Polymeric Nanocomposites and Nanocoatings for Food Packaging: A Review
Abstract
1. Introduction
- (I)
- Improved packaging which offers improved mechanical properties as flexibility, enhanced barrier properties against water, gases, taint, durability, temperature/moisture stability, and so forth;
- (II)
- Active/bioactive food packaging offers antimicrobial, antioxidant or biocatalytic functions. It can be obtained by the incorporation of active/bioactive compounds into matrices used in existing packaging materials, or by the application of coatings with the mentioned functionality through physical or chemical surface modification. Active packaging is applied in food packaging, pharmaceuticals and consumer goods in order to improve shelf life, safety, or quality of packaged foods. Coating option is advantageous because the bulk properties of the packaging materials are preserved almost intact by using a minimum amount of active agent required to impart efficacy and therefore also cost is reduced.
- (III)
- Smart/intelligent packaging as a promising area for active packaging coating is developed by manufacture of nano(bio)sensors which can indicate quality of foodstuffs, of nano(bio)switch to release preservatives and nano-coatings as antimicrobial, antifungal, antioxidant, barrier coatings, external stimuli responsive materials and self-cleaning food contact surfaces. Intelligent inks such as nanoparticles and reactive nanolayers allow analyte recognition at nanoscale. Printed labels are applied to indicate: temperature, time, pathogen, freshness, humidity, integrity [8]. Smart packaging may monitor various parameters such as: temperature, oxygen, pH, moisture and so forth [9,10,11] of packaged products.
2. Polymeric Nanocomposites
2.1. Preparation Methods
2.2. Types of Polymer Nanocomposites Used in Food Packaging
2.2.1. Montmorilonites (MMT) Containing Nanocomposites
2.2.2. Bionanofibrils
2.2.3. Other Types of Nanofillers
2.2.4. Bioplastics–Biopackaging
2.2.5. Degradability and Recyclability of Nanocomposites
3. Polymer Nano-Coatings in Food Packaging
3.1. Types of Nanocoatings
3.2. Coatings Procedures
3.2.1. Solution Casting
3.2.2. Extrusion
3.2.3. Sol-Gel Procedure
3.2.4. Spraying Solution with Compressor Gun
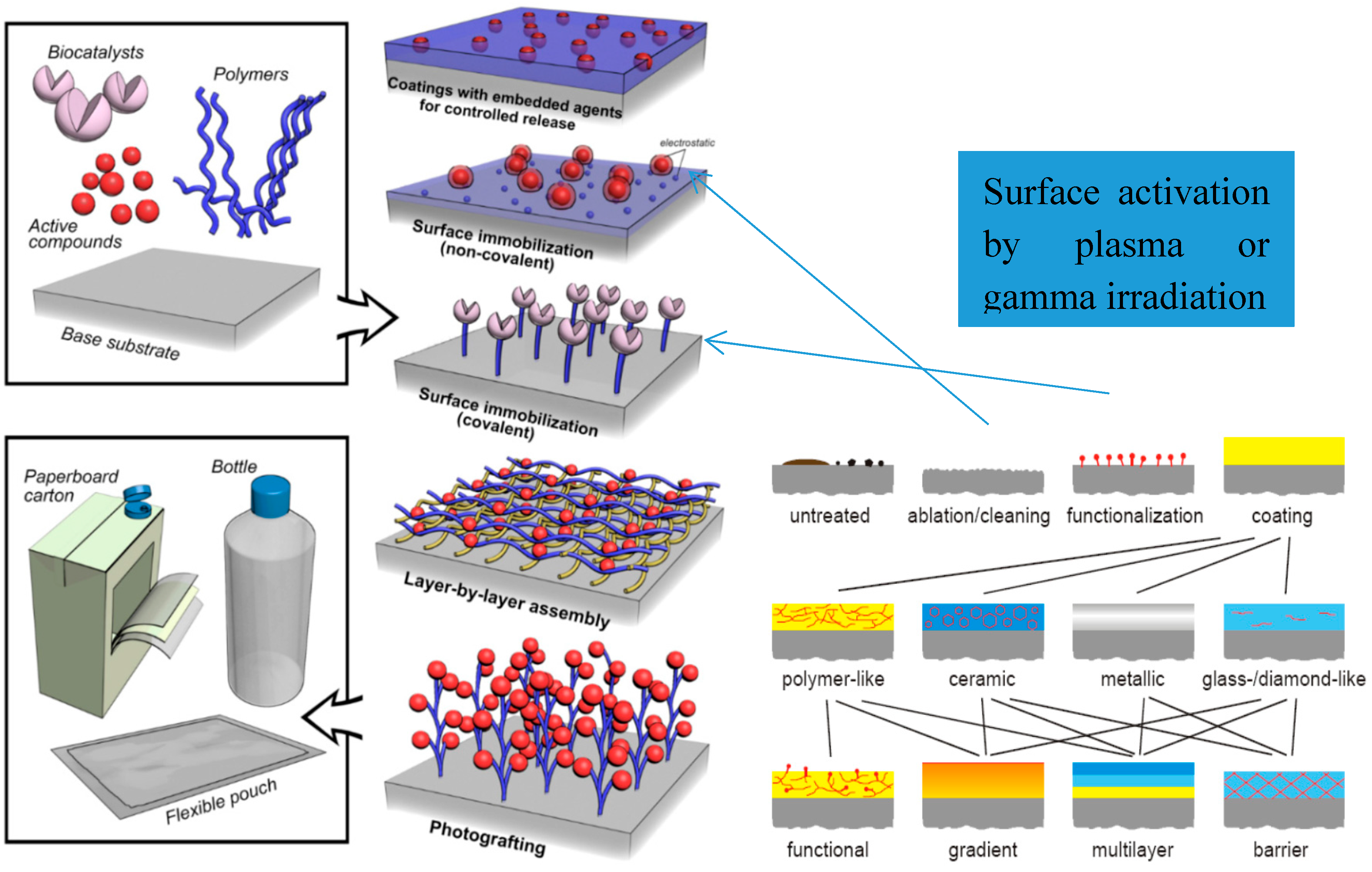
3.2.5. Surface Immobilization
- Activation of polymeric substrates by non-solvent, environment friendly methods by using of gamma-ionizing radiation or cold plasma gas discharge.
- Stable layers have been deposited onto activated polymeric substrates using different coupling agents for covalent linking of active/bioactive formulations.
- Selected bioactive compounds were: chitosan/chitin, lactoferrin, vitamin E, natural vegetable oils with high content of antioxidant compounds as phenols or flavonoids mixtures.
3.2.6. Wet Methods
3.2.7. Photografting
3.2.8. Biological Methods
3.2.9. Chemical Vapour Deposition (CVD)
3.2.10. Atomic Layer Deposition (ALD) Method
3.2.11. Layer-by-Layer Assembly
3.2.12. Ultrasonic Nozzle Systems for Nanotechnology Coating Applications
3.2.13. Plasma Nano-Coating of Beverage Cans
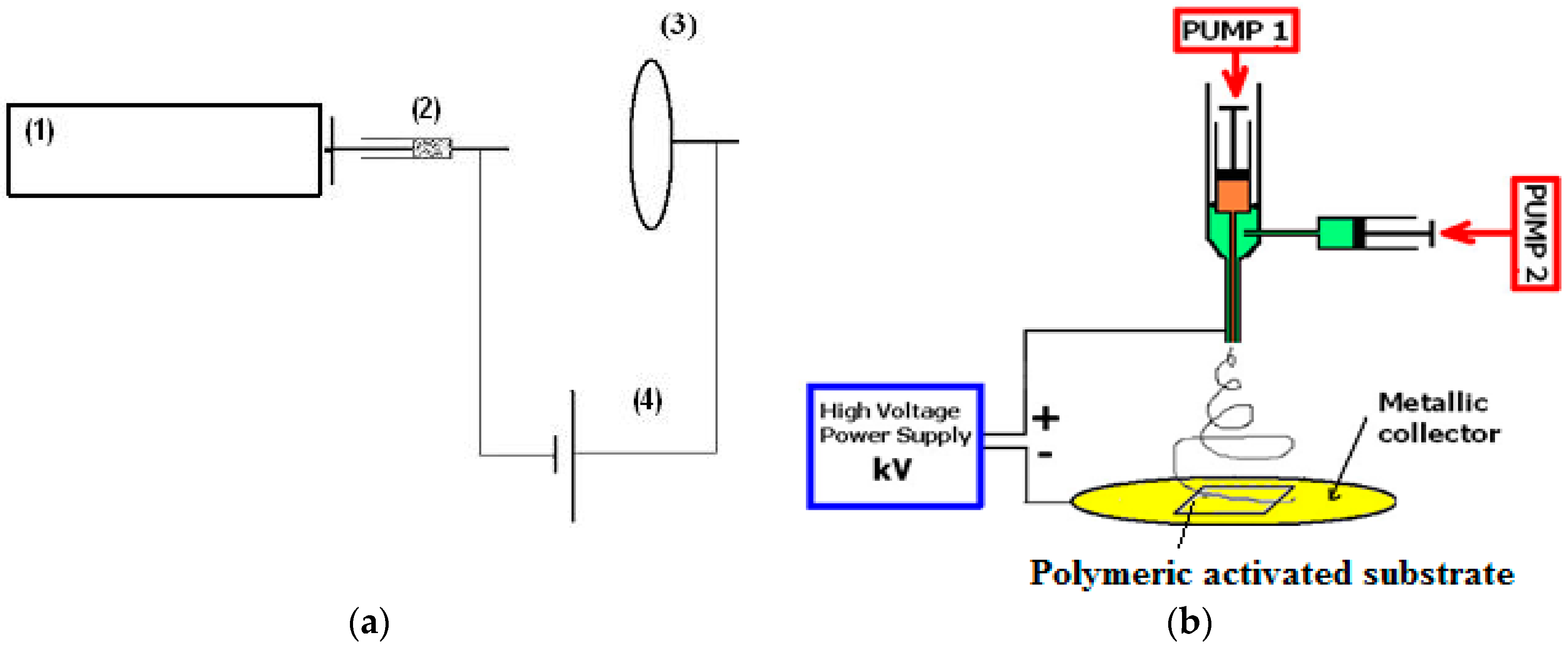
3.2.14. Electrospinning/Electrospraying
4. Applications
4.1. Antimicrobial/Antibacterial
4.2. Antioxidant
4.3. Biocatalytic
4.4. Barrier Applications of Polymer Nanocomposites
4.5. Stimuli Responsive Nanocomposites/Nanocoatings
5. Possible Risks
6. Commercial Level
7. Conclusion and Future Trends
Author Contributions
Funding
Conflicts of Interest
References
- Duncan, T.V. Applications of nanotechnology in food packaging and food safety: Barrier materials, antimicrobials and sensors. J. Colloid Interface Sci. 2011, 363, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Vasile, C. (Ed.) Polymeric Nanomaterials for Nanotherapeutics; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 978-0-12-813932-5. [Google Scholar]
- Grumazescu, A.M. (Ed.) Food Preservation, Nanitechnology in the Agri-Food Industry; Elsevier: Amsterdam, The Netherlands, 2017; Volume 5, ISBN Hardcover 9780128043035. [Google Scholar]
- Chaudhry, Q. Nanotechnology Applications for Food Packaging. 2017. Available online: http://ilsi.eu/wp-content/uploads/sites/3/2016/06/S2.3-WSPM12-Chaudhry.pdf (accessed on 21 June 2017).
- Huang, Q.; Given, P.; Qian, M. Micro/Nano-Encapsulation of Active Food Ingredients; American Chemical Society: Washington, DC, USA, 2009; Volume 1007, p. 314. [Google Scholar]
- Irshad, A. Applications of Nanotechnology in Food Packaging and Food Safety (Barrier Materials, Antimicrobials and Sensors). 2013. Available online: http://www.slideshare.net/irshad2k6/applications-of-nanotechnology-in-food-packaging-and-food-safety-barrier-materials-antimicrobials-and-sensors (accessed on 14 June 2017).
- Regulation (EC) No 1935/2004 of The European Parliament and of The Council of 27 October 2004 on Materials and Articles Intended to Come into Contact with Food and Repealing Directives 80/590/EEC and 89/109/EEC. Available online: https://www.interpack.com/cgi-bin/md_interpack/lib/pub/tt.cgi/Food_Safety.html?oid=55076&lang=2&ticket=g_u_e_s_t (accessed on 5 September 2018).
- Aliofkhazraei, M. Nanocoatings: Size Effect in Nanostructured Films; Springer: Berlin, Germany, 2011; p. 212. ISBN 978-3-642-17966-2. [Google Scholar]
- Brody, A.L. Nanocomposite technology in food packaging. Food Technol. 2007, 61, 80–83. [Google Scholar]
- Brody, A.L.; Strupinsky, E.R.; Kline, L.R. Active Packaging for Food Applications; Technomic Pub. Co.: Lancaster, PA, USA, 2001; p. 218. [Google Scholar]
- Kerry, J.; Butler, P. Smart Packaging Technologies for Fast Moving Consumer Goods; John Wiley: Chichester, UK; Hoboken, NJ, USA, 2008; p. 340. [Google Scholar]
- De Azeredo, H.M.C. Nanocomposites for food packaging applications. Food Res. Int. 2009, 42, 1240–1253. [Google Scholar] [CrossRef]
- Polyethylene Plastic Packaging Applications—Packaging Blog. Available online: https://packagingblog.org/2014/02/08/polyethylene-plastic-packaging-applications/ (accessed on 5 September 2018).
- Jin, T.; Zhang, H. Biodegradable Polylactic Acid Polymer with Nisin for Use in Antimicrobial Food Packaging. J. Food Sci. 2008, 73, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Mallegni, N.; Phuong, T.V.; Coltelli, M.-B.; Cinelli, P.; Lazzeri, A. Poly(lactic acid) (PLA) Based Tear Resistant and Biodegradable Flexible Films by Blown Film Extrusion. Materials 2018, 11, 148. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Wang, Z.; Zhao, Q.; Yang, Y.; Xu, J.; Waterhouse, G.I.N.; Zhang, K.; Li, S.; Jin, P.; Jin, G. Scale-Up Fabrication of Biodegradable Poly(butylene adipate-co-terephthalate)/Organophilic–clay Nanocomposite Films for Potential Packaging Applications. ACS Omega 2018, 3, 1187–1196. [Google Scholar] [CrossRef]
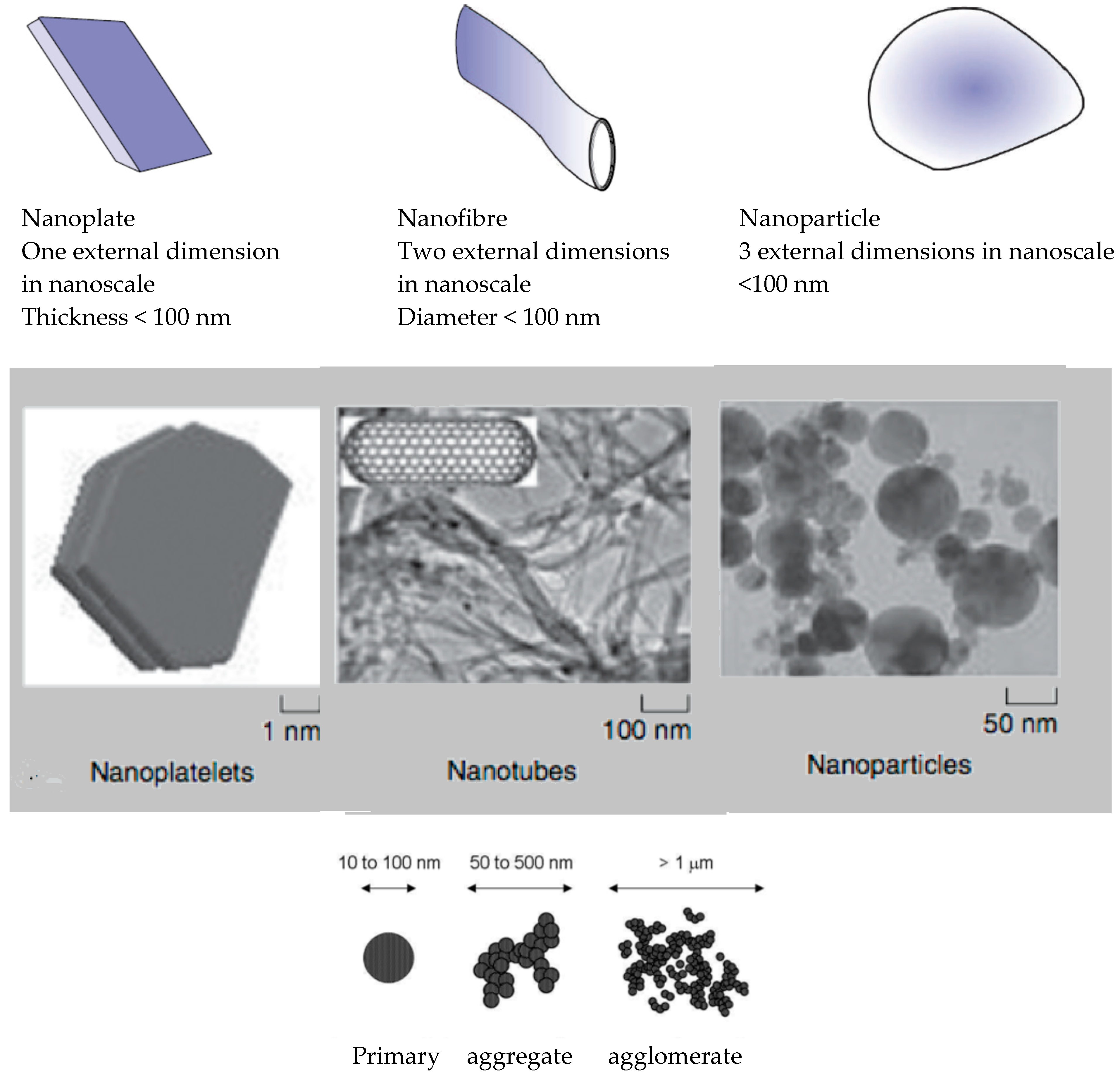
- ISO/TS27687:2008. This standard has been replaced by ISO/TS 80004-2:2015 ISO/TS 80004-2:2015(en) Nanotechnologies—Vocabulary—Part 2: Nano-objects: Nanotechnologies—Terminology and definitions for nano-objects—Nanoparticle, nanofibre and nanoplate.
- Schadler, L.S.; Brinson, L.C.; Sawyer, W.G. Polymer Nanocomposites: A Small Part of the Story. Overview Nanocomposite Materials. J. Mater. 2007, 59, 53–60. [Google Scholar] [CrossRef]
- Pedrazzoli, D. Understanding the Effect of Nanofillers on the Properties of Polypropylene and Glass Fiber/Polypropylene Multiscale Composites. Ph.D. Thesis, University of Trento, Rrento, Italy, October 2014. Available online: http://eprints-phd.biblio.unitn.it/1322/1/Thesis_PhD_Pedrazzoli_2014.pdf (accessed on 13 June 2017).
- Tiwari, J.N.; Tiwari, R.N.; Kim, K.S. Zero-dimensional, one-dimensional, two-dimensional and three-dimensional nanostructured materials for advanced electrochemical energy devices. Prog. Mater. Sci. 2012, 57, 724–803. [Google Scholar] [CrossRef]
- LeBaron, P.C.; Wang, Z.; Pinnavaia, T.J.Ž. Polymer-layered silicate nanocomposites: An overview. Appl. Clay Sci. 1999, 15, 11–29. [Google Scholar] [CrossRef]
- Pavlidou, S.; Papaspyrides, C.D. A review on polymer–layered silicate nanocomposites. Prog. Polym. Sci. 2008, 33, 1119–1198. [Google Scholar] [CrossRef]
- Esposito Corcione, C.; Frigione, M. Characterization of Nanocomposites by Thermal Analysis. Materials 2012, 5, 2960–2980. [Google Scholar] [CrossRef]
- Fernandez, A.; Torres-Giner, S.; Lagaron, J.M. Novel route to stabilization of bioactive antioxidants by encapsulation in electrospun fibres of zein prolamine. Food Hydrocoll. 2009, 23, 1427–1432. [Google Scholar] [CrossRef]
- Reig, C.S.; Lopez, A.D.; Ramos, M.H.; Ballester, V.A.C. Nanomaterials: A Map for Their Selection in Food Packaging Applications. Packag. Technol. Sci. 2014, 27, 839–866. [Google Scholar] [CrossRef]
- Sanchez-Garcia, M.D.; Lopez-Rubio, A.; Lagaron, J.M. Natural micro and nanobiocomposites with enhanced barrier properties and novel functionalities for food biopackaging applications. Trends Food Sci. Technol. 2010, 21, 528–536. [Google Scholar] [CrossRef]
- Siracusa, V.; Rocculi, P.; Romani, S.; Rosa, M.D. Biodegradable polymers for food packaging: A review. Trends Food Sci. Technol. 2008, 19, 634–643. [Google Scholar] [CrossRef]
- Smolander, M.; Chaudhry, Q. Nanotechnologies in Food Packaging. In Nanotechnologies in Food; Chaudhry, Q., Castle, L., Watkins, R., Eds.; RSC Publishing: London, UK, 2010; Chapter 6; pp. 86–101, ISBN 13: 978-0854041695, 10: 0854041699. [Google Scholar]
- Lvov, Y.; Abdullayev, E. Functional polymer–clay nanotube composites with sustained release of chemical agents. Prog. Polym. Sci. 2013, 38, 1690–1719. [Google Scholar] [CrossRef]
- Dufresne, A. Processing of Polymer Nanocomposites Reinforced with Polysaccharide Nanocrystals. Molecules 2010, 15, 4111–4128. [Google Scholar] [CrossRef] [PubMed]
- Azizi Samir, M.A.S.; Alloin, F.; Sanchez, J.Y.; Dufresne, A. Cross-linked nanocomposite polymer electrolytes reinforced with cellulose whiskers. Macromolecules 2004, 37, 4839–4844. [Google Scholar] [CrossRef]
- Bhattacharya, M. Review Polymer Nanocomposites—A Comparison between Carbon Nanotubes, Graphene, and Clay as Nanofillers. Materials 2016, 9, 262. [Google Scholar] [CrossRef] [PubMed]
- Kny, E. Polymer nanocomposite materials used for food packaging. In Ecosustainble polymer NANOMATERIALS for Food Packaging. Innovative Solutions, Characterisation Needs, Safety and Environmental Issues; Silvestre, C., Cimmino, S., Eds.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2013; Chapter 13; pp. 337–375. ISBN 9781138034266. [Google Scholar]
- Liu, J.; Boo, W.-J.; Clearfield, A.; Sue, H.-J. Intercalation and Exfoliation: A Review on Morphology of Polymer Nanocomposites Reinforced by Inorganic Layer Structures. Mater. Manuf. Process. 2006, 21, 143–151. [Google Scholar] [CrossRef]
- Oliveira, M.; Machado, A.V. Preparation of Polymer-Based Nanocomposites by Different Routes. 2013. Available online: https://repositorium.sdum.uminho.pt/bitstream/1822/26120/1/Chapter.pdf (accessed on 4 September 2018).
- Bottom-up Methods for Making Nanotechnology Products. Available online: https://www.azonano.com/article.aspx?ArticleID=1079 (accessed on 4 September 2018).
- Pal, S.L.; Jana, U.P.; Manna, K.; Mohanta, G.P.; Manava, R. Nanoparticle: An overview of preparation and characterization. J. Appl. Pharma. Sci. 2011, 01, 228–234. [Google Scholar]
- Mittal, V. In-Situ Synthesis of Polymer Nanocomposites; Wiley-VCH: Hoboken, NJ, USA, Chapter 1; pp. 1–26. Available online: https://application.wiley-vch.de/books/sample/3527328793_c01.pdf (accessed on 6 September 2018).
- Ahmad, M.B.; Gharayebi, Y.; Salit, M.S.; Hussein, M.Z.; Shameli, K. Comparison of In Situ Polymerization and Solution-Dispersion Techniques in the Preparation of Polyimide/Montmorillonite (MMT) Nanocomposite. Int. J. Mol. Sci. 2011, 12, 6040–6050. [Google Scholar] [CrossRef] [PubMed]
- Darie, R.N.; Pâslaru, E.; Sdrobis, A.; Pricope, G.M.; Hitruc, G.E.; Poiată, A.; Baklavaridis, A.; Vasile, C. Effect of Nanoclay Hydrophilicity on the Poly(lactic acid)/Clay Nanocomposites Properties. Ind. Eng. Chem. Res. 2014, 53, 7877–7890. [Google Scholar] [CrossRef]
- Reddy, T.R.K.; Kim, H.-J.; Park, J.-W. Bio-nanocomposite Properties and its Food Packaging Applications. In Ebook: Polymer Science: Research Advances, Practical Applications and Educational Aspects; Méndez-Vilas, A., Solano, A., Eds.; Formatex Research Center: Badajoz, Spain, 2016; ISBN 978-84-942134-8-9. Available online: http://www.formatex.org/polymerscience1/ (accessed on 26 September 2018).
- Lagarón, J.-M. (Ed.) Multifunctional and Nanoreinforced Polymers for Food Packaging; Woodhead Publishing Limited, Elsevier B.V.: Amsterdam, The Netherlands, 2011; pp. 485–497. [Google Scholar]
- Mohanty, F.; Swain, S.K. Bionanocomposites for Food Packaging Applications. In Nanotechnology Applications in Food. Flavor, Stability, Nutrition and Safety; Oprea, A.E., Grumezescu, A.M., Eds.; Elsevier B.V.: Amsterdam, The Netherlands, 2018; Chapter 18; ISBN 978-0-12-811942-6363-379. [Google Scholar]
- Wróblewska-Krepsztul, J.; Rydzkowski, T.; Borowski, G.; Szczypiński, M.; Klepka, T.; Thakur, V.K. Recent progress in biodegradable polymers and nanocomposite-based packaging materials for sustainable environment. Int. J. Polym. Anal. Charact. 2018, 23, 383–395. [Google Scholar] [CrossRef]
- Horst, M.F.; Quinzani, L.M.; Failla, M.D. Rheological and barrier properties of nanocomposites of HDPE and exfoliated montmorillonite. Thermoplast. Compos. Mater. 2014, 27, 106–125. [Google Scholar] [CrossRef]
- Lagaron, J.M.; Sanchez-Garcia, M.; Gimenez, E. Thermoplastic nanobiocomposites for rigid and flexible food packaging applications. In Environmentally Compatible Food Packaging; Chiellini, E., Ed.; Woodhead Publ. Ltd.: Cambridge, UK, 2008; Chapter 3; pp. 63–90. ISBN 9781845691943. [Google Scholar]
- Petersson, L.; Oksman, K. Biopolymer based nanocomposites: Comparing layered silicates and microcrystalline cellulose as nanoreinforcement. Compos. Sci. Technol. 2006, 66, 2187–2196. [Google Scholar] [CrossRef]
- Xu, Y.; Ren, X.; Hanna, M.A. Chitosan/clay nanocomposite film preparation and characterization. J. Appl. Polym. Sci. 2006, 99, 1684–1691. [Google Scholar] [CrossRef]
- Marras, S.I.; Kladi, K.P.; Tsivintzelis, I.; Zuburtikudis, I.; Panayiotou, C. Biodegradable polymer nanocomposites: The role of nanoclays on the thermomechanical characteristics and the electrospun fibrous structure. Acta Biomater. 2008, 4, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Jang, W.S.; Rawson, I.; Grunlan, J.C. Layer-by-layer assembly of thin film oxygen barrier. Thin Solid Films 2008, 516, 4819–4825. [Google Scholar] [CrossRef]
- Dean, K.; Yu, L.; Wu, D.Y. Preparation and characterization of melt extruded thermoplastic starch/clay nanocomposites. Compos. Sci. Technol. 2007, 67, 413–421. [Google Scholar] [CrossRef]
- Yu, J.; Yang, J.; Liu, B.; Ma, X. Preparation and characterization of glycerol plasticized-PEA starch/ZnO–carboxymethylcellulose sodium nanocomposite. Bioresour. Technol. 2009, 100, 2832–2841. [Google Scholar] [CrossRef] [PubMed]
- Fortunati, E.; Peltzer, M.; Armentano, I.; Torre, L.; Jiménez, A.; Kenny, J.M. Effects of modified cellulose nanocrystals on the barrier and migration properties of PLA nano-biocomposites. Carbohydr. Polym. 2012, 90, 948–956. [Google Scholar] [CrossRef] [PubMed]
- Sung, S.H.; Chang, Y.; Han, J. Development of polylactic acid nanocomposite films reinforced with cellulose nanocrystals derived from coffee silversk. Carbohydr. Polym. 2017, 169, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Kvien, I.; Tanem, B.S.; Oksman, K. Characterization of cellulose whiskers and their nanocomposites by atomic force and electron microscopy. Biomacromolecules 2005, 6, 3160–3165. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, C.; Chang, P.R.; Cao, X.; Anderson, D.P. Bionanocomposites based on PEA starch and cellulose nanowhiskers hydrolyzed from pea hull fibre: Effect of hydrolysis time. Carbohydr. Polym. 2009, 76, 607–615. [Google Scholar] [CrossRef]
- Svagan, A.J.; Hedenqvist, M.S.; Berglund, L. Reduced water vapour sorption in cellulose nanocomposites with starch matrix. Compos. Sci. Technol. 2009, 69, 500–506. [Google Scholar] [CrossRef]
- Kvien, I.; Oksman, K. Orientation of cellulose nanowhiskers in polyvinyl alcohol. Appl. Phys. A Mater. Sci. Process. 2007, 87, 641–643. [Google Scholar] [CrossRef]
- Martinez-Sanz, M.; Lopez-Rubio, A.; Lagaron, J.M. Cellulose nanowhiskers: Properties and applications as nanofiller in nanocomposites with interest in food packaging applications. In Ecosustainble Polymer Nanomaterials for Food Packaging. Innovative Solutions, Characterisation Needs, Safety and Environmental Issues; Silvestre, C., Cimmino, S., Eds.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2013; Chapter 8; pp. 195–219. ISBN 9781138034266. [Google Scholar]
- Helbert, W.; Cavaille, C.Y.; Dufresne, A. Thermoplastic nanocomposites filled with wheat straw cellulose whiskers. Part I: Processing and mechanical behaviour. Polym. Compos. 1996, 17, 604–611. [Google Scholar] [CrossRef]
- Chen, G.; Wei, M.; Chen, J.; Huang, J.; Dufresne, A.; Chang, P.R. Simultaneous reinforcing and toughening: New nanocomposites of waterborne polyurethane filled with low loading level of starch nanocrystals. Polymer 2008, 49, 1860–1870. [Google Scholar] [CrossRef]
- Ljungberg, N.; Bonini, C.; Bortolussi, F.; Boisson, C.; Heux, L.; Cavaillé, J.Y. New nanocomposite materials reinforced with cellulose whiskers in atactic polypropylene: Effect of surface and dispersion characteristics. Biomacromolecules 2005, 6, 2732–2739. [Google Scholar] [CrossRef] [PubMed]
- Ljungberg, N.; Cavaillé, J.Y.; Heux, L. Nanocomposites of isotactic polypropylene reinforced with rod-like cellulose whiskers. Polymer 2006, 47, 6285–6292. [Google Scholar] [CrossRef]
- Lu, Y.; Weng, L.; Zhang, L. Morphology and properties of soy protein isolate thermoplastics reinforced with chitin whiskers. Biomacromolecules 2004, 5, 1046–1051. [Google Scholar] [CrossRef] [PubMed]
- Sriupayo, J.; Supaphol, P.; Blackwell, J.; Rujiravanit, R. Preparation and characterization of a-chitin whisker-reinforced chitosan nanocomposite films with or without heat treatment. Carbohydr. Polym. 2005, 62, 130–136. [Google Scholar] [CrossRef]
- De Moura, M.R.; Aouada, F.A.; Avena-Bustillos, R.J.; McHugh, T.H.; Krochta, J.M.; Mattoso, L.H.C. Improved barrier and mechanical properties of novel hydroxypropyl methylcellulose edible films with chitosan/tripolyphosphate nanoparticlses. J. Food Eng. 2009, 92, 448–453. [Google Scholar] [CrossRef]
- Ramanathan, T.; Abdala, A.A.; Stankovich, S.; Dikin, D.A.; Herrera-Alonso, M.; Piner, R.D.; Adamson, D.H.; Schniep, H.C.; Ruoff, R.S.; Nguyen, S.T.; et al. Functionalized graphene sheets for polymer nanocomposites. Nat. Nanotechnol. 2008, 3, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Moraru, C.I.; Panchapakesan, C.P.; Huang, Q.; Takhistov, P.; Liu, S.; Kokini, J.L. Nanotechnology: A new frontier in Food Science. Food Technol. 2003, 57, 24–29. [Google Scholar]
- Brody, A.L. Nano and food packaging technologies converge. Food Technol. 2006, 60, 92–94. [Google Scholar]
- Ling, S.; Chen, W.; Fan, Y.; Ke, Z.; Jin, K.; Haipeng, Y.; Buehler, M.J.; Kaplan, D.L. Biopolymer nanofibrils: Structure, modeling, preparation, and applications. Prog. Polym. Sci. 2018, 85, 1–56. [Google Scholar] [CrossRef]
- Ling, S.; Kaplan, D.L.; Buehler, M.J. Nanofibrils in nature and materials engineering. Nat. Rev. Mater. 2018, 3, 18016. [Google Scholar] [CrossRef]
- Xiong, R.; Grant, A.M.; Ma, R. Naturally-derived biopolymer nanocomposites: Interfacial design, properties and emerging applications. Mater. Sci. Eng. R Rep. 2018, 125, 1–41. [Google Scholar] [CrossRef]
- You, J.; Zhu, L.; Wang, Z.; Zong, L.; Li, M.; Wu, X.; Li, C. Liquid Exfoliated Chitin Nanofibrils for Re-dispersibility and Hybridization of Two-Dimensional Nanomaterials. Chem. Eng. J. 2018, 44, 498–505. [Google Scholar] [CrossRef]
- Barbash, V.A.; Yaschenko, O.V.; Shniruk, O.M. Preparation and Properties of Nanocellulose from Organosolv Straw Pulp. Nanoscale Res. Lett. 2017, 12, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Wulandari, W.T.; Rochliadi, A.; Arcana, I.M. Nanocellulose prepared by acid hydrolysis of isolated cellulose from sugarcane bagasse. IOP Conf. Ser. Mater. Sci. Eng. 2016, 107, 012045. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Ioelovich, M.; Ahmad, I.; Thomas, S.; Dufresne, A. Methods for Extraction of Nanocellulose from Various Sources; Wiley-VCH Verlag GmbH & Co. KGaA: Hoboken, NJ, USA, 2017; Available online: https://application.wiley-vch.de/books/sample/3527338667_c01.pdf (accessed on 25 September 2018).
- Purdue University. Manufacturing Process Provides Low-Cost, Sustainable Option for Food Packaging. 2018. Available online: https://phys.org/news/2018-06-low-cost-sustainable-option-food-packaging.html#jCp (accessed on 25 September 2018).
- Rahman, M.; Netravali, A.N. Oriented bacterial cellulose-soy protein based fully ‘green’ nanocomposites. Compos. Sci. Technol. 2016, 136, 85–93. [Google Scholar] [CrossRef]
- Rahman, M.; Netravali, A.N. High-performance green nanocomposites using aligned bacterial cellulose and soy protein. Compos. Sci.Technol. 2017, 146, 183–190. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Davoudpour, Y.; Islam, M.N.; Mustapha, A.; Sudesh, K.; Dungani, R.; Jawaid, M. Production and modification of nanofibrillated cellulose using various mechanical processes: A review. Carbohydr. Polym. 2014, 99, 649–665. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Sanz, M.; Lopez-Rubio, A.; Lagaron, J.M. Optimization of the dispersion of unmodified bacterial cellulose nanowhiskers into polylactide via melt compounding to significantly enhance barrier and mechanical properties. Biomacromolecules 2012, 13, 3887–3899. [Google Scholar] [CrossRef] [PubMed]
- Hoeng, F.; Denneulin, A.; Neuman, C.; Bras, J. Charge density modification of carboxylated cellulose nanocrystals for stable silver nanoparticles suspension preparation. J. Nanopart. Res. 2015, 17, 244. [Google Scholar] [CrossRef]
- Trifol Guzman, J.; Szabo, P.; Daugaard, A.E.; Hassager, O. Hybrid Nanocellulose/Nanoclay Composites for Food Packaging Applications. Danmarks Tekniske Universitet, Kgs. Lyngby, Denmark, 2016. Available online: http://orbit.dtu.dk/files/128126567/Preprint_Jon_Trifol_Guzman.pdf (accessed on 4 September 2018).
- Trifol, J.; Plackett, D.; Sillard, C.; Szabo, P.; Bras, J.; Daugaard, A.E. Hybrid poly(lactic acid)/nanocellulose/nanoclay composites with synergistically enhanced barrier properties and improved thermomechanical resistance. Polym. Int. 2016, 65, 988–995. [Google Scholar] [CrossRef]
- Lopes, T.A.; Bufalino, L.; Cunha Claro, P.I.; Martins, M.A.; Tonoli, G.H.D.; Mendes, L.M. The effect of surface modifications with corona discharge in pinus and eucalyptus nanofibril films. Cellulose 2018, 25, 5017–5033. [Google Scholar] [CrossRef]
- Singh, A.A.; Wei, J.; Vargas, N.H.; Geng, S.; Oksman, N.K. Synergistic effect of chitin nanocrystals and orientations induced by solid-state drawing on PLA-based nanocomposite tapes. Compos. Sci. Technol. 2018, 162, 140–145. [Google Scholar] [CrossRef]
- Ling, S.; Qin, Z.; Li, C.; Huang, W.; Kaplan, D.L.; Buehler, M.J. Polymorphic Regenerated Silk Fibers Assembled Through Bioinspired Spinning. Available online: https://www.researchgate.net/publication/320959224_Polymorphic_regenerated_silk_fibers_assembled_through_bioinspired_spinning (accessed on 25 July 2018).
- Araki, J.; Wada, M.; Kuga, S. Steric stabilization of a cellulose microcrystal suspension by poly(ethylene glycol) grafting. Langmuir 2001, 17, 21–27. [Google Scholar] [CrossRef]
- Gopalan, N.K.; Dufresne, A.; Gandini, A.; Belgacem, M.N. Crab shells chitin whiskers reinforced natural rubber nanocomposites, 3. Effect of chemical modification of chitin whiskers. Biomacromolecules 2003, 4, 1835–1842. [Google Scholar] [CrossRef] [PubMed]
- Angellier, H.; Molina-Boisseau, S.; Belgacem, M.N.; Dufresne, A. Surface chemical modification of waxy maize starch nanocrystals. Langmuir 2005, 21, 2425–2433. [Google Scholar] [CrossRef] [PubMed]
- Nogi, M.; Abe, K.; Handa, K.; Nakatsubo, F.; Ifuku, S.; Yano, H. Property enhancement of optically transparent bionanofiber composites by acetylation. Appl. Phys. Lett. 2006, 89, 233123. [Google Scholar] [CrossRef]
- Ifuku, S.; Nogi, M.; Abe, K.; Handa, K.; Nakatsubo, F.; Yano, H. Surface modification of bacterial cellulose nanofibres for property enhancement of optically transparent composites: Dependence on acetyl-group DS. Biomacromolecules 2007, 8, 1973–1978. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cao, X.; Chang, P.R.; Huneault, M.A. Comparative study on the films of poly(vinyl alcohol)/pea starch nanocrystals and poly(vinyl alcohol)/native pea starch. Carbohydr. Polym. 2008, 73, 8–17. [Google Scholar] [CrossRef]
- Kristo, E.; Biliaderis, C.G. Physical properites of starch nanocrystal reinforced pullulan films. Carbohydr. Polym. 2007, 68, 146–158. [Google Scholar] [CrossRef]
- Bin, Y.; Mine, M.; Koganemaru, A.; Jiang, X.; Matsuo, M. Morphology and mechanical and electrical properties of oriented PVA–VGCF and PVA–MWNT composites. Polymer 2006, 47, 1308–1317. [Google Scholar] [CrossRef]
- Lopez Manchado, M.A.; Valentini, L.; Biagotti, J.; Kenny, J.M. Thermal and mechanical properties of single-walled carbon nanotubes–polypropylene composites prepared by melt processing. Carbon 2005, 43, 1499–1505. [Google Scholar] [CrossRef]
- Zeng, H.; Gao, C.; Wang, Y.; Watts, P.C.P.; Kong, H.; Cui, X.; Yan, D. In situ polymerization approach to multiwalled carbon nanotubes-reinforced nylon 10,10 composites: Mechanical properties and crystallization behavior. Polymer 2006, 47, 113–122. [Google Scholar] [CrossRef]
- Wu, C.L.; Zhang, M.Q.; Rong, M.Z.; Friedrick, K. Tensile performance improvement of low nanoparticles filled-polypropylene composites. Compos. Sci. Technol. 2002, 62, 1327–1340. [Google Scholar] [CrossRef]
- Vladimiriov, V.; Betchev, C.; Vassiliou, A.; Papageorgiou, G.; Bikiaris, D. Dynamic mechanical and morphological studies of isotactic polypropylene/fumed silica nanocomposites with enhanced gas barrier properties. Compos. Sci. Technol. 2006, 66, 2935–2944. [Google Scholar] [CrossRef]
- Tang, S.; Zou, P.; Xiong, H.; Tang, H. Effect of nano-SiO2 on the performance of starch/polyvinyl alcohol blend films. Carbohydr. Polym. 2008, 72, 521–526. [Google Scholar] [CrossRef]
- Xiong, H.G.; Tang, S.W.; Tang, H.L.; Zou, P. The structure and properties of a starch-based biodegradable film. Carbohydr. Polym. 2008, 71, 263–268. [Google Scholar] [CrossRef]
- Makaremi, M.; Pasbakhsh, P.; Cavallaro, G.; Lazzara, G.; Aw, Y.K.; Lee, S.M.; Milioto, S. Effect of Morphology and Size of Halloysite Nanotubes on Functional Pectin Bionanocomposites for Food Packaging Applications. ACS Appl. Mater. Interfaces 2017, 9, 17476–17488. [Google Scholar] [CrossRef] [PubMed]
- Darie-Niţă, R.N.; Vasile, C. Halloysite Containing Composites for Food Packaging Applications. In Composites Materials for Food Packaging; Cirillo, G., Kozlowski, M.A., Spizzirri, U.G., Eds.; Scrivener Publishing LLC: Beverly, MA, USA, 2018; Chapter 2; pp. 73–122. ISBN 978-1-119-16020-5. [Google Scholar]
- Gaikwad, K.K.; Singh, S.; Lee, Y.S. High adsorption of ethylene by alkali-treated halloysite nanotubes for food-packaging applications. Environ. Chem. Lett. 2018, 16, 1055–1062. [Google Scholar] [CrossRef]
- Tas, C.E.; Hendessi, S.; Baysal, M.; Unal, S.; Cebeci, F.Ç.; Menceloglu, Y.Z.; Unal, H. Halloysite Nanotubes/Polyethylene Nanocomposites for Active Food Packaging Materials with Ethylene Scavenging and Gas Barrier Properties. Food Bioprocess Technol. 2017, 10, 789–798. [Google Scholar] [CrossRef]
- Meister Meira, S.M.; Zehetmeyer, G.; Scheibel, J.M.; Orlandini Werner, J.; Brandelli, A. Starch-halloysite nanocomposites containing nisin: Characterization and inhibition of Listeria monocytogenes in soft cheese. LWT Food Sci. Technol 2016, 68, 226–234. [Google Scholar] [CrossRef]
- Bugatti, V.; Viscusi, G.; Naddeo, C.; Gorrasi, G. Nanocomposites Based on PCL and Halloysite Nanotubes Filled with Lysozyme: Effect of Draw Ratio on the Physical Properties and Release Analysis. Nanomaterials 2017, 7, 213. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Abdala, A.A.; Macosko, C.W. Graphene/Polymer Nanocomposites. Macromolecules 2010, 43, 6515–6530. [Google Scholar] [CrossRef]
- Allahbakhsh, A. High barrier graphene/polymer nanocomposite films. In Food Packaging; Academic Press: Cambridge, UK, 2017; Chapter 20; pp. 699–737. [Google Scholar]
- Dallasa, P.; Sharma, V.K.; Zborila, R. Silver polymeric nanocomposites as advanced antimicrobial agents: Classification, synthetic paths, applications, and perspectives. Adv. Colloid Interface Sci. 2011, 166, 119–135. [Google Scholar] [CrossRef] [PubMed]
- Llorensa, A.; Lloret, E.; Picouet, P.A.; Trbojevich, R.; Fernandeza, A. Review. Metallic-based micro and nanocomposites in food contact materials and active food packaging. Trends Food Sci. Technol. 2012, 24, 19–29. [Google Scholar] [CrossRef]
- Yang, W.; Owczarek, J.S.S.; Fortunati, E.; Kozanecki, M.; Mazzaglia, A.; Balestra, G.M.M.; Kenny, J.M.M.; Torre, L.; Puglia, D. Antioxidant and antibacterial lignin nanoparticles in polyvinyl alcohol/chitosan films for active packaging. Ind. Crops Prod. 2016, 94, 800–811. [Google Scholar] [CrossRef]
- Dumitriu, R.P.; Stoica, I.; Vasilescu, D.S.; Cazacu, G.; Vasile, C. Alginate/Lignosulfonate Blends with Photoprotective and Antioxidant Properties for Active Packaging Applications. J. Polym. Environ. 2018, 26, 1100–1112. [Google Scholar] [CrossRef]
- Sorrentino, A.; Gorrasi, G.; Vittoria, V. Review, Potential perspectives of bio-nanocomposites for food packaging applications. Trends Food Sci. Technol. 2007, 18, 84–95. [Google Scholar] [CrossRef]
- Kaci, M.; Benhamida, A.; Zaidi, L.; Touati, N.; Remili, C. Photodegradation of Poly(lactic acid)/organo-modified clay nanocomposites under natural weathering exposure. In Ecosustainble Polymer Nanomaterials for Food Packaging. Innovative solutions, Characterisation Needs, Safety and Environmental Issues; Silvestre, C., Cimmino, S., Eds.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2013; Chapter 11; pp. 281–315. ISBN 9781138034266. [Google Scholar]
- Kozlowski, M.A.; Macyszyn, J. Recycling of nanocomposites. In Ecosustainble Polymer Nanomaterials for Food Packaging. Innovative Solutions, Characterisation Needs, Safety and Envirinmental Issues; Silvestre, C., Cimmino, S., Eds.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2013; Chapter 12; pp. 313–335. ISBN 9781138034266. [Google Scholar]
- Bora, A.; Mishra, P. Characterization of casein and casein-silver conjugated nanoparticle containing multifunctional (pectin–sodium alginate/casein) bilayer film. J. Food Sci. Technol. 2016, 53, 3704–3714. [Google Scholar] [CrossRef] [PubMed]
- Tharanathan, R.N. Biodegradable films and composite coatings: Past, present and future. Trends Food Sci. Technol. 2003, 14, 71–78. [Google Scholar] [CrossRef]
- Arora, A.; Padua, G.W. Review: Nanocomposites in Food Packaging. J. Food Sci. 2010, 75, R43–R49. [Google Scholar] [CrossRef] [PubMed]
- Sothornvit, R.; Krochta, J.M. Plasticizers in edible films and coatings. In Innovation in Food Packaging, 2nd ed.; Han, J.H., Ed.; Elsevier Publishers: New York, NY, USA, 2005; ISBN 9780123948359, Hardcover ISBN 9780123946010. [Google Scholar]
- Zhou, J.J.; Wang, S.Y.; Gunasekaran, S. Preparation and characterization of whey protein film incorporated with TiO2 nanoparticles. J. Food Sci. 2009, 74, N50–N56. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Zhou, J.; Gunasekaran, S. Low temperature fabrication of ZnO-whey protein isolate nanocomposite. Mater. Lett. 2008, 62, 4383–4385. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, L. Interaction and properties of highly exfoliated soy protein/montmorillonite nanocomposites. Biomacromolecules 2006, 7, 1700–1706. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Cui, G.; Wei, M.; Huang, J. Facile exfoliation of rectorite nanoplatelets in soy protein matrix and reinforced bionanocomposites thereof. J. Appl. Polym. Sci. 2007, 104, 3367–3377. [Google Scholar] [CrossRef]
- Liu, X.; Sun, W.; Wang, H.; Zhang, L.; Wang, J.Y. Microspheres of corn, zein, for an ivermectin drug delivery system. Biomaterials 2005, 26, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Kijchavengkul, T.; Auras, R. Compostability of polymers. Polym. Int. 2008, 57, 793–804. [Google Scholar] [CrossRef]
- Zhao, R.; Torley, P.; Halley, P.J. Emerging biodegradable materials: Starch- and protein-based bio-nanocomposites. J. Mater. Sci. 2008, 43, 3058–3071. [Google Scholar] [CrossRef]
- Armentano, I.; Puglia, D.; Luzi, F.; Arciola, C.R.; Morena, F.; Martino, S.; Torre, L. Nanocomposites Based on Biodegradable Polymers. Materials 2018, 11, 795. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.R.; Xiao, C.M.; Xu, S.J. Starch-based completely biodegradable polymer materials. Express Polym. Lett. 2009, 3, 366–375. [Google Scholar] [CrossRef]
- Castro-Aguirre, E.; Auras, R.; Selke, S.; Rubino, M.; Marsh, T. Impact of Nanoclays on the Biodegradation of Poly(Lactic Acid) Nanocomposites. Polymers 2018, 10, 202. [Google Scholar] [CrossRef]
- Goodwin, D.D.; Boyer, I.; Devahif, T.; Gao, C.; Frank, B.P.; Lu, X.; Kuwama, L.; Gordon, T.B.; Wang, J.; Ranville, J.F.; et al. Biodegradation of Carbon Nanotube/Polymer Nanocomposites using a Monoculture. Environ. Sci. Technol. 2018, 52, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Nanocoatings, Nanowerk. Available online: https://www.nanowerk.com/nanotechnology-news/newsid=47370.php (accessed on 4 September 2018).
- Egodage, D.P.; Jayalath, H.T.S.; Samarasekara, A.M.P.B. Novel antimicrobial nano coated polypropylene based materials for food packaging systems. In Proceedings of the Moratuwa Engineering Research Conference (MERCon), Moratuwa, Sri Lanka, 29–31 May 2017. [Google Scholar]
- Müller, K.; Bugnicourt, E.; Latorre, M.; Jorda, M.; Echegoyen Sanz, Y.; Lagaron, J.M.; Miesbauer, O.; Bianchin, A.; Hankin, S.; Bölz, U.; et al. Review on the Processing and Properties of Polymer Nanocomposites and Nanocoatings and Their Applications in the Packaging, Automotive and Solar Energy Fields. Nanomaterials 2017, 7, 74. [Google Scholar] [CrossRef] [PubMed]
- Bastarrachea, L.J.; Wong, D.E.; Roman, M.J.; Lin, Z.; Goddard, J.M. Active Packaging Coatings. Coatings 2015, 5, 771–791. [Google Scholar] [CrossRef]
- Vasile, C.; Pâslaru, E.; Sdrobis, A.; Pricope, G.; Ioanid, G.E.; Darie, R.N. Plasma assisted functionalization of synthetic and natural polymers to obtain new bioactive food packaging materials in Ionizing Radiation and Plasma discharge Mediating Covalent Linking of Stratified Composites Materials for Food Packaging. In Proceedings of the Co-Ordinated Project: Application of Radiation Technology in the Development of Advanced Packaging Materials for Food Products, Vienna, Austria, 22–26 April 2013; Safrany, A., Ed.; pp. 100–110. Available online: http://www-naweb.iaea.org/napc/iachem/working_materials/F2-22063-CR-1-report.pdf (accessed on 19 June 2017).
- Cagri, A.; Ustunol, Z.; Ryser, E.T. Antimicrobial edible films and coatings. J. Food Prot. 2004, 67, 833–848. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, V.; Krasnoiarova, O.; Pridvorova, S.; Zherdev, A.; Gmoshinskii, I.; Kazydub, G.; Popov, K.; Khotimchenko, S. Characterization of silver nanoparticles migration from package materials destined for contact with foods. Voprosy Pitaniia 2012, 81, 34–39. (In Russian) [Google Scholar] [PubMed]
- Totolin, M. Plasma Chemistry and Natural Polymers; PIM Publisher: Iasi, Romania, 2007; pp. 15–25. ISBN 978-973-716-776-7. [Google Scholar]
- Vasile, C.; Stoleru, E.; Munteanu, B.S.; Zaharescu, T.; Ioanid, E.; Pamfil, D. Radiation Mediated Bioactive Compounds Immobilization on Polymers to Obtain Multifunctional Food Packaging Materials. In Proceedings of the International Conference on Applications of Radiation Science and Technology (ICARST’ 2017), Vienna, Austria, 23–28 April 2017. [Google Scholar]
- Riccardi, C.; Zanini, S.; Tassetti, D. A Polymeric Film Coating Method on a Substrate by Depositing and Subsequently Polymerizing a Monomeric Composition by Plasma Treatment. Patent WO2014191901 A1, 4 December 2014. [Google Scholar]
- Vasile, C. General survey of the properties of polyolefins. In Handbook of Polyolefins, 2nd ed.; Vasile, C., Ed.; Marcel Dekker: New York, NY, USA, 2000; pp. 401–416. ISBN 13: 978-0824786038, 10: 0824786033. [Google Scholar]
- Bastarrachea, L.; Dhawan, S.; Sablani, S.S. Engineering properties of polymeric-based antimicrobial films for food packaging. Food Eng. Rev. 2011, 3, 79–93. [Google Scholar] [CrossRef]
- Butnaru, E.; Stoleru, E.; Rapa, M.; Pricope, G.; Vasile, C. Development and manufacturing of formulations containing as active compounds CS and rosehip seeds oil by emulsion technique. In Proceedings of the 4th Technical Meeting of ActiBiosafe Project, Medias, Romania, 19–20 May 2016. [Google Scholar]
- Caruso, R.A.; Antonietti, M. Sol−Gel Nanocoating: An Approach to the Preparation of Structured Materials. Chem Mater. 2001, 13, 3272–3282. [Google Scholar] [CrossRef]
- Vasile, C.; Stoleru, E.; Irimia, A.; Zaharescu, T.; Dumitriu, R.P.; Ioanid, G.E.; Munteanu, B.S.; Oprica, L.; Pricope, G.M.; Hitruc, G.E. Ionizing Radiation and Plasma Discharge Mediating Covalent Linking of Bioactive Compounds onto Polymeric Substrate to Obtain Stratified Composites for Food Packing. In Report of the 3rd RCM: Application of Radiation Technology in the Development of Advanced Packaging Materials for Food Products; Safrany, A., Ed.; IAEA: Vienna, Austria, 2016; Chapter 15; pp. 11–15. [Google Scholar]
- Silvestre, C.; Cimmino, S.; Stoleru, E.; Vasile, C. Application of Radiation technology to food packaging. In Applications of Ionizing Radiation in Materials Processing; Sun, Y., Chmielewski, A., Eds.; Institute of Nuclear Chemistry and Technology: Warsaw, Poland, 2017; Chapter 20; pp. 461–485. [Google Scholar]
- Goddard, J.M.; Hotchkiss, J.H. Polymer surface modification for the attachment of bioactive compounds. Prog. Polym. Sci. 2007, 32, 698–725. [Google Scholar] [CrossRef]
- Moy, V.T.; Florin, E.L.; Gaub, H.E. Intermolecular forces and energies between ligands and receptors. Science 1994, 266, 257–259. [Google Scholar] [CrossRef] [PubMed]
- Stoleru, E.; Zaharescu, T.; Hitruc, E.G.; Vesel, A.; Ioanid, E.G.; Coroaba, A.; Safrany, A.; Pricope, G.; Lungu, M.; Schick, C.; et al. Lactoferrin-immobilized surfaces onto functionalized PLA assisted by the gamma-rays and nitrogen plasma to create materials with multifunctional properties. ACS Appl. Mater. Interfaces 2016, 8, 31902–31915. [Google Scholar] [CrossRef] [PubMed]
- Oniz-Magan, A.B.; Pastor-Blas, M.M.; Martin-Martinez, J.M. Different Performance of Ar, O2 and CO2 RF Plasmas in the Adhesion of Thermoplastic Rubber to Polyurethane Adhesive. In Plasma Processes and Polymers; D’Agostino, R., Favia, P., Oehr, C., Werheimer, M.E., Eds.; Wiley-VCH: Weinheim, Germany, 2005; pp. 177–192. [Google Scholar]
- Park, S.J.; Kim, J.S. Influence of Plasma Treatment on Microstructures and Acid–Base Surface Energetics of Nanostructured Carbon Blacks: N2 Plasma Environment. J Colloid Interface Sci. 2001, 244, 336–341. [Google Scholar] [CrossRef]
- Bryjak, M.; Gancarz, I.; Pozniak, G. Surface evaluation of plasma-modified polysulfone (Udel P-1700) films. Langmuir 1999, 15, 6400–6404. [Google Scholar] [CrossRef]
- Strobel, M.; Lyons, C.S.; Mittal, K.L. Plasma Surface Modification of Polymer: Relevance to Adhesion; VSP: Utrecht, Germany, 1994; ISBN 90-6764-164-2. [Google Scholar]
- Goddard, J.M.; Hotchkiss, J.H. Tailored functionalization of low-density polyethylene surfaces. J. Appl. Polym. Sci. 2008, 108, 2940–2949. [Google Scholar] [CrossRef]
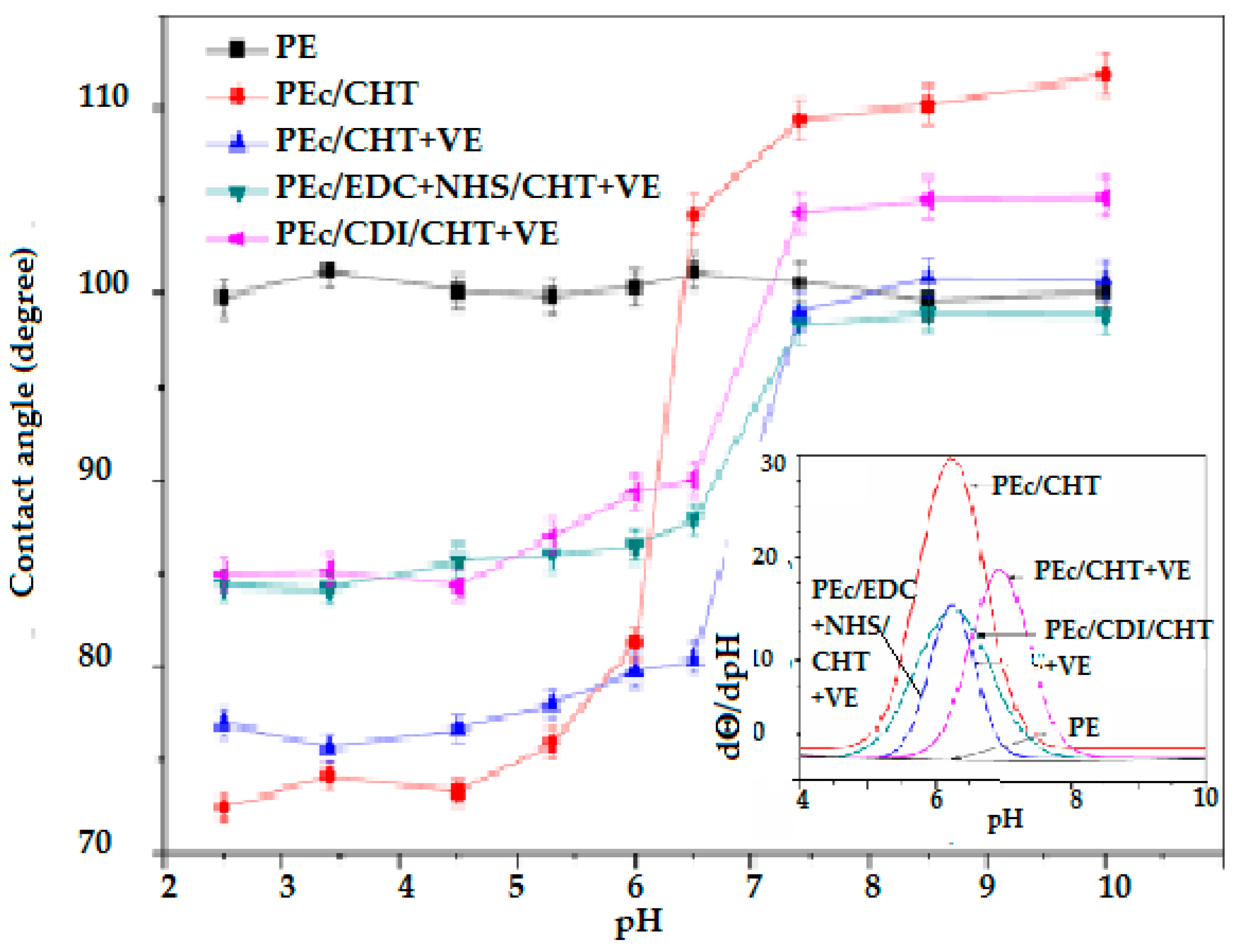
- Stoleru, E.; Dumitriu, R.P.; Munteanu, B.S.; Zaharescu, T.; Tanase, E.E.; Mitelut, A.; Ailiesei, G.-L.; Vasile, C. Novel Procedure to Enhance PLA Surface Properties By Chitosan Irreversible Immobilization. Appl. Surf. Sci. 2016, 367, 407–417. [Google Scholar] [CrossRef]
- Stoleru, E.; Munteanu, S.B.; Dumitriu, R.P.; Coroaba, A.; Drobotă, M.; Fras Zemljic, L.; Pricope, G.M.; Vasile, C. Polyethylene Materials with Multifunctional Surface Properties by Electrospraying Chitosan/Vitamin E Formulation Destined to Biomedical and Food Packaging Applications. Iran. Polym. J. 2016, 25, 295–307. [Google Scholar] [CrossRef]
- Munteanu, B.S.; Dumitriu, R.P.; Profire, L.; Sacarescu, L.; Hitruc, G.E.; Stoleru, E.; Dobromir, M.; Matricala, A.L.; Vasile, C. Hybrid Nanostructures Containing Sulfadiazine Modified Chitosan as Antimicrobial Drug Carriers. Nanomaterials 2016, 6, 207. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, B.S.; Stoleru, E.; Ioanid, E.G.; Zaharescu, T.; Secarescu, L.; Vasile, C. Plasma Discharge and Gamma Irradiation Mediating Covalent Linking of Stratified Composites Materials for Food Packaging. In Proceedings of the XX-th International Conference “Inventica 2016”, Iasi, Romania, 30 June–1 July 2016; pp. 213–221. [Google Scholar]
- Irimia, A.; Ioanid, G.E.; Zaharescu, T.; Coroabă, A.; Doroftei, F.; Safrany, A.; Vasile, C. Comparative study on Gamma Irradiation and Cold Plasma pretreatment for a Cellulosic substrate modification with phenolic compounds. Radiat. Phys. Chem. 2017, 130, 52–61. [Google Scholar] [CrossRef]
- Pâslaru (Stoleru), E.; Munteanu, B.S.; Vasile, C. Electrospun Nanostructures as Biodegradable Composite Materials for Biomedical Applications. In Biodegradable Polymeric Nanocomposites Advances in Biomedical Applications; Depan, D., Ed.; CRC Press, Taylor & Francis Group: Roca Baton, FL, USA, 2015; Chapter 3; pp. 49–73. ISBN 978-1-4822-6052-6. [Google Scholar]
- Irimia, A.; Vasile, C. Surface Functionalization of Cellulose Fibers. In Cellulose and Cellulose Derivatives: Synthesis, Modification, Nanostructure and Applications; Mondal, I.H., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2015; Chapter 5; ISBN 978-1-63483-150-5. [Google Scholar]
- Vasile, C.; Darie, R.N.; Sdrobis, A.; Pâslaru, E.; Pricope, G.; Baklavaridis, A.; Munteanu, B.S.; Zuburtikudis, I. Effectiveness of chitosan as antimicrobial agent in LDPE/CS composite films as minced poultry meat packaging materials. Cell. Chem. Technol. 2014, 48, 325–336. [Google Scholar]
- Munteanu, B.S.; Pâslaru, E.; Fras Zemljic, L.; Sdrobis, A.; Pricope, G.M.; Vasile, C. Chitosan coatings applied to polyethylene surface to obtain food-packaging materials. Cell. Chem. Technol. 2014, 48, 565–575. [Google Scholar]
- Paslaru, E.; Fras-Zemljic, L.; Bracic, M.; Vesel, A.; Petrinic, I.; Vasile, C. Stability of a Chitosan Layer Deposited onto a Polyethylene Surface. J. Appl. Polym. Sci. 2013, 130, 2444–2457. [Google Scholar] [CrossRef]
- Savard, T.; Beauliu, C.; Boucher, I.; Champagne, C.P. Antimicrobial action of hydrolyzed chitosan against spoilage yeasts and lactic acid bacteria of fermented vegetables. J. Food Prot. 2002, 65, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Rabea, E.I.; Badawy, M.E.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.W.; Traber, M.G. Vitamin E: Antioxidant activity, biokinetics, and bioavailability. Annu. Rev. Nutr. 1990, 10, 357–382. [Google Scholar] [CrossRef] [PubMed]
- Vasile, C.; Sivertsvik, M.; Mitelut, A.C.; Brebu, M.A.; Stoleru, E.; Rosnes, J.T.; Tanase, E.E.; Khan, W.; Pamfil, D.; Cornea, C.P.; et al. Comparative Analysis of the Composition and Active Property Evaluation of Certain Essential Oils to Assess their Potential Applications in Active Food Packaging. Materials 2017, 10, 45. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Hu, Y.F. Biomolecule immobilization techniques for bioactive paper fabrication. Anal. Bioanal. Chem. 2012, 403, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Sdrobiş, A.; Biederman, H.; Kylian, O.; Vasile, C. Modification of cellulose/chitin mix fibers under different cold plasma conditions. Cellulose 2012, 20, 509–524. [Google Scholar] [CrossRef]
- Barish, J.A.; Goddard, J.M. Topographical and chemical characterization of polymer surfaces modified by physical and chemical processes. J. Appl. Polym. Sci. 2011, 120, 2863–2871. [Google Scholar] [CrossRef]
- Carlini, C.; Angiolini, L. Polymers as free radical photoinitiators. In Synthesis and Photosynthesis; Springer: Heidelberg, Germany, 1995; pp. 127–214. Available online: https://link.springer.com/bookseries/12 (accessed on 25 September 2018).
- Hermanson, G.T. Bioconjugate Techniques, 3rd ed.; Academic Press: San Diego, CA, USA, 1996; 785p, ISBN 9780123822406. Hardcover ISBN 9780123822390. [Google Scholar]
- Maeda, T.; Hagiwara, K.; Hasebe, T.; Hotta, A. Polymers with DLC Nanocoating by CVD Method for Biomedical Devices and Food Packaging in Comprehensive Guide for Nanocoatings Technology; Volume 3: Properties and Development; Nova Science Publishers: New York, NY, USA, 2015; pp. 405–433. ISBN 978-1-63482-647-1. [Google Scholar]
- Technical Research Centre of Finland (VTT). Nanowerk News A Fully Recyclable Nanocoating for Food and Pharmaceuticals, 24 March 2010. Available online: http://www.nanowerk.com/news/newsid=15499.php (accessed on 23 June 2017).
- SPALASTM SPALASTM (Spray Assisted Layer-by-Layer Assembly) Coating System. Available online: http://www.agiltron.com/PDFs/SPALASTM.pdf (accessed on 23 June 2017).
- Bastarrachea, L.J.; Denis-Rohr, A.; Goddard, J.M. Antimicrobial food equipment coatings: Applications and challenges. Annu. Rev. Food Sci. Technol. 2015, 6, 97–118. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Haile, M.; Park, Y.T.; Malek, F.A.; Grunlan, J.C. Super gas barrier of all-polymer multilayer thin films. Macromolecules 2011, 44, 1450–1459. [Google Scholar] [CrossRef]
- Cerkez, I.; Kocer, H.B.; Worley, S.D.; Broughton, R.M.; Huang, T.S. N-Halamine biocidal coatings via a layer-by-layer assembly technique. Langmuir 2011, 27, 4091–4097. [Google Scholar] [CrossRef] [PubMed]
- Bastarrachea, L.J.; McLandsborough, L.A.; Peleg, M.; Goddard, J.M. Antimicrobial N-Halamine modified polyethylene: Characterization, biocidal efficacy, regeneration, and stability. J. Food Sci. 2014, 79, E887–E897. [Google Scholar] [CrossRef] [PubMed]
- Bastarrachea, L.J.; Peleg, M.; McLandsborough, L.A.; Goddard, J.M. Inactivation of Listeria Monocytogenes on a polyethylene surface modified by layer-by-layer deposition of the antimicrobial N-Halamine. Food Eng. 2013, 117, 52–58. [Google Scholar] [CrossRef]
- Jokar, M.; Abdul Rahman, R. Study of silver ion migration from melt-blended and layered deposited silver polyethylene nanocomposite into food simulants and apple juice. Food Addit. Contam. Part A 2014, 31, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Sono-Tek Corporation. Ultrasonic Nozzle Systems for Nanotechnology Coating Applications in Nanotechnology Coatings Overview. Available online: http://www.sono-tek.com/nanotechnology-overview/ (accessed on 23 June 2017).
- Food-Safe, Nano-Coating Appropriate for Food with for Optimal Corrosion Protection and Quality Imprinting on Tubes and Cans. Available online: http://www.plasmatreat.com/industrial-applications/packaging/glass-metal-packages/nano-coating-of-beverage-cans.html (accessed on 2 June 2017).
- Nobile, M.A.; Cannarsi, M.; Altieri, C.; Sinigaglia, M.; Favia, P.; Iacoviello, G.; D’Agostino, R. Effect of Ag-containing Nano-composite Active Packaging System on Survival of Alicyclobacillus acidoterrestris. J. Food Sci. 2004, 69, E379–E383. [Google Scholar] [CrossRef]
- Mirjalili, M.; Zohoori, S. Review for application of electrospinning and electrospun nanofibers technology in textile industry. J. Nanostruct. Chem. 2016, 6, 207–213. [Google Scholar] [CrossRef]
- Munteanu, B.S.; Aytac, Z.; Pricope, G.M.; Uyar, T.; Vasile, C. Polylactic acid (PLA)/Silver-NP/Vitamin E bionanocomposite electrospun nanofibers with antibacterial and antioxidant activity. J. Nanopart. Res. 2014, 16, 2643–2646. [Google Scholar] [CrossRef]
- Vasile, C.; Darie-Niță, R.; Brebu, M.; Râpă, M.; Ștefan, M.; Stan, M.; Macavei, S.; Barbu-Tudoran, L.; Borodi, G.; Vodnar, D.; et al. New PLA/ZnO:Cu/Ag bionanocomposites for food packaging. Express Polym. Lett. 2017, 11, 531–544. [Google Scholar] [CrossRef]
- Munteanu, B.S.; Ioanid, G.E.; Pricope, G.M.; Mitelut, A.C.; Tanase, E.E.; Vasile, C. Development and manufacturing of capsules with active substances by electrospinning technique. Encapsulated forms. In Proceedings of the 4th Technical Meeting of ActiBiosafe Project, Medias, Romania, 19–20 May 2016. [Google Scholar]
- Castro-Mayorga, J.L.; Fabra, M.J.; Cabedo, L.; Lagaron, J.M. On the Use of the Electrospinning Coating Technique to Produce Antimicrobial Polyhydroxyalkanoate Materials Containing In Situ-Stabilized Silver Nanoparticles. Nanomaterials 2017, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Valdes, S.; Ortega-Ortiz, H.; Ramos-de Valle, L.F.; Medellin-Rodriguez, F.J.; Guedea-Miranda, R. Mechanical and antimicrobial properties of multilayer films with a polyethylene/silver nanocomposite layer. J. Appl. Polym. Sci. 2009, 111, 953–962. [Google Scholar] [CrossRef]
- Garces, L.O.; de la Puerta, C.N. Antimicrobial Packaging Based on the Use of Natural Extracts and the Process to Obtain This Packaging. European Patent EP1657181-B1, 13 January 2010. [Google Scholar]
- Lacroix, M. Use of Irradiation, for the Development of Active Edible Coatings, Beads and Packaging to Assure Food Safety and to Prolong Preservation. 2017. Available online: https://media.superevent.com/documents/20170425/1c03bea5d97576aa37769311cb3ae83b/m.-lacroix.pdf (accessed on 25 September 2018).
- Min, M.; Shi, Y.; Ma, H.; Huang, H.; Shi, J.; Chen, X.; Liu, Y.; Wang, L. Polymer-nanoparticle composites composed of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and coated silver nanoparticles. J. Macromol. Sci. Part B 2015, 54, 411–423. [Google Scholar] [CrossRef]
- Martínez-Abad, A.; Lagarón, J.M.; Ocio, M.J. Characterization of transparent silver loaded poly(L-lactide) films produced by melt-compounding for the sustained release of antimicrobial silver ions in food applications. Food Control 2014, 43, 238–244. [Google Scholar] [CrossRef]
- George, M.; Shen, W.-Z.; Qi, Z.; Bhatnagar, A.; Montemagno, C. Development and Property Evaluation of Poly (Lactic) Acid and Cellulose Nanocrystals Based Films with Either Silver or Peptide Antimicrobial Agents: Morphological, Permeability, Thermal, and Mechanical Characterization. IOSR J. Polym. Text. Eng. 2017, 4, 8–24. [Google Scholar] [CrossRef]
- Girdthep, S.; Worajittiphon, P.; Leejarkpai, T.; Punyodom, W. Effect of Silver-loaded Kaolinite on Real Ageing, Hydrolytic Degradation, and Biodegradation of Composite Blown Films Based on Poly(lactic acid) and Poly(butylene adipate-co-terephthalate). Eur. Polym. J. 2016, 82, 244–259. [Google Scholar] [CrossRef]
- Mbhele, Z.H.; Salemane, M.G.; van Sitter, C.G.C.E.; Nedeljkov, J.M.; Djokovic, V.; Luyt, A.S. Fabrication and characterization of silver–polyvinyl alcohol nanocomposites. Chem. Mater. 2003, 15, 5019–5024. [Google Scholar] [CrossRef]
- Damm, C.; Munstedt, H.; Rosch, A. Long-term antimicrobial polyamide 6/silver-nanocomposites. J. Mater. Sci. 2007, 42, 6067–6073. [Google Scholar] [CrossRef]
- Damm, C.; Munstedt, H.; Rosch, A. The antimicrobial efficacy of polyamide 6/silver-nano- and microcomposites. Mater. Chem. Phys. 2008, 108, 61–66. [Google Scholar] [CrossRef]
- Cheng, Q.; Li, C.; Pavlinek, V.; Saha, P.; Wang, H. Surface-modified antibacterial TiO2/Ag+ nanoparticles: Preparation and properties. Appl. Surf. Sci. 2006, 252, 4154–4160. [Google Scholar] [CrossRef]
- Li, H.; Li, F.; Wang, L.; Sheng, J.; Xin, Z.; Zhao, L.; Xiao, H.; Zheng, Y.; Hu, Q. Effect of nano-packing on preservation quality of Chinese jujube. Food Chem. 2009, 114, 547–552. [Google Scholar] [CrossRef]
- Yu, H.; Sun, B.; Zhang, D.; Chen, G.; Yang, X.; Yao, J. Reinforcement of biodegradable poly(3-hydroxybutyrate-co-3-hydroxyvalerate) with cellulose nanocrystal/silver nanohybrids as bifunctional nanofillers. J. Mater. Chem. B 2014, 2, 8479–8489. [Google Scholar] [CrossRef]
- Manso, S.; Becerril, R.; Nerin, C.; Gomez-Lus, R. Influence of pH and temperature variations on vapor phase action of an antifungal food packaging against five mold strains. Food Control 2015, 47, 20–26. [Google Scholar] [CrossRef]
- Valderrama Solano, A.C.; Rojas de Gante, C. Two different processes to obtain antimicrobial packaging containing natural oils. Food Bioprocess Technol. 2012, 5, 2522–2528. [Google Scholar] [CrossRef]
- Minelli, M.; de Angelis, M.G.; Doghieri, F.; Rocchetti, M.; Montenero, A. Barrier properties of organic-inorganic hybrid coatings based on polyvinyl alcohol with improved water resistance. Polym. Eng. Sci. 2010, 50, 144–153. [Google Scholar] [CrossRef]
- Lantano, C.; Alfieri, I.; Cavazza, A.; Corradini, C.; Lorenzi, A.; Zucchetto, N.; Montenero, A. Natamycin based sol-gel antimicrobial coatings on polylactic acid films for food packaging. Food Chem. 2014, 165, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Jokar, M.; Alsing Pedersen, G.; Loeschner, K. Six open questions about the migration of engineered nano-objects from polymer-based food-contact materials: A review. Food Addit. Contam. Part A 2017, 34, 434–450. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Buonocore, G.G.; Lavorgna, M. Photocatalytic activity of PLA/TiO2 nanocomposites and TiO2-active multilayered hybrid coatings. Ital. J. Food Sci. 2012, 24, 102–106. [Google Scholar]
- Makwana, S.; Choudhary, R.; Dogra, N.; Kohli, P.; Haddock, J. Nanoencapsulation and immobilization of cinnamaldehyde for developing antimicrobial food packaging material. LWT Food Sci. Technol. 2014, 57, 470–476. [Google Scholar] [CrossRef]
- Theinsathid, P.; Visessanguan, W.; Kruenate, J.; Kingcha, Y.; Keeratipibul, S. Antimicrobial activity of lauric arginate-coated polylactic acid films against listeria monocytogenes and salmonella typhimurium on cooked sliced ham. J. Food Sci. 2012, 77, M142–M149. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Jin, T.Z.; Yang, R. Antimicrobial polylactic acid packaging films against listeria and salmonella in culture medium and on ready-to-eat meat. Food Bioprocess Technol. 2014, 7, 3293–3307. [Google Scholar] [CrossRef]
- Auxier, J.A.; Schilke, K.F.; McGuire, J. Activity retention after nisin entrapment in a polyethylene oxide brush layer. J. Food Prot. 2014, 77, 1624–1629. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, S.C.M.; Sadocco, P.; Causio, J.; Silvestre, A.J.D.; Mondragon, I.; Freire, C.S.R. Antimicrobial pullulan derivative prepared by grafting with 3-aminopropyltrimethoxysilane: Characterization and ability to form transparent films. Food Hydrocoll. 2014, 35, 247–252. [Google Scholar] [CrossRef]
- Muriel-Galet, V.; Talbert, J.N.; Hernandez-Munoz, P.; Gavara, R.; Goddard, J.M. Covalent immobilization of lysozyme on ethylene vinyl alcohol films for nonmigrating antimicrobial packaging applications. J. Agric. Food Chem. 2013, 61, 6720–6727. [Google Scholar] [CrossRef] [PubMed]
- Anthierens, T.; Billiet, L.; Devlieghere, F.; du Prez, F. Poly(butylene adipate) functionalized with quaternary phosphonium groups as potential antimicrobial packaging material. Innov. Food Sci. Emerg. Technol. 2012, 15, 81–85. [Google Scholar] [CrossRef]
- Mackiw, E.; Maka, L.; Sciezynska, H.; Pawlicka, M.; Dziadczyk, P.; Rzanek-Boroch, Z. The impact of plasma-modified films with sulfur dioxide, sodium oxide on food pathogenic microorganisms. Packag. Technol. Sci. 2015, 28, 285–292. [Google Scholar] [CrossRef]
- Pinheiro, A.C.; Bourbon, A.I.; Medeiros, B.G.D.S.; da Silva, L.H.M.; da Silva, M.C.H.; Carneiro-da-Cunha, M.G.; Coimbra, M.A.; Vicente, A.A. Interactions between κ-carrageenan and chitosan in nanolayered coatings-structural and transport properties. Carbohydr. Polym. 2012, 87, 1081–1090. [Google Scholar] [CrossRef]
- Carneiro-da-Cunha, M.G.; Cerqueira, M.A.; Souza, B.W.S.; Carvalhoc, S.; Quintas, M.A.C.; Teixeira, J.A.; Vicente, A.A. Physical and thermal properties of a chitosan/alginate nanolayered PET film. Carbohydr. Polym. 2010, 82, 153–159. [Google Scholar] [CrossRef]
- Medeiros, B.G.D.S.; Pinheiro, A.C.; Teixeira, J.A.; Vicente, A.A.; Carneiro-da-Cunha, M.G. Polysaccharide/protein nanomultilayer coatings: Construction, characterization and evaluation of their effect on “Rocha” pear (Pyrus communis L.) shelf-life. Food Bioprocess Technol. 2012, 5, 2435–2445. [Google Scholar] [CrossRef]
- Contini, C.; Álvarez, R.; O’Sullivan, M.; Dowling, D.P.; Gargan, S.O.; Monahan, F.J. Effect of an active packaging with citrus extract on lipid oxidation and sensory quality of cooked turkey meat. Meat Sci. 2014, 96, 1171–1176. [Google Scholar] [CrossRef] [PubMed]
- Contini, C.; Katsikogianni, M.G.; O’Neill, F.T.; O’Sullivan, M.; Boland, F.; Dowling, D.P.; Monahan, F.J. Storage stability of an antioxidant active packaging coated with citrus extract following a plasma jet pretreatment. Food Bioprocess Technol. 2014, 7, 2228–2240. [Google Scholar] [CrossRef]
- Bolumar, T.; Andersen, M.L.; Orlien, V. Antioxidant active packaging for chicken meat processed by high pressure treatment. Food Chem. 2011, 129, 1406–1412. [Google Scholar] [CrossRef]
- Lee, C.H.; An, D.S.; Lee, S.C.; Park, H.J.; Lee, D.S. A coating for use as an antimicrobial and antioxidative packaging material incorporating nisin and α-tocopherol. J. Food Eng. 2004, 62, 323–329. [Google Scholar] [CrossRef]
- Schreiber, S.B.; Bozell, J.J.; Hayes, D.G.; Zivanovic, S. Introduction of primary antioxidant activity to chitosan for application as a multifunctional food packaging material. Food Hydrocoll. 2013, 33, 207–214. [Google Scholar] [CrossRef]
- Struller, C.F.; Kelly, P.J.; Copeland, N.J. Aluminum oxide barrier coatings on polymer films for food packaging applications. Surf. Coat. Technol. 2014, 241, 130–137. [Google Scholar] [CrossRef]
- Chatham, H. Oxygen diffusion barrier properties of transparent oxide coatings on polymeric substrates. Surf. Coat. Technol. 1996, 78, 1–9. [Google Scholar] [CrossRef]
- Shutava, T.G.; Prouty, M.D.; Agabekov, V.E.; Lvov, Y.M. Antioxidant properties of layer-by-layer films on the basis of tannic acid. Chem. Lett. 2006, 35, 1144–1145. [Google Scholar] [CrossRef]
- Arrua, D.; Strumia, M.C.; Nazareno, M.A. Immobilization of Caffeic acid on a polypropylene film: Synthesis and antioxidant properties. J. Agric. Food Chem. 2010, 58, 9228–9234. [Google Scholar] [CrossRef] [PubMed]
- Roman, M.J.; Tian, F.; Decker, E.A.; Goddard, J.M. Iron chelating polypropylene films: Manipulating photoinitiated graft polymerization to tailor chelating activity. J. Appl. Polym. Sci. 2014, 131, 39948. [Google Scholar] [CrossRef]
- Ogiwara, Y.; Roman, M.J.; Decker, E.A.; Goddard, J.M. Iron chelating active packaging: Influence of competing ions and pH value on effectiveness of soluble and immobilized hydroxamate chelators. Food Chem. 2016, 196, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Decker, E.A.; Clements, D.J.; Goddard, J.M. Influence of non-migratory metal-chelating active packaging film on food quality: Impact on physical and chemical stability of emulsions. Food Chem. 2014, 151, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Decker, E.A.; Goddard, J.M. Controlling lipid oxidation via a biomimetic iron chelating active packaging material. J. Agric. Food Chem. 2013, 61, 12397–12404. [Google Scholar] [CrossRef] [PubMed]
- Roman, M.J.; Decker, E.A.; Goddard, J.M. Performance of nonmigratory iron chelating active packaging materials in viscous model food systems. J. Food Sci. 2015, 80, 1965–1973. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Roman, M.J.; Decker, E.A.; Goddard, J.M. Biomimetic design of chelating interfaces. J. Appl. Polym. Sci. 2015, 132, 41231–41239. [Google Scholar] [CrossRef]
- Wong, D.E.; Dai, M.; Talbert, J.N.; Nugen, S.R.; Goddard, J.M. Biocatalytic polymer nanofibers for stabilization and delivery of enzymes. J. Mol. Catal. B Enzym. 2014, 110, 16–22. [Google Scholar] [CrossRef]
- Johansson, K.; Winestrand, S.; Johansson, C.; Jarnstrom, L.; Jonsson, L.J. Oxygen-scavenging coatings and films based on lignosulfonates and laccase. J. Biotechnol. 2012, 161, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Goddard, J.M.; Talbert, J.N.; Hotchkiss, J.H. Covalent attachment of lactase to low-density polyethylene films. J. Food Sci. 2007, 72, E36–E41. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, K.W.; Talbert, J.N.; Goddard, J.M. Effect of polyethylene glycol tether size and chemistry on the attachment of lactase to polyethylene films. J. Appl. Polym. Sci. 2013, 127, 1203–1210. [Google Scholar] [CrossRef]
- Talbert, J.N.; Goddard, J.M. Influence of nanoparticle diameter on conjugated enzyme activity. Food Bioprod. Process. 2013, 91, 693–699. [Google Scholar] [CrossRef]
- Wong, D.E.; Talbert, J.N.; Goddard, J.M. Layer by layer assembly of a biocatalytic packaging film: Lactase covalently bound to low-density polyethylene. J. Food Sci. 2013, 78, E853–E860. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Zhao, Y.; Mo, T.; Li, J.; Li, P. Immobilization of glucose oxidase in electrospun nanofibrous membranes for food preservation. Food Control 2012, 26, 188–193. [Google Scholar] [CrossRef]
- Caseli, L.; Santos, D.S., Jr.; Foschini, M.; Goncalves, D.; Oliveira, O.N., Jr. Control of catalytic activity of glucose oxidase in layer-by-layer films of chitosan and glucose oxidase. Mater. Sci. Eng. C 2007, 27, 1108–1110. [Google Scholar] [CrossRef]
- Winestrand, S.; Johansson, K.; Järnström, L.; Jönsson, L.J. Co-immobilization of oxalate oxidase and catalase in films for scavenging of oxygen or oxalic acid. Biochem. Eng. J. 2013, 72, 96–101. [Google Scholar] [CrossRef]
- Shutava, T.G.; Kommireddy, D.S.; Lvov, Y.M. Layer-by-layer enzyme/polyelectrolyte films as a functional protective barrier in oxidizing media. J. Am. Chem. Soc. 2006, 128, 9926–9934. [Google Scholar] [CrossRef] [PubMed]
- Soares, N.F.F.; Hotchkiss, J.H. Bitterness reduction in grapefruit juice through active packaging. Packag. Technol. Sci. 1998, 11, 9–18. [Google Scholar] [CrossRef]
- Nunes, M.A.P.; Vila-Real, H.; Fernandes, P.C.B.; Ribeiro, M.H.L. Immobilization of naringinase in PVA-alginate matrix using an innovative technique. Appl. Biochem. Biotechnol. 2010, 160, 2129–2147. [Google Scholar] [CrossRef] [PubMed]
- Krenkova, J.; Lacher, N.A.; Svec, F. Highly efficient enzyme reactors containing trypsin and endoproteinase LysC immobilized on porous polymer monolith coupled to MS suitable for analysis of antibodies. Anal. Chem. 2009, 81, 2004–2012. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Iizawa, Y.; Yamada, J.; Hirata, M. Retention of activity of urease immobilized on grafted polymer films. J. Appl. Polym. Sci. 2006, 102, 4886–4896. [Google Scholar] [CrossRef]
- Andersson, M.; Andersson, T.; Adlercreutz, P.; Nielsen, T.; Hornsten, E. Toward an enzyme-based oxygen scavenging laminate. Influence of industrial lamination conditions on the performance of glucose oxidase. Biotechnol. Bioeng. 2002, 79, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Park, H.P.; Braun, P.V. Coaxial Electrospinning of Self-Healing Coatings. Adv. Mater. 2010, 22, 496–499. [Google Scholar] [CrossRef] [PubMed]
- Díez-Pascual, A.M. (Ed.) Antibacterial Activity of Nanomaterials; ISBN 978-3-03897-048-4 (Pbk); ISBN 978-3-03897-049-1 (PDF).
- Wanga, J.; Huang, N.; Pan, C.J.; Kwok, S.C.H.; Yang, P.; Leng, Y.X.; Chen, J.Y.; Sun, H.; Wan, G.J.; Liu, Z.Y.; et al. Bacterial repellence from polyethylene terephthalate surface modified by acetylene plasma immersion ion implantation-deposition. Surf. Coat. Technol. 2004, 186, 299–305. [Google Scholar] [CrossRef]
- Brobbey, K.J.; Saarinen, J.J.; Alakomi, H.-L.; Yang, B.; Toivakka, M. Efficacy Of Natural Plant Extracts In Antimicrobial Packaging Systems. J. Appl. Packag. Res. 2017, 9, 6. [Google Scholar]
- Miteluţ, A.C.; Popa, E.E.; Popescu, P.A.; Popa, M.E.; Munteanu, B.S.; Vasile, C.; Ştefănoiu, G. Research on chitosan and oil coated PLA as food packaging material. Presented at the International Worshop, “Progress in Antimicrobial Materials”, Iasi, Romania, 30 March 2017. [Google Scholar]
- Busolo, M.A.; Fernandez, P.; Ocio, M.J.; Lagaron, J.M. Novel silver-based nanoclay as an antimicrobial in polylactic acid food packaging coatings. Food Addit. Contam. Part A 2010, 27, 1617–1626. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.G.; Stutzenberger, F.J. Nanotechnology in the detection and control of microorganisms. In Advances in Applied Microbiology; Laskin, A.I., Sariaslani, S., Gadd, G.M., Eds.; Elsevier: London, UK, 2008; Volume 63, pp. 145–181. ISBN 9780120026623, 9780080468921. [Google Scholar]
- Makwana, S.; Choudhary, R.; Kohli, P. Advances in Antimicrobial Food Packaging with Nanotechnology and Natural Antimicrobials. Int. J. Food Sci. Nutr. Eng. 2015, 5, 169–175. [Google Scholar] [CrossRef]
- Li, Q.; Mahendra, S.; Lyon, D.Y.; Brunet, L.; Liga, M.V.; Li, D.; Alvarez, P.J.J. Antimicrobial nanomaterials for water disinfection and microbial control: Potential applications and implications. Water Res. 2008, 42, 4591–4602. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari-Moghaddam, M.; Eslahi, H. Synthesis, characterization and antibacterial properties of a novel nanocomposite based on polyaniline/polyvinyl alcohol/Ag. Arab. J. Chem. 2013, 7. [Google Scholar] [CrossRef]
- Kumar, R.; Munstedt, H. Silver ion release from antimicrobial polyamide/silver composites. Biomaterials 2005, 26, 2081–2088. [Google Scholar] [CrossRef] [PubMed]
- Hannon, J.C.; Cummins, E.; Kerry, J.; Cruz-Romero, M.; Morris, M. Advances and challenges for the use of engineered nanoparticles in food contact materials. Trends Food Sci. Technol. 2015, 43, 43–62. [Google Scholar] [CrossRef]
- Drew, R.; Hagen, T.; ToxConsult Pty Ltd. Nanotechnologies in Food Packaging: An Exploratory Appraisal of Safety and Regulation, Report Prepared for Food Standards Australia New Zealand. May 2016. Available online: https://www.foodstandards.gov.au/publications/Documents/Nanotech%20in%20food%20packaging.pdf (accessed on 10 June 2017).
- Qi, L.F.; Xu, Z.R.; Jiang, X.; Hu, C.; Zou, X. Preparation and antibacterial activity of chitosan nanoparticles. Carbohydr. Res. 2004, 339, 2693–2700. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Pinault, M.; Pfefferle, L.D.; Elimelech, M. Single-walled carbon nanotubes exhibit strong antimicrobial activity. Langmuir 2007, 23, 8670–8673. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Decker, E.A.; Goddard, J.M. Controlling lipid oxidation of food by active packaging technologies. Food Funct. 2013, 4, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Estaca, J.; Lopez-de-Dicastillo, C.; Hernandez-Munoz, P.; Catala, R.; Gavara, R. Advances in antioxidant active food packaging. Trends Food Sci. Technol. 2014, 35, 42–51. [Google Scholar] [CrossRef]
- Nerin, C.; Tovar, L.; Salafranca, J. Behaviour of a new antioxidant active film versus oxidizable model compounds. J. Food Eng. 2008, 84, 313–320. [Google Scholar] [CrossRef]
- Yemmireddy, V.K.; Farrell, G.D.; Hung, Y.C. Development of Titanium Dioxide (TiO2) Nanocoatings on Food Contact Surfaces and Method to Evaluate Their Durability and Photocatalytic Bactericidal Property. J. Food Sci. 2015, 80, N1903–N1911. [Google Scholar] [CrossRef] [PubMed]
- Barbiroli, A.; Bonomi, F.; Capretti, G.; Iametti, S.; Manzoni, M.; Piergiovanni, L.; Rollini, M. Antimicrobial activity of lysozyme and lactoferrin incorporated in cellulose-based food packaging. Food Control 2012, 26, 387–392. [Google Scholar] [CrossRef]
- Mendes de Souza, P.; Fernandez, A.; Lopez-Carballo, G.; Gavara, R.; Hernandez-Munoz, P. Modified sodium caseinate films as releasing carriers of lysozyme. Food Hydrocoll. 2010, 24, 300–306. [Google Scholar] [CrossRef]
- Moskovitz, Y.; Srebnik, S. Mean-field model of immobilized enzymes embedded in a grafted polymer layer. Biophys. J. 2005, 89, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Robertson, G.L. Food Packaging: Principles and Practice, 2nd ed.; Taylor & Francis/CRC Press: Boca Raton, FL, USA, 2006; p. 550. ISBN 9781439862414. [Google Scholar]
- Chan, C.M.; Ko, T.M.; Hiraoka, H. Polymer surface modification by plasmas and photons. Surf. Sci. Rep. 1996, 24, 3–54. [Google Scholar] [CrossRef]
- Jansen, B.; Kohnen, W. Prevention of Biofilm Formation by Polymer Modification. J. Ind. Microbiol. 1995, 15, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Karkhanisa, S.S.; Stark, N.M.; Sabo, R.C.; Matuana, L.M. Water vapor and oxygen barrier properties of extrusion-blown poly(lactic acid)/cellulose nanocrystals nanocomposite films. Compos. Part A Appl. Sci. Manuf. 2018, 114, 204–211. [Google Scholar] [CrossRef]
- López de Dicastillo, C.; Garrido, L.; Alvarado, N.; Romero, J.; Palma, J.L.; Galotto, M.J. Improvement of Polylactide Properties through Cellulose Nanocrystals Embedded in Poly(Vinyl Alcohol) Electrospun Nanofibers. Nanomaterials 2017, 7, 106. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarty, A.; Teramoto, Y. Review Recent Advances in Nanocellulose Composites with Polymers: A Guide for Choosing Partners and How to Incorporate Them. Polymers 2018, 10, 517. [Google Scholar] [CrossRef]
- Ratner, B.D.; Hoffman, A.S. Physichochemical surface modification of materials used in medicine. In Biomaterials Science: An Introduction to Materials in Medicine, 3rd ed.; Ratner, B.D., Hoffman, A.S., Schoen, F.J., Lemons, J.E., Eds.; Academic Press: Oxford, UK; Waltham, MA, USA, 2013; pp. 259–276. ISBN 9780123746269, 9780080877808. [Google Scholar]
- Wagner, J.R., Jr. Multilayer Flexible Packaging Technology and Applications for the Food, Personal Care and Over-the-Counter Pharmaceutical Industries, 1st ed.; Elsevier Science: Oxford, NY, USA, 2010; p. 258. ISBN 9780815520214, 9780815520221. [Google Scholar]
- Nistor, M.T.; Vasile, C.; Chiriac, A.P.; Rusu, A.; Zgardan, C.; Nita, L.E.; Neamtu, I. Hybrid Sensitive Hydrogels for Medical Applications. In Polymer Materials with Smart Properties; Bercea, M., Ed.; Nova Science Publishers: New York, NY, USA, 2013; Chapter 3; pp. 67–90. ISBN 978-1-62808-876-2. [Google Scholar]
- Cheaburu, C.N.; Vasile, C. pH-responsive Hydrogels based on Chitosan and its derivatives. In Material Science, Synthesis, Properties, Applications, Polyme Yearbook; Pethrick, R.A., Zaikov, G.E., Eds.; Nova Science Publishers: New York, NY, USA, 2010; Volume 24, ISBN 978-1-60876-872-1. [Google Scholar]
- Vasile, C.; Dumitriu, R.P. (Eds.) Polymeric Materials Responsive to External Stimuli—Smart Polymeric Materials; PIM Publishng House: Iasi, Romania, 2008; p. 285. ISBN 978-606-520-068-5. [Google Scholar]
- Pamfil, D.; Vasile, C. Responsive Polymeric Nanotherapeutics. In Polymeric Nanomaterials in Nanotherapeutics; Vasile, C., Ed.; Elsevier: Amstredam, The Netherlands, 2019; Chapter 2; p. 53. [Google Scholar]
- Cordeiro, A.L. Stimuli-Responsive Polymer Nanocoatings, Nanotechnologies for the Life Sciences. In Nanostructured Thin Films and Surfaces; Challa, S.S., Kumar, R., Eds.; John Wiley and Sons: New York, NY, USA, 2011; Volume 5. [Google Scholar]
- Nath, N.; Chilkoti, A. Creating “Smart” Surfaces Using Stimuli Responsive Polymers, Advanced Materials; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2002. [Google Scholar]
- Cohen Stuart, M.; Huck, W.; Genzer, J.; Müller, M.; Ober, C.; Stamm, M.; Sukhorukov, G.B.; Szleifer, I.; Tsukruk, V.V.; Urban, M.; et al. Emerging Applications of Stimuli-Responsive Polymer Materials. Nat. Mater. 2010, 9, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Gao, Y.; Li, X.; Serpe, M.J. Stimuli-responsive polymers and their applications. Polym. Chem. 2017, 8, 10–11. [Google Scholar] [CrossRef]
- Noonan, G.O.; Whelton, A.J.; Carlander, D.; Duncan, T.V. Measurement methods to evaluate engineered nanomaterial release from food contact materials. Compr. Rev. Food Sci. Food Saf. 2014, 13, 679–692. [Google Scholar] [CrossRef]
- Weiss, J.; Takhistov, P.; Mc Clements, D.J. Functional materials in food nanotechnology. J. Food Sci. 2006, 71, R107–R116. [Google Scholar] [CrossRef]
- Honarvar, Z.; Hadian, Z.; Mashayekh, M. Nanocomposites in food packaging applications and their risk assessment for health. Electron. Physician 2016, 8, 2531–2538. [Google Scholar] [CrossRef] [PubMed]
- Farhoodi, M. Nanocomposite Materials for Food Packaging Applications: Characterization and Safety Evaluation. Food Eng. Rev. 2016, 8, 35–51. [Google Scholar] [CrossRef]
- Lan, T. Nanocomposite Materials for Packaging Applications, ANTEC. 2007. Available online: http://www.nanocor.com/tech_papers/antec-nanocor-tie%20lan-5-07.pdf (accessed on 25 September 2018).
- BCC Research, The Advanced Packaging Solutions Market Value for 2017 Is Projected to Be Nearly $44.3 Billion. 2015. Available online: http://www.bccresearch.com (accessed on 20 June 2017).
- Realini, C.E.; Marcos, B. Active and intelligent packaging systems for a modern society. Meat Sci. 2014, 98, 404–419. [Google Scholar] [CrossRef] [PubMed]
- Day, B.P.F. Active packaging of foods. In Smart Packaging Technologies for Fast Moving Consumer Goods; Kerry, J.P., Butler, P., Eds.; Wiley & Sons, Ltd.: West Sussex, UK, 2008; pp. 1–18. ISBN 978-0-470-02802-5. [Google Scholar]
- Barnes, K.A.; Sinclair, C.R.; Watson, D.H. Chemical Migration and Food Contact Materials, 1st ed.; Woodhead Publishing: Amsterdam, The Netherlands, 2007; p. 464, ISBN 9781845692094; Hardcover ISBN 9781845690298. [Google Scholar]
- Farris, S.; Introzzi, L.; Piergiovanni, L.; Cozzolino, C.A. Effects of different sealing conditions on the seal strength of polypropylene films coated with a bio-based thin layer. Ital. J. Food Sci. 2011, 23, 111–114. [Google Scholar] [CrossRef]
- Siegrist, M.M.-E.; Cousin, H.; Kastenholz, A.; Wiek, A. Public acceptance of nanotechnology foods and food packaging: The influence of affect and trust. Appetite 2007, 49, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Siegrist, M.; Stampfli, N.; Kastenholz, H.; Keller, C. Perceived Risks and Perceived Benefits of Different Nanotechnology Foods and Nanotechnology Food Packaging. Appetite 2008, 51, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Siegrist, M.; Sütterlin, B. Importance of perceived naturalness for acceptance of food additives and cultured meat. Appetite 2017, 113, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Bearth, A.; Siegrist, M. Are risk or benefit perceptions more important for public acceptance of innovative food technologies: A meta-analysis. Trends Food Sci. Technol. 2016, 49, 14–23. [Google Scholar] [CrossRef]
- Marquis, D.M.; Guillaume, É.; Chivas-Joly, C. Properties of Nanofillers in Polymer. Chap 11. In Nanocomposites and Polymers with Analytical Methods; Cuppoletti, J., Ed.; IntechOpen: London, UK, 2011; Chapter 13; pp. 261–284. Available online: http://www.intechopen.com/books/nanocomposites-and-polymers-with-analyticalmethods/properties-of-nanofillers-in-polymer (accessed on 25 September 2018).
| Method | Method to Obtain Nanocomposites | Results |
|---|---|---|
| In situ polymerization: emulsion and miniemulsion polymerization | dispersing fine nanofiller and nanoreinforcement in a monomer | monomers interact with the nanofiller surface and form a uniform suspension |
| Solution casting and latex method or solvent processing | 1. filler dispersal in the polymer solution 2. solvent evaporation 3. freeze-drying and hot-pressing 4. freeze-drying, extruding and hot-pressing the mixture. 5. surfactant addition 6. grafting of long chains onto nanofiller surface | sandwiched multilayer structures forming of filler-rich layers polymer polarity-based results The dispersion of nanoparticles in the nanocomposite film strongly depends on the processing technique and conditions |
| Direct addition/extruder Blending, melt processing Shear mixing | 1.addition of filler directly into the melted polymer 2. blending either by (mechanical mixer and extruder) | target: to obtain uniform distribution of the nanofillers in a polymer matrix |
| Deposition or layer (LBL) assembly | Layer-by-layer deposition | sequential substrate dipping in clay and polycation solutions were adopted to make the coating Multilayer films |
| Dispersion and chemical reaction | UV-curing in presence of photo-initiator; casting, evaporating the solvent | cross-linked nanocomposites |
| Electrospinning | development of electrospun nanofibers | nanofibres with different morphologies |
| Type of Nanofiller | Matrix | Preparation | Properties/Applications | Ref |
|---|---|---|---|---|
| Organoclay | LDPE and HDPE | melt mixing using PE grafted with maleic anhydride as compatibilizer-exfoliation | oxygen permeability of PE decreases gradually with the clay concentration, reaching a maximum reduction of ∼30% for 15 wt % MMT; dynamic moduli increase showing pseudo solid-like behaviour at clay concentrations higher than 8 wt %. | [45] |
| Nanolayers of NanoterTM from NanoBioMatters LTD Spain | PE | melt processing | very good barrier properties | [46] |
| 4% MMT | EPDM | melt processing | decreased N2 permeability by 30% | [46] |
| Bentonite | PLA | solution casting | improve strength and modulus; decreased elongation at break | [47] |
| 5% MMT | PVOH | casting | 90% reduction in water permeability retaining optical clarity | [46] |
| MMT | proteins and polysaccharides | casting | 60% reduction in water permeability | |
| 1.1%–4%–10% Various unmodified and organically modified MMT, Cloisite 25A, NanoterTM | PLA, PCL, PHA, PHBV Strach | monolayer packaging | reduction of oxygen and water permeability | |
| MMT | chitosan films | solvent casting | exfoliated and intercalated structure depending on MMT amount, Tensile strength of a chitosan film was enhanced and elongation-at-break decreased | [48] |
| MMT | poly (ε-caprolactone) (PCL) | electrospinning | improved mechanical properties even elongation at break | [49] |
| Anionic sodium MMT exfoliated | cationic polyacrylamide on a PET substrate | Layer-by-layer (LbL) self-assembly multilayer film | oxygen transmission rate (OTR) decreased as a function of number of bilayers deposited, until a negligible value–below 0.005 cm3/(m2 day atm)—for a 30-bilayer film, microwaveable and with a good optical transparency (higher than 90%), it was presented as a good candidate for aluminium foil replacement in food packaging. | [50] |
| 5% Clay ZnO stabilized with sodium carboxymethylcellulose | thermoplastic starch; gelatinized starch film glycerol plasticized-PEA starch | melt extruded | improve the mechanical strength of biopolymers, decreased water vapour permeability by using only 5% (w/w) of clays; the highest exfoliation and best improvement in mechanical properties, exfoliated clay. | [51,52] |
| 1 wt % and 5 wt % of modified (surfactant-modified) and un-modified cellulose nanocrystals, | PLA | solvent casting method in the presence of surfactant | reductions of 34% in water permeability for the cast films with 1 wt % of surfacant modified-CNC; good oxygen barrier properties; the migration level of the studied nano-biocomposites was below the overall migration limits required by the current normative for food packaging materials in both non-polar and polar simulants. | [53] |
| Up to 3% cellulose nanocrystals CNCs | PLA | extrusion, twin-screw extruder | water vapour permeability decreased gradually with increasing addition of CNCs up to 3%; good oxygen barrier properties; enhanced barrier and mechanical properties | [54] |
| Cellulosic nanoparticles in chloroform and layered silicates; whiskers | PLA | dispersion in non-aqueous medium, casting; addition of PVOH | An improvement in storage modulus over the entire temperature range for both nanoreinforcements together with shifts in the tanδ peaks for both nanoreinforcements to higher temperatures; reduction in the oxygen permeability for the bentonite nanocomposite but not for the MCC nanocomposite. The amount of light being transmitted through the nanocomposites was reduced compared to pure PLA indicating that both nanoreinforcements were not fully exfoliated | [47,55] |
| cellulose whiskers extracted from PEA hull fibres with different hydrolysis times, which resulted in different aspect ratios. | PEA starch matrix | The composite produced by using the whiskers with the highest aspect ratio exhibited the highest transparency and best tensile properties; enhances thermomechanical properties, reduces the water sensitivity and keeps biodegradability; Tg increases; moisture resistance improved | [56,57] | |
| cellulose whiskers | PVOH | solution casting, water solvent | the modulus increased by orientation of reinforcement under magnetic field | [58] |
| cellulose nanowhiskers | k/l carrageenan | casting | high crystallinity, enhanced water barrier of carrageenan | [59] |
| PLA | casting | improved barrier properties to gases and vapours, fully renewable biocomposites for biopackaging | ||
| Bacterial cellulose nanowhiskers | EVOH or PLA | electrospinning | increased thermal stability | |
| Bacterial cellulose nanowhiskers | EVOH | melt compounding | enhanced barrier and mechanical properties of EVOH | |
| 30 wt % of Straw cellulose whiskers | poly(styrene-co-butyl acrylate) latex film | freeze-drying and moulding a mixture of aqueous suspensions | modulus more than a thousand times higher than that of the bulk matrix | [60] |
| Aqueous suspensions of polysaccharide (cellulose, chitin or starch) nanocrystals tunicin (the cellulose extracted from a tunicate–a sea animal) whiskers, wheat straw or sugar beet cellulose nanocrystals, potato starch nanocrystal and squid pen and Riftia tubes chitin whiskers | hydrophobic polymers as: styrene and butyl acrylate [poly(S-co-BuA)] poly(β-hydroxyoctanoate) (PHO) polyvinylchloride (PVC), waterborne epoxy, natural rubber (NR) and polyvinyl acetate (PVAc), poly(styrene-co-hexyl-acrylate) | dispersion of these nanocrystals in non-aqueous media is possible using surfactants or chemical grafting long chain surface chemical modification; mixing and casting the two aqueous suspensions, freeze-drying and hot-pressing or freeze-drying, extruding and hot pressing; mixture extrusion methods, miniemulsion polymerization | films; larger latex particle size results in higher mechanical properties | [30] |
| aqueous suspension of polysaccharide nanocrystals | hydrosoluble or hydrodispersible polymers as: reinforced starch, silk fibroin, poly(oxyethylene), polyvinyl alcohol, hydroxypropyl cellulose, carboxymethyl cellulose or soy protein isolate | mixing and casting the aqueous solutions, freeze-drying and hot-pressing | ||
| starch nanocrystals | waterborne polyurethane | solution casting; chemical grafting of starch nanocrystals | enhanced strength, elongation and Young’s modulus. The chemical grafting of the starch nanocrystals StNs did not affect positively the strength and elongation, because such a treatment inhibited the formation of physical interaction and increasing network density in nanocomposites | [61] |
| Coating of cotton and tunicin whiskers by a surfactant phosphoric ester of polyoxyethylene (9)-nonyl phenyl ether leads to stable suspensions in toluene and cyclohexane or chloroform | atactic polypropylene, isotactic polypropylene, or (EVA) | dispersion in non-aqueous medium, casting | decreased mechanical properties | [62,63] |
| Chitin whiskers | protein isolate thermoplastics | solution-casting technique | improved not only the tensile properties (tensile strength and elastic modulus) of the matrix but also its water resistance | [64] |
| chitin whiskers | chitosan films | solution-casting technique | improved chitosan films tensile strength until a whisker content of 2.96%, while higher increases of whiskers contents resulted in decreasing strength. Improved water resistance of the films. | [65] |
| chitosan–tripolyphosphate (CS–TPP) nanoparticles | hydroxypropyl methylcellulose (HPMC) films | solution-casting technique | improved mechanical and barrier properties of the films | [66] |
| carbon-based graphene, 20 to 60 nm in thickness and 0.5 to 25 μm in diameter, at 1 to 5 wt % loading | poly(methyl methacrylate) (PMMA) | dispersion at 30 °C by high speed shearing methods | heat resistant, high barrier nanocomposites promising in food packaging; increase the glass transition temperature of PMMA | [67] |
| Active Agent | Substrate or Matrix | Technique | Characteristics/Observations | Ref. |
|---|---|---|---|---|
| Antimicrobials | ||||
| Essential oils; adhesion promotors (e.g., acrylic or vinyl resins or nitrocellulose) and fixatives | common packaging materials | different coating or spraying | controlled release | [193] |
| Bacteriocins (ex: Nisaplin® nisin, pediocins); spice and herb extracts; Organic acids, microencapsulated nisin | oolycaprolactone, alginate, crosslinked chitosan/cellulose nanocrystal | crosslinking reaction under γ-irradiation | nanocellulose/PCL and alginate/cellulose nanocrystal based edible films and ready-to-eat meat | [194] |
| Silver nanoparticles AgNP | poly(3-hydroxybutyrate-co-3-hydroxyvalerate | extrusion/blending | [195] | |
| Silver | poly(l-lactide) | melt-compounding | sustained release of antimicrobial silver ions in food applications | [196] |
| AgNP/peptide/cellulose nanocrystals | PLA | solvent casting | significant inhibition of microbial growth; migration rates below values reported by international imposed limits | [197] |
| AgNP/kaolinite | PLA and poly(butylene adipate-co-terephthalate) | blow films | the composite showed its biodegradation extent of 69.94% (after 90 days), offering good biodegradability for use as a material for the production of degradable plastic bags. The ageing, hydrolytic degradation and biodegradation of PLA-based films could be tailored by Ag kaolinite incorporation | [198] |
| AgNPs | multilayer films of PHBV3 and electrospun fibres | compression-moulding, 180 °C and 1.8 MPa for 5 min; coated with PHBVs and PHBVs/AgNsP ultrathin fibre mats produced by electrospinning followed by an annealing step; electrospun coating (~0.71 ± 0.01 mg/cm2) | multilayer materials for food packaging and food contact surface applications; efficient antimicrobial materials; bactericidal effect against Salmonella enterica | [191] |
| AgNP | PVOH | mixing a colloidal solution | improved thermal properties, enhancing stability and increasing Tg. | [199] |
| 2 wt % Ag-NPs | polyamide 6 | thermal reduction of silver ions during the melt processing of a PA6/silver acetate mixture | effective against E. coli | [200,201] |
| TiO2/Ag grafted with γ-aminopropyltriethoxysilane | PVC | mixing | good antibacterial properties by photocatalytic bacterial inactivation | [202] |
| nano-Ag 35%, nano-TiO2 40%, kaolin 25%) (30%) | PE | high-speed mixer; extruded by a twin-screw extruder and then film was obtained | Ag-NPs retarded the senescence of jujube, a Chinese fruit. | [203] |
| ZnO:Cu/Ag | PLA | melt processing | Antimicrobial materials | [189] |
| cellulose nanocrystal/silver nanohybrids bifunctional nanofillers—10 wt % CNC-Ag | poly(3-hydroxybutyrate-co-3-hydroxyvalerate) | solution casting | high performance nanocomposites with improved thermal, mechanical and antibacterial properties against both Gram-negative E. coli and Gram-positive S. Aureus of PHBV. Reduced water uptake and water vapour permeability; lower migration level in both non-polar and polar simulants because of increased crystallinity and improved interfacial adhesion. Great potential applications in the fields of food, beverage packaging and disposable overwrap films. | [204] |
| 30 μm thick coating containing 2–6% cinnamon essential oil | PP | spreading | controlled release, total inhibition against Aspergillus flavus and niger and Penicillium roqueforti and Penicillium expansum | [205] |
| 4% thyme and oregano essential oils | corona treated LDPE | ionizing treatment and directly extrusion | controlled release, inhibit Escherichia coli 0157:H7, Salmonella Typhimurium and Listeria monocytogenes, changes in barrier properties | [206] |
| Organic-inorganic hybrid coatings, polyvinyl alcohol with improved water resistance | poly(ethylene terephthalate) and oriented polypropylene | sol-gel technique condensation of hydroxylated monomers and polymers into a network | multilayer materials for packaging applications | [207] |
| Antifungal agent natamycin was embedded in a tetraethyl orthosilicate/EVOH gel | plasma treated PLA films | sol-gel | antimicrobial coatings; controlled release; inhibit mould growth on cheese stored for 30 days | [208] |
| Nanosilver or chitosan | LDPE | melt-blended and layered deposited silver | silver ion migration from the nanocomposites into the food simulants and apple juice was less than the cytotoxicity-level concentration (10 mg kg−1) in all cases over 30 days | [209] |
| Antimicrobial photocatalysts (e.g., TiO2) | PLA multi-layered hybrid coatings | sol-gel technique | Controlled release, antimicrobial | [210] |
| Polydiacetylene liposomes containing cinnamaldehyde as liposome-encapsulated cinnamaldehyde | amine-functionalized silane monolayer on piranha treated glass or amine-functionalized PLA films | nanoencapsulation and immobilization of cinnamaldehyde | Controlled release, efficient against Bacillus cereus | [211] |
| Sorbic acid and/or lauric arginate ester and chitosan and nisin | PLA or corona treated PLA | directly coated with the solutions, or treated with solution-coated polylactic acid (PLA) films | 2–3 logarithmic reductions of Listeria innocua (2–3 logarithmic reductions), Listeria monocytogenes and Salmonella Tiphymurium/negative effect on CO2 gas barrier properties. There was no significant difference in the effectiveness of antimicrobial films versus the coatings. Antimicrobial packaging may be used alone, or in combination with flash pasteurization, in preventing foodborne illness due to post processing contamination of ready-to-eat meat products. | [212,213] |
| Peptide nisin entrapped in polyethylene oxide brushes grown on silane modified silicon wafers which protected nisin. | polyethylene oxide | entrapment | inhibition of Gram positive bacterium Pediococcus pentosaceous over a period of seven days | [214] |
| Pullalan powder was rendered cationic by reaction with an amine terminated silane as 3-aminopropyltrimethoxysilane | antimicrobial pullulan | immobilization | inhibits Staphylococcus aureus and Escherichia coli | [215] |
| Enzyme lysozyme | covalently attached onto UV-ozone treated EVOH films | immobilization via carbodiimide chemistry | inhibits Listeria monocytogenes | [216] |
| (3-Bromopropyl)triphenylphosphonium | poly(butylene adipate-co-terephthalate) functionalized with a quaternary phosphonium compound, (3-bromopropyl) triphenylphosphonium | immobilization by azide-alkyne “click” reaction | inhibits Escherichia coli | [217] |
| SO2 | multi-layered film made of PA and PE was subjected to atmospheric plasma treatment (Ar, Na2O and SO2) on the PA side of the films | immobilization | inhibits Escherichia coli (82%), Staphylococcus aureus (86%), Listeria monocytogenes (63%), Bacillus subtilis (79%) and Candida albicans (75%) | [218] |
| Chitosan (polycation) and κ-carrageenan (polyanion) | aminated PET | layer-by-layer assembly | improved gas barrier properties | [219,220] |
| Lysozyme: κ-carrageenan alternated with two layers of the antimicrobial enzyme lysozyme | amine-functionalized PET films | layer-by-layer assembly | improved oxygen and water vapour permeability | [221] |
| Antioxidants | ||||
| Citrus oil | plasma treated PET trays | spray deposition of citrus oil in methanol | controlled release; antioxidant activity with cooked turkey meat and retained activity after six months of storage | [222,223] |
| Rosemary extract | LDPE plastic wrap or a polymeric carrier | applied direct onto LDPE plastic wrap or with a polymeric carrier | controlled release; 0.45 mg rosemary cm−2 | [224] |
| α-Tocopherol | paperboard using a vinyl acetate-ethylene copolymer as a carrier for controlled release | solvent casting coating at a concentration of 3% | antimicrobial and antioxidant coating; controlled release | [225] |
| gallic acid | chitosan | immobilization by carbodiimide assisted reaction | reduced oxidation of peanuts | [226] |
| Metal oxide coatings Aluminium oxide or silicon oxide | biaxially oriented polypropylene and polyethylene terephthalate film substrates | reactive evaporation using an industrial high-speed vacuum deposition technique ‘boat-type’ roll-to-roll metallizer | transparent barrier coatings based on aluminium oxide or silicon oxide fulfil requirements such as product visibility, microwaveability or retortability reduce oxygen diffusion | [227,228] |
| Tannic acid and poly(allylamine hydrochloride) | glass slides | layer-by-layer assembly | the number of bilayers increases in overall scavenging activity | [229] |
| Caffeic acid | polypropylene packaging materials coating | photografting | prevent oxidative degradation of ascorbic acid in orange juice | [230] |
| Acrylic acid | metal chelating active packaging coatings PP films, PP-g-PAA | photografting | prevent lipid oxidation in food emulsions | [231,232,233] |
| Hydroxamic acid photografted polyhydroxamate chelators Plant-derived phenolic compounds, metal chelating | PP | in situ polymerization of a mixture of catechol and catechin and oxidative polymerization with laccase and in alkaline saline and photografting | surface adhesion properties upon polymerization; non-migratory iron chelating active packaging material biomimetic iron chelating active packaging material, inhibit oxidation of food | [234,235,236] |
| Lignosulfonate | Alginate | Solution casting | Antioxidant and UV protective films | [113] |
| Biocatalysts | ||||
| Lactase blended into polyethylene oxide nanofibers | oxygen scavenging | electrospinning-enzymes or other active agents are incorporated into polymer nanofibers | controlled release, retained up to 93% of free enzyme activity | [237,238] |
| Lactase | attachment of lactase to polyethylene films; | immobilization β-galactosidase bound to amine-functionalized PE films by a dialdehyde tether; polyethylene glycol tether size influences the attachment | reduce milk lactose in package; retained activity of immobilized lactase | [239,240] |
| Lactase conjugated to nanomaterials | lactase immobilization onto nanostructures | lactase was attached to carboxylic acid functionalized magnetic nanoparticles 18 nm, 50 nm and 200 nm in diameter using carbodiimide chemistry. | retained activity of immobilized lactase; reducing the particle size of magnetic nanoparticles can increase the activity retention of conjugated lactase | [241] |
| Lactase and polyethylenamine, glutaraldehyde | lactase covalently bound to low-density polyethylene | layer-by-layer assembly | more enzyme is immobilised but diffusion is difficult | [242] |
| Glucose oxidase | electrospun polyvinyl acetate/chitosan/tea extract fibres | electrospinning | reduce oxygen in packaged foods | [243] |
| Glucose oxidase | chitosan | LbL films | biosensors | [244] |
| Oxalate oxidase, oxygen-reducing enzymes in coatings and films for active packaging | extrusion-coated liner of polypropylene on top of the coating. | entrapment in a latex polymer matrix | protective packaging gas carbon dioxide; oxygen scavenging in active packaging; retained catalytic activity through entrapment in a latex polymer matrix | [245] |
| Laccase-catalysed reduction of oxygen | it was possible to use lignin derivatives as substrates for the enzymatic reaction. | laccase-catalysed reaction created a polymeric network by cross-linking of lignin-based entities, | resulted in increased stiffness and water-resistance of biopolymer films | [238] |
| Catalase | layered haemoglobin, PS | Immobilization | create a physical and chemical protective barrier | [229,246] |
| Fungal naringinase | cross-linking naringinase to polyvinyl alcohol and alginate | immobilization | bitterness reduction in grapefruit juice | [247,248] |
| Protease, trypsin and endoproteinases | PP, PVOH and PS | photografting, carboiimide chemistry -covalently couple enzymes (via amine groups) to carboxylic acid groups poly(ethylene) glycol methacrylate and 4-vinyl-2,2-dimethylazolactone | antibody analysis in enzyme reactors | [249] |
| Hydrolase urease | photografted polytetrafluoroethylene | photografting | remove urea from beverages and foods | [250] |
| Glucose oxidase catalase for oxygen scavenging activity | low-density polyethylene and paper board multilaminate or combinations of LDPE, PP and PLA | industial lamination | scaled-up production in tetra pack pilot plan, scavenge oxygen to improve food shell life | [251] |
| Polysiloxane-based healing agents | acrylate matrix | polymer coatings coaxial electrospinning | self-healing polymer coating systems | [252] |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasile, C. Polymeric Nanocomposites and Nanocoatings for Food Packaging: A Review. Materials 2018, 11, 1834. https://doi.org/10.3390/ma11101834
Vasile C. Polymeric Nanocomposites and Nanocoatings for Food Packaging: A Review. Materials. 2018; 11(10):1834. https://doi.org/10.3390/ma11101834
Chicago/Turabian StyleVasile, Cornelia. 2018. "Polymeric Nanocomposites and Nanocoatings for Food Packaging: A Review" Materials 11, no. 10: 1834. https://doi.org/10.3390/ma11101834
APA StyleVasile, C. (2018). Polymeric Nanocomposites and Nanocoatings for Food Packaging: A Review. Materials, 11(10), 1834. https://doi.org/10.3390/ma11101834