Pd-Free Activation Pretreatment for Electroless Ni-P Plating on NiFe2O4 Particles
Abstract
1. Introduction
2. Experimental Procedures
2.1. Sensitization and Activation Pretreatments
2.2. Pd-free Activation Pretreatment
2.3. Electroless Plating
2.4. Characterization
3. Results and Discussion
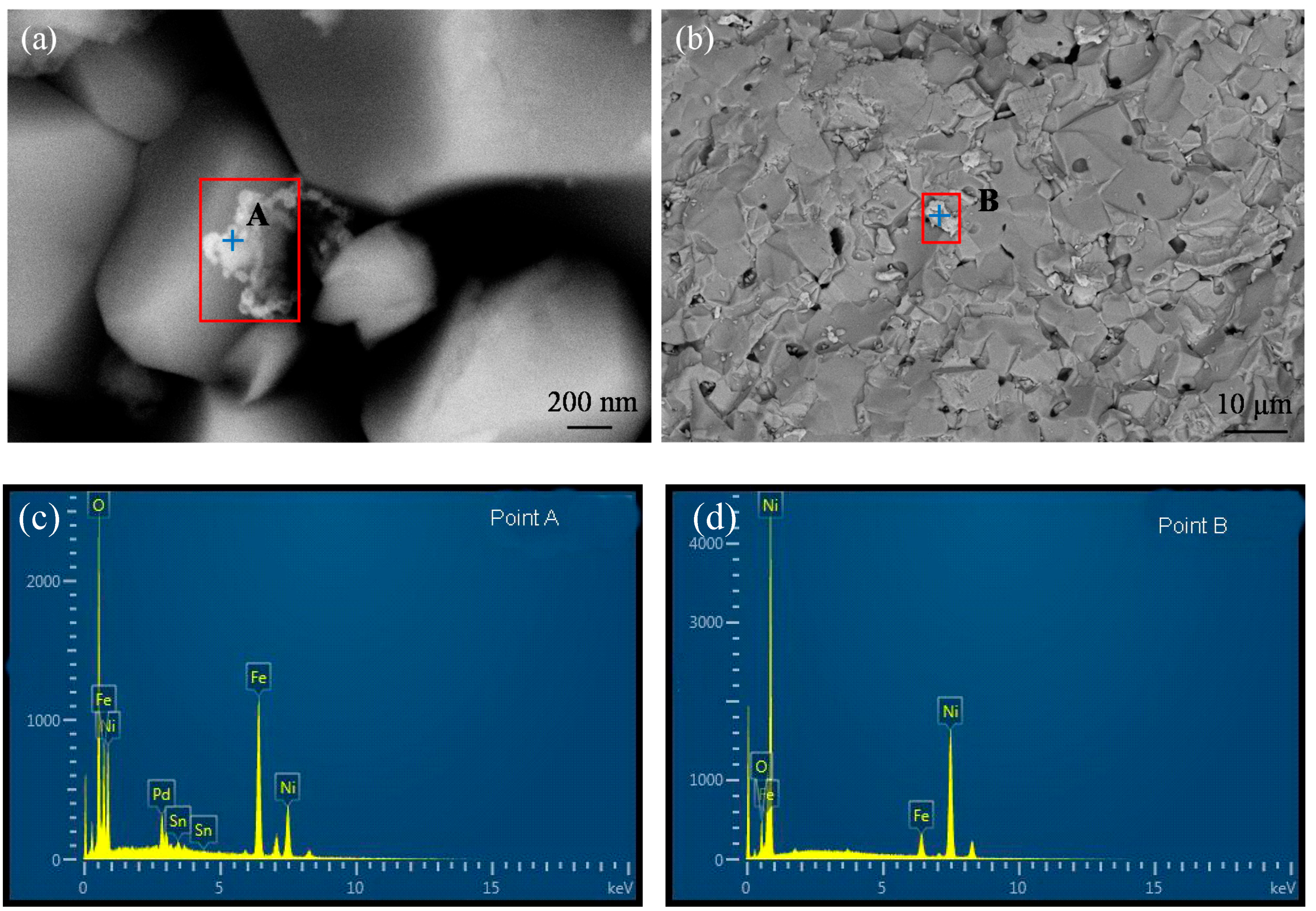
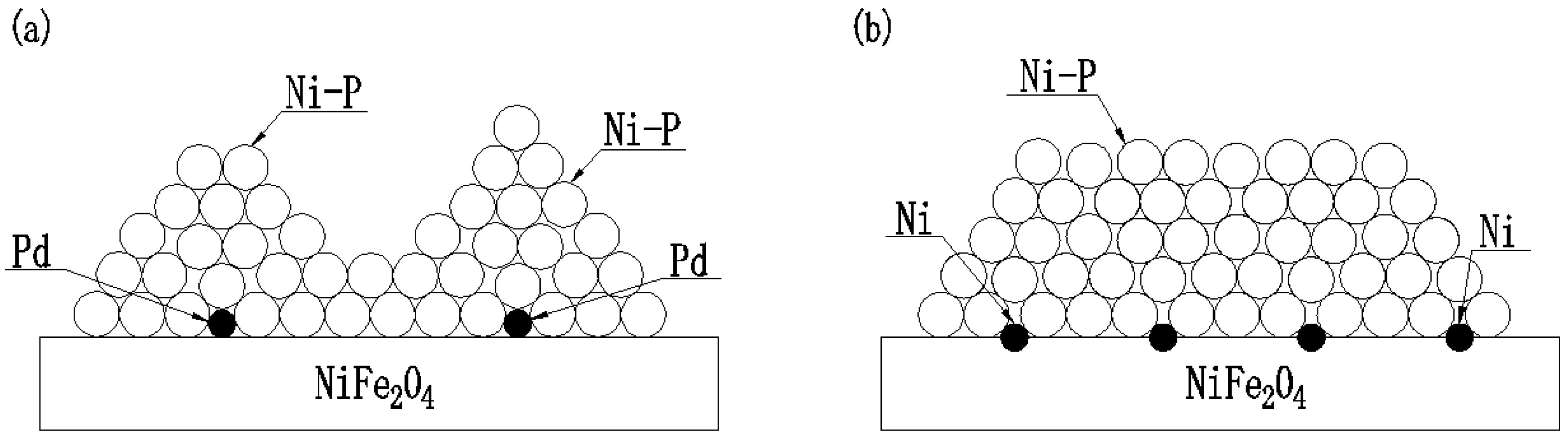
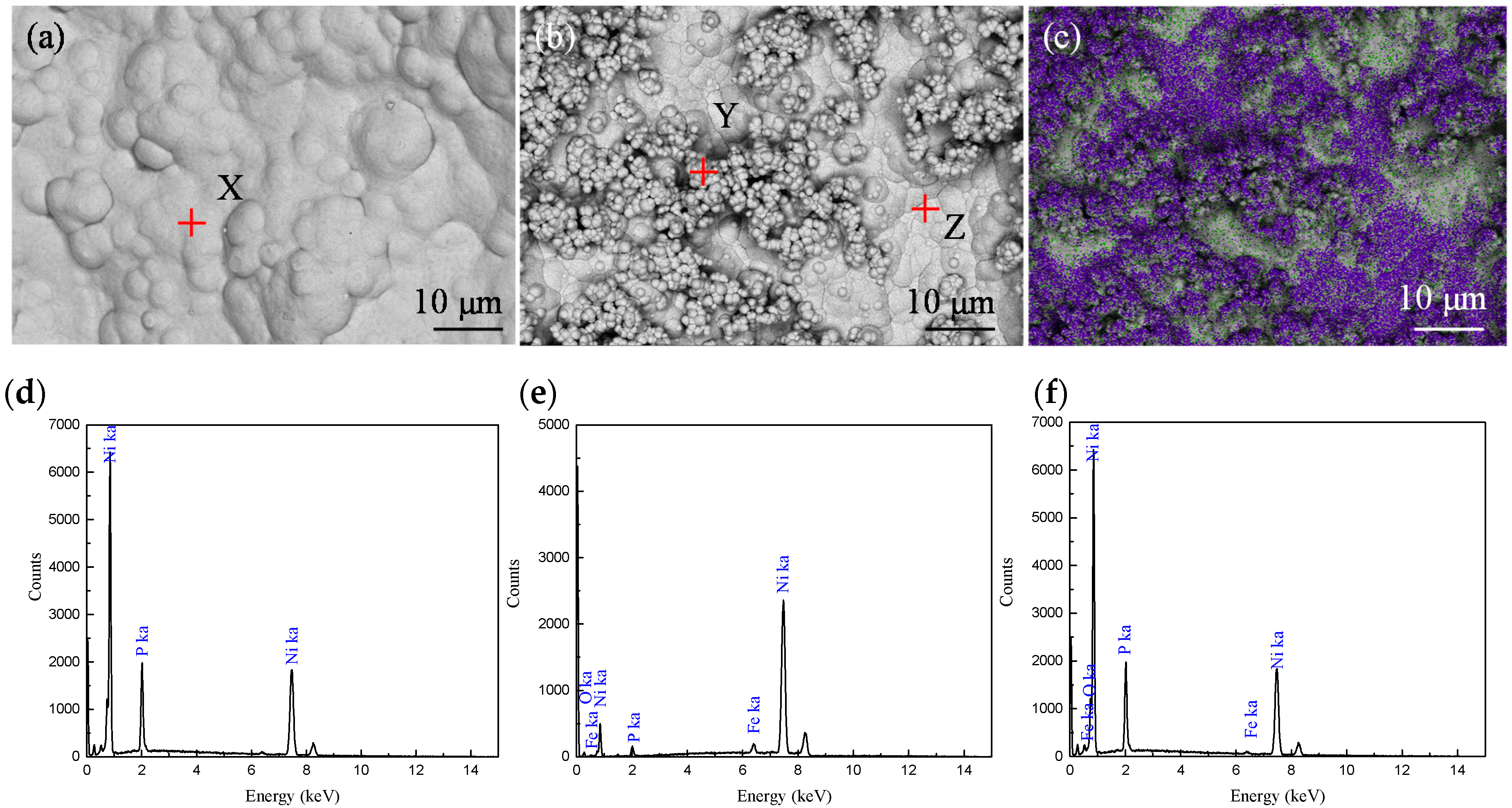
3.1. Pd Activation and Pd-Free Activation Pretreatment
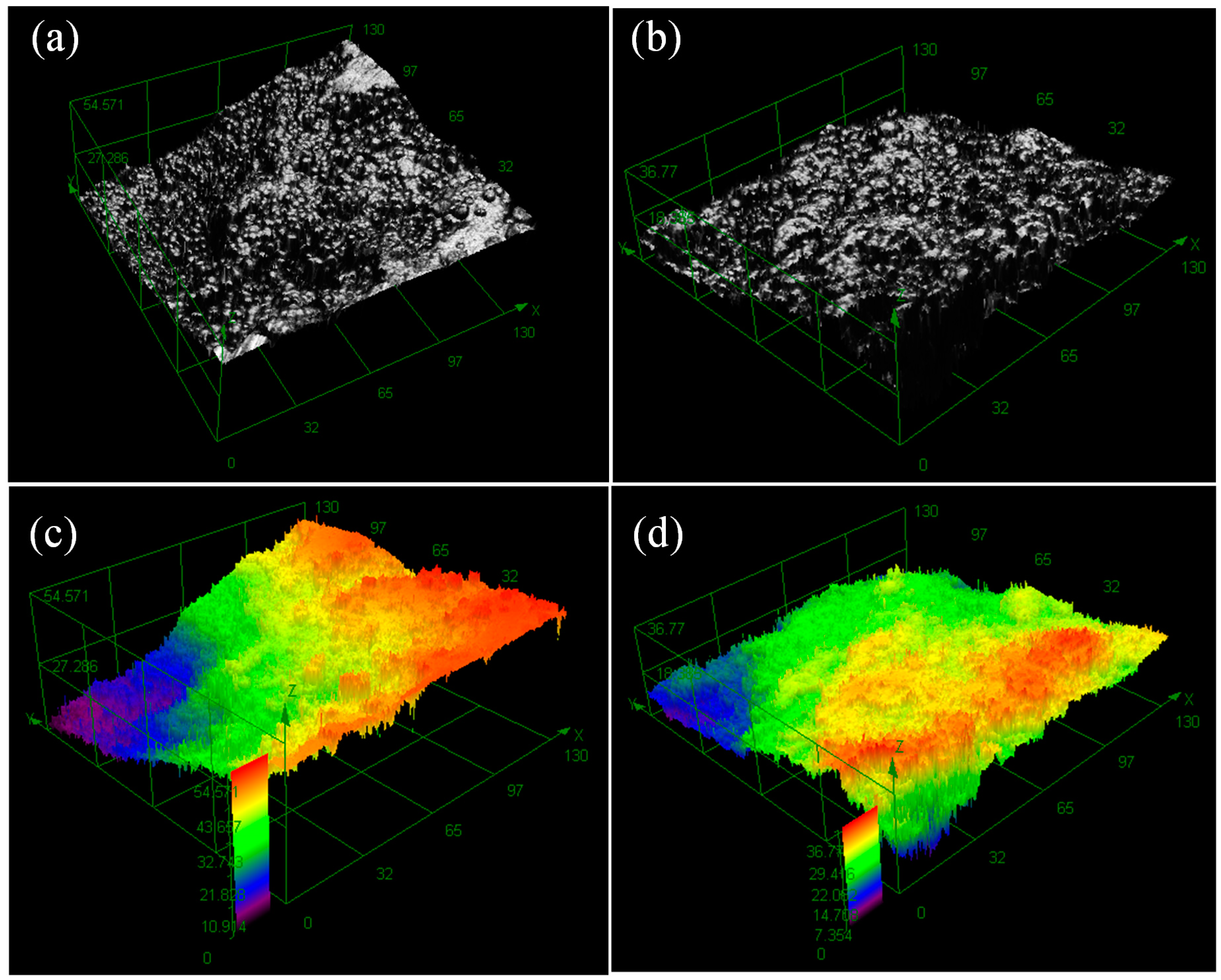
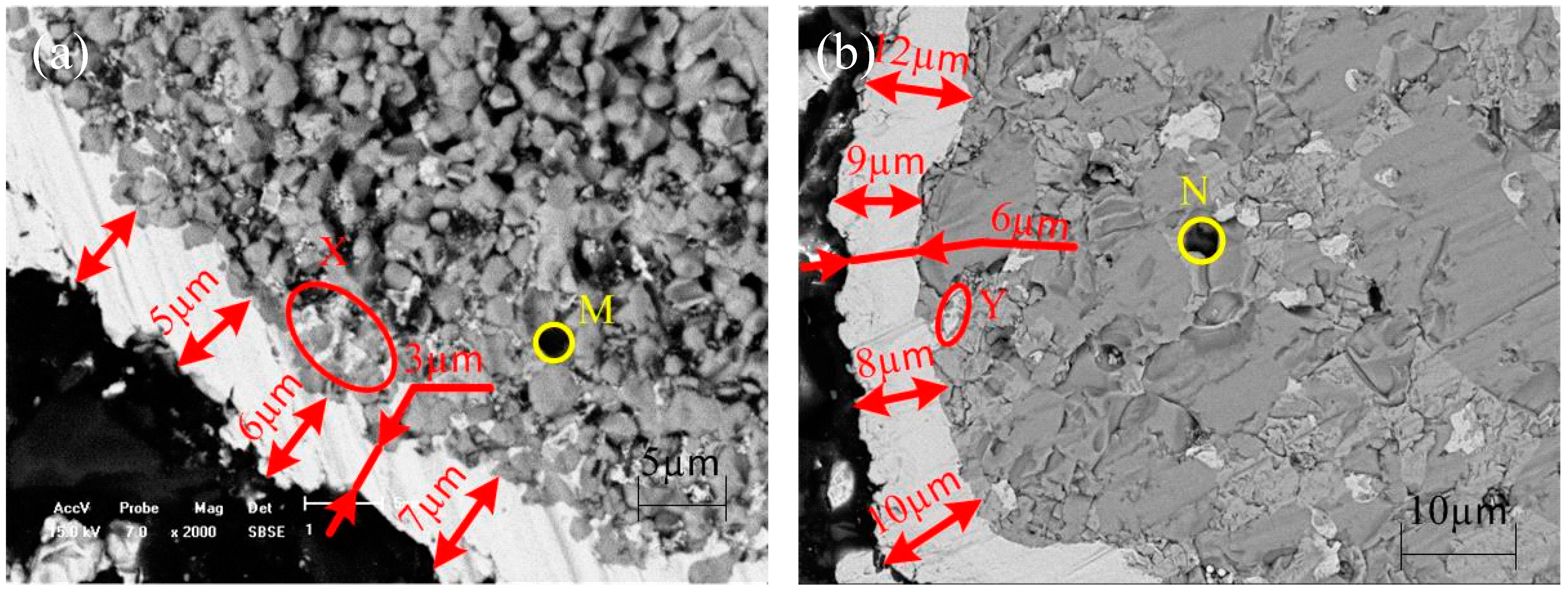
3.2. Electroless Ni-P Plating
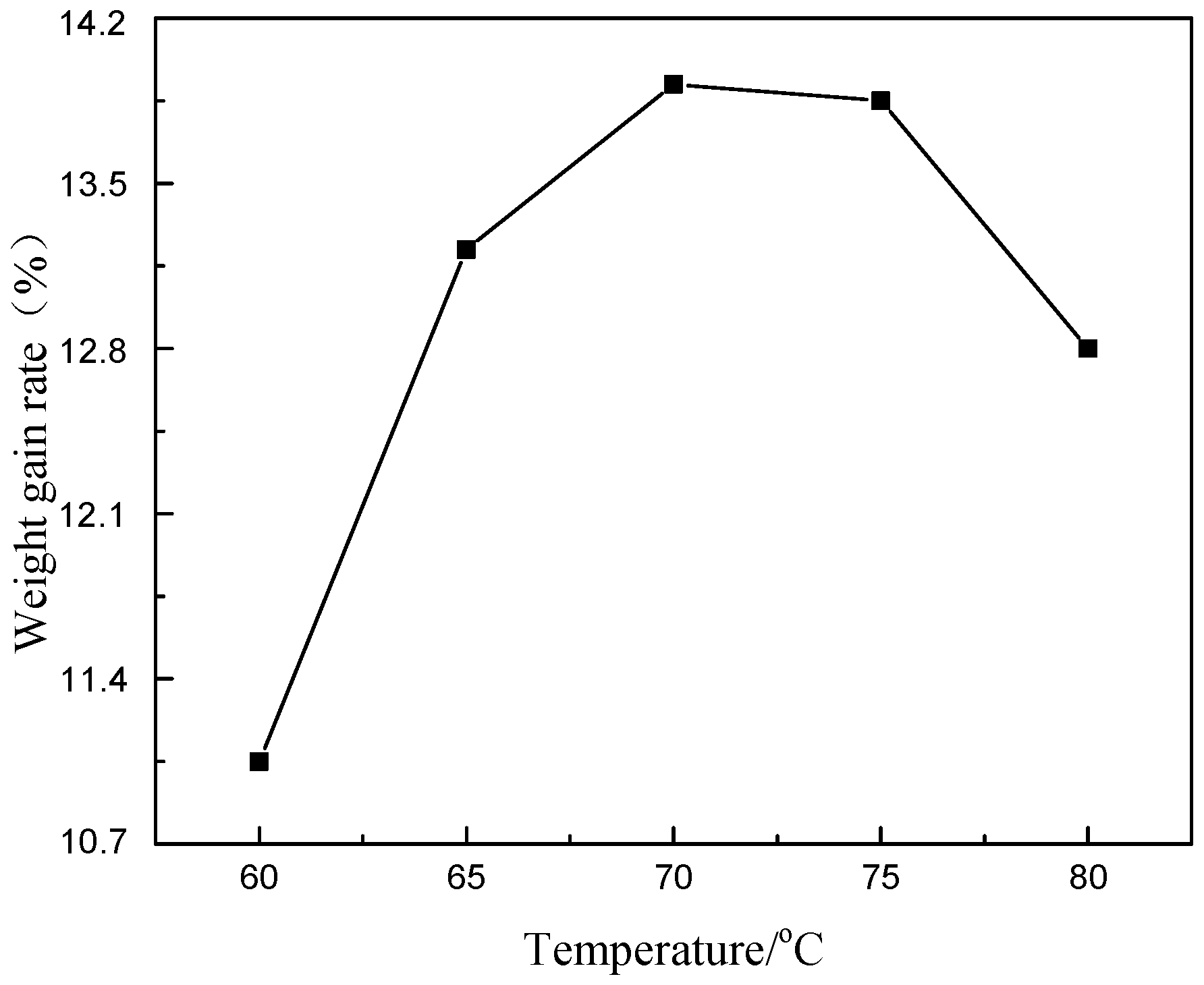
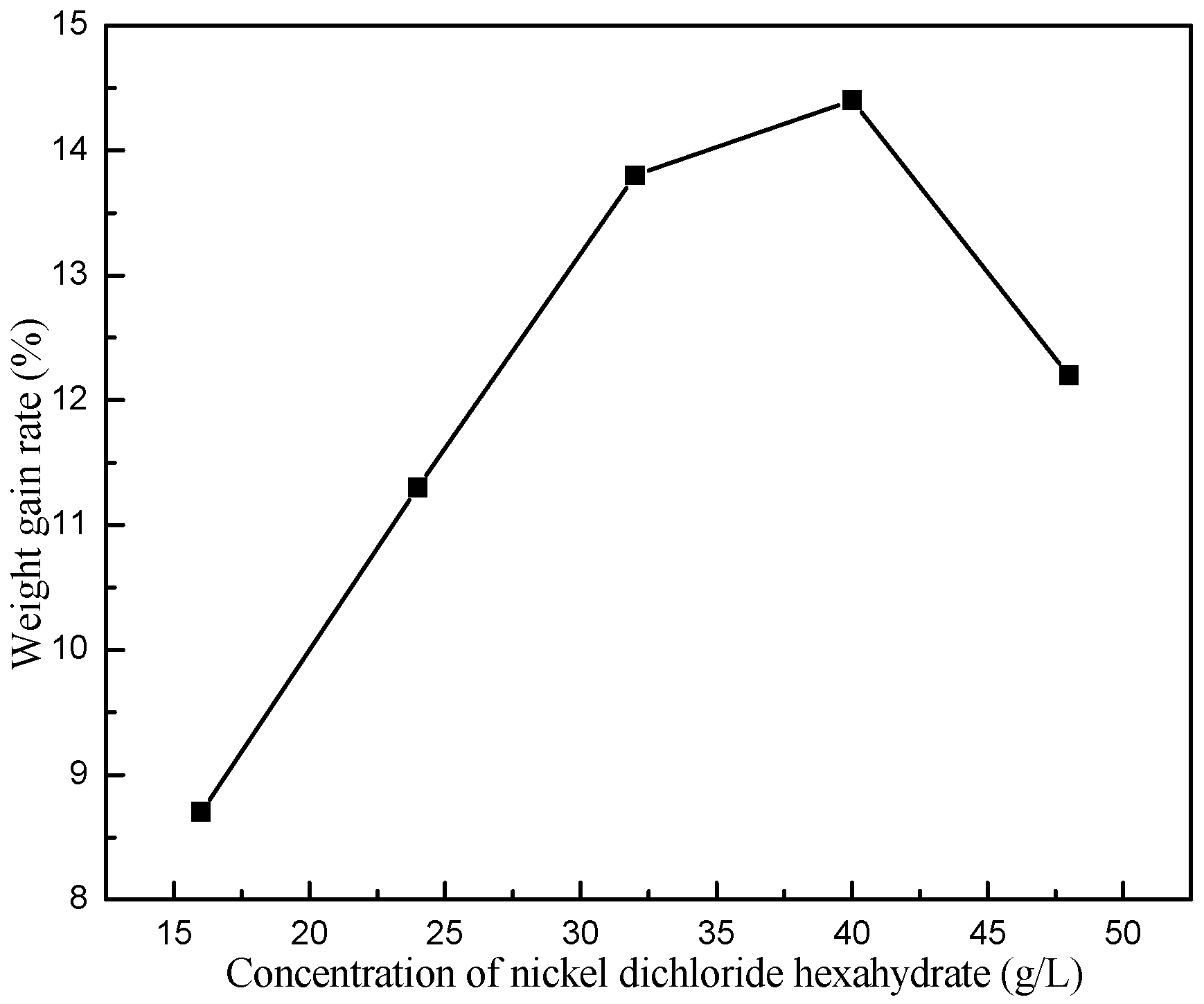
3.3. Influences of Main Factors on Pd-Free Electroless Plating
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sadowy, D.R. Inert anodes for the Hall-Héroult cell: The ultimate materials challenge. JOM 2001, 53, 34–35. [Google Scholar] [CrossRef]
- Glucina, M.; Hyland, M. Laboratory-scale performance of a binary Cu-Al alloy as an anode for aluminiumelectrowinning. Corros. Sci. 2006, 48, 2457–2469. [Google Scholar] [CrossRef]
- Shi, Z.N.; Xu, J.L.; Qiu, Z.X.; Wang, Z.W. Copper-nickel superalloys as inert alloy anodes for aluminum electrolysis. JOM 2003, 55, 63–65. [Google Scholar] [CrossRef]
- Constantin, V. Influence of the operating parameters over the current efficiency and corrosion rate in the Hall-Heroult aluminum cell with tin oxide anode substrate material. Chin. J. Chem. Eng. 2015, 23, 722–726. [Google Scholar] [CrossRef]
- Wang, G.H.; Sun, X.F. Electrochemical behavior of cermet anodes in Na3AlF6–K3AlF6-based low-melting electrolytes for aluminium electrolysis. In Light Metals 2013; Sadler, B., Ed.; Springer: Cham, Switzerland, 2016; pp. 1295–1298. [Google Scholar]
- Li, W.X.; Zhang, G.; Li, J.; Lai, Y.Q. NiFe2O4-based cermet inert anodes for aluminum electrolysis. JOM 2009, 61, 39–43. [Google Scholar] [CrossRef]
- Pawlek, R.P. Inert anodes: An update. In Light Metals 2014; Grandfield, J., Ed.; Springer: Cham, Switzerland, 2016; pp. 1309–1313. [Google Scholar]
- Olsen, E.; Thonstad, J. Nickel ferrite as inert anodes in aluminium electrolysis Part II material performance and long-time testing. J. Appl. Electrochem. 1999, 29, 301–311. [Google Scholar] [CrossRef]
- Xi, J.H.; Xie, Y.J.; Yao, G.C.; Liu, Y.H. Effect of additive on corrosion resistance of NiFe2O4 ceramics as inert anodes. Trans. Nonferrous Met. Soc. China 2008, 18, 356–360. [Google Scholar] [CrossRef]
- He, H.B.; Wang, Y. Corrosion of NiFe2O4–10NiO-based cermet inert anodes for aluminium electrolysis. Trans. Nonferrous Met. Soc. China 2013, 23, 3816–3821. [Google Scholar] [CrossRef]
- Tian, Z.L.; Lai, Y.Q.; Li, J.; Liu, Y.X. Effect of Ni content on corrosion behavior of Ni/(10NiO-90NiFe2O4) cermet inert anode. Trans. Nonferrous Met. Soc. China 2008, 18, 361–365. [Google Scholar] [CrossRef]
- Lai, Y.Q.; Tian, Z.L.; Li, J.; Ye, S.L.; Liu, Y.X. Preliminary testing of NiFe2O4-NiO-Ni cermet as inert anode in Na3AlF6-AlF3 melts. Trans. Nonferrous Met. Soc. China 2006, 16, 654–658. [Google Scholar] [CrossRef]
- Song, J.L.; Chen, W.G.; Dong, L.L.; Wang, J.J.; Deng, N. An electroless plating and planetary ball milling process for mechanical properties enhancement of bulk CNTs/Cu composites. J. Alloys Compd. 2017, 720, 54–62. [Google Scholar] [CrossRef]
- Li, G.J.; Huang, X.X.; Guo, J.K.; Chen, D.M. Ni-coated Al2O3 powders. Ceram. Int. 2002, 28, 623–626. [Google Scholar] [CrossRef]
- He, W.B.; Zhang, B.L.; Zhuang, H.R.; Li, W.L. Preparation and sintering of Ni-coated Si3N4 composite powders. Ceram. Int. 2005, 31, 811–815. [Google Scholar]
- Wang, H.; Jia, J.F.; Song, H.Z.; Hu, X.; Sun, H.W.; Yang, D.L. The preparation of Cu-coated Al2O3 composite powders by electroless plating. Ceram. Int. 2011, 37, 2181–2184. [Google Scholar] [CrossRef]
- Zhang, R.; Gao, L.; Guo, J.K. Preparation and characterization of coated nanoscale Cu-SiCp composite particles. Ceram. Int. 2004, 30, 401–404. [Google Scholar] [CrossRef]
- Vincenzino, V.; Mojtaba, G. Modelling of auto-agglomeration of cohesive powders. Chem. Eng. Res. Des. 2018, 133, 137–141. [Google Scholar]
- Li, S.; Xiong, D.G.; Liu, M.; Bai, S.X.; Zhao, X. Thermophysical properties of SiC/Al composites with three dimensional interpenetrating network structure. Ceram. Int. 2014, 40, 7539–7544. [Google Scholar] [CrossRef]
- Lai, Y.Q.; Zhang, Y.; Tian, Z.L.; Sun, X.G.; Zhang, G.; Li, J. Effect of adding methods of metallic phase on microstructure and thermal shock resistance of Ni/(90NiFe2O4-10NiO) cermets. Trans. Nonferrous Met. Soc. China 2007, 17, 681–685. [Google Scholar] [CrossRef]
- Huang, C.W. Friction Stir Processing of Copper-Coated SiC Particulate-Reinforced Aluminum Matrix Composite. Materials 2018, 11, 599. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.Q.; Cao, M.S.; Yuan, J.; Zhang, L.; Wen, B.; Fang, X.Y. Preparation and microwave absorption properties of basalt fiber/nickel core-shell hetero structures. J. Alloys Compd. 2010, 495, 254–259. [Google Scholar] [CrossRef]
- Zou, G.Z.; Cao, M.S.; Zhang, L.; Li, J.G.; Xu, H.; Chen, Y.J. A nanoscale core-shell of β-SiCp-Ni prepared by electroless plating at lower temperature. Surf. Coat. Technol. 2006, 201, 108–112. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, H.K. Electroless Ni-P metallization on palladium activated polyacrylonitrile (PAN) fiber by using a drying process. Mater. Chem. Phys. 2018, 204, 257–261. [Google Scholar] [CrossRef]
- Luo, L.M.; Lu, Z.L.; Huang, X.M.; Tan, X.Y.; Ding, X.Y.; Cheng, J.G.; Zhu, L.; Wu, Y.C. Electroless copper plating on PC engineering plastic with a novel palladium-free surface activation process. Surf. Coat. Technol. 2014, 251, 69–73. [Google Scholar] [CrossRef]
- Song, D.; Zhou, J.D.; Jiang, W.; Zhang, X.J.; Yan, Y.; Li, F.S. A novel activation for electroless plating on preparing Ni/PS microspheres. Mater. Lett. 2009, 63, 282–284. [Google Scholar] [CrossRef]
- Tang, X.J.; Bi, C.L.; Han, C.X.; Zhang, B.G. A new palladium-free surface activation process for Ni electroless plating on ABS plastic. Mater. Lett. 2009, 63, 840–842. [Google Scholar] [CrossRef]
- Wang, X.; Miao, Z. Environmentally Friendly Copper Metallization of ABS by Cu-Catalysed Electroless Process. Rare Met. Mater. Eng. 2016, 45, 1709–1713. [Google Scholar]
- Li, J.M.; Xue, X.N.; Cai, H.; Jiang, B.L. Preparation and characterization of electroless Ni coating on the surface of MgO with porous structure. Acta Metall. Sin. 2010, 46, 1103–1108. [Google Scholar] [CrossRef]
- Tian, D.; Li, D.Y. A Pd-free activation method for electroless nickel deposition on copper. Surf. Coat. Technol. 2013, 228, 27–33. [Google Scholar] [CrossRef]
- Nobaria, N.; Behboudnia, M. Palladium-free electroless deposition of pure copper film on glass substrate using hydrazine as reducing agent. Appl. Surf. Sci. 2016, 385, 9–17. [Google Scholar] [CrossRef]
- Li, X.L.; Wang, X.B.; Gao, R.; Sun, L. Study of deposition patterns of plating layers in SiC/Cu composites by electro-brush plating. Appl. Surf. Sci. 2011, 257, 10294–10299. [Google Scholar] [CrossRef]
- Malecki, A.; Micek-Ilnicka, A. Electroless nickel plating from acid bath. Surf. Coat. Technol. 2002, 123, 8–13. [Google Scholar]

| Electroless Plating Bath | |||||
|---|---|---|---|---|---|
| Reagents | NiCl2·6H2O | NaH2PO2·H2O | C6H5Na3O7·2H2O | C3H6O3 | CH3COONa |
| Concentration | 16–48 g/L | 28 g/L | 25 g/L | 20 mL/L | 15 g/L |
| Process time | 70 min | ||||
| Temperature | 60–80 °C | ||||
| pH | 7.5–9.5 | ||||
| Stirring speed | 250 rpm | ||||
| Characteristics | NiFe2O4 | Ni-NiFe2O4 |
|---|---|---|
| D10 | 152 μm | 153 μm |
| D50 | 236 μm | 234 μm |
| D90 | 350 μm | 346 μm |
| Specific surface area | 0.008 m2/g | 0.008 m2/g |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Zhang, Z.; Liu, Y.; Zhang, X.; Luo, H.; Yao, G. Pd-Free Activation Pretreatment for Electroless Ni-P Plating on NiFe2O4 Particles. Materials 2018, 11, 1810. https://doi.org/10.3390/ma11101810
Ma J, Zhang Z, Liu Y, Zhang X, Luo H, Yao G. Pd-Free Activation Pretreatment for Electroless Ni-P Plating on NiFe2O4 Particles. Materials. 2018; 11(10):1810. https://doi.org/10.3390/ma11101810
Chicago/Turabian StyleMa, Junfei, Zhigang Zhang, Yihan Liu, Xiao Zhang, Hongjie Luo, and Guangchun Yao. 2018. "Pd-Free Activation Pretreatment for Electroless Ni-P Plating on NiFe2O4 Particles" Materials 11, no. 10: 1810. https://doi.org/10.3390/ma11101810
APA StyleMa, J., Zhang, Z., Liu, Y., Zhang, X., Luo, H., & Yao, G. (2018). Pd-Free Activation Pretreatment for Electroless Ni-P Plating on NiFe2O4 Particles. Materials, 11(10), 1810. https://doi.org/10.3390/ma11101810





