Further Enhancement of Mechanical Properties of Conducting Rubber Composites Based on Multiwalled Carbon Nanotubes and Nitrile Rubber by Solvent Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Dispersion of Carbon Nanotubes
2.3. UV-VIS Measurements
2.4. Casting of NBR/CNT Nanocomposites
2.5. Surface Resistance Measurements
2.6. Thermal Conductivity Measurements
2.7. Stress–Strain Measurements
2.8. Dynamical Mechanical Analysis
3. Results and Discussion
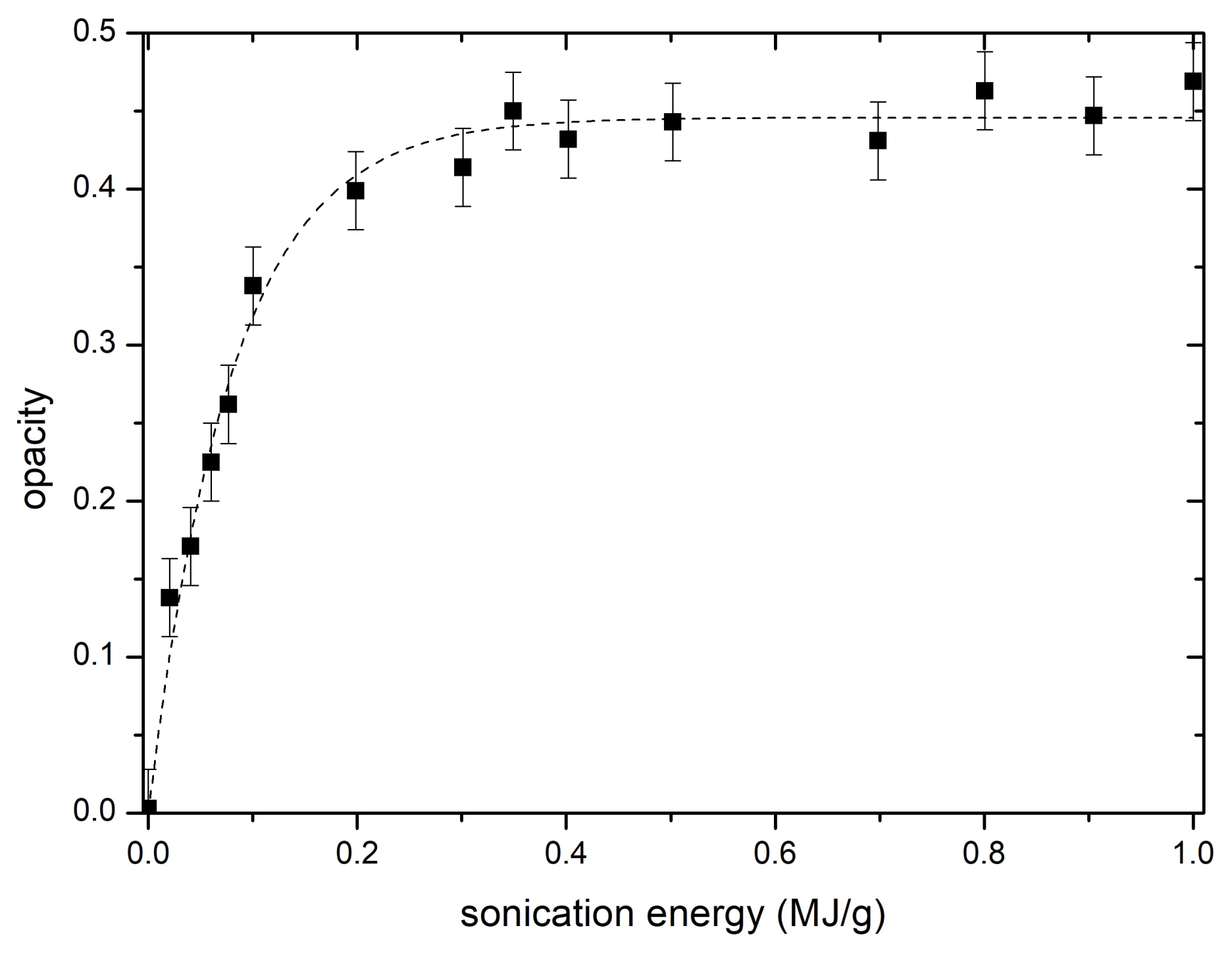
3.1. UV-VIS Measurements
3.2. Acetone Immersion
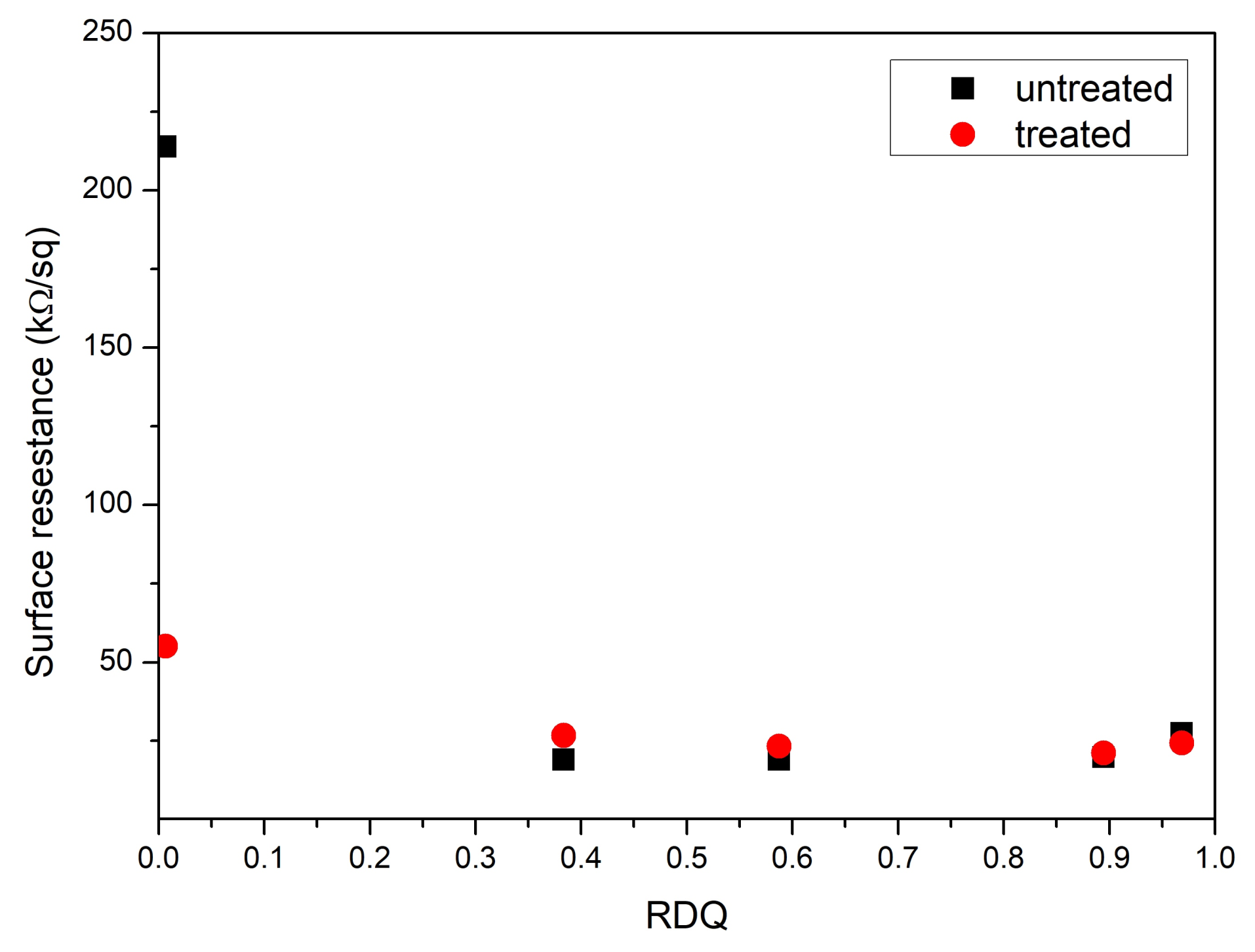
3.3. Surface Resistance Measurements
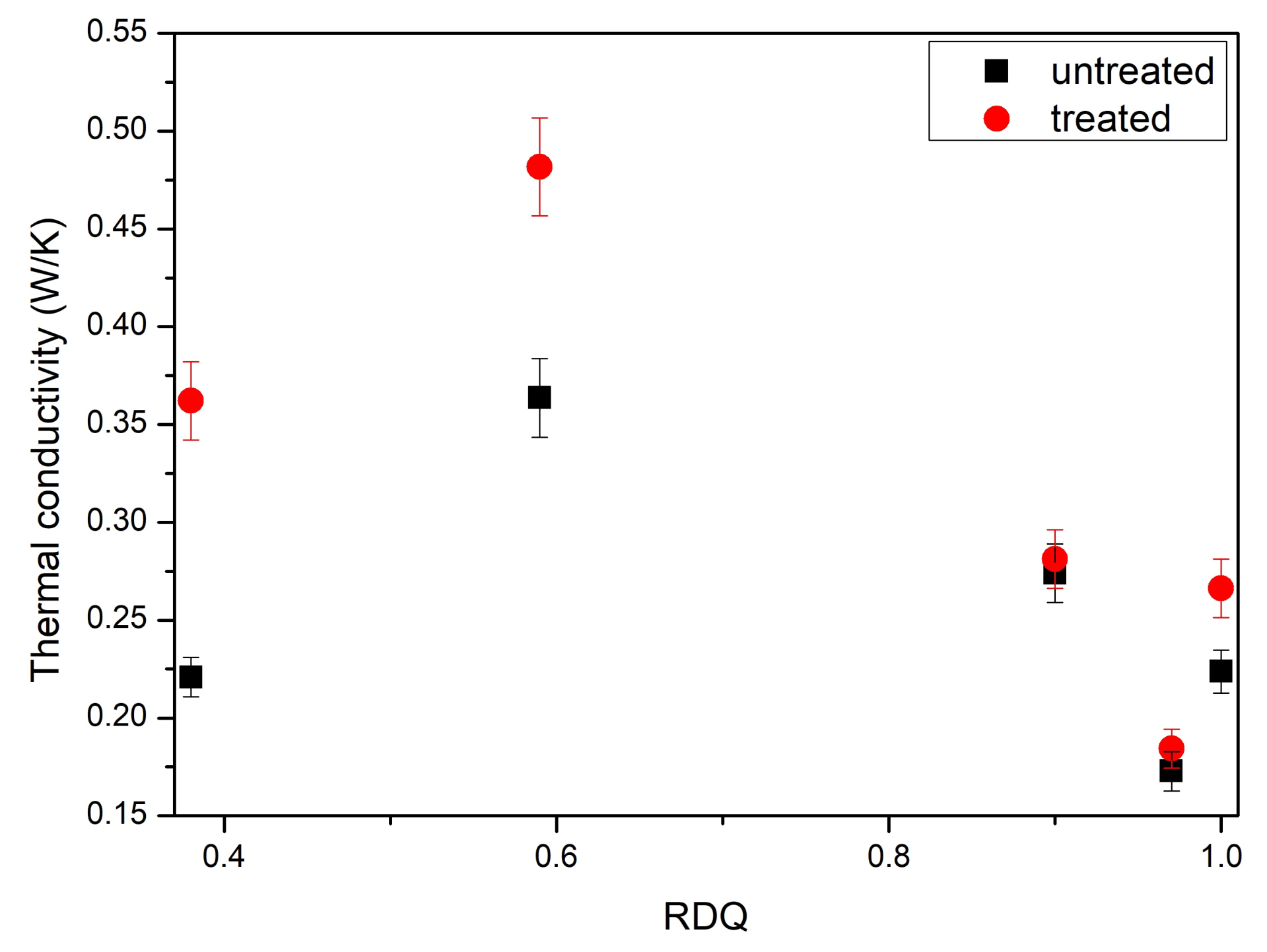
3.4. Thermal Conductivity Measurements
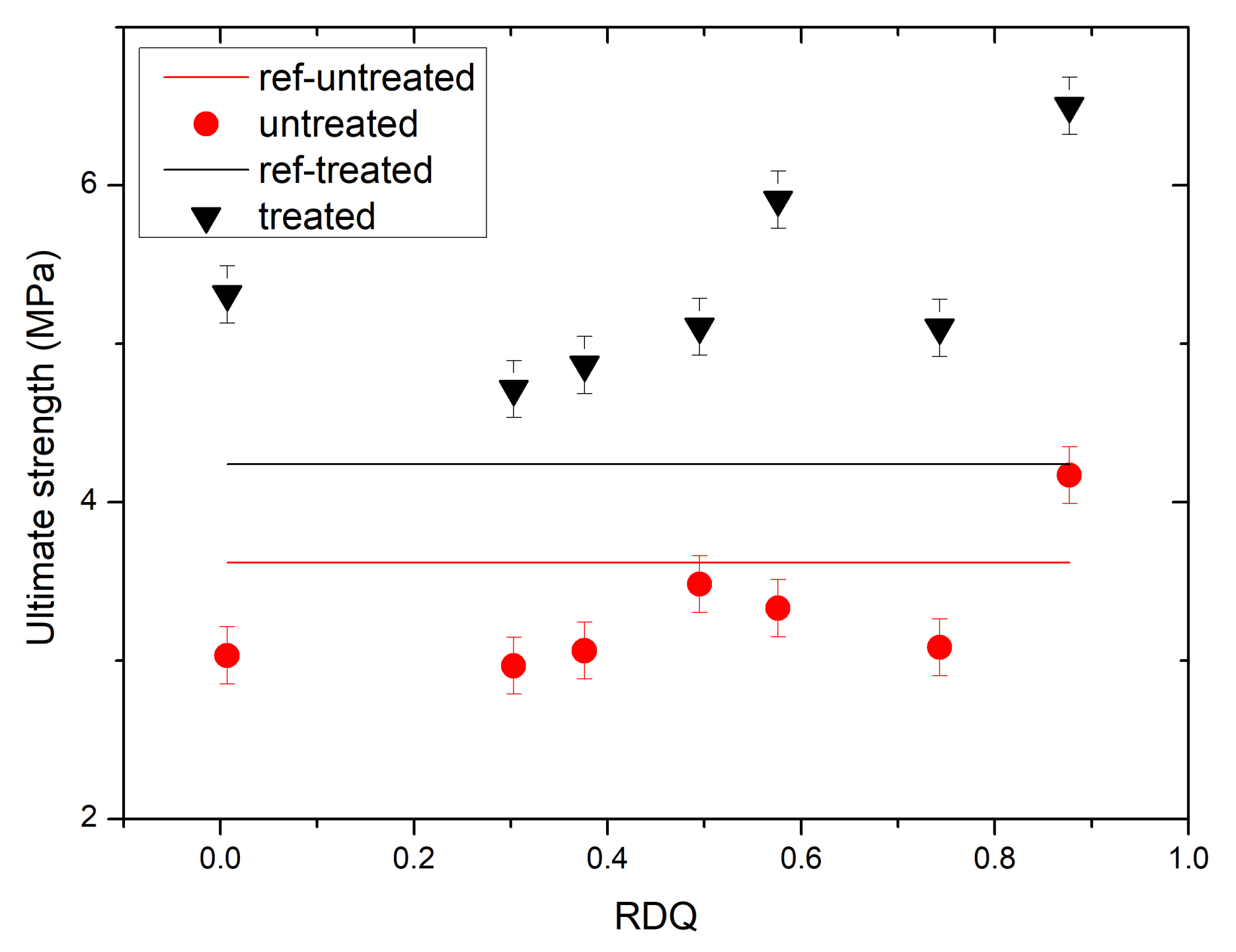
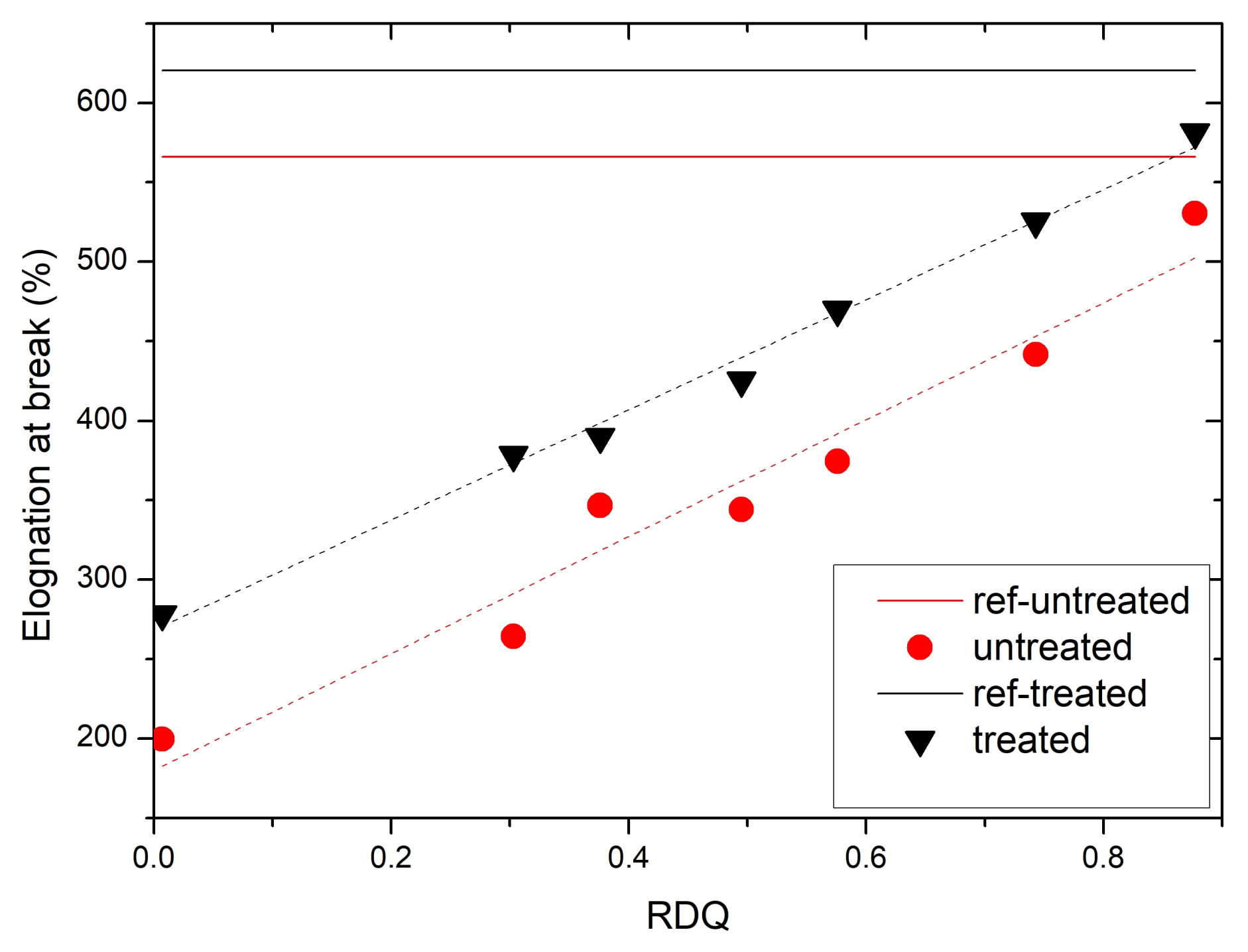
3.5. Stress–Strain
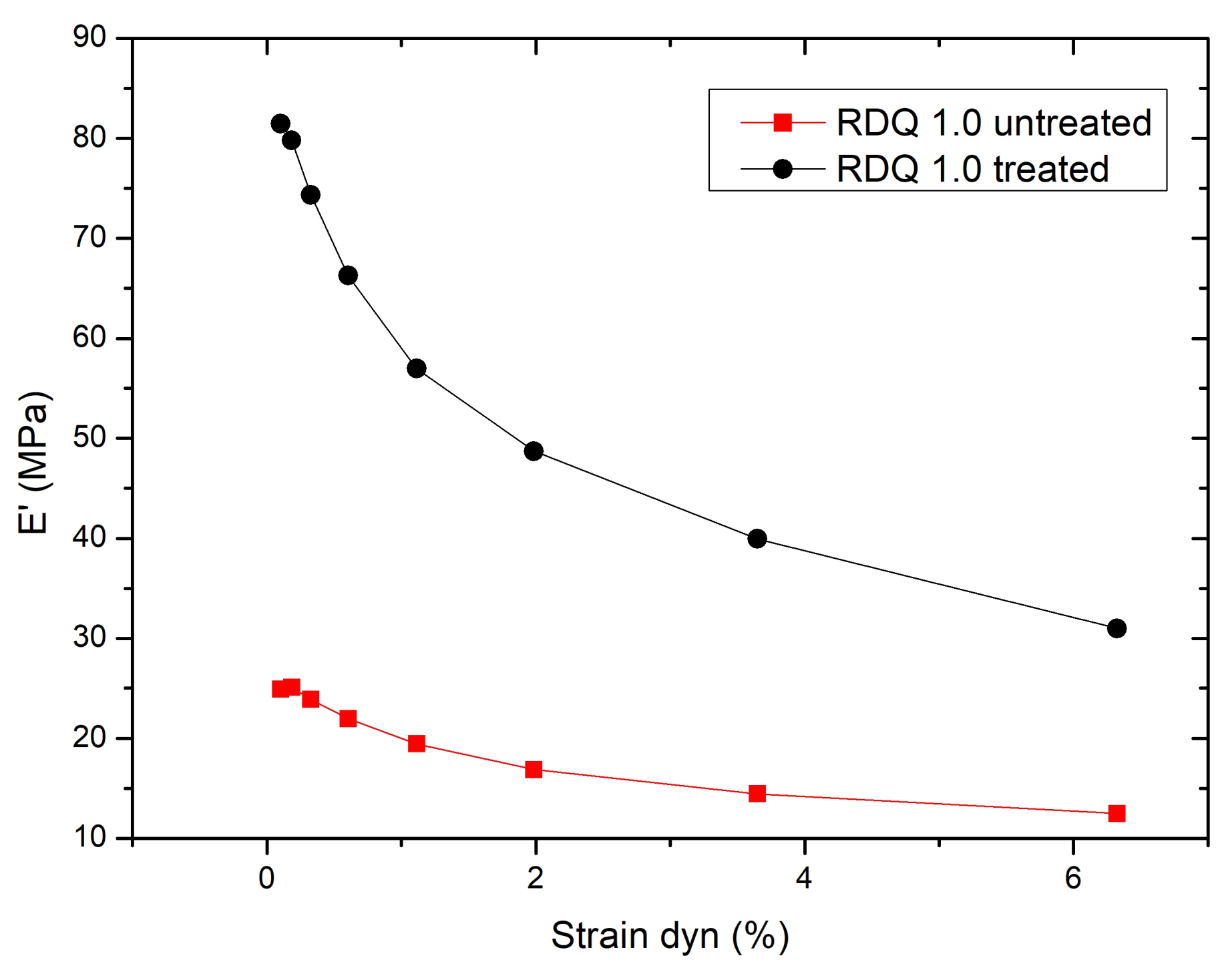
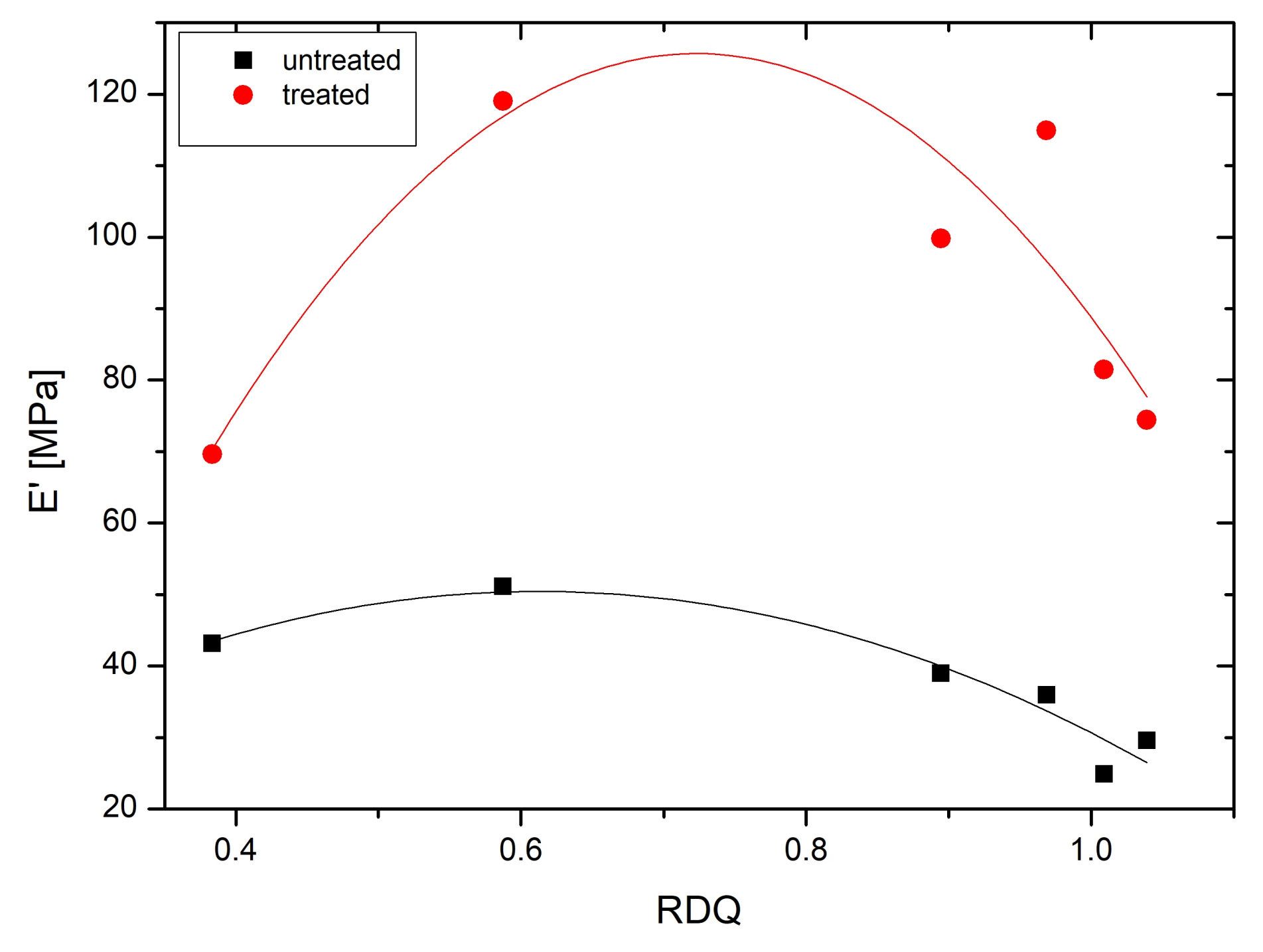
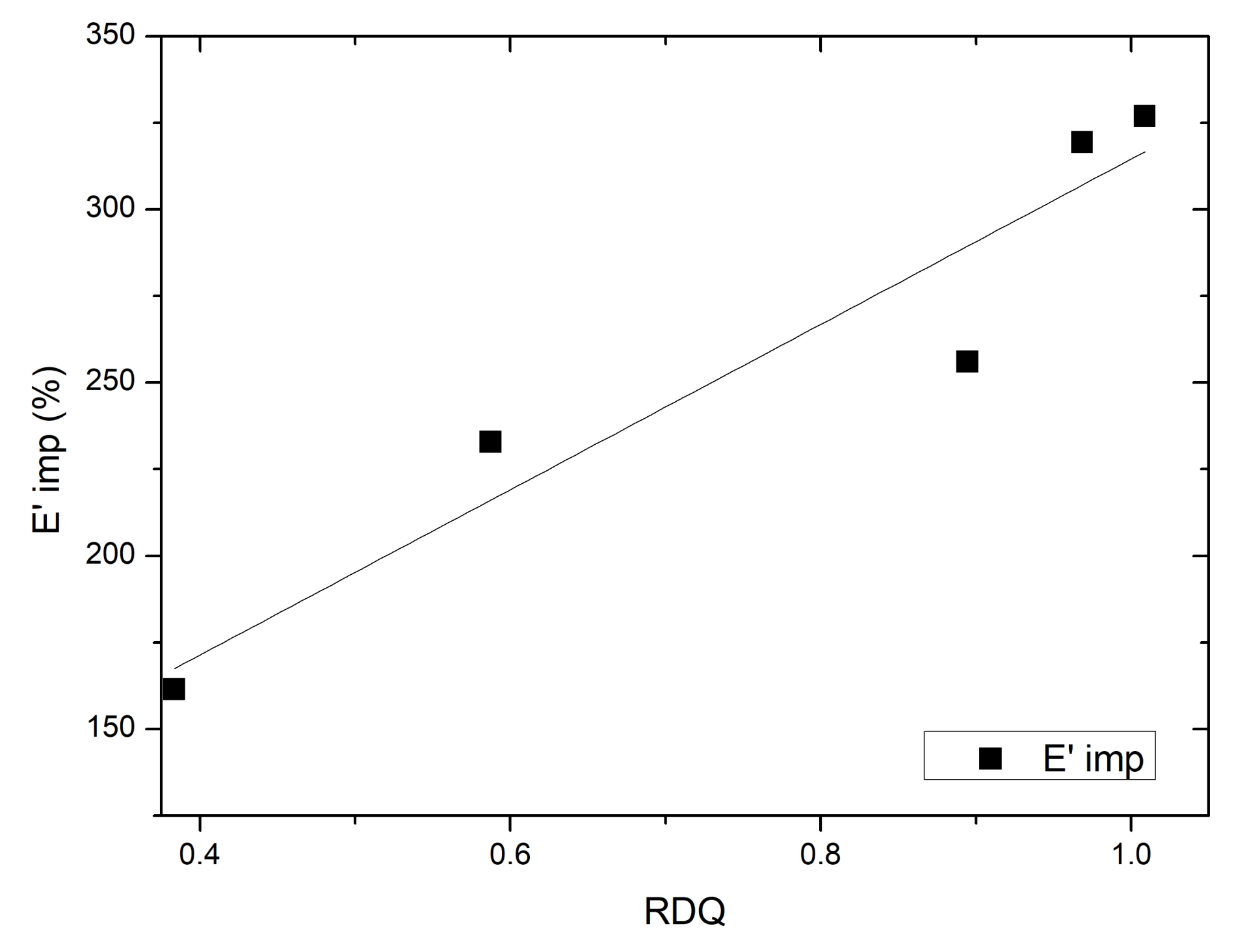
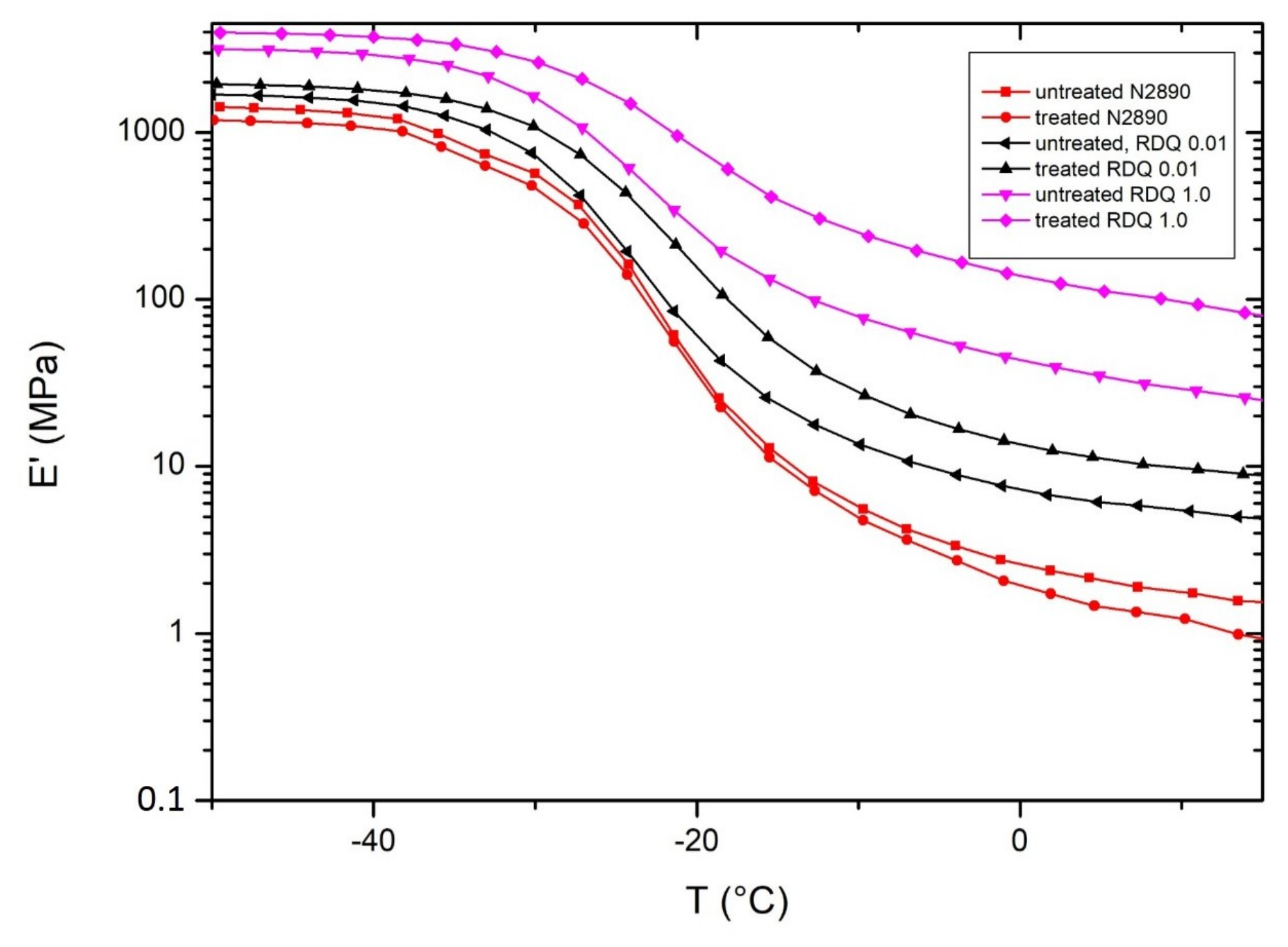
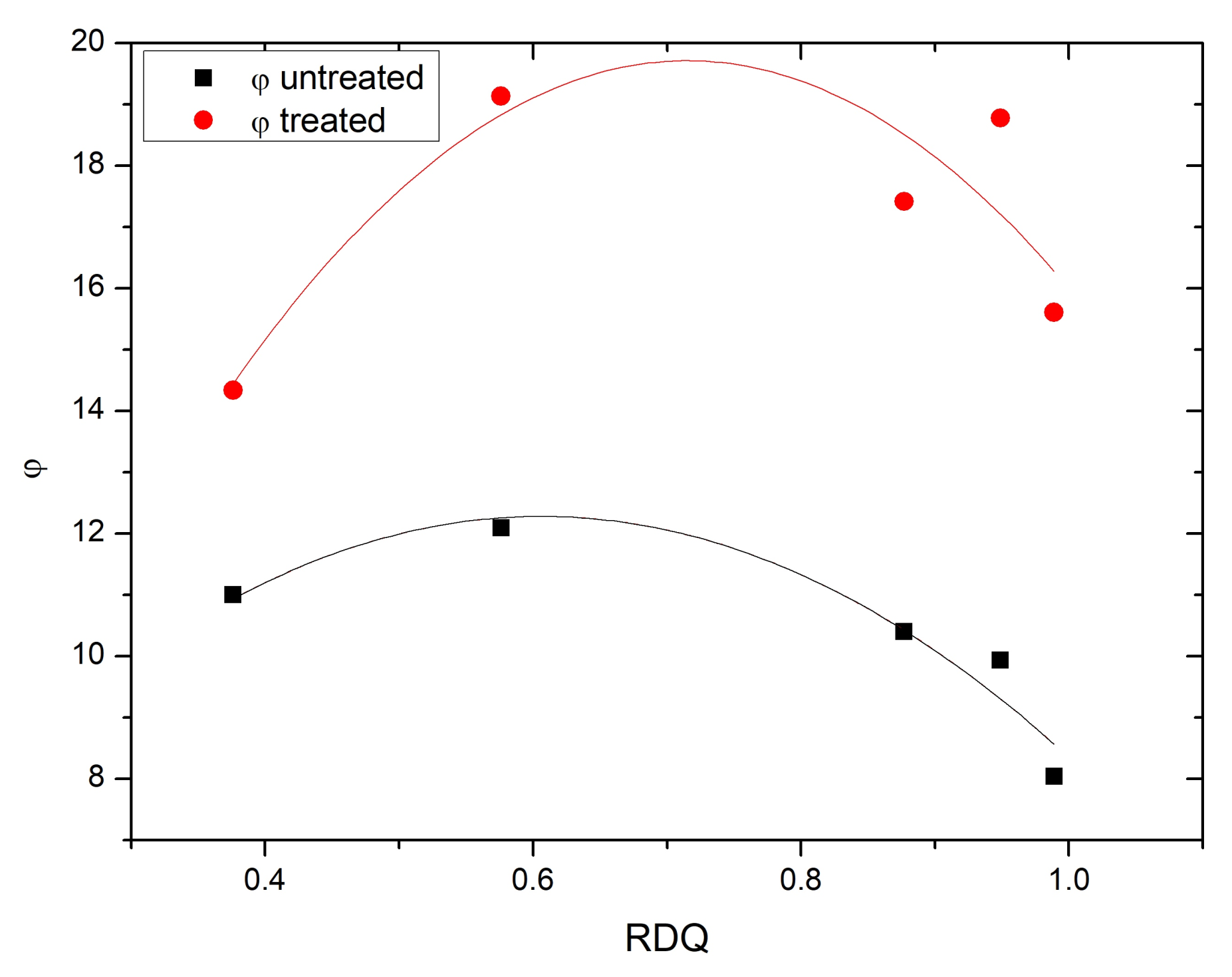
3.6. Dynamical Mechanical Analysis
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- McCarthy, D.; Mark, J.; Schaefer, D. Synthesis, structure, and properties of hybrid organic–inorganic composites based on polysiloxanes. I. Poly (dimethylsiloxane) elastomers containing silica. J. Polym. Sci. Part B Polym. Phys. 1998, 36, 1167–1189. [Google Scholar] [CrossRef]
- Joly, S.; Garnaud, G.; Ollitrault, R.; Bokobza, L.; Mark, J. Organically modified layered silicates as reinforcing fillers for natural rubber. Chem. Mater. 2002, 14, 4202–4208. [Google Scholar] [CrossRef]
- Frogley, M.D.; Ravich, D.; Wagner, H.D. Mechanical properties of carbon nanoparticle-reinforced elastomers. Compos. Sci. Technol. 2003, 63, 1647–1654. [Google Scholar] [CrossRef]
- Andrews, R.; Jacques, D.; Minot, M.; Rantell, T. Fabrication of carbon multiwall nanotube/polymer composites by shear mixing. Macromol. Mater. Eng. 2002, 287, 395–403. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Terentjev, E.M. Dispersion of carbon nanotubes: Mixing, sonication, stabilization, and composite properties. Polymers 2012, 4, 275–295. [Google Scholar] [CrossRef]
- Sethi, J.; Sarlin, E.; Meysami, S.S.; Suihkonen, R.; Kumar, A.R.S.S.; Honkanen, M.; Keinänen, P.; Grobert, N.; Vuorinen, J. The effect of multi-wall carbon nanotube morphology on electrical and mechanical properties of polyurethane nanocomposites. Compos. Part A Appl. Sci. Manuf. 2017, 102, 305–313. [Google Scholar] [CrossRef]
- Lavilla, I.; Bendicho, C. Fundamentals of Ultrasound-Assisted Extraction. In Water Extraction of Bioactive Compounds; Elsevier: New York, NY, USA, 2018; pp. 291–316. [Google Scholar]
- Yang, D.Q.; Rochette, J.F.; Sacher, E. Functionalization of multiwalled carbon nanotubes by mild aqueous sonication. J. Phys. Chem. B 2005, 109, 7788–7794. [Google Scholar] [CrossRef] [PubMed]
- Shahshahan, M.; Keinänen, P.; Vuorinen, J. The effect of ultrasonic dispersion on the surface chemistry of carbon nanotubes in the Jeffamine D-230 polyetheramine medium. IEEE Trans. Nanotechnol. 2017, 16, 741–744. [Google Scholar] [CrossRef]
- Liu, J.; Rinzler, A.G.; Dai, H.; Hafner, J.H.; Bradley, R.K.; Boul, P.J.; Lu, A.; Iverson, T.; Shelimov, K.; Huffman, C.B.; et al. Fullerene pipes. Science 1998, 280, 1253–1256. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.; Rojas, E.; Bergey, D.; Johnson, A.; Yodh, A. High weight fraction surfactant solubilization of single-wall carbon nanotubes in water. Nano Lett. 2003, 3, 269–273. [Google Scholar] [CrossRef]
- Geng, H.Z.; Kim, K.K.; So, K.P.; Lee, Y.S.; Chang, Y.; Lee, Y.H. Effect of acid treatment on carbon nanotube-based flexible transparent conducting films. J. Am. Chem. Soc. 2007, 129, 7758–7759. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, J.; Gao, L.; Wang, Y.; Zhang, J.; Kajiura, H.; Li, Y.; Noda, K. Removal of the residual surfactants in transparent and conductive single-walled carbon nanotube films. J. Phys. Chem. C 2009, 113, 17685–17690. [Google Scholar] [CrossRef]
- Siljander, S.; Keinänen, P.; Räty, A.; Ramakrishnan, K.R.; Tuukkanen, S.; Kunnari, V.; Harlin, A.; Vuorinen, J.; Kanerva, M. Effect of Surfactant Type and Sonication Energy on the Electrical Conductivity Properties of Nanocellulose-CNT Nanocomposite Films. Int. J. Mol. Sci. 2018, 19, 1819. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Chen, Z.; Du, X.; Logan, J.M.; Sippel, J.; Nikolou, M.; Kamaras, K.; Reynolds, J.R.; Tanner, D.B.; Hebard, A.F.; et al. Transparent, conductive carbon nanotube films. Science 2004, 305, 1273–1276. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.D.; Subramanian, S.; Krishnamoorti, R. Understanding surfactant aided aqueous dispersion of multi-walled carbon nanotubes. J. Colloid Interface Sci. 2011, 354, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Kasaliwal, G.R.; Jurk, R.; Boldt, R.; Fischer, D.; Stöckelhuber, K.W.; Heinrich, G. Rubber composites based on graphene nanoplatelets, expanded graphite, carbon nanotubes and their combination: A comparative study. Compos. Sci. Technol. 2012, 72, 1961–1967. [Google Scholar] [CrossRef]
- Keinänen, P.; Siljander, S.; Koivula, M.; Sethi, J.; Sarlin, E.; Vuorinen, J.; Kanerva, M. Optimized dispersion quality of aqueous carbon nanotube colloids as a function of sonochemical yield and surfactant/CNT ratio. Heliyon 2018, 4, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, R. Influence of Temperature on the Tensile Strength of Carbon Filled Vulcanizates. Rubber Chem. Technol. 1971, 44, 728–743. [Google Scholar] [CrossRef]

| Property | Value | Unit | Method |
|---|---|---|---|
| Solids content | 41.0 | % | ISO 124 |
| pH | 7.3 | - | ISO 976 |
| Viscosity | <100 | mPas | ISO 1652 |
| Surface tension | 31.9 | mNm | DIN 1409 |
| Property | Value | Unit | Method |
|---|---|---|---|
| Average diameter | 10 | nm | TEM |
| Average length | 1.5 | TEM | |
| Carbon purity | 90 | % | TGA |
| Transitional metal oxide | <1 | % | ICP-MS |
| Surface area | 250–300 | mg | BET |
| RDQ | Mass of the Film (g) | Mass Difference From Acetone Treatment (g) |
|---|---|---|
| 0.0 | 1.36 | 0.48 |
| 0.4 | 2.06 | 0.60 |
| 0.6 | 1.82 | 0.51 |
| 0.9 | 1.70 | 0.51 |
| 1.0 | 1.89 | 0.53 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keinänen, P.; Das, A.; Vuorinen, J. Further Enhancement of Mechanical Properties of Conducting Rubber Composites Based on Multiwalled Carbon Nanotubes and Nitrile Rubber by Solvent Treatment. Materials 2018, 11, 1806. https://doi.org/10.3390/ma11101806
Keinänen P, Das A, Vuorinen J. Further Enhancement of Mechanical Properties of Conducting Rubber Composites Based on Multiwalled Carbon Nanotubes and Nitrile Rubber by Solvent Treatment. Materials. 2018; 11(10):1806. https://doi.org/10.3390/ma11101806
Chicago/Turabian StyleKeinänen, Pasi, Amit Das, and Jyrki Vuorinen. 2018. "Further Enhancement of Mechanical Properties of Conducting Rubber Composites Based on Multiwalled Carbon Nanotubes and Nitrile Rubber by Solvent Treatment" Materials 11, no. 10: 1806. https://doi.org/10.3390/ma11101806
APA StyleKeinänen, P., Das, A., & Vuorinen, J. (2018). Further Enhancement of Mechanical Properties of Conducting Rubber Composites Based on Multiwalled Carbon Nanotubes and Nitrile Rubber by Solvent Treatment. Materials, 11(10), 1806. https://doi.org/10.3390/ma11101806





