A Novel Approach for Assessing the Fatigue Behavior of PEEK in a Physiologically Relevant Environment
Abstract
1. Introduction
2. Material and Methods
3. Results and Discussion
4. Conclusions
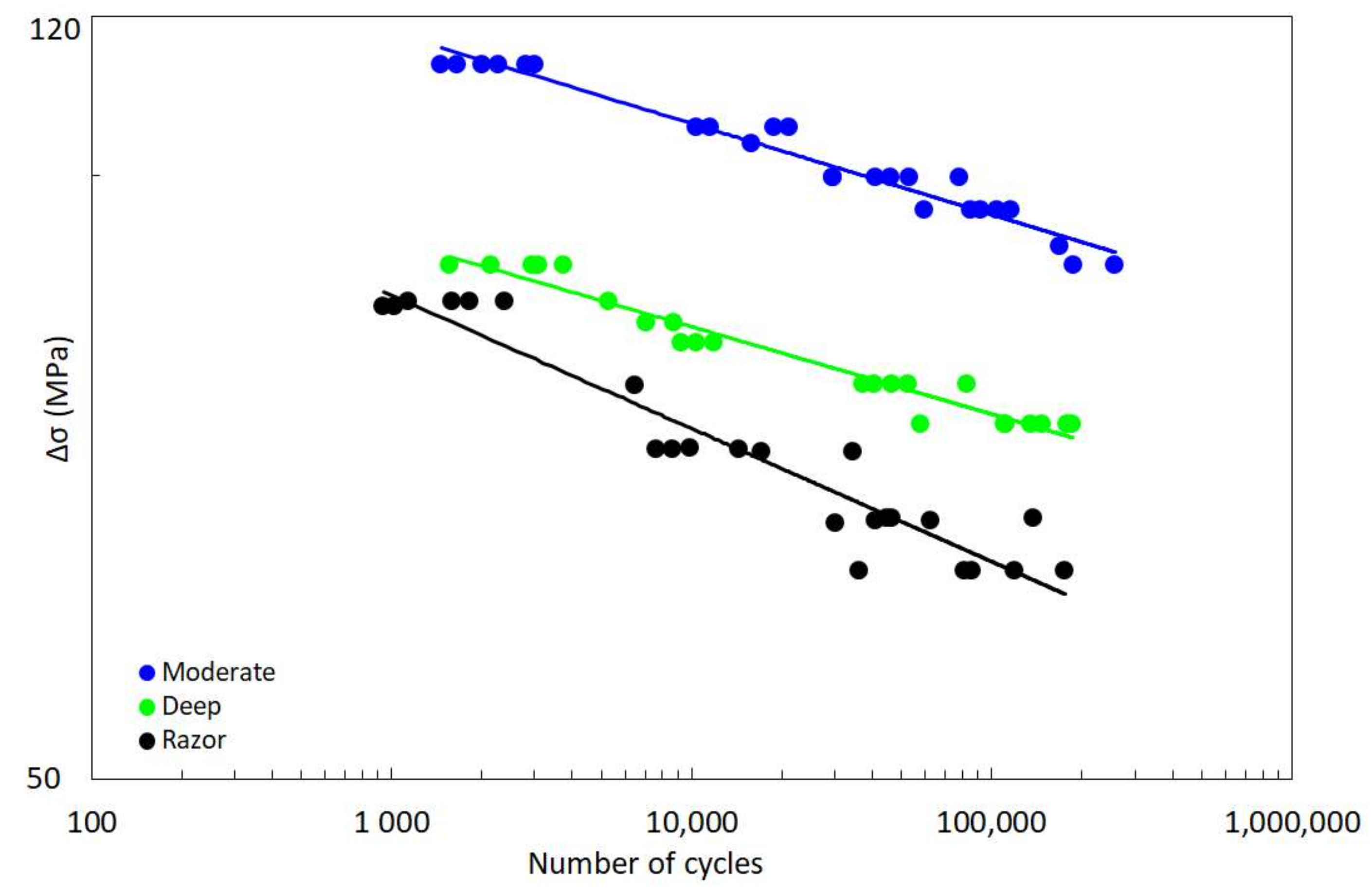
- With respect to NSIFs approach, SED criterion has been shown to summarize fatigue data for different notch geometries within a single narrow scatter band.
- Compared to LM, SED approach is characterized by a lower scatter, i.e., Tσ = 1.33 and 1.22 for LM and SED criterion, respectively.
Author Contributions
Funding
Conflicts of Interest
References
- Ginebra, M.P.; Traykova, T.; Planell, J.A. Calcium phosphate cements as bone drug delivery systems: A review. J. Control. Release 2006, 113, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Regar, E.; Sianos, G.; Serruys, P.W. Stent development and local drug delivery. Br. Med. Bull. 2001, 59, 227–248. [Google Scholar] [CrossRef] [PubMed]
- Greatbatch, W.; Holmes, C.F. History of implantable devices. IEEE Eng. Med. Biol. Mag. 1991, 10, 38–41. [Google Scholar] [CrossRef] [PubMed]
- Long, P.H. Medical Devices in Orthopedic Applications. Toxicol. Pathol. 2008, 36, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Khan, W.; Muntimadugu, E.; Jaffe, M.; Domb, A.J. Implantable Medical Devices; Springer: Berlin/Heidelberg, Germany, 2014; pp. 33–59. [Google Scholar]
- Pruitt, L.; Furmanski, J. Polymeric biomaterials for load-bearing medical devices. JOM 2009, 61, 14–20. [Google Scholar] [CrossRef]
- Evans, S.L.; Gregson, P.J. Composite technology in load-bearing orthopaedic implants. Biomaterials 1998, 19, 1329–1342. [Google Scholar] [CrossRef]
- Nagels, J.; Stokdijk, M.; Rozing, P.M. Stress shielding and bone resorption in shoulder arthroplasty. J. Shoulder Elbow Surg. 2003, 12, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Peron, M.; Torgersen, J.; Berto, F. Mg and Its Alloys for Biomedical Applications: Exploring Corrosion and Its Interplay with Mechanical Failure. Metals (Basel) 2017, 7, 252. [Google Scholar] [CrossRef]
- Huiskes, R.; Weinans, H.; van Rietbergen, B. The relationship between stress shielding and bone resorption around total hip stems and the effects of flexible materials. Clin. Orthop. Relat. Res. 1992, 124–134. [Google Scholar] [CrossRef]
- Niinomi, M. Metals for Biomedical Devices; CRC Press: Bocaton, FL, USA, 2010; ISBN 9781845699246. [Google Scholar]
- Black, J. Biological Performance of Materials: Fundamentals of Biocompatibility; CRC Taylor & Francis: Bocaton, FL, USA, 2006; ISBN 9781420057843. [Google Scholar]
- Ramakrishna, S.; Mayer, J.; Wintermantel, E.; Leong, K.W. Biomedical applications of polymer-composite materials: A review. Compos. Sci. Technol. 2001, 61, 1189–1224. [Google Scholar] [CrossRef]
- Fraldi, M.; Esposito, L.; Perrella, G.; Cutolo, A.; Cowin, S.C.; Esposito, L. Topological optimization in hip prosthesis design. Biomech. Model. Mechanobiol. 2010, 9, 389–402. [Google Scholar] [CrossRef] [PubMed]
- E Murr, L.; M Gaytan, S.; Martinez, E.; Medina, F.R.; Wicker, R.B. Fabricating Functional Ti-Alloy Biomedical Implants by Additive Manufacturing Using Electron Beam Melting. J. Biotechnol. Biomater. 2012, 2. [Google Scholar] [CrossRef]
- Obaton, A.F.; Fain, J.; Djemai, M.; Meinel, D.; Leonard, F.; Mahe, E.; Leculelle, B.; Fouchet, J.J.; Bruno, G. In vivo XCT bone characterization of lattice structured implants fabricated by additive manufacturing. Heliyon 2017, 3. [Google Scholar] [CrossRef] [PubMed]
- Smeets, R.; Stadlinger, B.; Schwarz, F.; Beck-Broichsitter, B.; Jung, O.; Precht, C.; Kloss, F.; Gröbe, A.; Heiland, M.; Ebker, T.; et al. Impact of Dental Implant Surface Modifications on Osseointegration. Biomed. Res. Int. 2016, 2016, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Wennerberg, A. Oral implant surfaces: Part 1—Review focusing on topographic and chemical properties of different surfaces and in vivo responses to them. Int. J. Prosthodont. 2004, 17, 536–543. [Google Scholar] [PubMed]
- Osman, R.; Swain, M. A Critical Review of Dental Implant Materials with an Emphasis on Titanium versus Zirconia. Materials (Basel) 2015, 8, 932–958. [Google Scholar] [CrossRef] [PubMed]
- Gil, F.J.; Herrero-Climent, M.; Lázaro, P.; Rios, J.V. Implant–abutment connections: Influence of the design on the microgap and their fatigue and fracture behavior of dental implants. J. Mater. Sci. Mater. Med. 2014, 25, 1825–1830. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, K.; Ichikawa, T.; Murakami, H.; Miyamoto, Y.; Asaoka, K. Fracture mechanisms of retrieved titanium screw thread in dental implant. Biomaterials 2002, 23, 2459–2465. [Google Scholar] [CrossRef]
- Chao, J.; Lopez, V. Failure analysis of a Ti6Al4V cementless HIP prosthesis. Eng. Fail. Anal. 2007, 14, 822–830. [Google Scholar] [CrossRef]
- Prawoto, Y. Integration of Mechanics into Materials Science Research: A Guide for Material Researchers in Analytical, Computational and Experimental Methods; Nova science: Hauppauge, NY, USA, 2013; ISBN 9781257841042. [Google Scholar]
- Verreman, Y.; Nie, B. Early development of fatigue cracking at manual fillet welds. Fatigue Fract. Eng. Mater. Struct. 1996, 19, 669–681. [Google Scholar] [CrossRef]
- Lazzarin, P.; Tovo, R. A notch intensity factor approach to the stress analysis of welds. Fatigue Fract. Eng. Mater. Struct. 1998, 21, 1089–1103. [Google Scholar] [CrossRef]
- Williams, M.L. Stress singularities resulting from various boundary conditions in angular corners on plates in extension. J. Appl. Mech. 1952, 19, 526–528. [Google Scholar]
- Kasiri, S.; Taylor, D. A critical distance study of stress concentrations in bone. J. Biomech. 2008, 41, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D. Geometrical effects in fatigue: A unifying theoretical model. Int. J. Fatigue 1999, 21, 413–420. [Google Scholar] [CrossRef]
- Razavi, S.M.J.; Peron, M.; Torgersen, J.; Berto, F.; Mutignani, F. Effect of hot dip galvanization on the fatigue strength of steel bolted connections. Frat. Integr. Strutt. 2017, 11. [Google Scholar] [CrossRef]
- Razavi, S.M.J.; Peron, M.; Torgersen, J.; Berto, F. Static Multiaxial Fracture Behavior of Graphite Components: A Review of Recent Results. Key Eng. Mater. 2017, 754, 35–38. [Google Scholar] [CrossRef]
- Razavi, S.M.J.; Peron, M.; Mutignani, F.; Torgersen, J.; Berto, F. A Study on the Fatigue Behavior of Hot Dip Galvanized Steel Connections. Key Eng. Mater. 2017, 754, 241–243. [Google Scholar] [CrossRef]
- Berto, F.; Lazzarin, P. A review of the volume-based strain energy density approach applied to V-notches and welded structures. Theor. Appl. Fract. Mech. 2009, 52, 183–194. [Google Scholar] [CrossRef]
- Peron, M.; Razavi, S.M.J.; Berto, F.; Torgersen, J.; Colussi, M. Fracture assessment of magnetostrictive materials. Frat. Integr. Strutt. 2017, 11. [Google Scholar] [CrossRef]
- Campagnolo, A.; Razavi, S.M.J.; Peron, M.; Torgersen, J.; Berto, F. Mode II brittle fracture: Recent developments. Frat. Integr. Strutt. 2017, 11. [Google Scholar] [CrossRef]
- Razavi, S.M.J.; Peron, M.; Torgersen, J.; Berto, F. Notched graphite under multiaxial loading. Frat. Integr. Strutt. 2017, 11. [Google Scholar] [CrossRef]
- Peron, M.; Razavi, S.M.J.; Berto, F.; Torgersen, J. Notch stress intensity factor under mixed mode loadings: An overview of recent advanced methods for rapid calculation. Frat. Integr. Strutt. 2017, 42, 196–204. [Google Scholar] [CrossRef]
- Peron, M.; Razavi, S.M.J.; Berto, F.; Torgersen, J.; Mutignani, F. Local strain energy density for the fatigue assessment of hot dip galvanized welded joints: Some recent outcomes. Frat. Integr. Strutt. 2017, 11. [Google Scholar] [CrossRef]
- Razavi, S.M.J.; Peron, M.; Mutignani, F.; Torgersen, J.; Berto, F. Fatigue Strength of Hot-Dip Galvanized Welded Steel Connections. Key Eng. Mater. 2017, 754, 244–247. [Google Scholar] [CrossRef]
- Chebat, F.; Peron, M.; Viespoli, L.; Welo, T.; Berto, F. Fatigue Strength Assessment of Steel Rollers: On the Reliability of the Strain Energy Density Approach on Real Components. Appl. Sci. 2018, 8, 1015. [Google Scholar] [CrossRef]
- Peron, M.; Torgersen, J.; Berto, F. Rupture Predictions of Notched Ti-6Al-4V Using Local Approaches. Materials (Basel) 2018, 11, 663. [Google Scholar] [CrossRef] [PubMed]
- Berto, F.; Cendon, D.A.; Lazzarin, P.; Elices, M. Fracture behaviour of notched round bars made of PMMA subjected to torsion at −60 °C. Eng. Fract. Mech. 2013. [Google Scholar] [CrossRef]
- Berto, F.; Cendon, D.A.; Lazzarin, P.; Elices, M. Fracture behaviour of notched round bars made of PMMA subjected to torsion at room temperature. Eng. Fract. Mech. 2012, 90, 143–160. [Google Scholar] [CrossRef]
- Peron, M.; Razavi, S.M.J.; Berto, F.; Torgersen, J.; Marsavina, L. Local strain energy density for the fracture assessment of polyurethane specimens weakened by notches of different shape. Frat. Integr. Strutt. 2017, 42, 214–222. [Google Scholar] [CrossRef]
- Peron, M.; Razavi, S.; Torgersen, J.; Berto, F. Fracture Assessment of PEEK under Static Loading by Means of the Local Strain Energy Density. Materials 2017, 10, 1423. [Google Scholar] [CrossRef] [PubMed]
- Ulery, B.D.; Nair, L.S.; Laurencin, C.T. Biomedical Applications of Biodegradable Polymers. J. Polym. Sci. B Polym. Phys. 2011, 49, 832–864. [Google Scholar] [CrossRef] [PubMed]
- Maitz, M.F. Applications of synthetic polymers in clinical medicine. Biosurf. Biotribol. 2015, 1, 161–176. [Google Scholar] [CrossRef]
- Williams, D.F. On the nature of biomaterials. Biomaterials 2009, 30, 5897–5909. [Google Scholar] [CrossRef] [PubMed]
- Lendlein, A.; Rehahn, M.; Buchmeiser, M.R.; Haag, R. Polymers in Biomedicine and Electronics. Macromol. Rapid Commun. 2010, 31, 1487–1491. [Google Scholar] [CrossRef] [PubMed]
- Platt, D.K.; Rapra Technology Limited. Engineering and High Performance Plastics Market Report: A Rapra Market Report; Rapra Technology Ltd.: Brussels, Belgium, 2003; ISBN 1859573800. [Google Scholar]
- Walter, J.; Kuhn, S.A.; Reichart, R.; Kalff, R.; Ewald, C. PEEK cages as a potential alternative in the treatment of cervical spondylodiscitis: A preliminary report on a patient series. Eur. Spine J. 2010, 19, 1004–1009. [Google Scholar] [CrossRef] [PubMed]
- Schwitalla, A.; Müller, W.-D. PEEK Dental Implants: A Review of the Literature. J. Oral Implantol. 2013, 39, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Rahmitasari, F.; Ishida, Y.; Kurahashi, K.; Matsuda, T.; Watanabe, M.; Ichikawa, T. PEEK with Reinforced Materials and Modifications for Dental Implant Applications. Dent. J. 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- Sagomonyants, K.B.; Jarman-Smith, M.L.; Devine, J.N.; Aronow, M.S.; Gronowicz, G.A. The in vitro response of human osteoblasts to polyetheretherketone (PEEK) substrates compared to commercially pure titanium. Biomaterials 2008, 29, 1563–1572. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-T.; Koak, J.-Y.; Lim, Y.-J.; Kim, S.-K.; Kwon, H.-B.; Kim, M.-J. Stress shielding and fatigue limits of poly-ether-ether-ketone dental implants. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100B, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Sobieraj, M.C.; Murphy, J.E.; Brinkman, J.G.; Kurtz, S.M.; Rimnac, C.M. Notched fatigue behavior of PEEK. Biomaterials 2010, 31, 9156–9162. [Google Scholar] [CrossRef] [PubMed]
- Sobieraj, M.C.; Kurtz, S.M.; Rimnac, C.M. Notch sensitivity of PEEK in monotonic tension. Biomaterials 2009, 30, 6485–6494. [Google Scholar] [CrossRef] [PubMed]
- Dowling, N.E. Mechanical Behavior of Materials: Engineering Methods for Deformation, Fracture and Fatigue, 3rd ed.; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2007; ISBN 0131863126. [Google Scholar]
- Lazzarin, P.; Zambardi, R. A finite-volume-energy based approach to predict the static and fatigue behavior of components with sharp V-shaped notches. Int. J. Fract. 2001, 112, 275–298. [Google Scholar] [CrossRef]
- Gallo, P.; Razavi, S.M.J.; Peron, M.; Torgersen, J.; Berto, F. Creep behavior of v-notched components. Frat. Integr. Strutt. 2017, 11. [Google Scholar] [CrossRef]
- Razavi, S.M.J.; Peron, M.; Torgersen, J.; Berto, F.; Welo, T. 40CrMoV13.9 notched specimens under multiaxial fatigue: An overview of recent results. Frat. Integr. Strutt. 2017, 11, 440–446. [Google Scholar] [CrossRef]
- Gross, B.; Mendelson, A. Plane elastostatic analysis of V-notched plates. Int. J. Fract. Mech. 1972, 8, 267–276. [Google Scholar] [CrossRef]
- Lazzarin, P.; Filippi, S. A generalized stress intensity factor to be applied to rounded V-shaped notches. Int. J. Solids Struct. 2006, 43, 2461–2478. [Google Scholar] [CrossRef]
- Lazzarin, P.; Berto, F.; Zappalorto, M. Rapid calculations of notch stress intensity factors based on averaged strain energy density from coarse meshes: Theoretical bases and applications. Int. J. Fatigue 2010, 32, 1559–1567. [Google Scholar] [CrossRef]
- Kurtz, S.M.; Devine, J.N. PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials 2007, 28, 4845–4869. [Google Scholar] [CrossRef] [PubMed]






| Parameters | Moderate | Deep | Razor |
|---|---|---|---|
| A (MPa) | 152 | 120 | 131 |
| d | −0.043 | −0.043 | −0.063 |
| R2 | 0.95 | 0.90 | 0.92 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peron, M.; Torgersen, J.; Berto, F. A Novel Approach for Assessing the Fatigue Behavior of PEEK in a Physiologically Relevant Environment. Materials 2018, 11, 1923. https://doi.org/10.3390/ma11101923
Peron M, Torgersen J, Berto F. A Novel Approach for Assessing the Fatigue Behavior of PEEK in a Physiologically Relevant Environment. Materials. 2018; 11(10):1923. https://doi.org/10.3390/ma11101923
Chicago/Turabian StylePeron, Mirco, Jan Torgersen, and Filippo Berto. 2018. "A Novel Approach for Assessing the Fatigue Behavior of PEEK in a Physiologically Relevant Environment" Materials 11, no. 10: 1923. https://doi.org/10.3390/ma11101923
APA StylePeron, M., Torgersen, J., & Berto, F. (2018). A Novel Approach for Assessing the Fatigue Behavior of PEEK in a Physiologically Relevant Environment. Materials, 11(10), 1923. https://doi.org/10.3390/ma11101923







