Mesoporous Titanium Dioxide: Synthesis and Applications in Photocatalysis, Energy and Biology
Abstract
1. Introduction
2. Synthesis
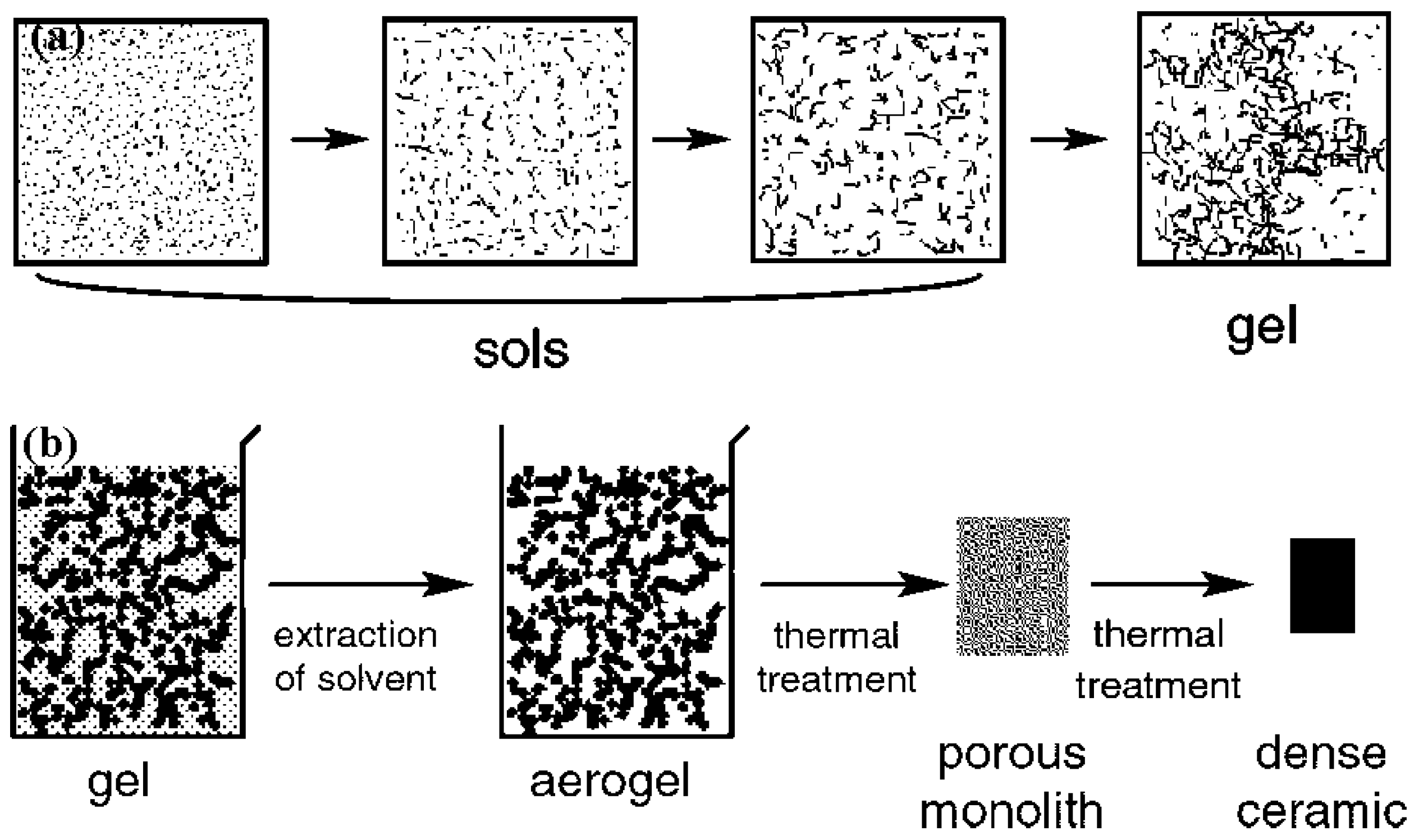
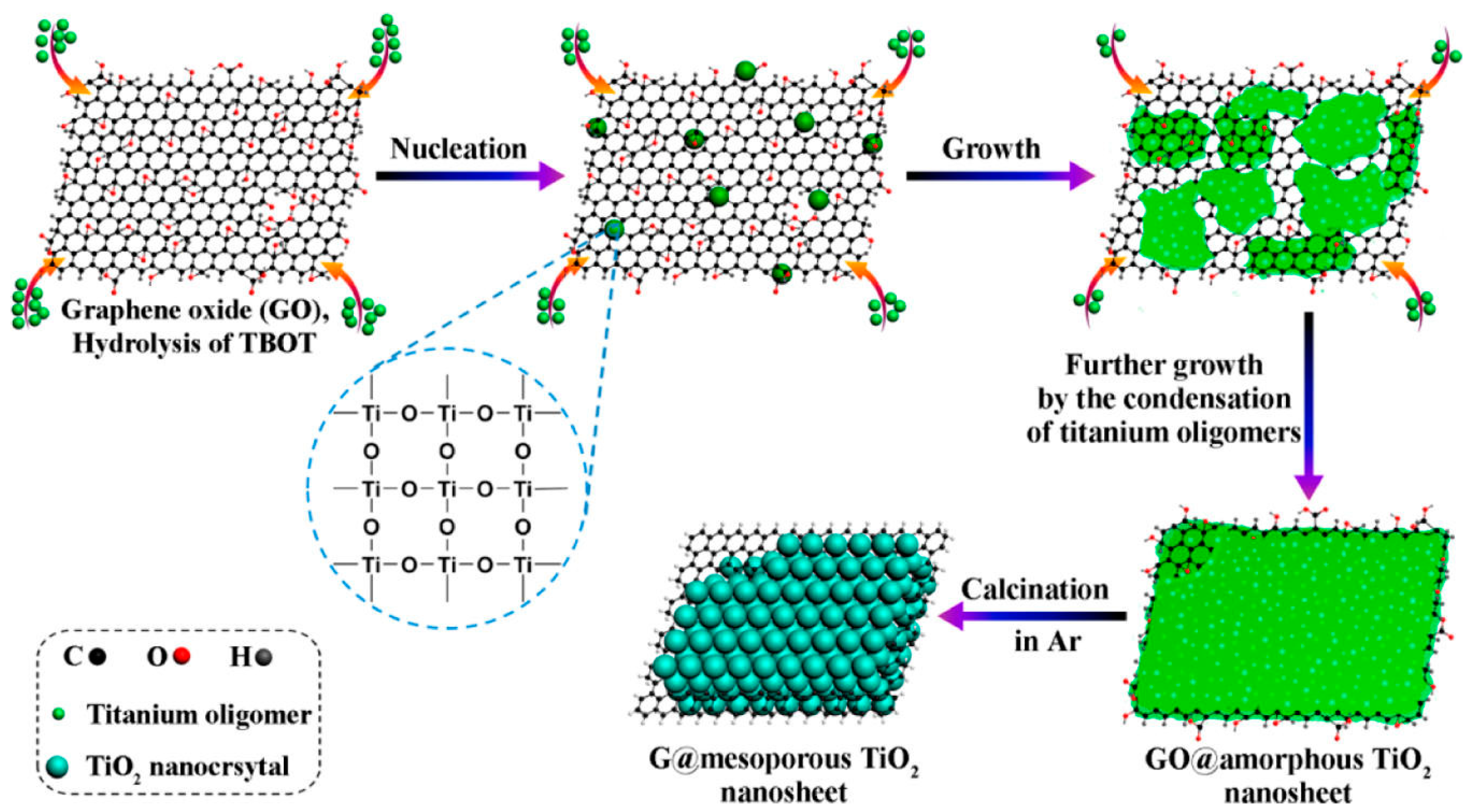
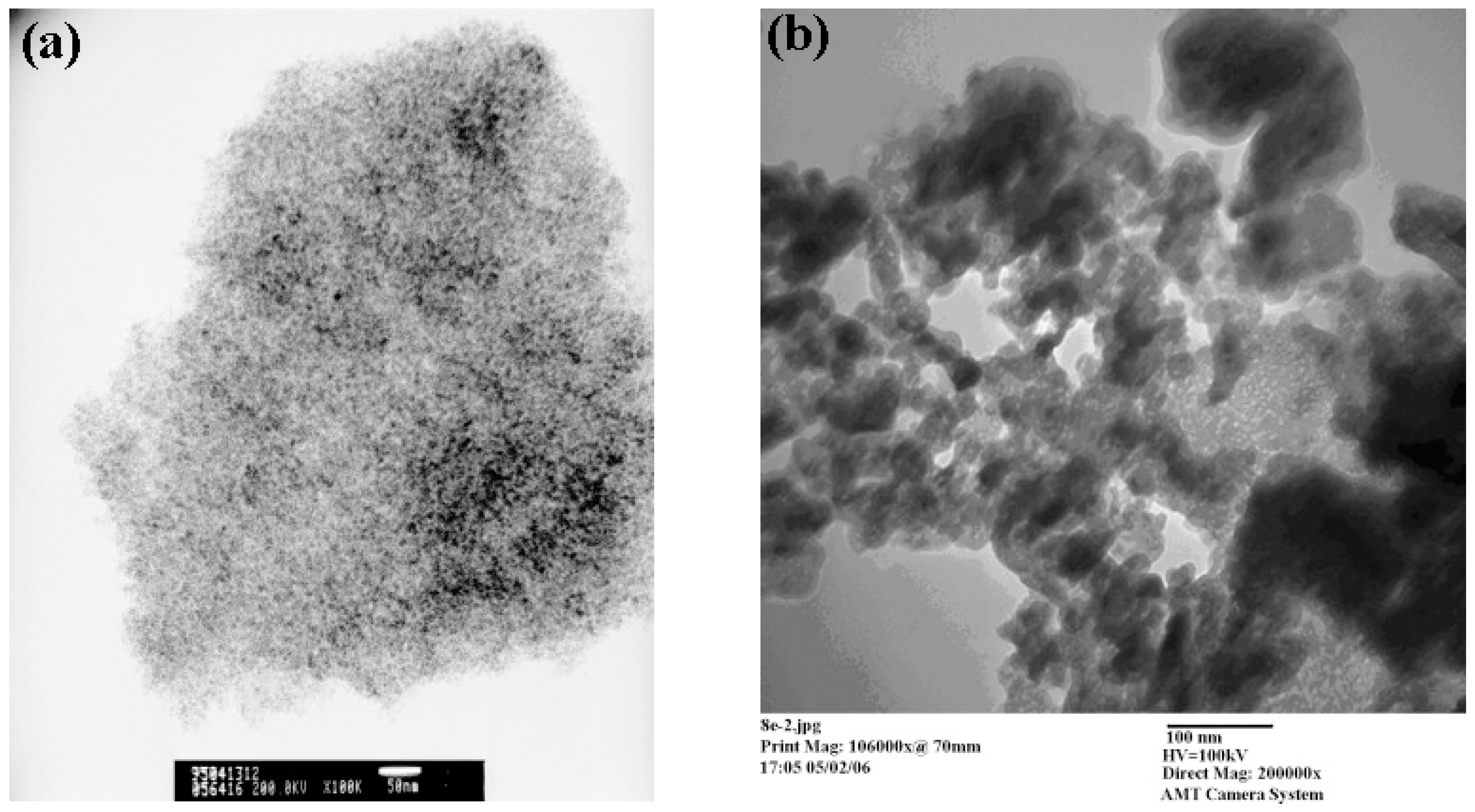
2.1. Sol–Gel Method
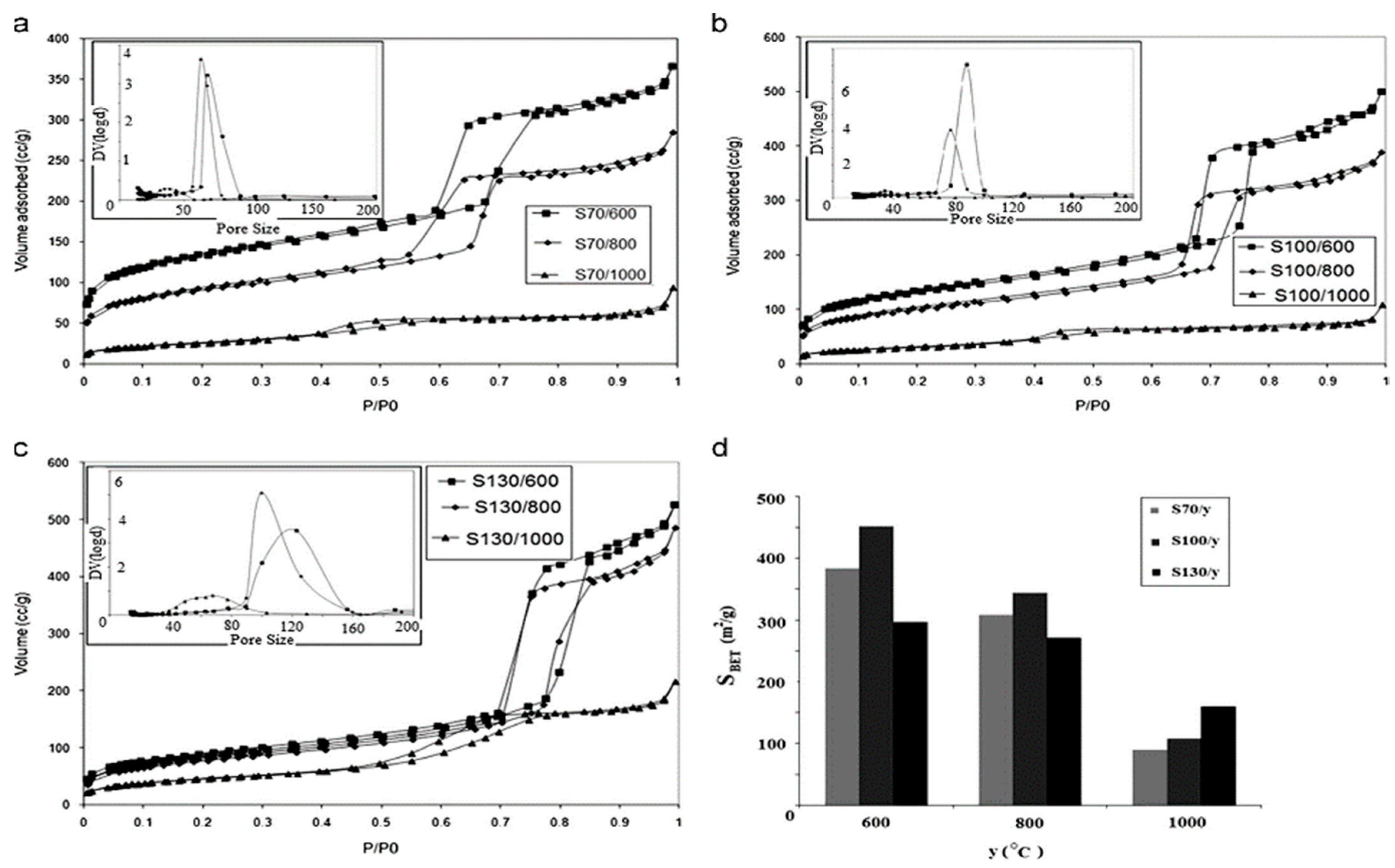
2.2. Hydrothermal Method
2.3. Solvothermal Method
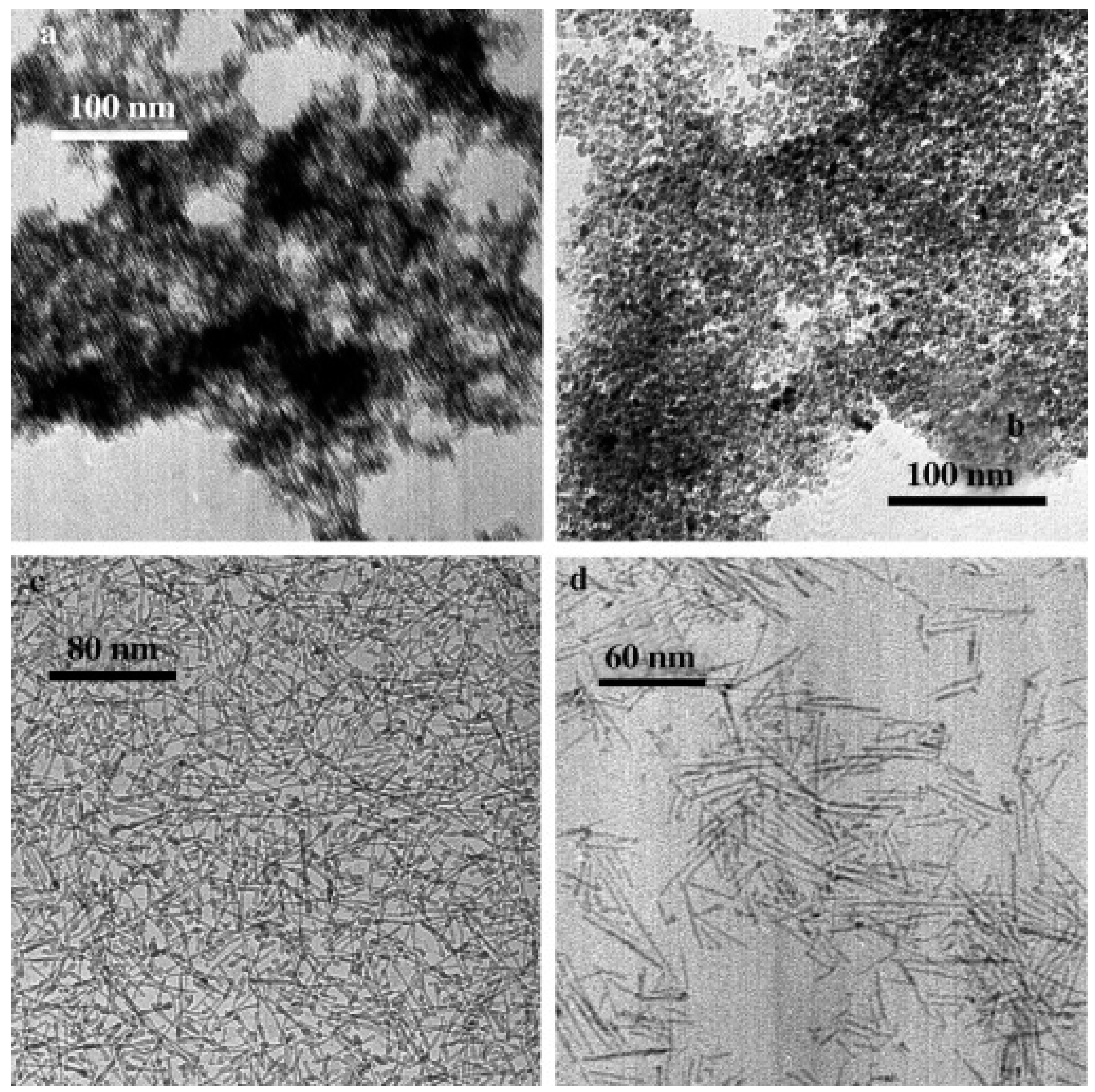
2.4. Template Method
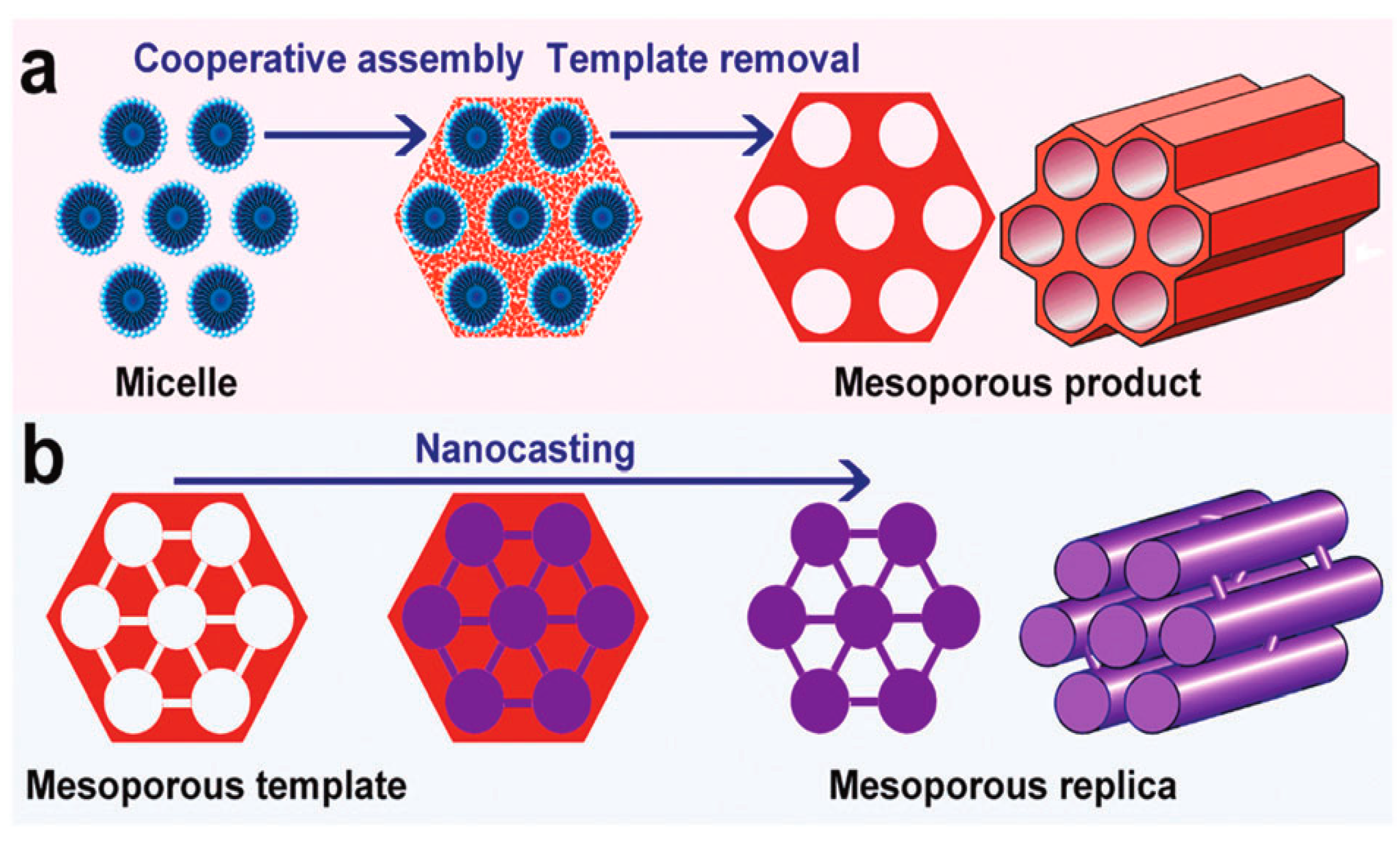
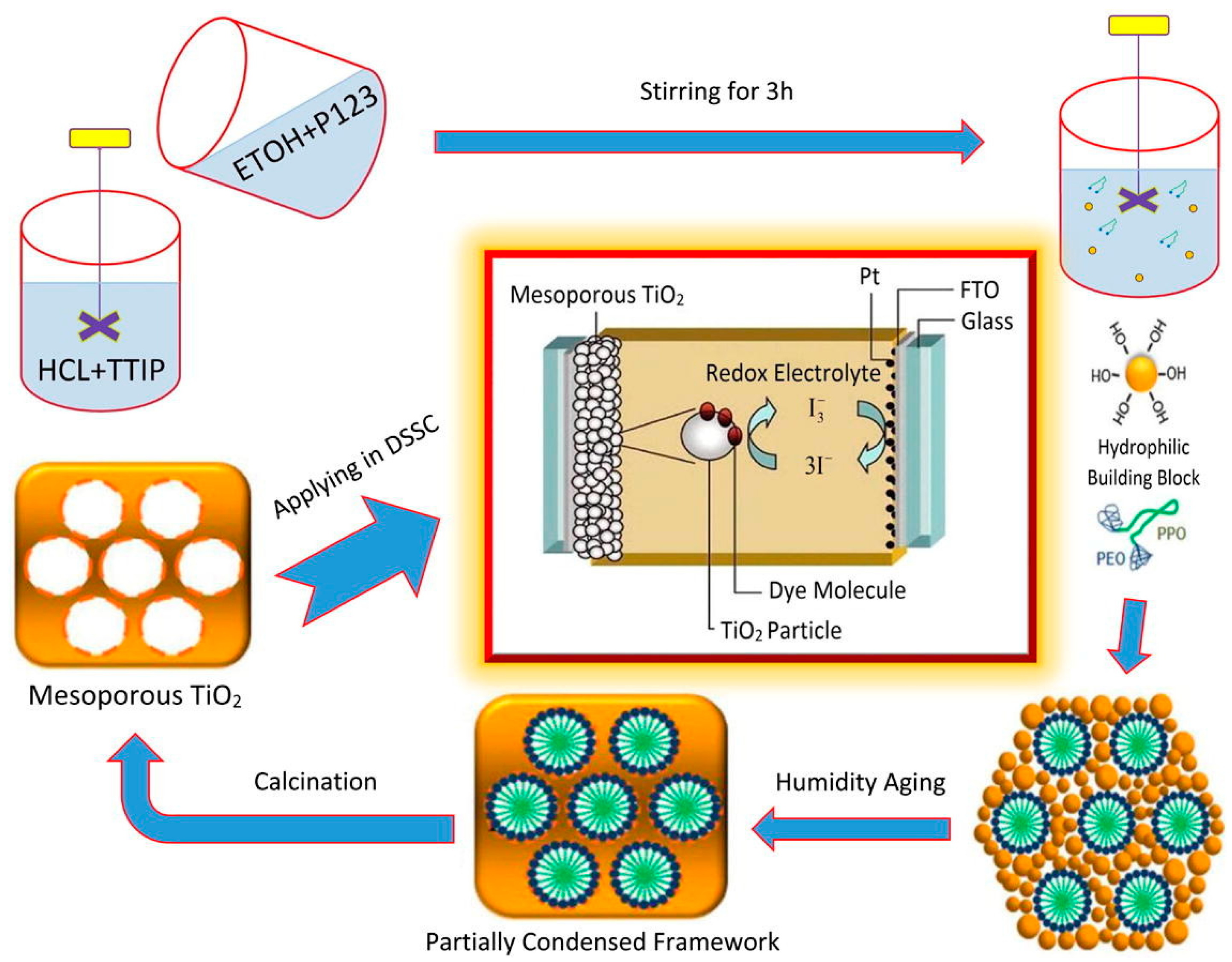
2.4.1. Soft-Templating Method
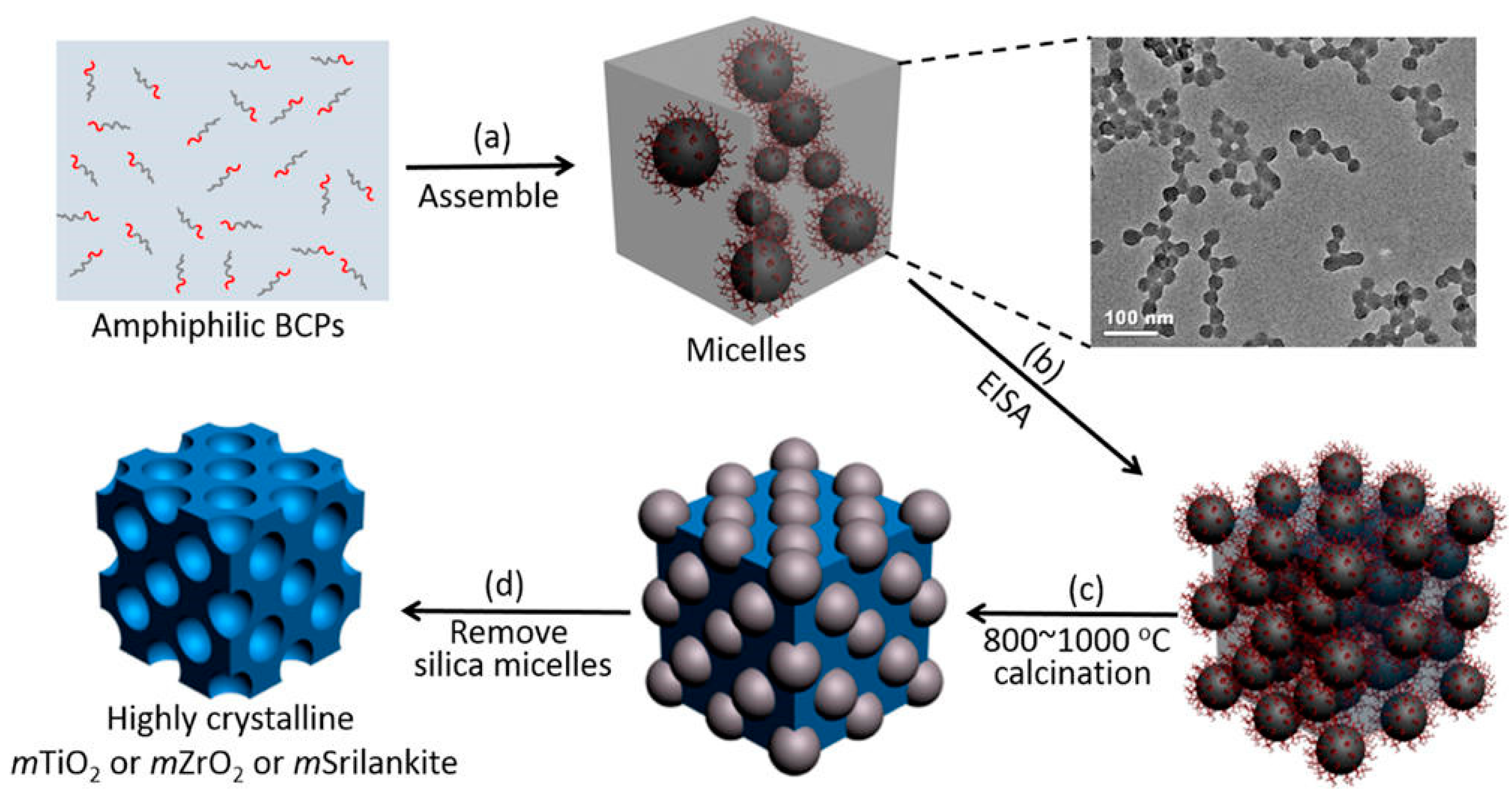
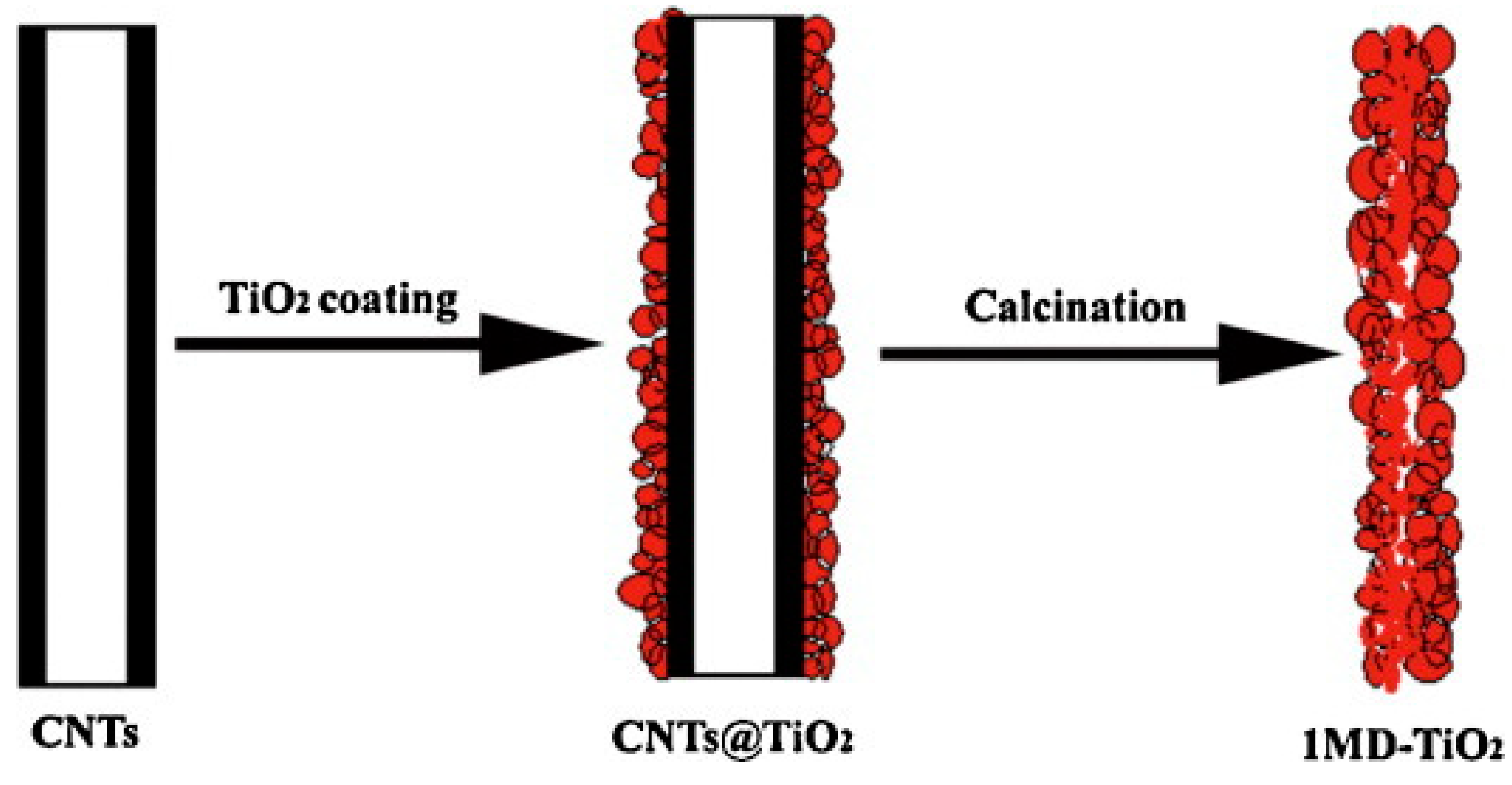
2.4.2. Hard-Templating Method
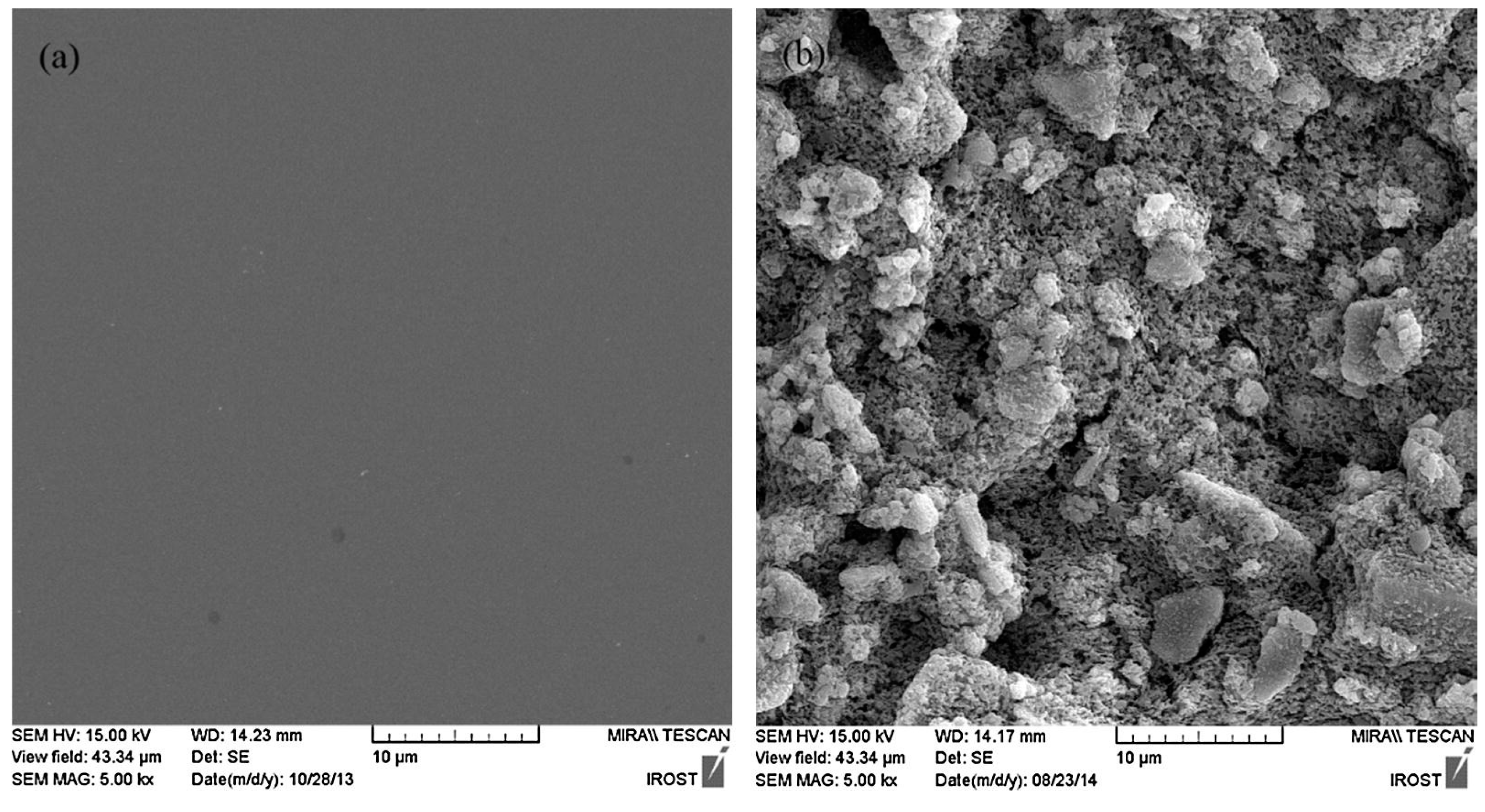
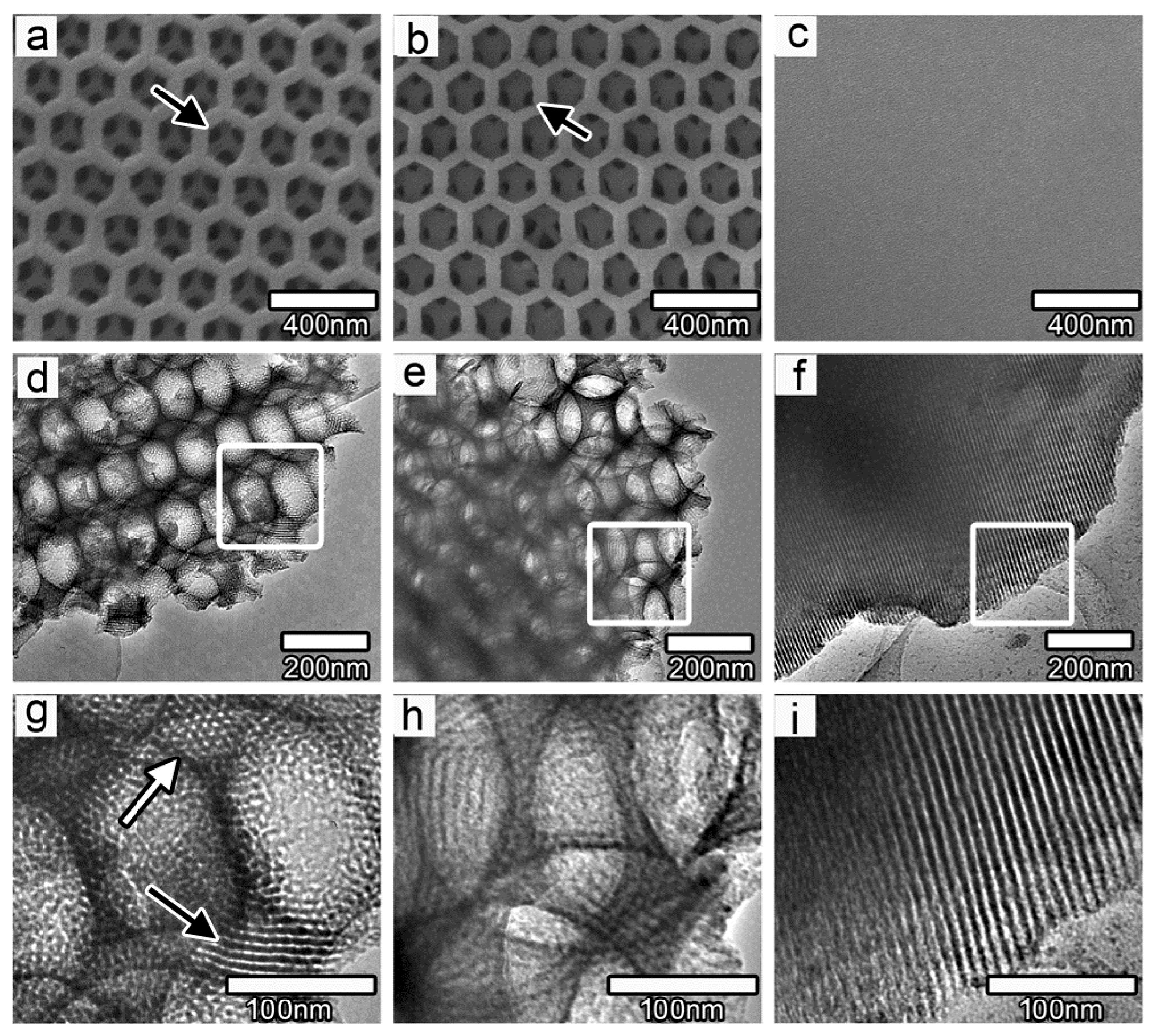
2.5. Morphology
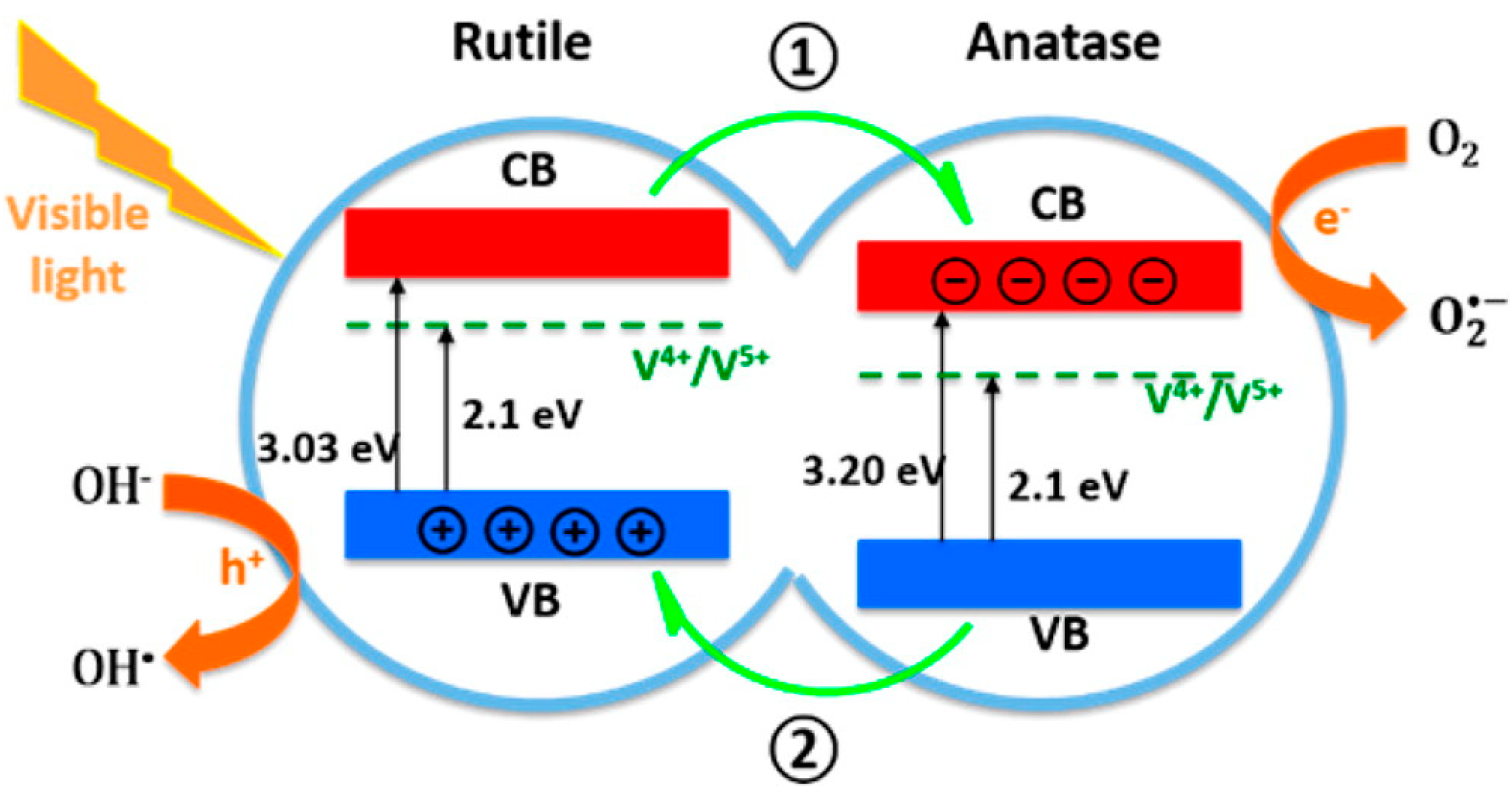
2.6. Crystallization
2.7. Doping
3. Application
3.1. Photocatalysis
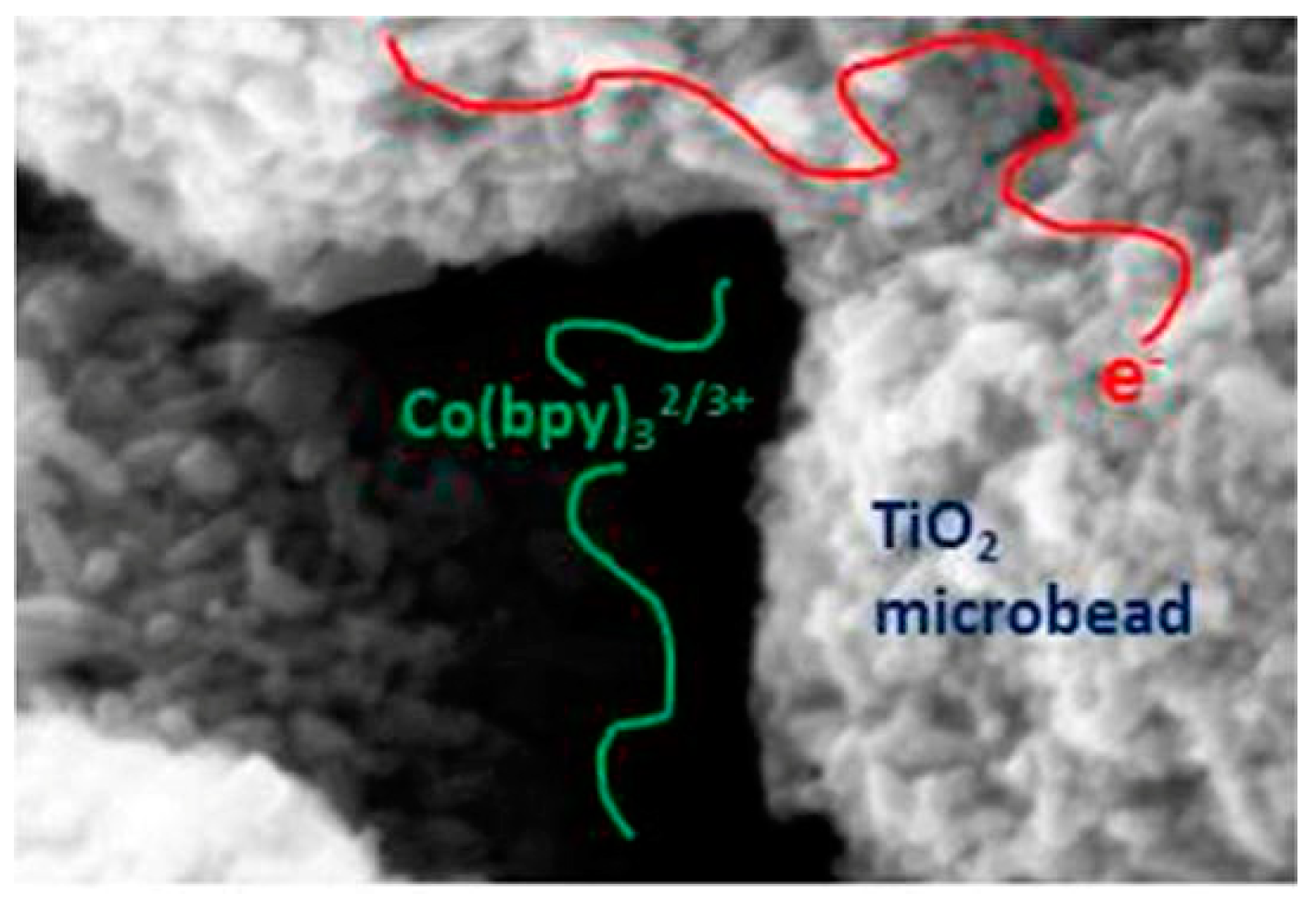
3.2. Solar Cells
3.3. Lithium-Ion Batteries
3.4. Biological Applications
3.4.1. Biosensors
3.4.2. Cancer Therapy
4. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Beck, J.S.; Vartuli, J.C.; Roth, W.J.; Leonowicz, M.E.; Kresge, C.T.; Schmitt, K.D.; Chu, C.T.W.; Olson, D.H.; Sheppard, E.W.; Mccullen, S.B. A new family of mesoporous molecular sieves prepared with liquid crystal templates. J. Am. Chem. Soc. 1992, 114, 10834–10843. [Google Scholar] [CrossRef]
- Antonelli, D.M.; Ying, J.Y. Synthesis of Hexagonally Packed Mesoporous TiO2 by a Modified Sol–Gel Method. Angew. Chem. Int. Ed. 1995, 34, 2014–2017. [Google Scholar] [CrossRef]
- Vu, T.T.D.; Mighri, F.; Ajji, A. Synthesis of Titanium Dioxide/Cadmium Sulfide Nanosphere Particles for Photocatalyst Applications. Ind. Eng. Chem. Res. 2014, 53, 3888–3897. [Google Scholar] [CrossRef]
- Zhao, J.; Liao, C.; Liu, J.; Shen, X.; Tong, H. Development of mesoporous titanium dioxide hybrid poly(vinylidene fluoride) ultrafiltration membranes with photocatalytic properties. J. Appl. Polym. Sci. 2016, 133, 43427. [Google Scholar] [CrossRef]
- Singh, N.; Mondal, K.; Misra, M.; Sharma, A.; Gupta, R.K. Quantum dot sensitized electrospun mesoporous titanium dioxide hollow nanofibers for photocatalytic applications. RSC Adv. 2016, 6, 48109–48119. [Google Scholar] [CrossRef]
- Carp, O.; Huisman, C.L.; Reller, A. Photoinduced reactivity of titanium dioxide. Prog. Solid State Chem. 2004, 32, 33–177. [Google Scholar] [CrossRef]
- And, X.C.; Mao, S.S. Titanium Dioxide Nanomaterials: Synthesis, Properties, Modifications, and Applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar]
- Sharon, M.; Modi, F.; Sharon, M. Titania based nanocomposites as a photocatalyst: A review. Mater. Sci. 2016, 3, 1236–1254. [Google Scholar] [CrossRef]
- Zhao, D.; Ranjit, S.B.; Koodali, T. Mesoporous Titanium Dioxide. ACS 2010, 1045, 97–123. [Google Scholar]
- Zhang, R.; Elzatahry, A.A.; Al-Deyab, S.S.; Zhao, D. Mesoporous titania: From synthesis to application. Nano Today 2012, 7, 344–366. [Google Scholar] [CrossRef]
- Hu, J.; Yang, Q.; Jing, C.; Wang, T.; He, L.; Qian, H. Synthesis and Applications of Mesoporous TiO2 Functional Nanomaterials. Prog. Chem. 2013, 25, 2080–2092. [Google Scholar]
- Bagheri, S.; Hir, Z.A.M.; Yousefi, A.T.; Hamid, S.B.A. Progress on mesoporous titanium dioxide: Synthesis, modification and applications. Microporous Mesoporous Mater. 2015, 218, 206–222. [Google Scholar] [CrossRef]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef]
- Gaya, U.I.; Abdullah, A.H. Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: A review of fundamentals, progress and problems. J. Photochem. Photobiol. C 2008, 9, 1–12. [Google Scholar] [CrossRef]
- Li, W.; Wu, Z.; Wang, J.; Elzatahry, A.A.; Zhao, D. A Perspective on Mesoporous TiO2 Materials. Chem. Mater. 2014, 26, 287–298. [Google Scholar] [CrossRef]
- Zhou, W.; Fu, H. Mesoporous TiO2: Preparation, doping, and as a composite for photocatalysis. Chem. Cat. Chem. 2013, 5, 885–894. [Google Scholar] [CrossRef]
- Celzard, A. Applications of the Sol–gel Process Using Well-Tested Recipes. J. Chem. Educ. 2002, 79, 854–859. [Google Scholar] [CrossRef]
- Liu, C.; Fu, L.; Economy, J. A simple, template-free route for the synthesis of mesoporous titanium dioxide materials. J. Mater. Chem. 2004, 14, 1187–1189. [Google Scholar] [CrossRef]
- Castañeda, C.; Tzompantzi, F.; Gómez, R.; Rojas, H. Enhanced photocatalytic degradation of 4-chlorophenol and 2,4-dichlorophenol on in situ phosphated sol-gel TiO2. J. Chem. Technol. Biotechnol. 2016, 91, 2170–2178. [Google Scholar] [CrossRef]
- Ahirwar, D.; Bano, M.; Khan, F. Synthesis of mesoporous TiO2 and its role as a photocatalyst in degradation of indigo carmine dye. J. Sol–Gel Sci. Technol. 2016, 79, 228–237. [Google Scholar] [CrossRef]
- Li, W.; Wang, F.; Liu, Y.; Wang, J.; Yang, J.; Zhang, L.; Elzatahry, A.A.; Al-Dahyan, D.; Xia, Y.; Zhao, D. General strategy to synthesize uniform mesoporous TiO2/graphene/mesoporous TiO2 sandwich-like nanosheets for highly reversible lithium storage. Nano Lett. 2015, 15, 2186–2193. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.C.; Lin, R.H.; Yang, C.Y.; Lin, M.H.; Wang, W.Y. Preparations and characterization of novel photocatalysts with mesoporous titanium dioxide (TiO2) via a sol–gel method. Mater. Chem. Phys. 2008, 109, 275–280. [Google Scholar] [CrossRef]
- Mazinani, B.; Masrom, A.K.; Beitollahi, A.; Luque, R. Photocatalytic activity, surface area and phase modification of mesoporous SiO2–TiO2 prepared by a one-step hydrothermal procedure. Ceram. Int. 2014, 40, 11525–11532. [Google Scholar] [CrossRef]
- Li, X.L.; Peng, Q.; Yi, J.X.; Wang, X.; Li, Y. Near monodisperse TiO2 nanoparticles and nanorods. Chemistry 2006, 12, 2383–2391. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Luo, G.S.; Wang, Y.J. Using phosphoric acid as a catalyst to control the structures of mesoporous titanium dioxide materials. Microporous Mesoporous Mater. 2005, 84, 27–33. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Y.; Shi, L.; Yuan, S.; Fang, J.; Wang, Z.; Zhang, M. Solvothermal synthesis of crystalline phase and shape controlled Sn(4+)-doped TiO2 nanocrystals: Effects of reaction solvent. ACS Appl. Mater. Interfaces 2011, 3, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Nam, C.T.; Le, M.D.; Le, M.D. Solvothermal synthesis of TiO2 photocatalysts in ketone solvents with low boiling points. J. Nanomater. 2013. [Google Scholar] [CrossRef]
- Shi, Y.; Wan, Y.; Zhao, D. Ordered mesoporous non-oxide materials. Chem. Soc. Rev. 2011, 40, 3854–3878. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, L.; Koodali, R.T. Versatility of Evaporation-Induced Self-Assembly (EISA) Method for Preparation of Mesoporous TiO2 for Energy and Environmental Applications. Materials 2014, 7, 2697–2746. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A.; Bhaumik, A.; Paul, B.K. Synthesis and characterization of mesoporous titanium dioxide using self-assembly of sodium dodecyl sulfate and benzyl alcohol systems as templates. Microporous Mesoporous Mater. 2008, 109, 66–72. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, L.; Zheng, Z.; Zhao, J. Synthesis and Characterization of Phosphated Mesoporous Titanium Dioxide with High Photocatalytic Activity. Chem. Mater. 2003, 15, 2280–2286. [Google Scholar] [CrossRef]
- Ismail, A.A.; Bahnemann, D.W.; Bannat, I.; Wark, M. Gold Nanoparticles on Mesoporous Interparticle Networks of Titanium Dioxide Nanocrystals for Enhanced Photonic Efficiencies. J. Phys. Chem. C 2009, 113, 7429–7435. [Google Scholar] [CrossRef]
- Mao, L.; Liu, J.; Zhu, S.; Zhang, D.; Chen, Z.; Chen, C. Sonochemical fabrication of mesoporous TiO2 inside diatom frustules for photocatalyst. Ultrason. Sonochem. 2014, 21, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Zhou, S.; You, B.; Wu, L. Facile Fabrication and High Photoelectric Properties of Hierarchically Ordered Porous TiO2. Chem. Mater. 2012, 24, 3800–3810. [Google Scholar] [CrossRef]
- Madhugiri, S.; Sun, B.; Smirniotis, P.G.; Ferraris, J.P.; Balkus, K.J., Jr. Electrospun mesoporous titanium dioxide fibers. Microporous Mesoporous Mater. 2015, 69, 77–83. [Google Scholar] [CrossRef]
- Wang, T.; Meng, X.; Li, P.; Ouyang, S.; Chang, K.; Liu, G.; Mei, Z.; Ye, J. Photoreduction of CO2 over the well-crystallized ordered mesoporous TiO2 with the confined space effect. Nano Energy 2014, 9, 50–60. [Google Scholar] [CrossRef]
- Sadatlu, M.A.A.; Mozaffari, N. Synthesis of mesoporous TiO2 structures through P123 copolymer as the structural directing agent and assessment of their performance in dye-sensitized solar cells. Sol. Energy 2016, 133, 24–34. [Google Scholar] [CrossRef]
- Liu, B.; Luo, Z.; Federico, A.; Song, W.; Suib, S.L.; He, J. Colloidal Amphiphile-Templated Growth of Highly Crystalline Mesoporous Nonsiliceous Oxides. Chem. Mater. 2015, 27, 6918–6928. [Google Scholar] [CrossRef]
- Tian, Q.; Zhang, Z.; Yang, L.; Hirano, S.I. Facile fabrication of one-dimensional mesoporous titanium dioxide composed of nanocrystals for lithium storage. Electrochim. Acta 2014, 138, 155–162. [Google Scholar] [CrossRef]
- Du, J.; Lai, X.; Yang, N.; Zhai, J.; Kisailus, D.; Su, F.; Wang, D.; Jiang, L. Hierarchically ordered macro-mesoporous TiO2-graphene composite films: Improved mass transfer, reduced charge recombination, and their enhanced photocatalytic activities. ACS Nano 2011, 5, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.G.; Hu, Y.S.; Sigle, W.; Maier, J. Superior Electrode Performance of Nanostructured Mesoporous TiO2 (Anatase) through Efficient Hierarchical Mixed Conducting Networks. Adv. Mater. 2010, 19, 2087–2091. [Google Scholar] [CrossRef]
- Ghosh, M.; Liu, J.; Chuang, S.S.C.; Jana, S.C. Fabrication of Hierarchical V2O5 Nanorods on TiO2 Nanofibers and Their Enhanced Photocatalytic Activity under Visible Light. ChemCatChem 2018, 10, 3305–3318. [Google Scholar] [CrossRef]
- Ghosh, M.; Jana, S.C. Bi-component inorganic oxide nanofibers from gas jet fiber spinning process. RSC Adv. 2015, 5, 105313–105318. [Google Scholar] [CrossRef]
- Li, Y.; Bastakoti, B.P.; Imura, M.; Hwang, S.M.; Sun, Z.; Kim, J.H.; Dou, S.X.; Yamauchi, Y. Synthesis of mesoporous TiO2/SiO2 hybrid films as an efficient photocatalyst by polymeric micelle assembly. Chemistry 2014, 20, 6027–6032. [Google Scholar] [CrossRef] [PubMed]
- Mahshid, S.; Askari, M.; Ghamsari, M.S. Effect of brookite presence on nanocrystalline anatase—Rutile phase transformation. Int. J. Nanotechnol. 2009, 6, 961–972. [Google Scholar] [CrossRef]
- Reyes-Coronado, D.; Rodríguez-Gattorno, G.; Espinosa-Pesqueira, M.E.; Cab, C.; De, C.R.; Oskam, G. Phase-pure TiO2 nanoparticles: Anatase, brookite and rutile. Nanotechnology 2008, 19, 145605. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Grampp, G.; Liu, Y.; Wang, B.X.; Tao, F.F.; Wang, L.J.; Liang, X.Z.; Xiao, H.Q.; Shen, Y.M. Visible Light Mediated Cyclization of Tertiary Anilines with Maleimides Using Nickel(II) Oxide Surface-Modified Titanium Dioxide Catalyst. J. Org. Chem. 2015, 80, 2724–2732. [Google Scholar] [CrossRef] [PubMed]
- Youngmin, K.; Min, H.H.; Wang, L.; Ikjoon, K.; Yeoheung, Y.; Hyoyoung, L. Solar-light photocatalytic disinfection using crystalline/amorphous low energy bandgap reduced TiO2. Sci. Rep. 2016, 6, 25212. [Google Scholar]
- Moon, B.C.; Park, J.H.; Lee, D.K.; Tsvetkov, N.; Ock, I.; Choi, K.M.; Kang, J.K. Broadband Light Absorption and Efficient Charge Separation Using a Light Scattering Layer with Mixed Cavities for High-Performance Perovskite Photovoltaic Cells with Stability. Small 2017, 13. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Li, X.; Zhao, Q.; Ke, J.; Zhang, D. Novel V2O5 /BiVO4 /TiO2 Nanocomposites with High Visible-Light-Induced Photocatalytic Activity for the Degradation of Toluene. J. Phys. Chem. C 2014, 118, 10113–10121. [Google Scholar] [CrossRef]
- Lebedev, V.A.; Sudin, V.V.; Kozlov, D.A.; Garshev, A.V. Photocatalytic properties of nanocrystalline TiO2 modified with CuO and WO3. Nanotechnol. Russ. 2016, 11, 20–28. [Google Scholar] [CrossRef]
- Sakthivel, S.; Kisch, H. Daylight photocatalysis by carbon-modified titanium dioxide. Angew. Chem. 2003, 42, 4908–4911. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Wang, H.; Wu, Z. One-Step “Green” Synthetic Approach for Mesoporous C-Doped Titanium Dioxide with Efficient Visible Light Photocatalytic Activity. J. Phys. Chem. C 2009, 113, 16717–16723. [Google Scholar] [CrossRef]
- Venditti, F.; Cuomo, F.; Ceglie, A.; Avino, P.; Russo, M.V.; Lopez, F. Visible light caffeic acid degradation by carbon-doped titanium dioxide. Langmuir 2015, 31, 3627–3634. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.A.; Wan, N.W.S.; Jaafar, J.; Rosmi, M.S.; Hir, Z.A.M.; Mutalib, M.A.; Ismail, A.F.; Tanemura, M. Carbon as amorphous shell and interstitial dopant in mesoporous rutile TiO2: Bio-template assisted sol–gel synthesis and photocatalytic activity. Appl. Surf. Sci. 2017, 393, 46–59. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Ai, Z.; Jia, F.; Zhang, L.; Fan, X.; Zou, Z. Low temperature preparation and visible light photocatalytic activity of mesoporous carbon-doped crystalline TiO2. Appl. Catal. B Environ. 2007, 69, 138–144. [Google Scholar] [CrossRef]
- Zhou, M.; Yu, J.; Cheng, B.; Yu, H. Preparation and photocatalytic activity of Fe-doped mesoporous titanium dioxide nanocrystalline photocatalysts. Mater. Chem. Phys. 2005, 93, 159–163. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, T.; Xie, Y.; Wang, Q.; Gao, X.; Zhang, R.; Zhang, Y.; Su, H. Synthesis and characterization of iron-based catalyst on mesoporous titania for photo-thermal F-T synthesis. Int. J. Hydrogen Energy 2015, 40, 870–877. [Google Scholar] [CrossRef]
- Lv, X.; Gao, F.; Yang, Y.; Wang, T. A Facile Electrochemical Approach to form TiO2/Ag Heterostructure Films with Enhanced Photocatalytic Activity. Ind. Eng. Chem. Res. 2015, 55. [Google Scholar] [CrossRef]
- Mermana, J.; Sutthivaiyakit, P.; Blaise, C.; Gagné, F.; Charnsethikul, S.; Kidkhunthod, P.; Sutthivaiyakit, S. Photocatalysis of S-metolachlor in aqueous suspension of magnetic cerium-doped mTiO2 core-shell under simulated solar light. Environ. Sci. Pollut. Res. Int. 2017. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.S.; Yeom, E.J.; Yang, W.S.; Hur, S.; Kim, M.G.; Im, J.; Seo, J.; Noh, J.H.; Seok, S.I. Colloidally prepared La-doped BaSnO3 electrodes for efficient, photostable perovskite solar cells. Science 2017, 356, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Siwinska-Stefanska, K.; Paukszta, D.; Piasecki, A.; Jesionowski, T. Synthesis and physicochemical characteristics of titanium dioxide doped with selected metals. Physicochem. Probl. Miner. Process. 2014, 50, 265–276. [Google Scholar]
- Zhang, K.; Wang, X.; Guo, X.; He, T.; Feng, Y. Preparation of highly visible light active Fe–N co-doped mesoporous TiO2 photocatalyst by fast sol–gel method. J. Nanopart. Res. 2014, 16, 2246. [Google Scholar] [CrossRef]
- Cant, A.M.; Huang, F.; Zhang, X.L.; Chen, Y.; Cheng, Y.B.; Amal, R. Tailoring the conduction band of titanium oxide by doping tungsten for efficient electron injection in a sensitized photoanode. Nanoscale 2014, 6, 3875–3880. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Thind, S.S.; Wu, G.; Wen, J.; Chen, A. Synthesis and photoelectrochemical studies of N, Zr co-doped mesoporous titanium dioxide. J. Electroanal. Chem. 2015, 736, 93–100. [Google Scholar] [CrossRef]
- Nasir, M.; Lei, J.; Iqbal, W.; Zhang, J. Study of synergistic effect of Sc and C co-doping on the enhancement of visible light photo-catalytic activity of TiO2. Appl. Surf. Sci. 2016, 364, 446–454. [Google Scholar] [CrossRef]
- Men, J.; Gao, Q.; Sun, S.; Zhang, X.; Duan, L.; Wei, L. Carbon nitride doped TiO2 photoelectrodes for photocatalysts and quantum dot sensitized solar cells. Mater. Res. Bull. 2017, 85, 209–215. [Google Scholar] [CrossRef]
- Rasalingam, S.; Wu, C.M.; Koodali, R.T. Modulation of pore sizes of titanium dioxide photocatalysts by a facile template free hydrothermal synthesis method: Implications for photocatalytic degradation of rhodamine B. ACS Appl. Mater. Interfaces 2015, 7, 4368–4380. [Google Scholar] [CrossRef] [PubMed]
- Akhter, P.; Hussain, M.; Saracco, G.; Russo, N. Novel nanostructured-TiO2 materials for the photocatalytic reduction of CO2 greenhouse gas to hydrocarbons and syngas. Fuel 2015, 149, 55–65. [Google Scholar] [CrossRef]
- Samsudin, E.M.; Hamid, S.B.A.; Juan, J.C.; Wan, J.B. Influence of triblock copolymer (pluronic F127) on enhancing the physico-chemical properties and photocatalytic response of mesoporous TiO2. Appl. Surf. Sci. 2015, 355, 959–968. [Google Scholar] [CrossRef]
- Cao, S.; Zhao, Y.; Qu, T.; Wang, P.; Guan, S.; Xu, Y.; Rao, F.; Li, Y.; Chen, A.; Iyoda, T. Ordered mesoporous crystalline titania with high thermal stability from comb-like liquid crystal block copolymers. RSC Adv. 2016, 6, 55834–55841. [Google Scholar] [CrossRef]
- Ghosh, M.; Lohrasbi, M.; Chuang, S.S.C.; Jana, S.C. Mesoporous Titanium Dioxide Nanofibers with a Significantly Enhanced Photocatalytic Activity. ChemCatChem 2016, 8, 2525–2535. [Google Scholar] [CrossRef]
- Yu, J.; Zhou, M.; Cheng, B.; Yu, H.; Zhao, X. Ultrasonic preparation of mesoporous titanium dioxide nanocrystalline photocatalysts and evaluation of photocatalytic activity. J. Mol. Catal. A Chem. 2005, 227, 75–80. [Google Scholar] [CrossRef]
- Shao, G.N.; Jeon, S.J.; Haider, M.S.; Abbass, N.; Kim, H.T. Investigation of the influence of vanadium, iron and nickel dopants on the morphology, and crystal structure and photocatalytic properties of titanium dioxide based nanopowders. J. Colloid Interface Sci. 2016, 474, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Liu, P.; Yu, M.; Xing, M.; Zhang, J. Preparation of homogeneous nitrogen-doped mesoporous TiO2 spheres with enhanced visible-light photocatalysis. Appl. Catal. B Environ. 2015, 164, 352–359. [Google Scholar]
- Fang, Q.; Tang, J.; Zou, H.; Cai, T.; Deng, Q. Preparation of N-Doped Mesoporous TiO2 Using Nitromethane as Nitrogen Source and Their High Photocatalytic Performance. Synth. React. Inorg. Met. Org. Chem. 2016, 46, 766–774. [Google Scholar] [CrossRef]
- Chiang, L.F.; Doong, R.A. Cu-TiO2 nanorods with enhanced ultraviolet- and visible-light photoactivity for bisphenol A degradation. J. Hazard. Mater. 2014, 277, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Ameen, S.; Akhtar, M.S.; Seo, H.K.; Shin, H.S. Solution-processed CeO2/TiO2 nanocomposite as potent visible light photocatalyst for the degradation of bromophenol dye. Chem. Eng. J. 2014, 247, 193–198. [Google Scholar] [CrossRef]
- Chaker, H.; Chérif-Aouali, L.; Khaoulani, S.; Bengueddach, A.; Fourmentin, S. Photocatalytic degradation of methyl orange and real wastewater by silver doped mesoporous TiO2 catalysts. J. Photochem. Photobiol. A Chem. 2016, 318, 142–149. [Google Scholar] [CrossRef]
- Hir, Z.A.M.; Moradihamedani, P.; Abdullah, A.H.; Mohamed, M.A. Immobilization of TiO2 into polyethersulfone matrix as hybrid film photocatalyst for effective degradation of methyl orange dye. Mater. Sci. Semicond. Process. 2017, 57, 157–165. [Google Scholar] [CrossRef]
- Abdullah, A.M.; Al-Thani, N.J.; Tawbi, K.; Al-Kandari, H. Carbon/nitrogen-doped TiO2: New synthesis route, characterization and application for phenol degradation. Arab. J. Chem. 2016, 9, 229–237. [Google Scholar] [CrossRef]
- Elsalamony, R.A.; Mahmoud, S.A. Preparation of nanostructured ruthenium doped titania for the photocatalytic degradation of 2-chlorophenol under visible light. Arab. J. Chem. 2017, 10, 194–205. [Google Scholar] [CrossRef]
- Lin, X.; Li, Y.; Chen, F.; Xu, P.; Li, M. Facile synthesis of mesoporous titanium dioxide doped by Ag-coated graphene with enhanced visible-light photocatalytic performance for methylene blue degradation. RSC Adv. 2017, 7, 25314–25324. [Google Scholar]
- Liu, Y.; Becker, B.; Burdine, B.; Sigmon, G.E.; Burns, P.C. Photocatalytic decomposition of Rhodamine B on uranium-doped mesoporous titanium dioxide. RSC Adv. 2017, 7, 21273–21280. [Google Scholar] [CrossRef]
- Luo, Z.; Poyraz, A.S.; Kuo, C.H.; Miao, R.; Meng, Y.; Chen, S.Y.; Jiang, T.; Wenos, C.; Suib, S.L. Crystalline Mixed Phase (Anatase/Rutile) Mesoporous Titanium Dioxides for Visible Light Photocatalytic Activity. Chem. Mater. 2014, 27. [Google Scholar] [CrossRef]
- Ovcharov, M.L.; Shvalagin, V.V.; Granchak, V.M. Photocatalytic Reduction of CO2 on Mesoporous TiO2 Modified with Ag/Cu Bimetallic Nanostructures. Theor. Exp. Chem. 2014, 50, 175–180. [Google Scholar] [CrossRef]
- Chowdhury, S.; Parshetti, G.K.; Balasubramanian, R. Post-combustion CO2 capture using mesoporous TiO2/graphene oxide nanocomposites. Chem. Eng. J. 2015, 263, 374–384. [Google Scholar] [CrossRef]
- He, D.; Li, Y.; Wang, I.; Wu, J.; Yang, Y.; An, Q. Carbon wrapped and doped TiO2 mesoporous nanostructure with efficient visible-light photocatalysis for NO removal. Appl. Surf. Sci. 2016, 391, 318–325. [Google Scholar] [CrossRef]
- Shang, M.; Hou, H.; Gao, F.; Wang, L.; Yang, W. Mesoporous Ag@TiO2 nanofibers and their photocatalytic activity for hydrogen evolution. RSC Adv. 2017, 7, 30051–30059. [Google Scholar] [CrossRef]
- Atabaev, T.S.; Hossain, M.A.; Lee, D.; Kim, H.K.; Hwang, Y.H. Pt-coated TiO2 nanorods for photoelectrochemical water splitting applications. Results Phys. 2016, 6, 373–376. [Google Scholar] [CrossRef]
- Yun, T.K.; Park, S.S.; Kim, D.; Hwang, Y.K.; Huh, S.; Bae, J.Y.; Yong, S.W. Pore-size effect on photovoltaic performance of dye-sensitized solar cells composed of mesoporous anatas e-titania. J. Power Sources 2011, 196, 3678–3682. [Google Scholar] [CrossRef]
- Tiwana, P.; Parkinson, P.; Johnston, M.B.; Snaith, H.J.; Herz, L.M. Ultrafast Terahertz Conductivity Dynamics in Mesoporous TiO2: Influence of Dye Sensitization and Surface Treatment in Solid-State Dye-Sensitized Solar Cells. J. Phys. Chem. C 2010, 114, 1365–1371. [Google Scholar] [CrossRef]
- Pazoki, M.; Taghavinia, N.; Hagfeldt, A.; Boschloo, G. Mesoporous TiO2 Microbead Electrodes for Cobalt-Mediator-Based Dye-Sensitized Solar Cells. J. Phys. Chem. C 2014, 118, 16472–16478. [Google Scholar] [CrossRef]
- Hou, W.; Xiao, Y.; Han, G.; Zhou, H.; Chang, Y.; Zhang, Y. Preparation of mesoporous titanium dioxide anode by a film- and pore-forming agent for the dye-sensitized solar cell. Mater. Res. Bull. 2016, 76, 140–146. [Google Scholar] [CrossRef]
- Chandiran, A.K.; Nazeeruddin, M.K.; Grätzel, M. The Role of Insulating Oxides in Blocking the Charge Carrier Recombination in Dye-Sensitized Solar Cells. Adv. Funct. Mater. 2014, 24, 1615–1623. [Google Scholar] [CrossRef]
- Colella, S.; Orgiu, E.; Bruder, I.; Liscio, A.; Palermo, V.; Bruchmann, B.; Samorì, P.; Erk, P. Titanium Dioxide Mesoporous Electrodes for Solid-State Dye-Sensitized Solar Cells: Cross-Analysis of the Critical Parameters. Adv. Energy Mater. 2014, 4, 1289–1295. [Google Scholar] [CrossRef]
- Yan, W.; Li, Y.; Sun, W.; Peng, H.; Ye, S.; Liu, Z.; Bian, Z.; Huang, C. High-performance hybrid perovskite solar cells with polythiophene as hole-transporting layer via electrochemical polymerization. RSC Adv. 2014, 4, 33039–33046. [Google Scholar] [CrossRef]
- Choi, J.J.; Yang, X.; Norman, Z.M.; Billinge, S.J.; Owen, J.S. Structure of methylammonium lead iodide within mesoporous titanium dioxide: Active material in high-performance perovskite solar cells. Nano Lett. 2014, 14, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Chandiran, A.K.; Yella, A.; Mayer, M.T.; Gao, P.; Nazeeruddin, M.K.; Grätzel, M. Sub-nanometer conformal TiO2 blocking layer for high efficiency solid-state perovskite absorber solar cells. Adv. Mater. 2014, 26, 4309–4312. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Ma, J.; Wang, Z.; Mu, C.; Fan, Z.; Du, L.; Bai, Y.; Fan, L.; Yan, H.; Phillips, D.L. Efficiency enhancement of perovskite solar cells through fast electron extraction: The role of graphene quantum dots. J. Am. Chem. Soc. 2014, 136, 3760–3763. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, B.; Bhatt, P.; Kanth, P.C.; Yadav, P.; Desai, B.; Pandey, M.K.; Kumar, M. Temperature induced structural, electrical and optical changes in solution processed perovskite material: Application in photovoltaics. Sol. Energy Mater. Sol. Cells 2015, 132, 615–622. [Google Scholar] [CrossRef]
- Xue, H.; Fu, K.; Wong, L.H.; Birgersson, E.; Stangl, R. Modelling and loss analysis of meso-structured perovskite solar cells. J. Appl. Phys. 2017, 122, 083105. [Google Scholar] [CrossRef]
- Tian, Q.; Zhang, Z.; Yang, L.; Hirano, S.I. Fabrication of mesoporous titanium dioxide/tin dioxide/carbon hollow microspheres as high performance anode for lithium-ion batteries. J. Power Sources 2015, 279, 528–532. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, Y.; Cui, Y.; Zhang, J.; Zhang, G.; Luo, W.; Zheng, W. A Facile Synthesis of Mesoporous TiO2 Sub-Microsphere Host for Long Life Lithium-Sulfur Battery Cathodes. Electrochim. Acta 2017. [Google Scholar] [CrossRef]
- Wu, L.; Liu, Y.; Chi, B.; Xu, Z.; Feng, X.; Li, S.; Xu, H. An innovative method for immobilizing sucrose isomerase on ε-poly-L-lysine modified mesoporous TiO2. Food Chem. 2015, 187, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wu, S.; Xu, Z.; Qiu, Y.; Li, S.; Xu, H. Modified nanoporous titanium dioxide as a novel carrier for enzyme immobilization. Biosens. Bioelectron. 2016, 80, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Bown, S.G.; Rogowska, A.Z.; Whitelaw, D.E.; Lees, W.R.; Lovat, L.B.; Ripley, P.; Jones, L.; Wyld, P.; Gillams, A. Photodynamic therapy for cancer of the pancreas. Gut 2002, 50, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Dolmans, D.E.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Sun, J.; Zhu, X.; Qiu, L.; Xu, M.; Liu, S.; Ouyang, J.M.; Liu, J. Mesoporous Titanium Dioxide Nanocarrier with Magnetic-Targeting and High Loading Efficiency for Dual-Modal Imaging and Photodynamic Therapy. J. Mater. Chem. B 2017, 5, 6081–6096. [Google Scholar] [CrossRef]
- Yumita, N.; Kawabata, K.; Sasaki, K.; Umemura, S. Sonodynamic effect of erythrosin B on sarcoma 180 cells in vitro. Ultrason. Sonochem. 2002, 9, 259–265. [Google Scholar] [CrossRef]
- Yumita, N.; Iwase, Y.; Nishi, K.; Komatsu, H.; Takeda, K.; Onodera, K.; Fukai, T.; Ikeda, T.; Umemura, S.; Okudaira, K. Involvement of Reactive Oxygen Species in Sonodynamically Induced Apoptosis Using a Novel Porphyrin Derivative. Theranostics 2012, 2, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Varchi, G.; Foglietta, F.; Canaparo, R.; Ballestri, M.; Arena, F.; Sotgiu, G.; Guerrini, A.; Nanni, C.; Cicoria, G.; Cravotto, G. Engineered porphyrin loaded core-shell nanoparticles for selective sonodynamic anticancer treatment. Nanomedicine 2015, 10, 3483–3494. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, W.; Yu, L.; Tang, Y.; Cao, J.; Chen, Y. Site-specific sonocatalytic tumor suppression by chemically engineered single-crystalline mesoporous titanium dioxide sonosensitizers. J. Mater. Chem. B 2017, 5, 4579–4586. [Google Scholar] [CrossRef]
- Lai, M.; Cai, K.; Zhao, L.; Chen, X.; Hou, Y.; Yang, Z. Surface functionalization of TiO2 nanotubes with bone morphogenetic protein 2 and its synergistic effect on the differentiation of mesenchymal stem cells. Biomacromolecules 2011, 12, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Wang, Y.; Cao, L.; Wang, Y.; Lin, B.; Lan, W.; Cao, B. Preparation and antimicrobial assay of ceramic brackets coated with TiO2 thin films. Korean J. Orthod. 2016, 46, 146–154. [Google Scholar] [CrossRef] [PubMed]
| Sample | Eg (eV) | Surface Area (m2·g−1) | Pore Size (Å) | Pore Volume (cm3·g−1) | Crystallite Size (nm) |
|---|---|---|---|---|---|
| TiO2 | 3.01 | 126 | 56 | 0.23 | 10.1 |
| PTi 0.5% | 3.08 | 185 | 34 | 0.23 | 7.3 |
| PTi 1% | 3.11 | 198 | 34 | 0.22 | 7.1 |
| PTi 3% | 3.09 | 269 | 34 | 0.24 | 5.6 |
| PTi 5% | 3.10 | 285 | 34 | 0.26 | 5.8 |
| H3PO4 Concentration (mol/L) | BET Surface Area (m2/g) | Total Pore Volume (cm3/g) | Average Pore Size (nm) |
|---|---|---|---|
| 0.28 | 163 | 0.316 | 7.8 |
| 0.5 | 161 | 0.368 | 9.2 |
| 0.8 | 207 | 0.472 | 9.1 |
| 1.0 | 196 | 0.462 | 9.4 |
| 1.5 | 294 | 0.545 | 7.4 |
| 2.0 | 227 | 0.506 | 8.9 |
| Crystal Structure | System | Density (g/cm3) | Band Gap (eV) |
|---|---|---|---|
| Anatase | Tetragonal | 3.82–3.98 | 3.62 |
| Rutile | Tetragonal | 4.2–4.3 | 3.05 |
| Brookite | Rhombohedral | 4.1–4.2 | - |
| TiO2 (B) | Monoclinic | 3.6–3.8 | 3–3.22 |
| Ti Precursor | Method | Cal. Tempt. | BET (m2/g) | Pore Size (nm) | Ref. |
|---|---|---|---|---|---|
| TTIP | Hydrothermal | 500 °C | 24–110 | 4–17.5 | [70] |
| TBT | Sol–gel | 550 °C | 190 | 3.2 | [71] |
| TTIP | Soft-template | 500 °C | 30.46 | 11.1 | [72] |
| TiCl4 | Hard-template | 450 °C | 239 | 4.87 | [73] |
| TTIP | Sol–gel | 500–700 °C | 3.4–30 | 5–23 | [74] |
| TBOT | Hydrothermal | 500 °C | 97.3 | 9.1 | [75] |
| TiOCl2 | Sol–gel | 600 °C | 38–88 | 4.8–19 | [76] |
| Ti(SO4)2 | Solvothermal | 400 °C | >94.9 | >7.0 | [77] |
| TiCl4, TBOT | Soft-template | 470 °C | 77.77 | 6.60 | [78] |
| Method | Pore Size (nm) | BET (m2/g) | Morphology | Crystalline Structure | Application | Reference |
|---|---|---|---|---|---|---|
| Sol–gel | 5.5 | 187 | nanofiber | anatase | Immobilizing enzymes for biosensor | [107] |
| Template | 6.2 | - | core/shell | anatase | As nanocarrier for PDT | [110] |
| EISA | 3.80 | 96.10 | sphere | anatase | As sonosensitizers for SDT | [115] |
| Electrochemistry | 30 | - | nanotube | - | Implant surface modification | [116] |
| Sol–gel | - | - | film | rutile | Photocatalytic antibacterial agent | [117] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, B.; Wang, X.; Wu, K.; He, X.; Zhang, R. Mesoporous Titanium Dioxide: Synthesis and Applications in Photocatalysis, Energy and Biology. Materials 2018, 11, 1910. https://doi.org/10.3390/ma11101910
Niu B, Wang X, Wu K, He X, Zhang R. Mesoporous Titanium Dioxide: Synthesis and Applications in Photocatalysis, Energy and Biology. Materials. 2018; 11(10):1910. https://doi.org/10.3390/ma11101910
Chicago/Turabian StyleNiu, Ben, Xin Wang, Kai Wu, Xianru He, and Rui Zhang. 2018. "Mesoporous Titanium Dioxide: Synthesis and Applications in Photocatalysis, Energy and Biology" Materials 11, no. 10: 1910. https://doi.org/10.3390/ma11101910
APA StyleNiu, B., Wang, X., Wu, K., He, X., & Zhang, R. (2018). Mesoporous Titanium Dioxide: Synthesis and Applications in Photocatalysis, Energy and Biology. Materials, 11(10), 1910. https://doi.org/10.3390/ma11101910





