Low-Temperature CVD Graphene Nanostructures on Cu and Their Corrosion Properties
Abstract
1. Introduction
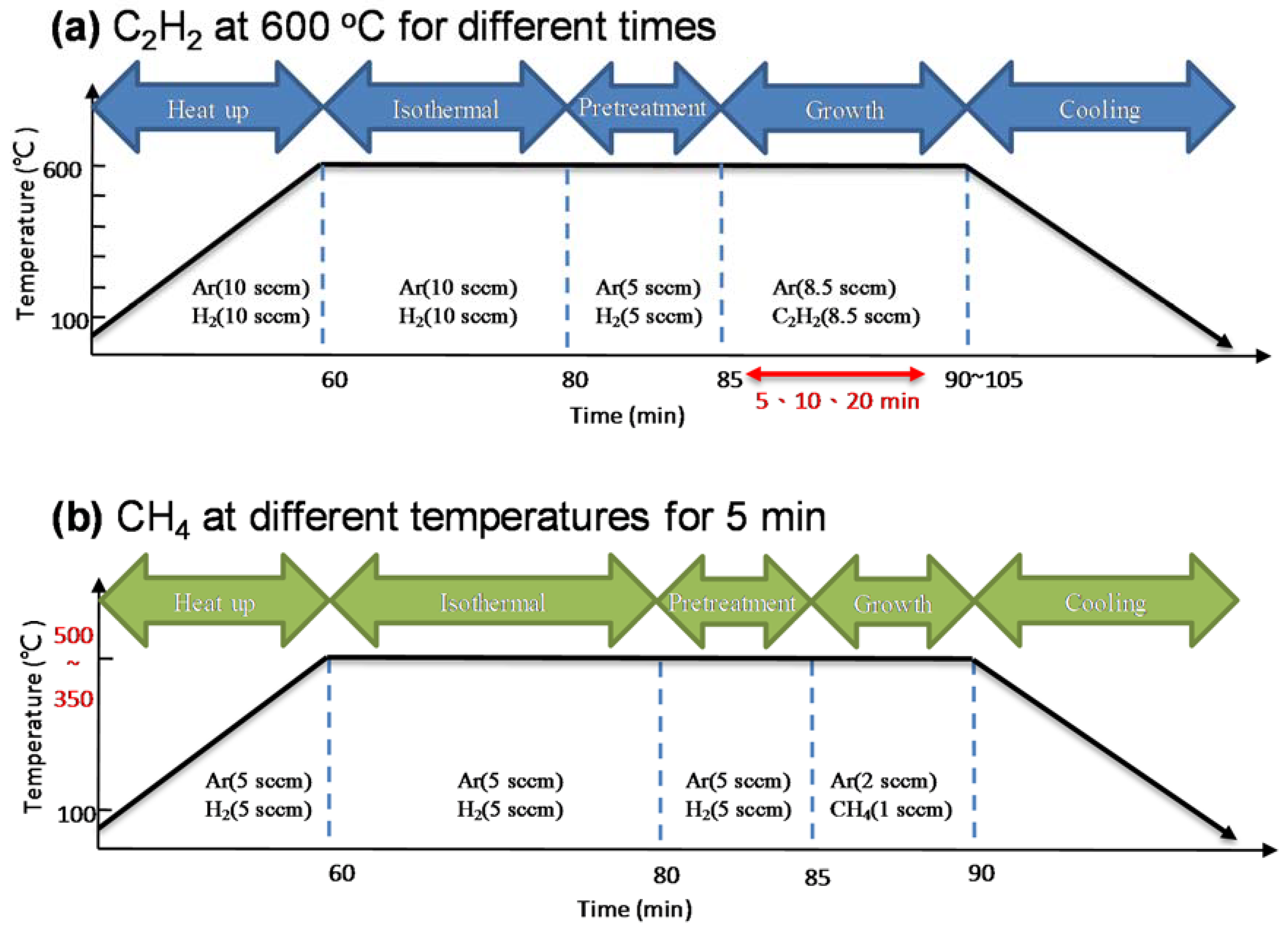
2. Materials and Methods
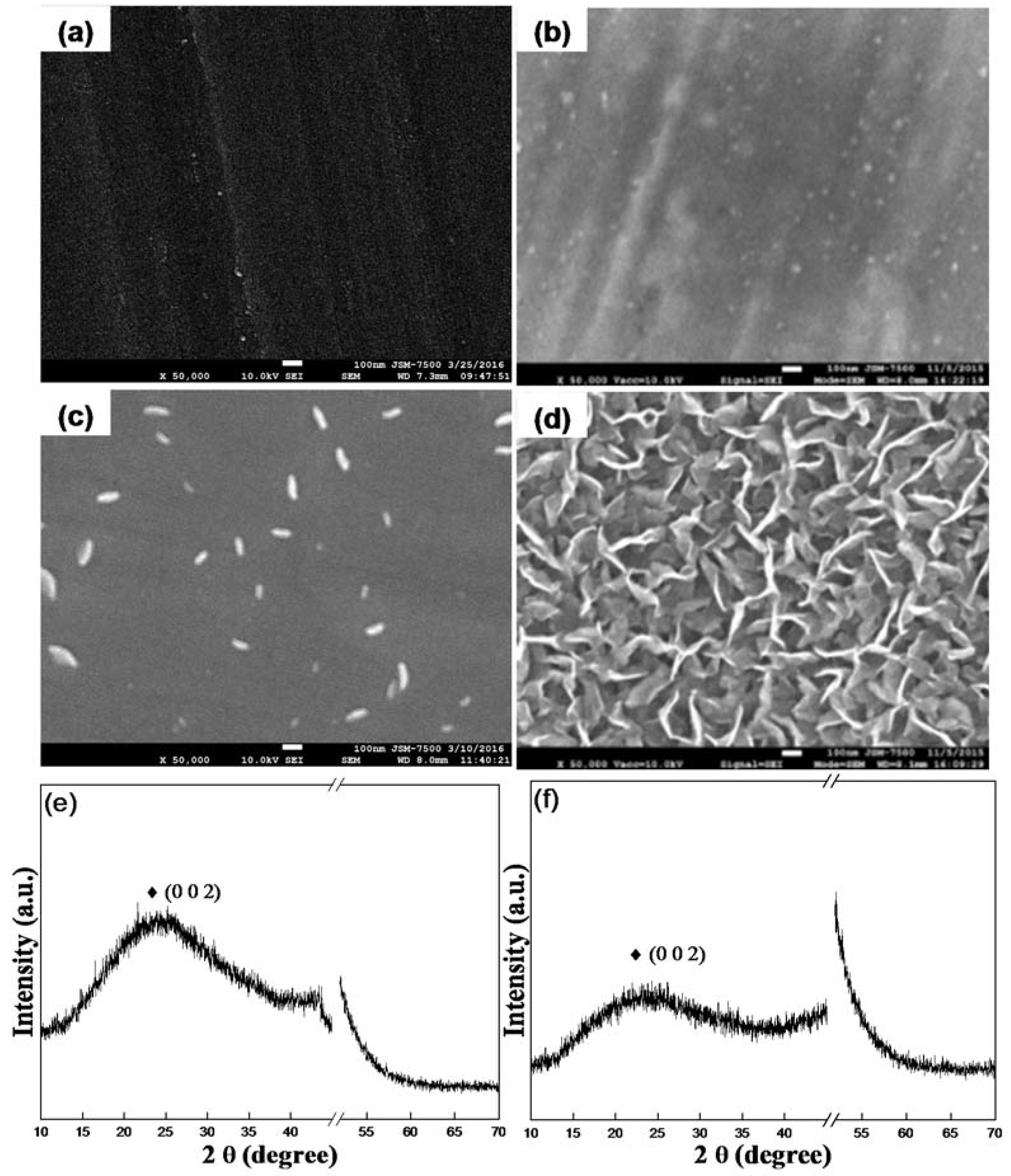
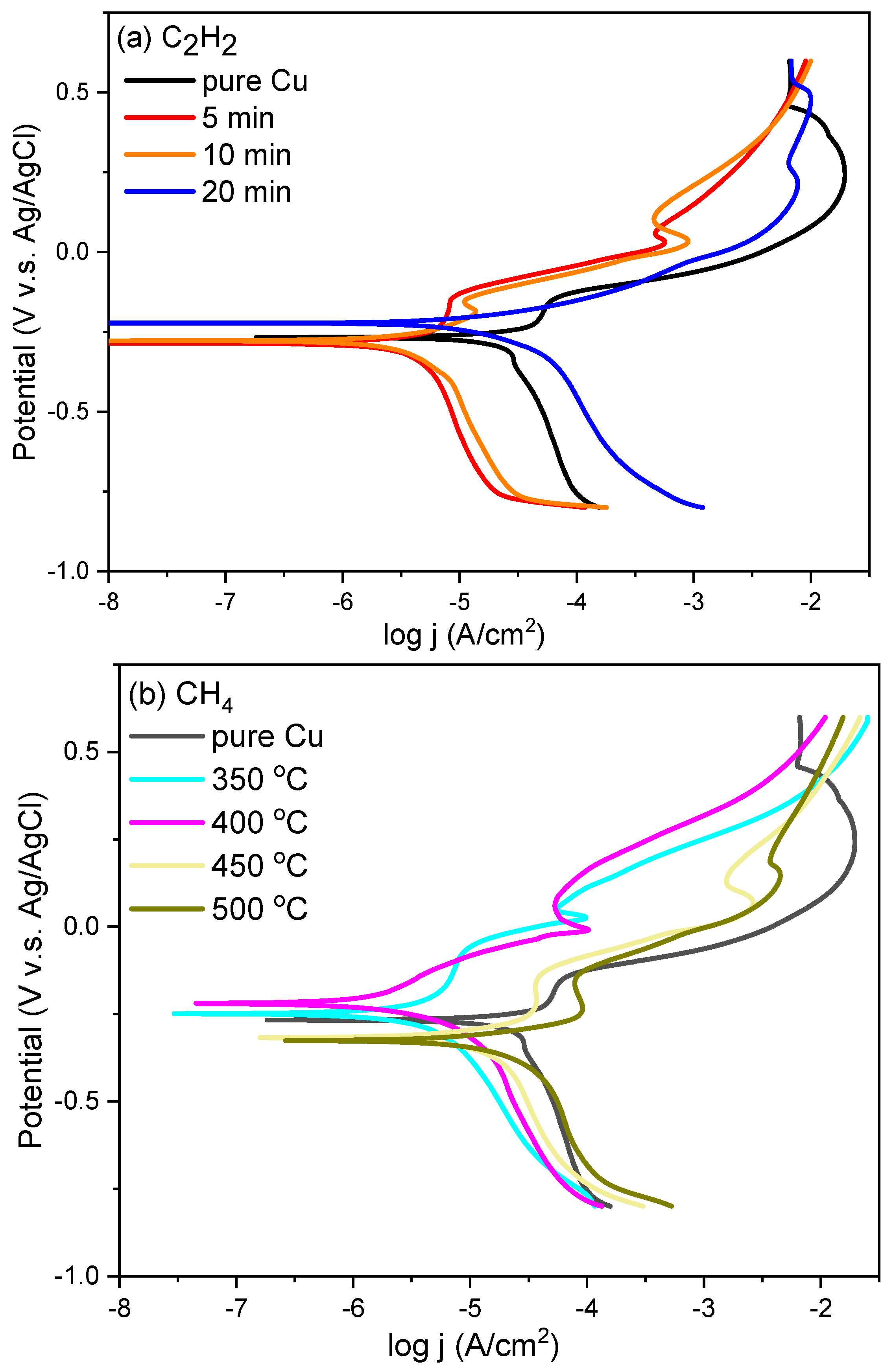
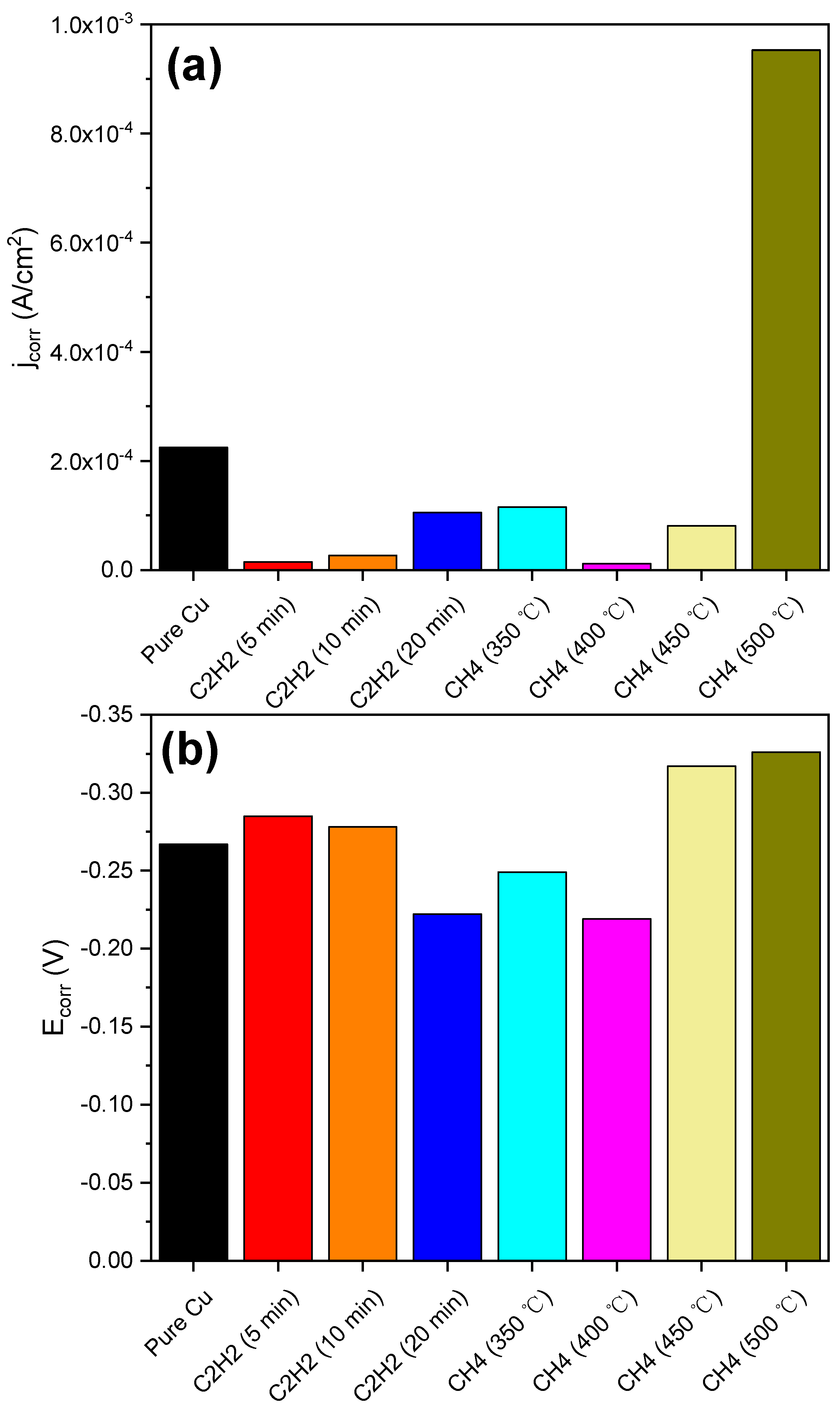
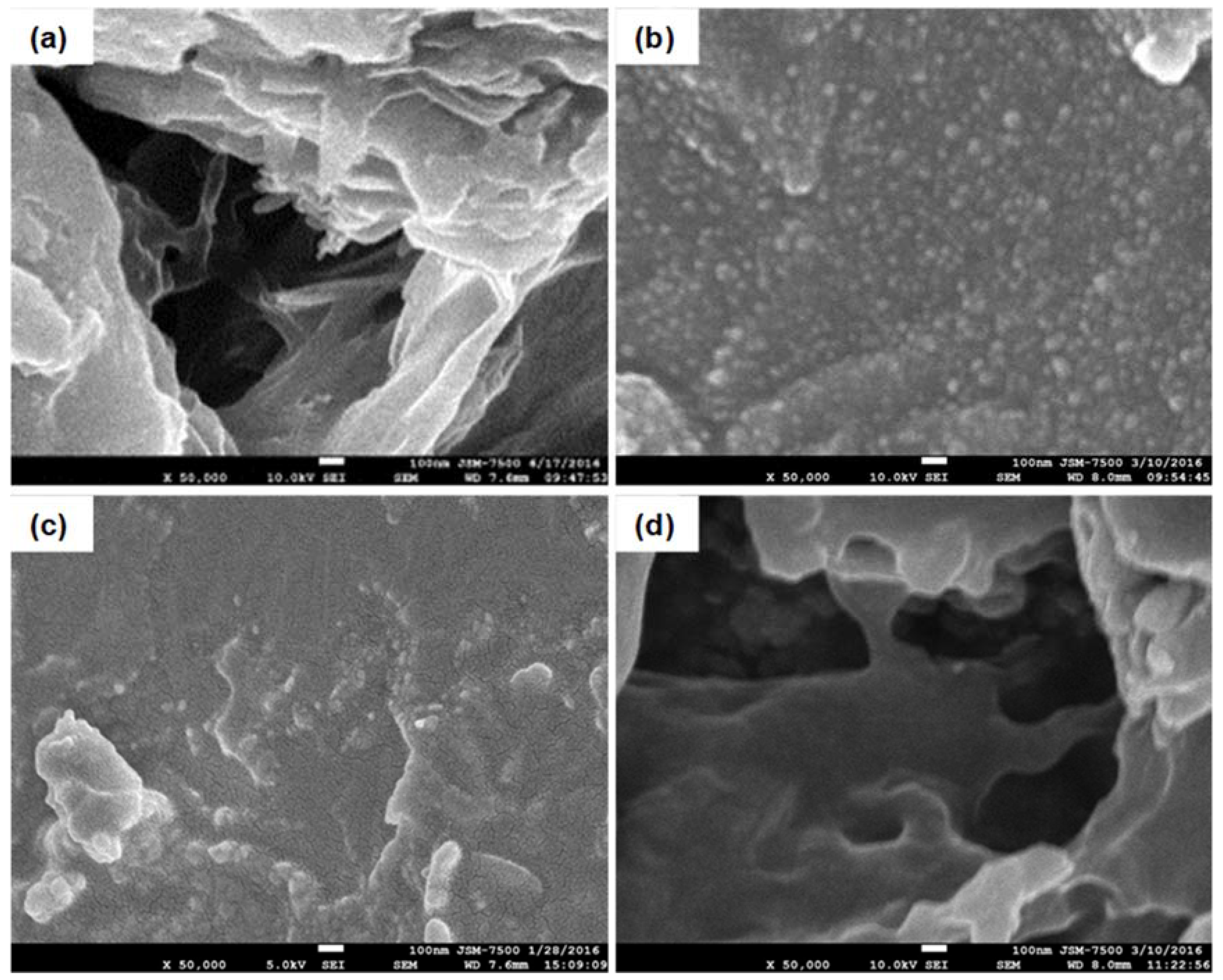
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Katsnelson, M.I.; Grigorieva, I.V.; Dubonos, S.V.; Firsov, A.A. Two-dimensional gas of massless Dirac fermions in graphene. Nature 2005, 438, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Su, C.Y.; Lu, A.Y.; Wu, C.Y.; Li, Y.T.; Liu, K.K.; Zhang, W.; Lin, S.Y.; Juang, Z.Y.; Zhong, Y.L.; Chen, F.R.; et al. Direct formation of wafer scale graphene thin layers on insulating substrates by chemical vapor deposition. Nano Lett. 2011, 11, 3612–3616. [Google Scholar] [CrossRef] [PubMed]
- Su, C.Y.; Lu, A.Y.; Xu, Y.; Chen, F.R.; Khlobystov, A.N.; Li, L.J. High-quality thin graphene films from fast electrochemical exfoliation. ACS Nano 2011, 5, 2332–2339. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.L.; Lee, H.H.; Yang, J.M.; Wu, C.C. The simultaneous electrochemical detection of ascorbic acid, dopamine, and uric acid using graphene/size-selected Pt nanocomposites. Biosens. Bioelectron. 2011, 26, 3450–3455. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.-W.; Hsu, T.-K.; Sun, C.-L.; Nien, Y.-T.; Pu, N.-W.; Ger, M.-D. Synthesis of CuO/graphene nanocomposites for nonenzymatic electrochemical glucose biosensor applications. Electrochim. Acta 2012, 82, 152–157. [Google Scholar] [CrossRef]
- Yeh, M.-H.; Sun, C.-L.; Su, J.-S.; Lin, L.-Y.; Lee, C.-P.; Chen, C.-Y.; Wu, C.-G.; Vittal, R.; Ho, K.-C. A low-cost counter electrode of ITO glass coated with a graphene/Nafion® composite film for use in dye-sensitized solar cells. Carbon 2012, 50, 4192–4202. [Google Scholar] [CrossRef]
- Sun, C.-L.; Cheng, W.-L.; Hsu, T.-K.; Chang, C.-W.; Chang, J.-L.; Zen, J.-M. Ultrasensitive and highly stable nonenzymatic glucose sensor by a CuO/graphene-modified screen-printed carbon electrode integrated with flow-injection analysis. Electrochem. Commun. 2013, 30, 91–94. [Google Scholar] [CrossRef]
- Wang, L.; Yang, R.T.; Sun, C.-L. Graphene and other carbon sorbents for selective adsorption of thiophene from liquid fuel. AICHE J. 2013, 59, 29–32. [Google Scholar] [CrossRef]
- Sun, C.-L.; Su, J.-S.; Tang, J.-H.; Lin, M.-C.; Wu, J.-J.; Pu, N.-W.; Shi, G.-N.; Ger, M.-D. Investigation of the adsorption of size-selected Pt colloidal nanoparticles on high-surface-area graphene powders for methanol oxidation reaction. J. Taiwan Inst. Chem. Eng. 2014, 45, 1025–1030. [Google Scholar] [CrossRef]
- Yang, J.-M.; Wang, S.-A.; Sun, C.-L.; Ger, M.-D. Synthesis of size-selected Pt nanoparticles supported on sulfonated graphene with polyvinyl alcohol for methanol oxidation in alkaline solutions. J. Power Source 2014, 254, 298–305. [Google Scholar] [CrossRef]
- Sun, C.-L.; Tang, J.-S.; Brazeau, N.; Wu, J.-J.; Ntais, S.; Yin, C.-W.; Chou, H.-L.; Baranova, E.A. Particle size effects of sulfonated graphene supported Pt nanoparticles on ethanol electrooxidation. Electrochim. Acta 2015, 162, 282–289. [Google Scholar] [CrossRef]
- Pu, N.-W.; Shi, G.-N.; Liu, Y.-M.; Sun, X.; Chang, J.-K.; Sun, C.-L.; Ger, M.-D.; Chen, C.-Y.; Wang, P.-C.; Peng, Y.-Y.; et al. Graphene grown on stainless steel as a high-performance and ecofriendly anti-corrosion coating for polymer electrolyte membrane fuel cell bipolar plates. J. Power Source 2015, 282, 248–256. [Google Scholar] [CrossRef]
- Liu, B.; Liu, Z.; Chiu, I.S.; Di, M.; Wu, Y.; Wang, J.C.; Hou, T.H.; Lai, C.S. Programmable Synaptic Metaplasticity and below Femtojoule Spiking Energy Realized in Graphene-Based Neuromorphic Memristor. ACS Appl. Mater. Interfaces 2018, 10, 20237–20243. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ye, C.; Li, X.; Cui, N.; Wu, T.; Du, S.; Wei, Q.; Fu, L.; Yin, J.; Lin, C.T. Highly Sensitive and Selective Potassium Ion Detection Based on Graphene Hall Effect Biosensors. Materials 2018, 11, 399. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Bachmatiuk, A.; Ibrahim, I.; Fu, L.; Placha, D.; Martynkova, G.S.; Trzebicka, B.; Gemming, T.; Eckert, J.; Rümmeli, M.H. CVD growth of 1D and 2D sp2 carbon nanomaterials. J. Mater. Sci. 2016, 51, 640–667. [Google Scholar] [CrossRef]
- Hofmann, S.; Braeuninger-Weimer, P.; Weatherup, R.S. CVD-Enabled Graphene Manufacture and Technology. J. Phys. Chem. Lett. 2015, 6, 2714–2721. [Google Scholar] [CrossRef] [PubMed]
- Naghdi, S.; Rhee, K.Y.; Park, S.J. A catalytic, catalyst-free, and roll-to-roll production of graphene via chemical vapor deposition: Low temperature growth. Carbon 2018, 127, 1–12. [Google Scholar] [CrossRef]
- Chen, K.; Shi, L.; Zhang, Y.; Liu, Z. Scalable chemical-vapour-deposition growth of three-dimensional graphene materials towards energy-related applications. Chem. Soc. Rev. 2018, 47, 3018–3036. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.-S.; Kuo, C.-C.; Wu, C.-T.; Chen, C.-W.; Ang, P.K.; Loh, K.P.; Chen, K.-H.; Chen, L.-C. The production of SiC nanowalls sheathed with a few layers of strained graphene and their use in heterogeneous catalysis and sensing applications. Carbon 2011, 49, 4911–4919. [Google Scholar] [CrossRef]
- Parra, C.; Montero-Silva, F.; Gentil, D.; Del Campo, V.; Henrique Rodrigues da Cunha, T.; Henriquez, R.; Haberle, P.; Garin, C.; Ramirez, C.; Fuentes, R.; et al. The Many Faces of Graphene as Protection Barrier. Performance under Microbial Corrosion and Ni Allergy Conditions. Materials 2017, 10, 1406. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Hou, B.; Dai, D.; Shu, S.; Wu, M.; Li, A.; Han, Y.; Zhu, Z.-X.; Chen, B.-A.; Ding, Y.; et al. CVD Synthesis of Monodisperse Graphene/Cu Microparticles with High Corrosion Resistance in Cu Etchant. Materials 2018, 11, 1459. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Brown, L.; Levendorf, M.; Cai, W.; Ju, S.Y.; Edgeworth, J.; Li, X.; Magnuson, C.W.; Velamakanni, A.; Piner, R.D.; et al. Oxidation Resistance of Graphene-Coated Cu and Cu-Ni Alloy. ACS Nano 2011, 5, 1321–1327. [Google Scholar] [CrossRef] [PubMed]
- Kalita, G.; Ayhan, M.E.; Sharma, S.; Shinde, S.M.; Ghimire, D.; Wakita, K.; Umeno, M.; Tanemura, M. Low temperature deposited graphene by surface wave plasma CVD as effective oxidation resistive barrier. Corros. Sci. 2014, 78, 183–187. [Google Scholar] [CrossRef]
- Singh Raman, R.K.; Chakraborty Banerjee, P.; Lobo, D.E.; Gullapalli, H.; Sumandasa, M.; Kumar, A.; Choudhary, L.; Tkacz, R.; Ajayan, P.M.; Majumder, M. Protecting copper from electrochemical degradation by graphene coating. Carbon 2012, 50, 4040–4045. [Google Scholar] [CrossRef]
- Prasai, D.; Tuberquia, J.C.; Harl, R.R.; Jennings, G.K.; Bolotin, K.I. Graphene: Corrosion-Inhibiting Coating. ACS Nano 2012, 6, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.P.; Hofmann, M.; Chang, K.W.; Jhu, J.G.; Li, Y.Y.; Chen, K.Y.; Yang, C.C.; Chang, W.S.; Chen, L.C. Complete Corrosion Inhibition through Graphene Defect Passivation. ACS Nano 2014, 8, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Mišković-Stanković, V.; Jevremović, I.; Jung, I.; Rhee, K. Electrochemical study of corrosion behavior of graphene coatings on copper and aluminum in a chloride solution. Carbon 2014, 75, 335–344. [Google Scholar] [CrossRef]
- Dong, Y.; Liu, Q.; Zhou, Q. Corrosion behavior of Cu during graphene growth by CVD. Corros. Sci. 2014, 89, 214–219. [Google Scholar] [CrossRef]
- Qi, K.; Sun, Y.; Duan, H.; Guo, X. A corrosion-protective coating based on a solution-processable polymer-grafted graphene oxide nanocomposite. Corros. Sci. 2015, 98, 500–506. [Google Scholar] [CrossRef]
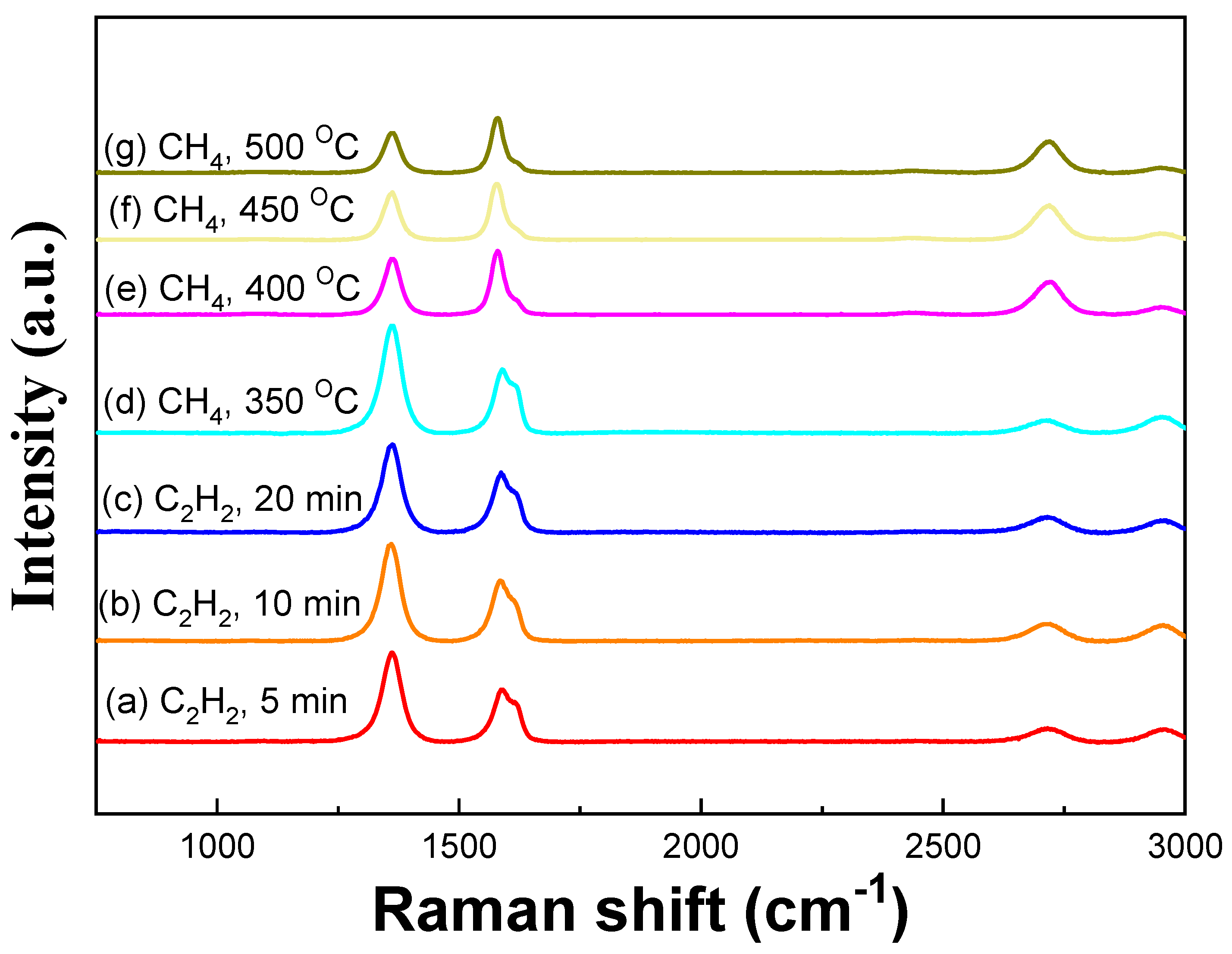
| Sample | ID/IG | I2D/IG |
|---|---|---|
| C2H2, 5 min (600 °C) | 1.70 | 0.24 |
| C2H2, 10 min (600 °C) | 1.60 | 0.28 |
| C2H2, 20 min (600 °C) | 1.47 | 0.25 |
| CH4, 350 °C (5 min) | 1.69 | 0.19 |
| CH4, 400 °C (5 min) | 0.87 | 0.51 |
| CH4, 450 °C (5 min) | 0.84 | 0.61 |
| CH4, 500 °C (5 min) | 0.72 | 0.51 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, W.-H.; Lin, C.-H.; Lin, B.-S.; Sun, C.-L. Low-Temperature CVD Graphene Nanostructures on Cu and Their Corrosion Properties. Materials 2018, 11, 1989. https://doi.org/10.3390/ma11101989
Huang W-H, Lin C-H, Lin B-S, Sun C-L. Low-Temperature CVD Graphene Nanostructures on Cu and Their Corrosion Properties. Materials. 2018; 11(10):1989. https://doi.org/10.3390/ma11101989
Chicago/Turabian StyleHuang, Wei-Hao, Cheng-Hsuan Lin, Ben-Son Lin, and Chia-Liang Sun. 2018. "Low-Temperature CVD Graphene Nanostructures on Cu and Their Corrosion Properties" Materials 11, no. 10: 1989. https://doi.org/10.3390/ma11101989
APA StyleHuang, W.-H., Lin, C.-H., Lin, B.-S., & Sun, C.-L. (2018). Low-Temperature CVD Graphene Nanostructures on Cu and Their Corrosion Properties. Materials, 11(10), 1989. https://doi.org/10.3390/ma11101989




