The Safety Properties of a Potential Kind of Novel Green Primary Explosive: Al/Fe2O3/RDX Nanocomposite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Materials
2.2. Sensitivity Tests of the Al/Fe2O3/RDX Nanocomposites
3. Results and Discussion
3.1. Mechanical Sensitivity Analysis
3.2. Static Discharges Sensitivity Analysis
3.3. Flame Sensitivity Analysis
3.4. Hot Bridge Wire Sensitivity Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Danilov, Y.N.; Ilyushin, M.A.; Tselinsky, I.V. Industrial Explosives: Primary Explosives; State Inst. of Technol. (Technic. Univ.): St. Petersburg, Russia, 2004. [Google Scholar]
- Baev, V.I.; Lvov, C.N.; Shugalei, I.V. Influence of the Environment Internal Factors on Warm-blooded Organisms. In Proceedings of the Problems of Military and Emergency Medicine, St. Petersburg, Russia, 1998; pp. 62–63. [Google Scholar]
- Tsvetkova, O.B.; Shatrova, N.M.; Scheglov, A.N. Storage of radio-nucleases and heavy metals by basidiomycetes of forest ecosystems. Bull. Inst. Nucl. Invest. Kiev 2001, 3, 171–176. [Google Scholar]
- Talawar, M.B.; Sivabalan, R.; Mukundan, T.; Muthurajan, H.; Sikder, A.K.; Gandhe, B.R.; Rao, A.S. Environmentally compatible next generation green energetic materials (GEMs). J. Hazard. Mater. 2009, 161, 589–607. [Google Scholar] [CrossRef] [PubMed]
- Ilyushin, M.A.; Tselinsky, I.V.; Shugalei, I.V. Environmentally friendly energetic materials for initiation devices. Cent. Eur. J. Energ. Mater. 2012, 9, 293–327. [Google Scholar]
- Huynh, M.H.V.; Coburn, M.D.; Meyer, T.J.; Wetzler, M. Green primary explosives: 5-Nitrotetrazolato-N2-ferrate hierarchies. PNAS 2006, 103, 10322–10327. [Google Scholar] [CrossRef] [PubMed]
- Klapötke, T.M.; Stierstorfer, J.; Weber, B. New energetic materials: Synthesis and characterization of copper 5-nitriminotetrazolates. Inorg. Chem. Acta 2009, 362, 2311–2320. [Google Scholar] [CrossRef]
- Klapötke, T.M.; Mehta, N. Lead-Free primary explosives. Propellants Explos. Pyrotech. 2014, 39, 7–8. [Google Scholar] [CrossRef]
- Li, Z.M.; Zhang, J.G.; Zhang, T.L.; Shu, Y.J. Nitro-tetrazole and its high nitrogen-contented compounds. Prog. Chem. 2010, 22, 639–647. [Google Scholar]
- Fronabarger, J.W.; Williams, M.D.; Sanborn, W.B.; Bragg, J.G.; Parrish, D.A.; Bichay, M. DBX-1-A Lead-free replacement for lead azide. Propellants Explos. Pyrot. 2011, 36, 541–550. [Google Scholar] [CrossRef]
- Fischer, N.; Klapoetke, T.M.; Stierstorfer, J. Calcium 5-Nitriminotetrazolate—A green replacement for lead azide in priming charges. J. Energ. Mater. 2011, 29, 61–74. [Google Scholar] [CrossRef]
- Zhu, S.G.; Sun, Y.L.; Zhang, L.; Li, Y. A new green primary explosive: Zinc 5,5’-azotetrazole. In Proceedings of the 38th International Pyrotechnics Seminar and Symposium, Littleton, CO, USA, 10–15 June 2012; pp. 696–704. [Google Scholar]
- Fronabarger, J.; Williams, M.; Sanborn, W.; Sitzmann, M.; Bichay, M.; Gilardi, R. Characterization and output testing of the novel primary explosive, bis(furoxano)nitrophenol, potassium salt. In Proceedings of the 41st AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, Tucson, AZ, USA, 10–13 July 2005. [Google Scholar]
- Asay, B.W.; Son, S.F.; Busse, J.R.; Oschwald, D.M. Ignition characteristics of metastable intermolecular composites. Propellants Explos. Pyrot. 2004, 29, 216–219. [Google Scholar] [CrossRef]
- Puszynski, J.A.; Bulian, C.J.; Swiatkiewicz, J.J. The effect of nanopowder attributes on reaction mechanism and ignition sensitivity of nanothermites. MRS Proc. 2005, 896, 0896-H04-01. [Google Scholar] [CrossRef]
- Levitas, V.I.; Asay, B.W.; Son, S.F.; Pantoya, M. Melt dispersion mechanism for fast reaction of nanothermites. Appl. Phys. Lett. 2006, 89, 071909. [Google Scholar] [CrossRef]
- Lafontaine, E.; Comet, M. Nanothermites; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Comet, M.; Martin, C.; Schnell, F.; Spitzer, D. Nanothermite foams: From nanopowder to object. Chem. Eng. J. 2017, 316, 807–812. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, Y.; Cheng, Z.; Ke, X.; Jiang, W. Facile preparation and energetic characteristics of core-shell Al/CuO metastable intermolecular composite thin film on silicon substrate. Chem. Eng. J. 2017, 328, 585–590. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, N.; Li, J.; Gong, H.; An, T.; Zhao, F.; Ma, H. Thermal behavior of nitrocellulose-based superthermites: Effects of nano-Fe2O3 with three morphologies. RSC Adv. 2017, 7, 23583–23590. [Google Scholar] [CrossRef]
- Thiruvengadathan, R.; Bezmelnitsyn, A.; Apperson, S.; Staley, C.; Redner, P.; Balas, W.; Nicolich, S.; Kapoor, D.; Gangopadhyay, K.; Gangopadhyay, S. Combustion characteristics of novel hybrid nanoenergetic formulations. Combust. Flame 2011, 158, 964–978. [Google Scholar] [CrossRef]
- Qiao, Z.; Shen, J.; Wang, J.; Wang, J.; Huang, B.; Yang, Z.; Yang, G.; Zhang, K. Fast deflagration to detonation transition of energetic material based on a quasi-core/shell structured nanothermite composite. Compos. Sci. Technol. 2015, 107, 113–119. [Google Scholar] [CrossRef]
- Comet, M.; Martin, C.; Klaumünzer, M.; Schnell, F.; Spitzer, D. Energetic nanocomposites for detonation initiation in high explosives without primary explosives. Appl. Phys. Lett. 2015, 107, 113–119. [Google Scholar] [CrossRef]
- Luo, Q.; Nie, F.; Long, X.; Qiao, Z.; Yang, G.; Ma, Y. Preparation of nano-Fe2O3 by CO2-supercritical-process-assisted sol-gel method. Curr. Nanosci. 2014, 10, 722–729. [Google Scholar] [CrossRef]
- Plantier, K.B.; Pantoya, M.L.; Gash, A.E. Combustion wave speeds of nanocomposite Al/Fe2O3: The effects of Fe2O3 particle synthesis technique. Combust. Flame 2005, 140, 299–309. [Google Scholar] [CrossRef]
- Liu, C.L.; Wang, S.P.; Wu, Z.C.; Li, Y.M. Study on the test method of the electrostatic sensitivity of the non-initiating explosive devices. J. Electrost. 2011, 69, 501–503. [Google Scholar] [CrossRef]
- Wei, Y.J.; Lu, C.L.; Zhou, D.C.; Geng, X.H.; Zhang, J. Influence of particle size on RDX thermal sensitivity and flame sensitivity. Chem. Propellants Polym. Mater. 2011, 9, 89–91. [Google Scholar]
- Yao, L.N.; Feng, X.S.; Zhao, S.X.; Wang, C.L.; Wang, S.P. Influence of nano Al on mechanical sensitivity and flame sensitivity of RDX-based explosive. Chin. J. Explos. Propellants 2012, 35, 15–18. [Google Scholar]
- Weir, C.; Pantoya, M.L.; Ramachandran, G.; Dallas, T.; Prentice, D.; Daniels, M. Electrostatic discharge sensitivity and electrical conductivity of composite energetic materials. J. Electrost. 2013, 71, 77–83. [Google Scholar] [CrossRef]
- Zeman, S. New aspects of initiation reactivities of energetic materials demonstrated on nitramines. J. Hazard. Mater. 2006, A132, 155–164. [Google Scholar] [CrossRef] [PubMed]
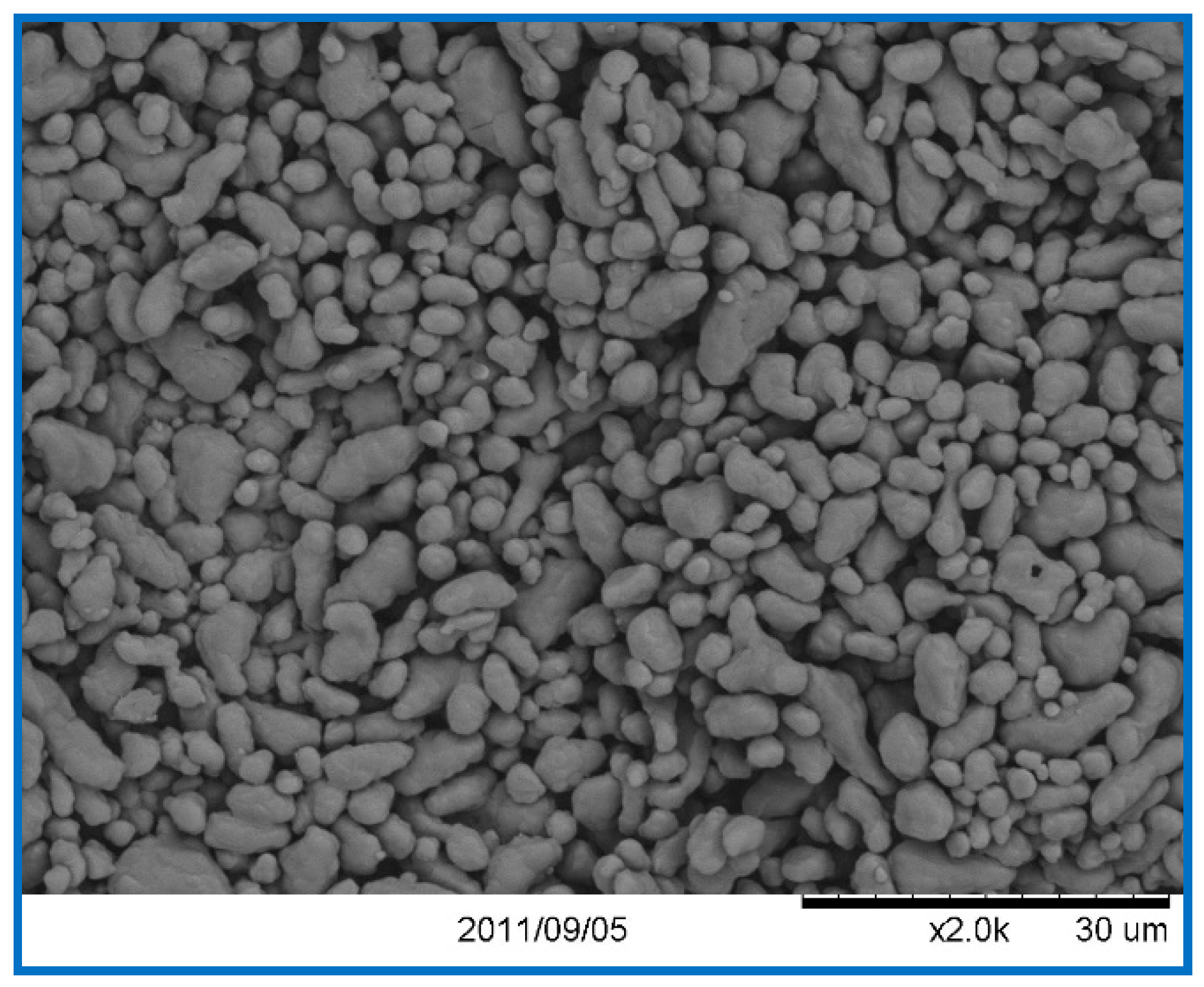


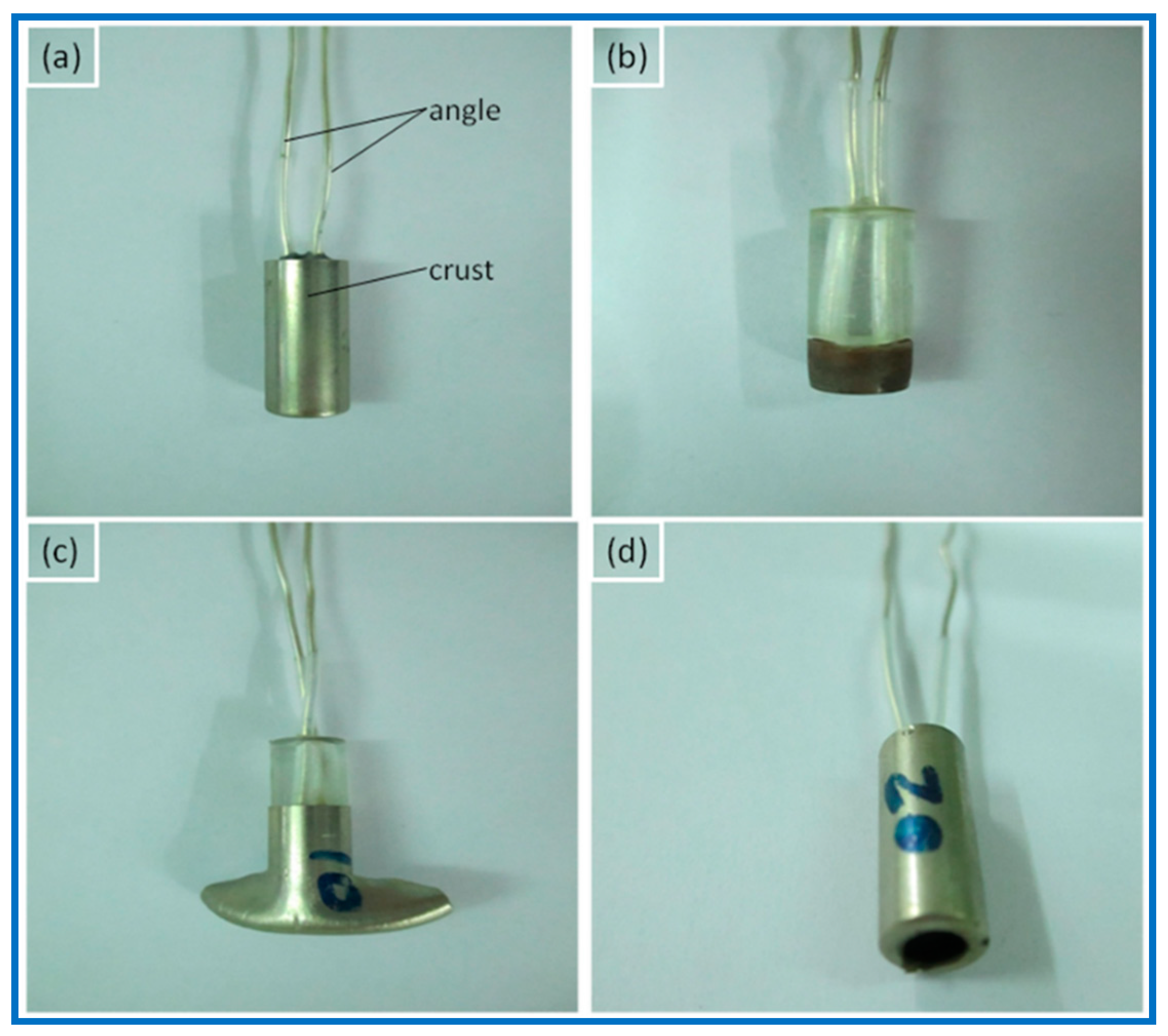
| Sample | Specific Surface Area of Fe2O3 (m2/g) | Impact Sensitivity (%) | Friction Sensitivity (%) |
|---|---|---|---|
| R-100 | - | 8 | 8 |
| R-70 | 43.2 | 16 | 100 |
| R-70 | 230 | 12 | 100 |
| R-50 | 10.3 | 16 | 100 |
| R-50 | 43.2 | 12 | 100 |
| R-50 | 230 | 8 | 100 |
| R-30 | 43.2 | 8 | 100 |
| R-30 | 230 | 8 | 100 |
| R-0 | 230 | 0 | 100 |
| Sample | Specific Surface Area of Fe2O3 (m2/g) | Solvent | V50 (kV) | E50 (mJ) |
|---|---|---|---|---|
| R-100 | - | - | 4.824 | 355.1 |
| R-70 | 43.2 | cyclohexane | 3.367 | 173 |
| R-70 | 230 | cyclohexane | 3.1 | 147 |
| R-70 | 230 | acetone + cyclohexane | 2.5 | 95.3 |
| R-50 | 230 | cyclohexane | 1.9 | 55.05 |
| R-50 | 230 | acetone + cyclohexane | <1.8 | <49.41 |
| R-30 | 230 | acetone + cyclohexane | 2.8 | 119.6 |
| R-0 | 230 | cyclohexane | 6.04 | 560 |
| Sample | Specific Surface Area of Fe2O3 (m2/g) | Solvent | Ignition Distance (mm) |
|---|---|---|---|
| R-100 | - | - | <1.2 mm |
| R-70 | 43.2 | Cyclohexane | 9.0 |
| R-70 | 230 | Cyclohexane | 15 |
| R-70 | 230 | acetone + cyclohexane | 30 |
| R-50 | 230 | Cyclohexane | 35 |
| R-50 | 230 | acetone + cyclohexane | 70 |
| R-30 | 230 | acetone + cyclohexane | >80 |
| R-0 | 230 | Cyclohexane | >80 |
| Sample | Specific Surface Area of Fe2O3 (m2/g) | Solvent | Firing Property at Static Discharge | |
|---|---|---|---|---|
| Angle–Crust | Angle–Angle | |||
| R-100 | - | - | no | no |
| R-70/50/30/0 | 10.3/43.2/230 | cyclohexane/acetone + cyclohexane | no | yes |
| Sample | Specific Surface Area of Fe2O3 (m2/g) | Solvent | Firing Conditions | Electrical Resistance of Bridge Wire (Ω) | Firing Energy (mJ) |
|---|---|---|---|---|---|
| R-100 | - | - | - | 0.995 | - |
| R-70 | 43.2 | cyclohexane | 5 A/14.8 ms | 1.082 | 400.3 |
| R-70 | 230 | cyclohexane | 5 A/10.2 ms | 0.965 | 245.3 |
| R-70 | 230 | Acetone + cyclohexane | 5 A/4.5 ms | 1.001 | 112.6 |
| R-50 | 230 | cyclohexane | 5 A/3.9 ms | 1.040 | 101.4 |
| R-50 | 230 | Acetone + cyclohexane | 5 A/3.5 ms | 1.005 | 87.9 |
| R-30 | 230 | cyclohexane | 5 A/9.3 ms | 0.975 | 226.7 |
| R-30 | 230 | Acetone + cyclohexane | 5 A/6.6 ms | 0.946 | 156.1 |
| R-0 | 230 | cyclohexane | 5 A/17.8 ms | 1.002 | 445.9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Q.; Long, X.; Nie, F.; Liu, G.; Zhu, M. The Safety Properties of a Potential Kind of Novel Green Primary Explosive: Al/Fe2O3/RDX Nanocomposite. Materials 2018, 11, 1930. https://doi.org/10.3390/ma11101930
Luo Q, Long X, Nie F, Liu G, Zhu M. The Safety Properties of a Potential Kind of Novel Green Primary Explosive: Al/Fe2O3/RDX Nanocomposite. Materials. 2018; 11(10):1930. https://doi.org/10.3390/ma11101930
Chicago/Turabian StyleLuo, Qingping, Xinping Long, Fude Nie, Guixiang Liu, and Mingshui Zhu. 2018. "The Safety Properties of a Potential Kind of Novel Green Primary Explosive: Al/Fe2O3/RDX Nanocomposite" Materials 11, no. 10: 1930. https://doi.org/10.3390/ma11101930
APA StyleLuo, Q., Long, X., Nie, F., Liu, G., & Zhu, M. (2018). The Safety Properties of a Potential Kind of Novel Green Primary Explosive: Al/Fe2O3/RDX Nanocomposite. Materials, 11(10), 1930. https://doi.org/10.3390/ma11101930





