Yb2O3 Doped Zr0.92Y0.08O2-α(8YSZ) and Its Composite Electrolyte for Intermediate Temperature Solid Oxide Fuel Cells
Abstract
1. Introduction
2. Experimental
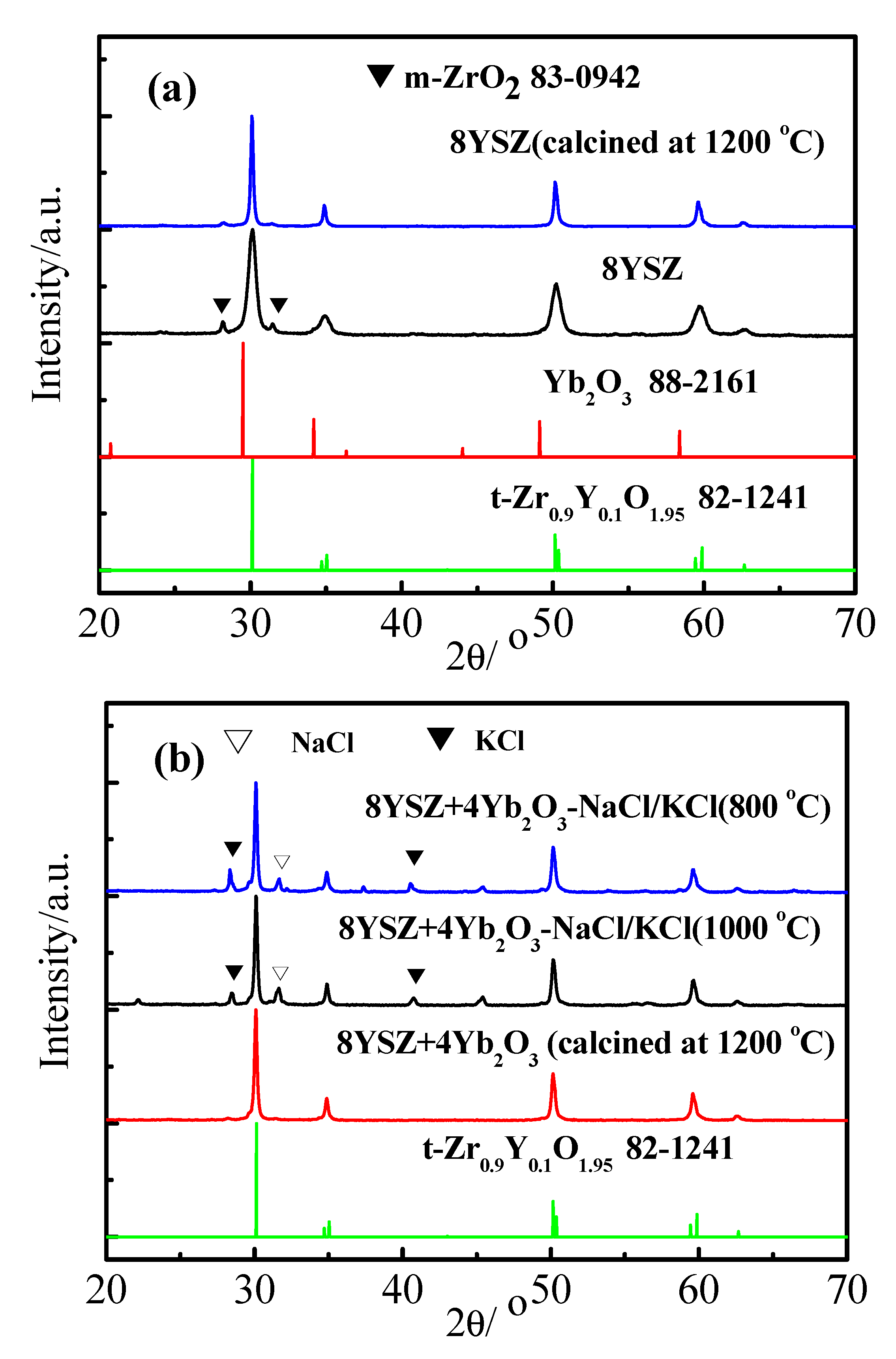
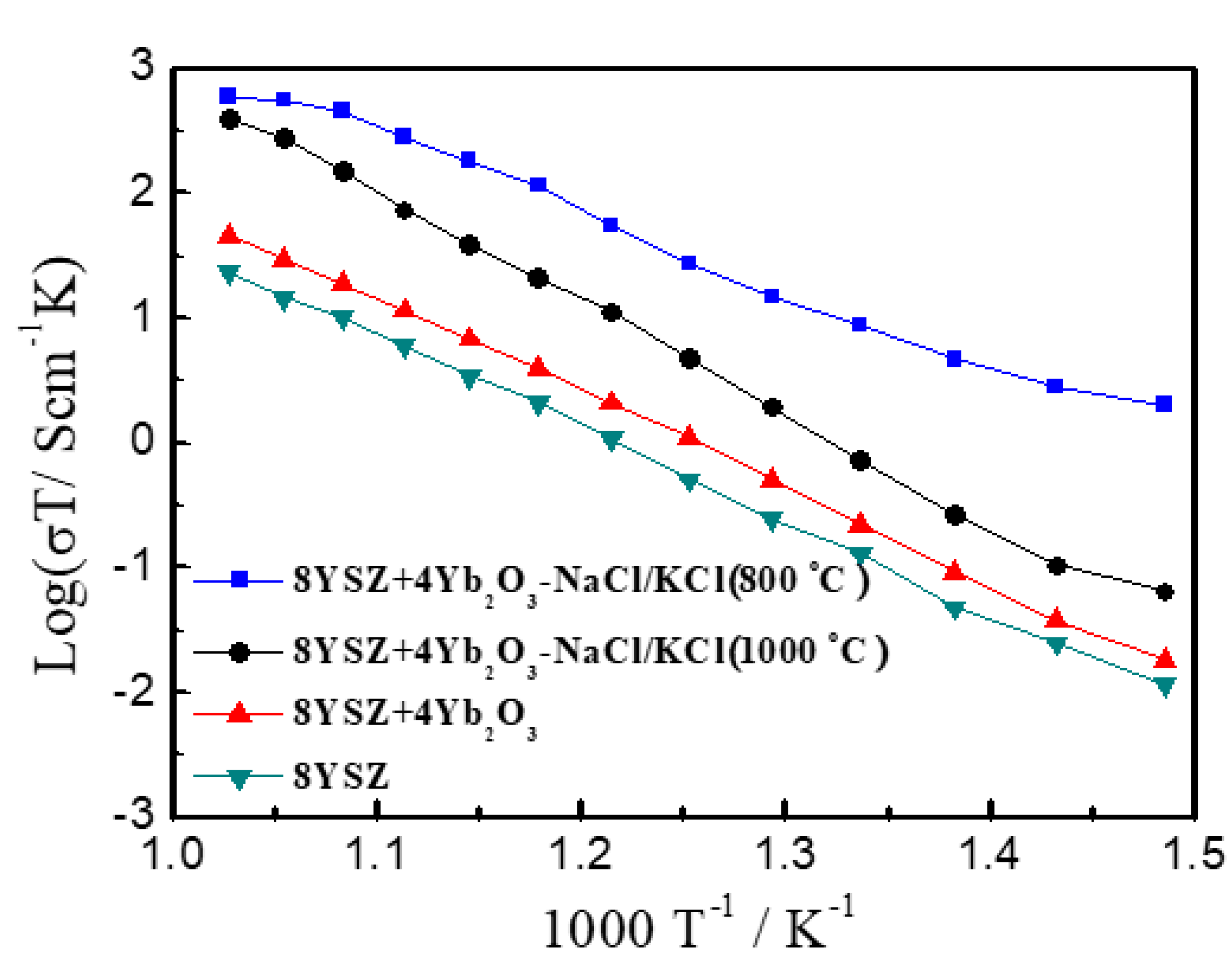
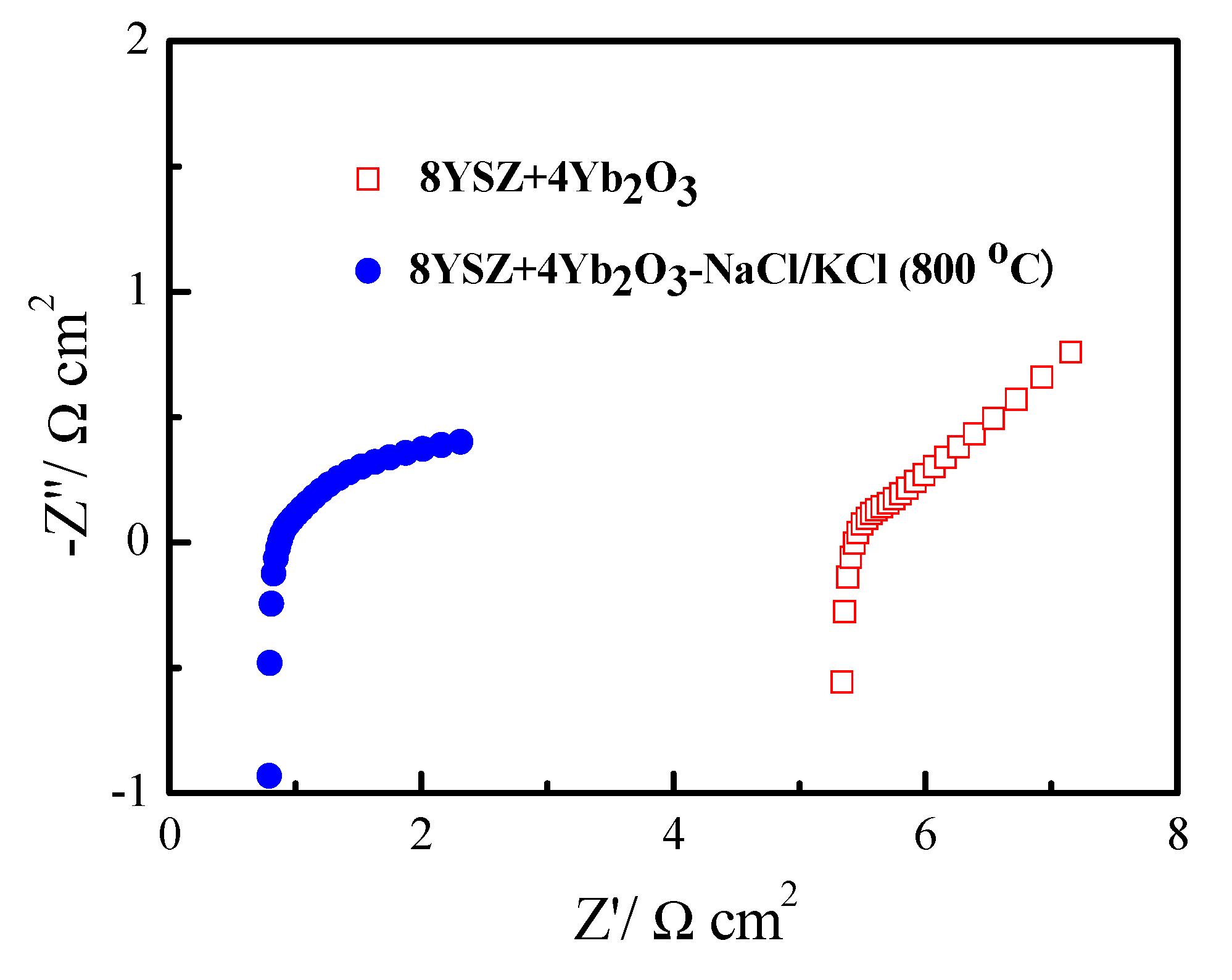
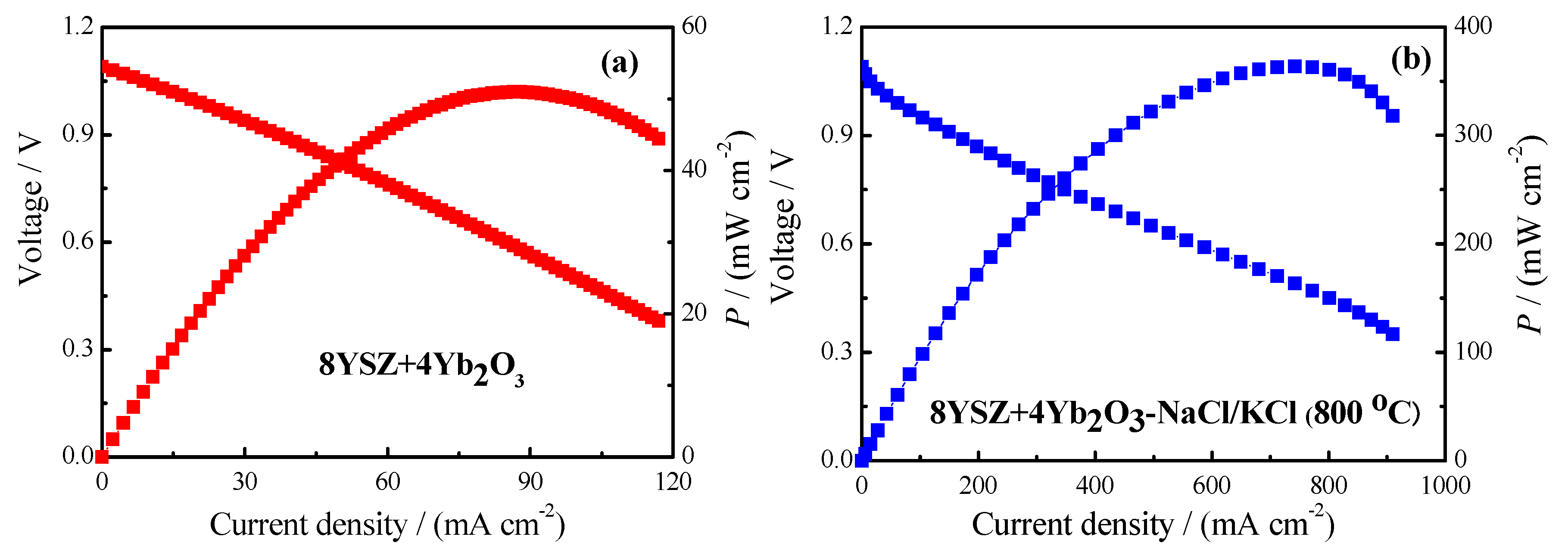
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hibino, T.; Kobayashi, K.; Lv, P.; Nagao, M.; Teranishi, S.; Mori, T. An intermediate- temperature biomass fuel cell using wood sawdust and pulp directly as fuel. J. Electrochem. Soc. 2017, 164, F557–F563. [Google Scholar] [CrossRef]
- Sadeghifar, H. In-plane and through-plane electrical conductivities and contact resistances of a Mercedes-Benz catalyst-coated membrane, gas diffusion and micro-porous layers and a Ballard graphite bipolar plate: Impact of humidity, compressive load and polytetrafluoroethylene. Energy Convers. Manag. 2017, 154, 191–202. [Google Scholar]
- Irshad, M.; Siraj, K.; Raza, R.; Ali, A.; Tiwari, P.; Zhu, B.; Asia, R.; Ali, A.; Ullah, M.K.; Usman, A. A brief description of high temperature solid oxide fuel cell’s operation, materials, design, fabrication technologies and performance. Appl. Sci. 2016, 6, 75. [Google Scholar] [CrossRef]
- Sadeghifar, H.; Bahrami, M.; Djilali, N. A statistically-based thermal conductivity model for fuel cell Gas Diffusion Layers. J. Power Sources 2013, 233, 369–379. [Google Scholar] [CrossRef]
- Xia, C.; Qiao, Z.; Feng, C.; Kim, J.; Wang, B.; Zhu, B. Study on zinc oxide-based electrolytes in low-temperature solid oxide fuel cells. Materials 2018, 11, 40–53. [Google Scholar] [CrossRef]
- Fang, X.; Zhu, J.; Lin, Z. Effects of electrode composition and thickness on the mechanical performance of a solid oxide fuel cell. Energies 2018, 11, 1735. [Google Scholar] [CrossRef]
- Sadeghifar, H.; Djilali, N.; Bahrami, M. Effect of Polytetrafluoroethylene (PTFE) and micro porous layer (MPL) on thermal conductivity of fuel cell gas diffusion layers: Modeling and experiments. J. Power Sources 2014, 248, 632–641. [Google Scholar] [CrossRef]
- Sadeghifar, H.; Djilali, N.; Bahrami, M. A new model for thermal contact resistance between fuel cell gas diffusion layers and bipolar plates. J. Power Sources 2014, 266, 51–59. [Google Scholar] [CrossRef]
- Bernuy-Lopez, C.; Rioja-Monllor, L.; Nakamura, T.; Ricote, S.; O’Hayre, R.; Amezawa, K.; Einarsrud, M.; Grande, T. Effect of cation ordering on the performance and chemical stability of layered double perovskite cathodes. Materials 2018, 11, 196–212. [Google Scholar] [CrossRef] [PubMed]
- Vilela, C.; Martins, A.P.C.; Sousa, N.; Silvestre, A.J.D.; Figueiredo, F.M.L.; Freire, C.S.R. Poly(bis [2-(methacryloyloxy) ethyl] phosphate)/bacterial cellulose nanocomposites: Preparation, characterization and application as polymer electrolyte membranes. Appl. Sci. 2018, 8, 1145. [Google Scholar] [CrossRef]
- Hibino, T.; Kobayashi, K.; Nagao, M.; Teranishi, S. Hydrogen production by direct lignin electrolysis at intermediate temperatures. ChemElectroChem 2017, 4, 3032–3036. [Google Scholar] [CrossRef]
- Sadeghifar, H.; Djilali, N.; Bahrami, M. Thermal conductivity of a graphite bipolar plate (BPP) and its thermal contact resistance with fuel cell gas diffusion layers: Effect of compression, PTFE, micro porous layer (MPL), BPP out-of-flatness and cyclic load. J. Power Sources 2015, 273, 96–104. [Google Scholar] [CrossRef]
- Luo, J.; Jensen, A.H.; Brooks, N.R.; Sniekers, J.; Knipper, M.; Aili, D.; Li, Q.; Vanroy, B.; Wübbenhorst, M.; Yan, F.; et al. 1,2,4-Triazolium perfluorobutanesulfonate as an archetypal pure protic organic ionic plastic crystal electrolyte for all-solid-state fuel cells. Energy Environ. Sci. 2015, 8, 1276–1291. [Google Scholar] [CrossRef]
- Rendtorff, N.M.; Suarez, G.; Aglietti, E.F.; Rivas, P.C.; Martinez, J.A. Phase evolution in the mechanochemical synthesis of stabilized nanocrystalline (ZrO2)0.97(Y2O3)0.03 solid solution by PAC technique. Ceram. Int. 2013, 39, 5577–5583. [Google Scholar] [CrossRef]
- Liu, X.Y.; Xu, Z.H.; Liang, G.Y. Comparative study of the sintering behaviors between YSZ and LZ/YSZ composite. Mater. Lett. 2017, 191, 108–111. [Google Scholar] [CrossRef]
- Dankeaw, A.; Poungchan, G.; Panapoy, M.; Ksapabutr, B. In-situ one-step method for fabricating three-dimensional grass-like carbon-doped ZrO2 films for room temperature alcohol and acetone sensors. Sens. Actuators B Chem. 2017, 242, 202–214. [Google Scholar] [CrossRef]
- Mamana, N.; Díaz-Parralejo, A.; Ortiz, A.L.; Sánchez-Bajo, F.; Caruso, R. Influence of the synthesis process on the features of Y2O3-stabilized ZrO2 powders obtained by the sol-gel method. Ceram. Int. 2014, 40, 6421–6426. [Google Scholar] [CrossRef]
- Park, K.-Y.; Lee, T.-H.; Kim, J.-T.; Lee, N.; Seo, Y.; Song, S.-J.; Park, J.-Y. Highly conductive barium zirconate-based carbonate composite electrolytes for intermediate temperature-protonic ceramic fuel cells. J. Alloys Compd. 2014, 585, 103–110. [Google Scholar] [CrossRef]
- Slim, C.; Baklouti, L.; Cassir, M.; Ringuedé, A. Structural and electrochemical performance of gadolinia-doped ceria mixed with alkali chlorides (LiCl-KCl) for Intermediate Temperature-Hybrid Fuel Cell applications. Electrochim. Acta 2014, 123, 127–134. [Google Scholar] [CrossRef]
- Zhang, W.; Yuan, M.; Wang, H.; Liu, J. High-performance intermediate temperature fuel cells of new SrCe0.9Yb0.1O3-α-inorganic salt composite electrolytes. J. Alloys Compd. 2016, 677, 38–41. [Google Scholar] [CrossRef]
- Ojha, A.K.; Ponnilavan, V.; Kannan, S. Structural, morphological and mechanical investigations of in situ synthesized c-CeO2/α-Al2O3 composites. Ceram. Int. 2017, 43, 686–692. [Google Scholar] [CrossRef]
- Martins, N.C.T.; Rajesh, S.; Marques, F.M.B. Synthesis and electrochemical assessment of Ce0.5Yb0.5O1.75 ceramics and derived composite electrolytes. Mater. Res. Bull. 2015, 70, 449–455. [Google Scholar] [CrossRef]
- Kim, J.-T.; Lee, T.-H.; Park, K.-Y.; Seo, Y.; Kim, K.B.; Song, S.-J.; Park, B.; Park, J.-Y. Electrochemical properties of dual phase neodymium-doped ceria alkali carbonate composite electrolytes in intermediate temperature. J. Power Sources 2015, 275, 563–572. [Google Scholar] [CrossRef]
- Fu, Q.X.; Zhang, W.; Peng, R.R.; Peng, D.K.; Meng, G.Y.; Zhu, B. Doped ceria–chloride composite electrolyte for intermediate temperature ceramic membrane fuel cells. Mater. Lett. 2002, 53, 186–192. [Google Scholar] [CrossRef]
- Kravchyk, K.V.; Bohnke, O.; Gunes, V.; Belous, A.G.; Pashkova, E.V.; Lannic, J.L.; Gouttefangeas, F. Ionic and electronic conductivity of 3 mol% Fe2O3-substituted cubic Y-stabilized ZrO2. Solid State Ion. 2012, 226, 53–58. [Google Scholar] [CrossRef]
- Lei, L.; Bai, Y.; Liu, J. Ni-based anode-supported Al2O3-doped-Y2O3-stabilized ZrO2 thin electrolyte solid oxide fuel cells with Y2O3-stabilized ZrO2 buffer layer. J. Power Sources 2014, 248, 1312–1319. [Google Scholar] [CrossRef]
- Yuan, F.; Wang, J.X.; Miao, H.; Guo, C.; Wang, W. Investigation of the crystal structure and ionic conductivity in the ternary system (Yb2O3)x–(Sc2O3)(0.11−x)–(ZrO2)0.89 (x = 0–0.11). J. Alloys Compd. 2013, 549, 200–205. [Google Scholar] [CrossRef]
- Bohnke, O.; Gunes, V.; Kravchyk, K.V.; Belous, A.G.; Yanchevskii, O.Z.; V’Yunov, O.I. Ionic and electronic conductivity of 3 mol% Fe2O3-substituted cubic yttria-stabilized ZrO2 (YSZ) and scandia-stabilized ZrO2 (ScSZ). Solid State Ion. 2014, 262, 517–521. [Google Scholar] [CrossRef]
- Chen, Y.; Orlovskaya, N.; Payzant, E.A.; Graule, T.; Kuebler, J. A search for temperature induced time-dependent structural transitions in 10 mol% Sc2O3–1 mol% CeO2–ZrO2 and 8mol% Y2O3–ZrO2 electrolyte ceramics. J. Eur. Ceram. Soc. 2015, 35, 951–958. [Google Scholar]
- Zeeshan, N.; Rafiuddin. Solid electrolytes based on {1 − (x + y)}ZrO2-(x)MgO-(y)CaO ternary system: Preparation, characterization, ionic conductivity, and dielectric properties. J. Adv. Res. 2018, 9, 35–41. [Google Scholar] [CrossRef]
- Liu, X.; Fechler, N.; Antonietti, M. Salt melt synthesis of ceramics, semiconductors and carbon nanostructures. Chem. Soc. Rev. 2013, 42, 8237–8265. [Google Scholar] [CrossRef] [PubMed]
- Sadeghifar, H.; Djilali, N.; Bahrami, M. Counter-intuitive reduction of thermal contact resistance with porosity: A case study of polymer electrolyte membrane fuel cells. Int. J. Hydrog. Energy 2014, 41, 6833–6841. [Google Scholar] [CrossRef]
- Shi, R.; Chen, W.; Hu, W.; Liu, J.; Wang, H. SrCe0.9Sm0.1O3-α Compounded with NaCl-KCl as a Composite Electrolyte for Intermediate Temperature Fuel Cell. Materials 2018, 11, 1583. [Google Scholar] [CrossRef]
- Sadeghifar, H. An optimized microstructure to minimizing in-plane and through-plane pressure drops of fibrous materials: Counter-intuitive reduction of gas diffusion layer permeability with porosity. J. Power Sources 2018, 385, 100–113. [Google Scholar] [CrossRef]
- Sadeghifar, H. In-plane and through-plane local and average Nusselt numbers in fibrous porous materials with different fiber layer temperatures: Gas diffusion layers for fuel cells. J. Power Sources 2016, 325, 311–321. [Google Scholar] [CrossRef]
- Zhu, B.; Li, S.; Mellander, B.E. The oretical approach on ceria-based two-phase electrolytes for low temperature (300–600 °C) solid oxide fuel cells. Electrochem. Commun. 2008, 10, 302–305. [Google Scholar] [CrossRef]
- Shi, R.; Liu, J.; Wang, H.; Wu, F.; Miao, H.; Cui, Y. Low temperature synthesis of SrCe0.9Eu0.1O3-α by sol-gel method and SrCe0.9Eu0.1O3-α-NaCl-KCl composite electrolyte for intermediate temperature fuel cells. Int. J. Electrochem. Sci. 2017, 12, 11594–11601. [Google Scholar] [CrossRef]
- Sun, L.; Miao, H.; Wang, H. Novel SrCe1-xYbxO3-α-(Na/K)Cl composite electrolytes for intermediate temperature solid oxide fuel cells. Solid State Ion. 2017, 311, 41–45. [Google Scholar] [CrossRef]
- Afzal, M.; Raza, R.; Du, S.; Lima, R.B.; Zhu, B. Synthesis of Ba0.3Ca0.7Co0.8Fe0.2O3-α composite material as novel catalytic cathode for ceria-carbonate electrolyte fuel cells. Electrochim. Acta 2015, 178, 385–391. [Google Scholar] [CrossRef]
- Sadeghifar, H. Reconstruction and analysis of fuel cell gas diffusion layers using fiber spacing rather than pore size data: Questioned validity of widely-used porosity-based thermal conductivity models. J. Power Sources 2016, 307, 673–677. [Google Scholar] [CrossRef]
- Liu, J.; Du, R.; Shi, R.; Wang, H. Facile Synthesis and Enhanced Intermediate Temperature Electrical Properties of Novel Sn0.9Mg0.1P2O7/KSn2(PO4)3 Composite Electrolyte. Int. J. Electrochem. Sci. 2018, 13, 5061–5067. [Google Scholar] [CrossRef]
- Ma, G.; Shimura, T.; Iwahara, H. Simultaneous doping with La3+ and Y3+ for Ba2+- and Ce4+-sites in BaCeO3 and the ionic conduction. Solid State Ion. 1999, 120, 51–60. [Google Scholar] [CrossRef]
- Baek, S.-S.; Park, K.-Y.; Lee, T.-H.; Lee, N.; Seo, Y.; Song, S.-J.; Park, J.-Y. PdO-doped BaZr0.8Y0.2O3−δ electrolyte for intermediate-temperature protonic ceramic fuel cells. Acta Mater. 2014, 66, 273–283. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, B.; Yang, Q.; Chen, C.; Wang, W.; Ma, G. Preparation via microemulsion method and proton conduction at intermediate-temperature of BaCe1-xYxO3-α. Electrochem. Commun. 2009, 11, 153–156. [Google Scholar] [CrossRef]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, Y.; Shi, R.; Liu, J.; Wang, H.; Li, H. Yb2O3 Doped Zr0.92Y0.08O2-α(8YSZ) and Its Composite Electrolyte for Intermediate Temperature Solid Oxide Fuel Cells. Materials 2018, 11, 1824. https://doi.org/10.3390/ma11101824
Cui Y, Shi R, Liu J, Wang H, Li H. Yb2O3 Doped Zr0.92Y0.08O2-α(8YSZ) and Its Composite Electrolyte for Intermediate Temperature Solid Oxide Fuel Cells. Materials. 2018; 11(10):1824. https://doi.org/10.3390/ma11101824
Chicago/Turabian StyleCui, Yumin, Ruijuan Shi, Junlong Liu, Hongtao Wang, and Huiquan Li. 2018. "Yb2O3 Doped Zr0.92Y0.08O2-α(8YSZ) and Its Composite Electrolyte for Intermediate Temperature Solid Oxide Fuel Cells" Materials 11, no. 10: 1824. https://doi.org/10.3390/ma11101824
APA StyleCui, Y., Shi, R., Liu, J., Wang, H., & Li, H. (2018). Yb2O3 Doped Zr0.92Y0.08O2-α(8YSZ) and Its Composite Electrolyte for Intermediate Temperature Solid Oxide Fuel Cells. Materials, 11(10), 1824. https://doi.org/10.3390/ma11101824




