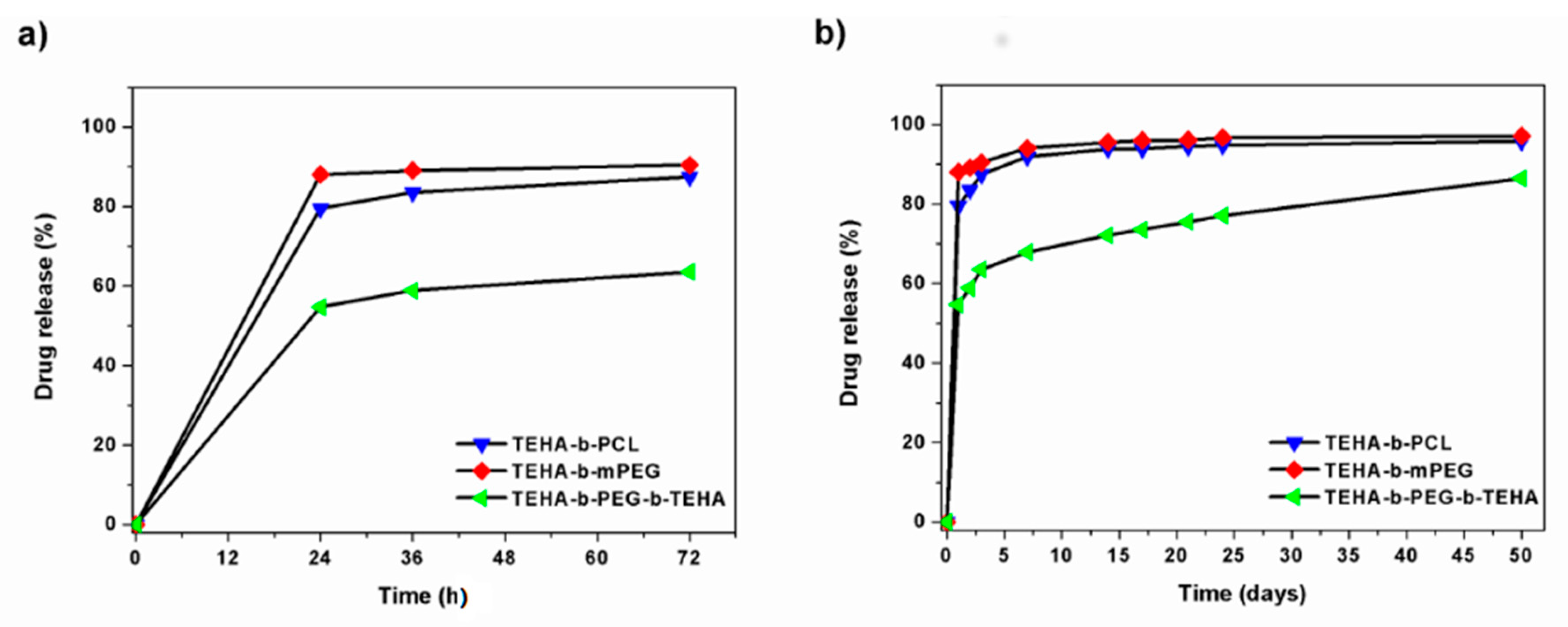
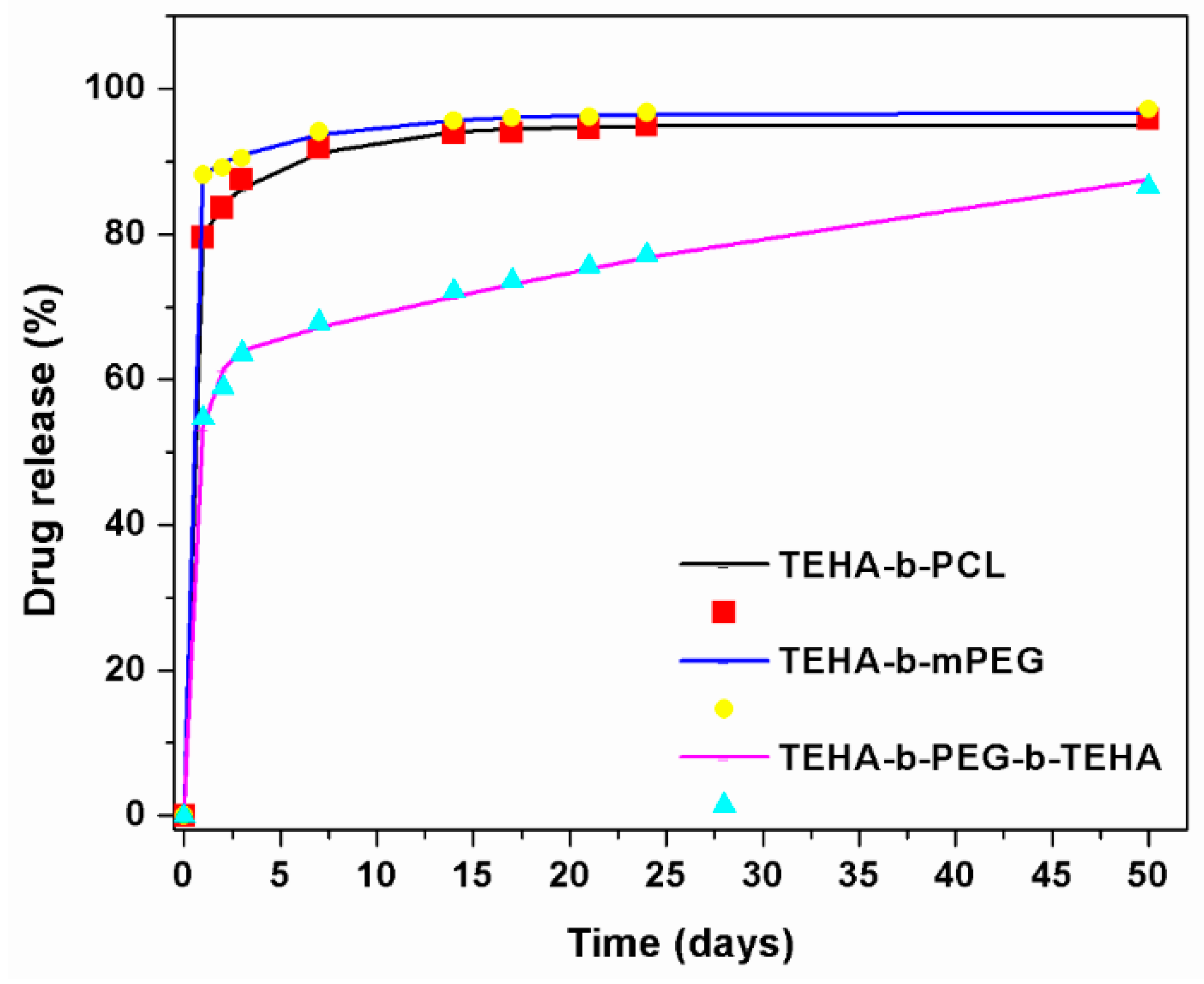
3.2.3. In Vitro Release Properties
3.2.4. Drug Release Kinetics
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Bader, H.; Ringsdorf, H.; Schmidt, B. Watersoluble polymers in medicine. Die Angew. Makromol. Chem. 1984, 123, 457–485. [Google Scholar] [CrossRef]
- Cho, H.K.; Cheong, I.W.; Lee, J.M.; Kim, J.H. Polymeric nanoparticles, micelles and polymersomes from amphiphilic block copolymer. Korean J. Chem. Eng. 2010, 27, 731–740. [Google Scholar] [CrossRef]
- Hu, X.; Jing, X. Biodegradable amphiphilic polymer-drug conjugate micelles. Expert Opin. Drug Deliv. 2009, 6, 1079–1090. [Google Scholar] [CrossRef] [PubMed]
- Helstrom, A.W.; Blow, F.C.; Slaymaker, V.; Kranzler, H.R.; Leong, S.; Oslin, D. Reductions in alcohol craving following naltrexone treatment for heavy drinking. Alcohol. Alcohol. 2016, 51, 562–566. [Google Scholar] [CrossRef] [PubMed]
- Callahan, E.J.; Rawson, R.A.; McCleave, B.; Arias, R.; Glazer, M.; Liberman, R.P. The Treatment of Heroin Addiction: Naltrexone Alone and with Behavior Therapy. Int. J. Addict. 1980, 15, 795–807. [Google Scholar] [CrossRef] [PubMed]
- Hulse, G.K. Improving clinical outcomes for naltrexone as a management of problem alcohol use. Br. J. Clin. Pharmacol. 2013, 76, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Krupitsky, E.M.; Blokhina, E.A. Long-acting depot formulations of naltrexone for heroin dependence: A review. Curr. Opin. Psychiatry 2010, 23, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Goonoo, N.; Bhaw-Luximon, A.; Ujoodha, R.; Jhugroo, A.; Hulse, G.K.; Jhurry, D. Naltrexone: A review of existing sustained drug delivery systems and emerging nano-based systems. J. Control. Release 2014, 183, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Comer, S.D.; Sullivan, M.A.; Yu, E.; Rothenberg, J.L.; Kleber, H.D.; Kampman, K.; Dackis, C.; O’Brien, C.P. Injectable, Sustained-Release Naltrexone for the Treatment of Opioid Dependence: A Randomized, Placebo-Controlled Trial. Arch. Gen. Psychiatry 2006, 63, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Hulse, G.K.; Stalenberg, V.; McCallum, D.; Smit, W.; O’Neil, G.; Morris, N.; Tait, R.J. Histological changes over time around the site of sustained release naltrexone-poly(dl-lactide) implants in humans. J. Control. Release 2005, 108, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Volpicelli, J.R.; Fenton, M. Sustained-release naltrexone formulations for the treatment of alcohol and opioid dependence. Future Neurol. 2006, 1, 389–398. [Google Scholar] [CrossRef]
- Akala, E.O.; Wiriyacoonkasem, P.; Pan, G. Studies on in vitro availability, degradation, and thermal properties of naltrexone-loaded biodegradable microspheres. Drug Dev. Ind. Pharm. 2011, 37, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Salehi, R.; Nowruzi, K.; Salehi, S.; Khandaghi, A.A.; Davaran, S.; Entezami, A.A. Smart poly (N-isopropylacrylamide)-block-poly (l-Lactide) nanoparticles for prolonged release of naltrexone. Int. J. Polym. Mater. Polym. Biomater. 2013, 62, 686–694. [Google Scholar] [CrossRef]
- Pagar, K.P.; Vavia, P.R. Naltrexone-loaded poly[La-(Glc-Leu)] polymeric microspheres for the treatment of alcohol dependence: In vitro characterization and in vivo biocompatibility assessment. Pharm. Dev. Technol. 2014, 19, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Park, K. Polymeric micelles and alternative nanonized delivery vehicles for poorly soluble drugs. Int. J. Pharm. 2013, 453, 198–214. [Google Scholar] [CrossRef] [PubMed]
- Vassiliou, A.A.; Papadimitriou, S.A.; Bikiaris, D.N.; Mattheolabakis, G.; Avgoustakis, K. Facile synthesis of polyester-PEG triblock copolymers and preparation of amphiphilic nanoparticles as drug carriers. J. Control. Release 2010, 148, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Nerantzaki, M.; Adam, K.V.; Koliakou, I.; Skoufa, E.; Avgeropoulos, A.; Papageorgiou, G.Z.; Bikiaris, D. Novel Castor Oil-Derived Block Copolymers as Promising Candidates for Biological Applications: Biorelevant and Biocompatible. Macromol. Chem. Phys. 2017, 218, 1–13. [Google Scholar] [CrossRef]
- Beyazkilic, Z.; Lligadas, G.; Ronda, J.C.; Galià, M.; Cádiz, V. Fully biobased triblock copolyesters from l-lactide and sulfur-containing castor oil derivatives: Preparation, oxidation and characterization. Polymer 2015, 68, 101–110. [Google Scholar] [CrossRef]
- Türünç, O.; Firdaus, M.; Kleina, G.; Meier, M.A.R. Fatty acid derived renewable polyamides via thiol–ene additions. Green Chem. 2012, 14, 2577–2583. [Google Scholar] [CrossRef]
- Kim, S.Y.; Ha, J.C.; Lee, Y.M. Poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide)/poly(epsilon-caprolactone) (PCL) amphiphilic block copolymeric nanospheres. II. Thermo-responsive drug release behaviors. J. Control. Release 2000, 65, 345–358. [Google Scholar] [CrossRef]
- Wang, J.; Yao, K.; Wang, C.; Tang, C.; Jiang, X. Synthesis and drug delivery of novel amphiphilic block copolymers containing hydrophobic dehydroabietic moiety. J. Mater. Chem. B 2013, 1, 2324–2332. [Google Scholar] [CrossRef]
- Desroches, M.; Caillol, S.; Lapinte, V.; Auvergne, R.; Boutevin, B. Synthesis of biobased polyols by thiol-ene coupling from vegetable oils. Macromolecules 2011, 44, 2489–2500. [Google Scholar] [CrossRef]
- Danafar, H. MPEG–PCL copolymeric nanoparticles in drug delivery systems. Cogent Med. 2016, 3, 1142411. [Google Scholar] [CrossRef]
- Callari, M.; De Souza, P.L.; Rawal, A. The Effect of Drug Loading on Micelle Properties: Solid-State NMR as a Tool to Gain Structural Insight. Angew. Chem. In.t Ed. 2017, 56, 8441–8445. [Google Scholar] [CrossRef] [PubMed]
- Gaucher, G.; Dufresne, M.H.; Sant, V.P.; Kang, N.; Maysinger, D.; Leroux, J.C. Block copolymer micelles: Preparation, characterization and application in drug delivery. J. Control. Release 2005, 109, 169–188. [Google Scholar] [CrossRef] [PubMed]
- Riess, G. Micellization of block copolymers. Prog. Polym. Sci. 2003, 28, 1107–1170. [Google Scholar] [CrossRef]
- Tan, Q.; Chu, Y.; Bie, M.; Wang, Z.; Xu, X. Preparation and Investigation of Amphiphilic Block Copolymers/Fullerene Nanocomposites as Nanocarriers for Hydrophobic Drug. Materials 2017, 10, 192. [Google Scholar] [CrossRef] [PubMed]
- Rösler, A.; Vandermeulen, G.W.; Klok, H.A. Advanced drug delivery devices via self-assembly of amphiphilic block copolymers. Adv. Drug Deliv. Rev. 2001, 53, 95–108. [Google Scholar] [CrossRef]
- Saralidze, K.; Koole, L.H.; Knetsch, M.L.W. Polymeric microspheres for medical applications. Materials 2010, 3, 537–564. [Google Scholar] [CrossRef]
- Kyu, S.; Dukjoon, C. Drug-releasing behavior of MPEG/PLA block copolymer micelles and solid particles controlled by component block length. J. Appl. Polym. Sci. 2002, 83, 435–445. [Google Scholar] [CrossRef]
- Li, G.; Li, D.; Niu, Y.; He, T.; Chen, K.C.; Xu, K. Alternating block polyurethanes based on PCL and PEG as potential nerve regeneration materials. J. Biomed. Mater. Res. 2014, 102, 685–697. [Google Scholar] [CrossRef] [PubMed]
- Nidhi, K.; Indrajeet, S.; Khushboo, M.; Gauri, K.; Sen, D.J. Hydrotropy: A promising tool for solubility enhancement: A review. Int. J. Drug Dev. Res. 2011, 3, 26–33. [Google Scholar]
- Chen, C.; Xie, X.; Li, Y.; Zhou, C.; Song, Y.; Yan, Z.; Yan, X. Influence of different polymers on crystallization tendency and dissolution behavior of cilnidipine in solid dispersions. Drug Dev. Ind. Pharm. 2014, 40, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Karavelidis, V.; Karavas, E.; Giliopoulos, D.; Papadimitriou, S.; Bikiaris, D. Evaluating the effects of crystallinity in new biocompatible polyester nanocarriers on drug release behavior. Int. J. Nanomed. 2011, 6, 3021–3032. [Google Scholar] [CrossRef]
- Ahmad, Z.; Shah, A.; Siddiq, M.; Kraatz, H.B. Polymeric micelles as drug delivery vehicles. RSC Adv. 2014, 4, 17028–17038. [Google Scholar] [CrossRef]
- Ke, X.; Ng, V.W.L.; Ono, R.J.; Chan, J.M.W.; Krishnamurthy, S.; Wang, Y.; Hedrick, J.L.; Yang, Y.Y. Role of non-covalent and covalent interactions in cargo loading capacity and stability of polymeric micelles. J. Control. Release 2014, 193, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.; Van Colen, G.; Sander, B.; Golas, M.M.; Uezguen, S.; Weigandt, M.; Goepferich, A. Drug loading of polymeric micelles. Pharm. Res. 2013, 30, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Karavelidis, V.; Bikiaris, D.; Avgoustakis, K. New thermosensitive nanoparticles prepared by biocompatible pegylated aliphatic polyester block copolymers for local cancer treatment. J. Pharm. Pharmacol. 2015, 67, 215–230. [Google Scholar] [CrossRef] [PubMed]
- Karavelidis, V.; Giliopoulos, D.; Karavas, E.; Bikiaris, D. Nanoencapsulation of a water soluble drug in biocompatible polyesters. Effect of polyesters melting point and glass transition temperature on drug release behavior. Eur. J. Pharm. Sci. 2010, 41, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Casalini, T.; Rossi, F.; Lazzari, S.; Perale, G.; Masi, M. Mathematical Modeling of PLGA Microparticles: From Polymer Degradation to Drug Release. Mol. Pharm. 2014, 11, 4036–4048. [Google Scholar] [CrossRef] [PubMed]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion, 2nd ed.; Clarendon Press Oxford University Press: Oxford, UK, 1976; ISBN 10-0198534116. [Google Scholar]
- Tien, C. Adsorption Calculations and Modeling; Series in Chemical Engineering; Butterworth Heinemann: Boston, MA, USA, 1994; ISBN 9780750691215. [Google Scholar]
- Parker, A.; Vigouroux, F.; Reed, W.F. Dissolution kinetics of polymer powders. Fluid Mech. Transp. Phenom. 1999, 218, 1290–1299. [Google Scholar] [CrossRef]

| Sample | Feed Molar Ratio 1 | Mn 2 (g/mol) | Mw 2 (g/mol) | PDI 2 |
|---|---|---|---|---|
| TEHA-b-PCL | TEHA:ε-CL, 1:5 | 9800 | 14.800 | 1.55 |
| THEA-b-mPEG | TEHA:mPEG, 1:0.05 | 4.800 | 6.700 | 1.40 |
| TEHA-b-PEG-b-TEHA | TEHA:PEG, 1:0.04 | 2.500 | 3.675 | 1.47 |
| Sample | Yield (%) | Drug Loading (%) | Encapsulation Efficiency (%) |
|---|---|---|---|
| NLX-loaded/TEHA-b-PCL | 41.5 ± 2.1 | 1.19 ± 0.6 | 25.6 ± 1.5 |
| NLX-loaded/THEA-b-mPEG | 32.3 ± 3.2 | 0.27 ± 0.07 | 7.6 ± 0.9 |
| NLX-loaded/TEHA-b-PEG-b-TEHA | 89.9 ± 5.2 | 2.71 ± 0.5 | 25.9 ± 1.4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nerantzaki, M.; Skoufa, E.; Adam, K.-V.; Nanaki, S.; Avgeropoulos, A.; Kostoglou, M.; Bikiaris, D. Amphiphilic Block Copolymer Microspheres Derived from Castor Oil, Poly(ε-carpolactone), and Poly(ethylene glycol): Preparation, Characterization and Application in Naltrexone Drug Delivery. Materials 2018, 11, 1996. https://doi.org/10.3390/ma11101996
Nerantzaki M, Skoufa E, Adam K-V, Nanaki S, Avgeropoulos A, Kostoglou M, Bikiaris D. Amphiphilic Block Copolymer Microspheres Derived from Castor Oil, Poly(ε-carpolactone), and Poly(ethylene glycol): Preparation, Characterization and Application in Naltrexone Drug Delivery. Materials. 2018; 11(10):1996. https://doi.org/10.3390/ma11101996
Chicago/Turabian StyleNerantzaki, Maria, Eirini Skoufa, Kyriakos-Vasileios Adam, Stavroula Nanaki, Apostolos Avgeropoulos, Margaritis Kostoglou, and Dimitrios Bikiaris. 2018. "Amphiphilic Block Copolymer Microspheres Derived from Castor Oil, Poly(ε-carpolactone), and Poly(ethylene glycol): Preparation, Characterization and Application in Naltrexone Drug Delivery" Materials 11, no. 10: 1996. https://doi.org/10.3390/ma11101996
APA StyleNerantzaki, M., Skoufa, E., Adam, K.-V., Nanaki, S., Avgeropoulos, A., Kostoglou, M., & Bikiaris, D. (2018). Amphiphilic Block Copolymer Microspheres Derived from Castor Oil, Poly(ε-carpolactone), and Poly(ethylene glycol): Preparation, Characterization and Application in Naltrexone Drug Delivery. Materials, 11(10), 1996. https://doi.org/10.3390/ma11101996