High-Temperature Raman Spectroscopy of Nano-Crystalline Carbon in Silicon Oxycarbide
Abstract
1. Introduction
2. Materials and Methods
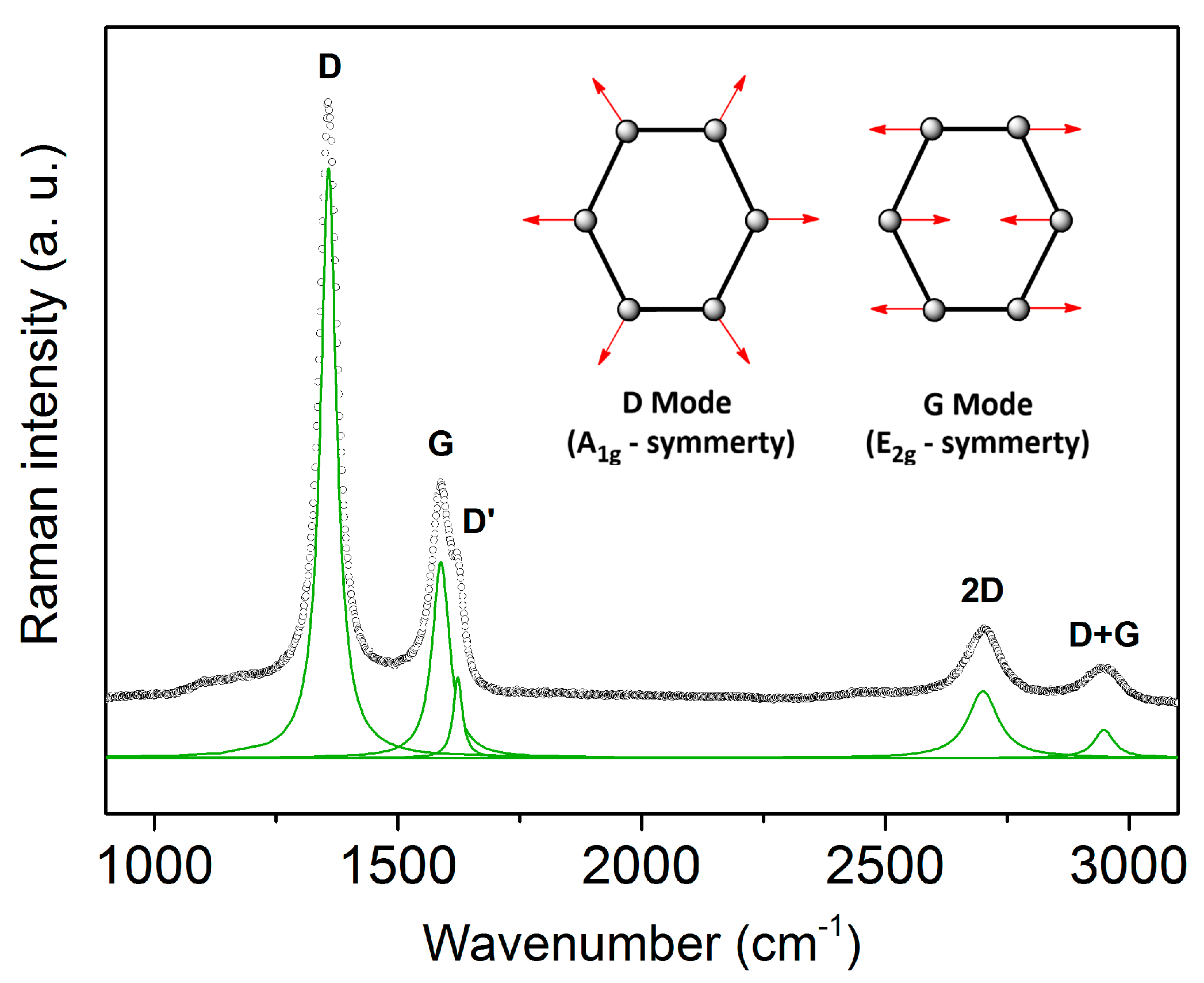
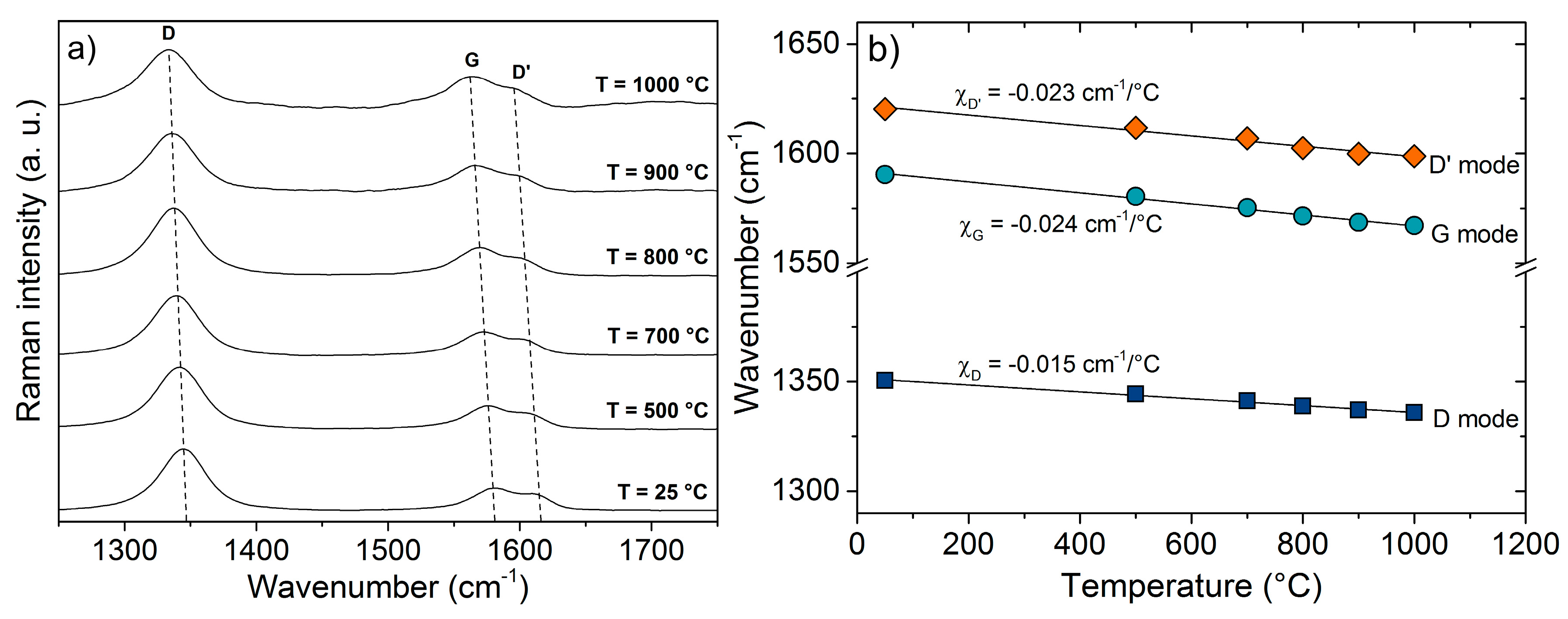
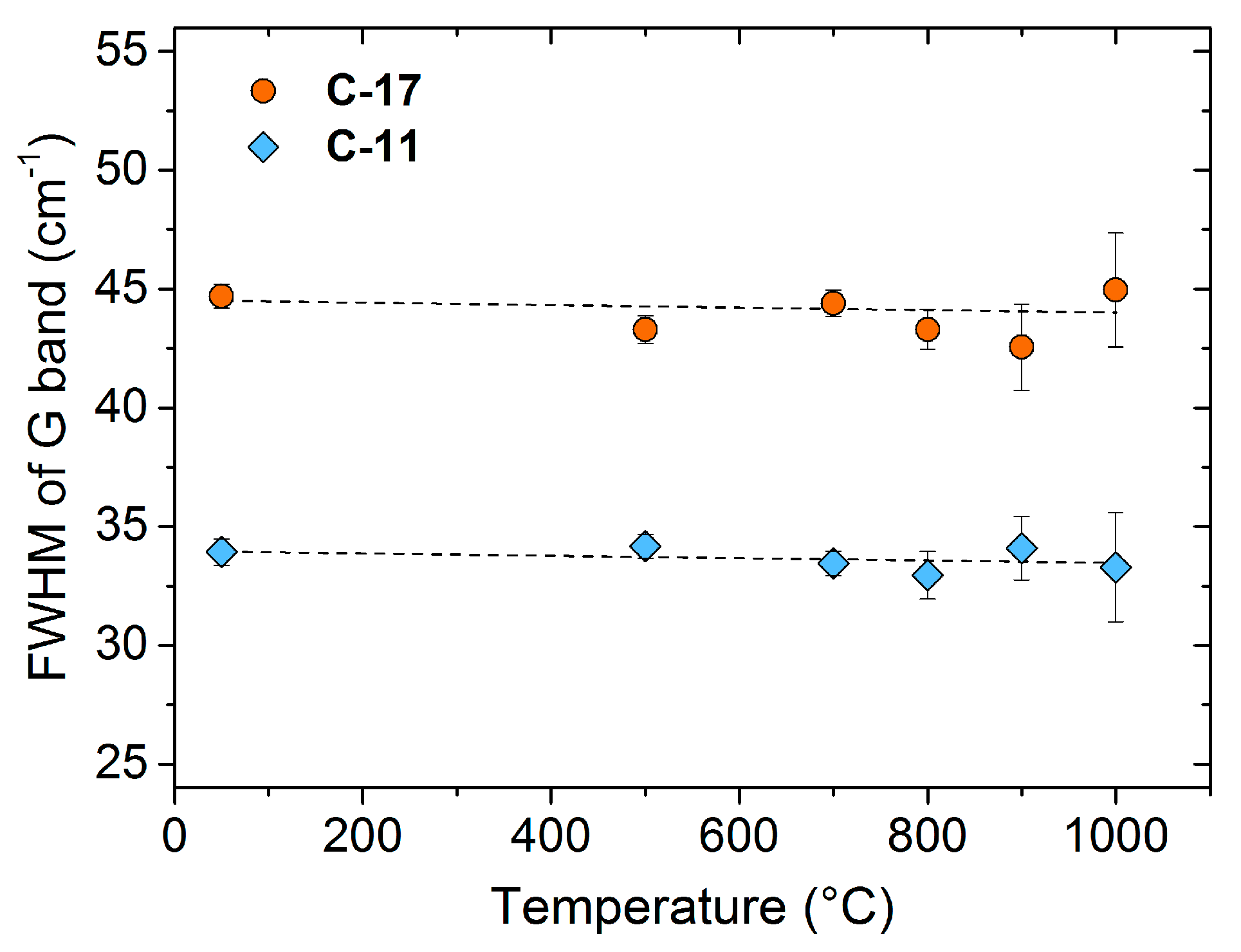
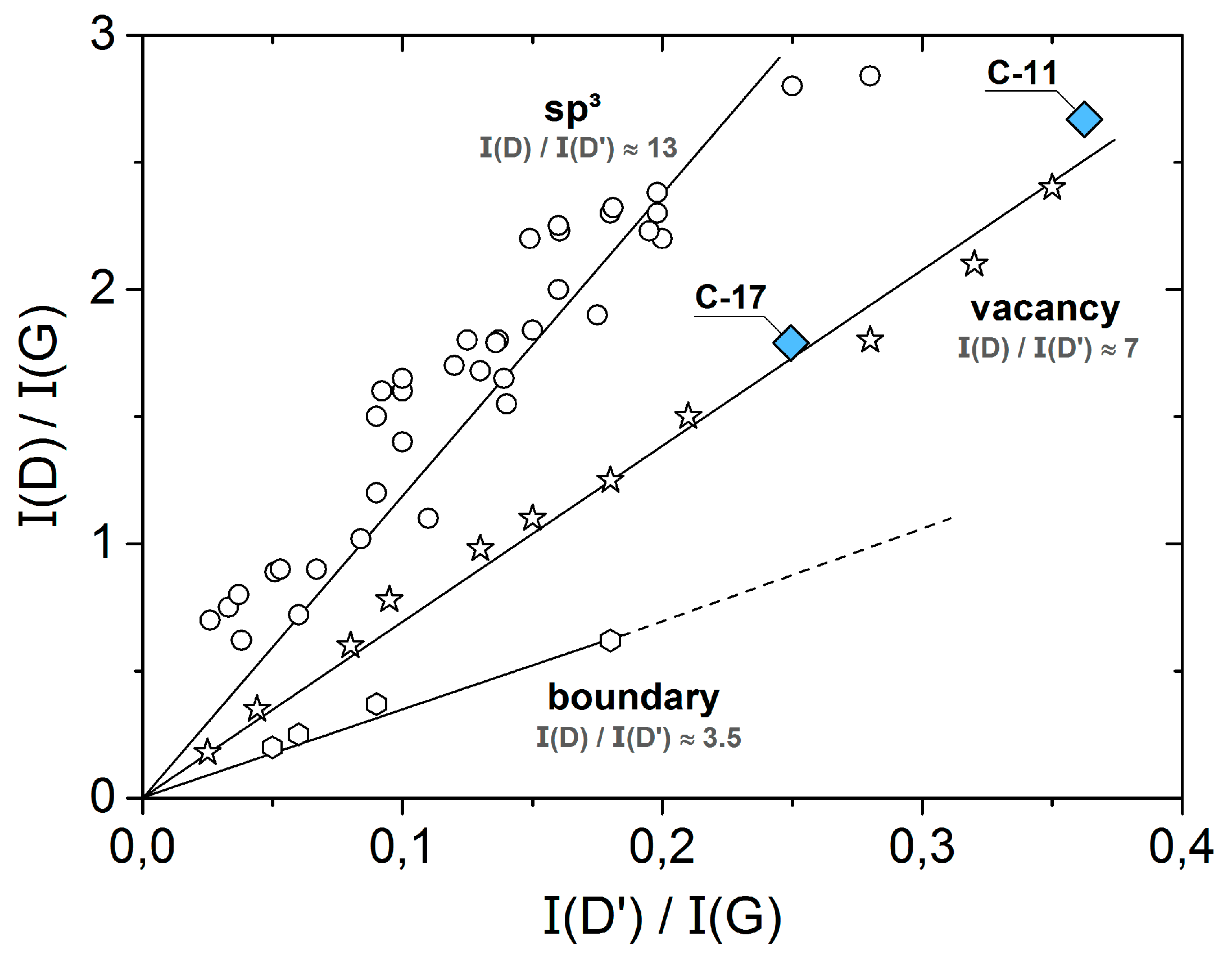
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron-phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Tuinstra, F.; Koenig, J.L. Raman Spectrum of Graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef]
- Tessonnier, J.-P.; Rosenthal, D.; Hansen, T.W.; Hess, C.; Schuster, M.E.; Blume, R.; Girgsdies, F.; Pfänder, N.; Timpe, O.; ShengSu, D. Analysis of the structure and chemical properties of some commercial carbon nanostructures. Carbon 2009, 47, 1779–1798. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Dresselhaus, G.; Jorio, A.; Filho, A.G.S.; Saitof, R. Raman spectroscopy on isolated single wall carbon nanotubes. Carbon 2002, 40, 2043–2061. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Dresselhaus, G.; Saito, R.; Joriod, A. Raman spectroscopy of carbon nanotubes. Phys. Rep. 2005, 409, 47–99. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Basko, D.M. Raman spectroscopy as a versatile tool for studying the properties of graphene. Nat. Nanotechnol. 2013, 8, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Malard, L.M.; Pimenta, M.A.; Dresselhaus, G.; Dresselhauscd, M.S. Raman spectroscopy in graphene. Phys. Rep. 2009, 473, 51–87. [Google Scholar] [CrossRef]
- Schwan, J.; Ulrich, S.; Batori, V.; Ehrhardt, H. Raman spectroscopy on amorphous carbon films. J. Appl. Phys. 1996, 80, 440. [Google Scholar] [CrossRef]
- Tamor, M.A.; Vassell, W.C. Raman “fingerprinting” of amorphous carbon films. J. Appl. Phys. 1994, 76, 3823. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Rodil, S.E.; Robertson, J.; Milne, W.I. Is stress necessary to stabilise sp3 bonding in diamond-like carbon? Diam. Relat. Mater. 2002, 11, 994–999. [Google Scholar] [CrossRef]
- Irmer, G.; Dorner-Reisel, A. Micro-Raman Studies on DLC coatings. Adv. Eng. Mater. 2005, 7, 694–705. [Google Scholar] [CrossRef]
- Calizo, I.; Balandin, A.A.; Bao, W.; Miao, F.; Lau, C.N. Temperature dependence of the Raman spectra of graphene and graphene multilayers. Nano Lett. 2007, 7, 2645–2649. [Google Scholar] [CrossRef] [PubMed]
- Colombo, P.; Mera, G.; Riedel, R.; Soraru, G.D. Polymer-Derived Ceramics: 40 Years of Research and Innovation in Advanced Ceramics. J. Am. Ceram. Soc. 2010, 93, 1805–1837. [Google Scholar] [CrossRef]
- Mera, G.; Navrotsky, A.; Sen, S.; Kleebe, H.; Riedel, R. Polymer-derived SiCN and SiOC ceramics—Structure and energetics at the nanoscale. J. Mater. Chem. A 2013, 1, 3826–3836. [Google Scholar] [CrossRef]
- Linck, C.; Ionescu, E.; Papendorf, B.; Galuskova, D.; Galusek, D.; Ŝajgalík, P.; Riedel, R. Corrosion behavior of silicon oxycarbide-based ceramic nanocomposites under hydrothermal conditions. Int. J. Mater. Res. 2012, 103, 31–39. [Google Scholar] [CrossRef]
- Modena, S.; Soraru, G.D.; Blum, Y.; Raj, R. Passive Oxidation of an Effluent System: The Case of Polymer-Derived SiCO. J. Am. Ceram. Soc. 2005, 88, 339–345. [Google Scholar] [CrossRef]
- Ionescu, E.; Balan, C.; Kleebe, H.J.; Raj, R.; Müller, M.M.; Guillon, O.; Schliephake, D.; Heilmaier, M. High-Temperature Creep Behavior of SiOC Glass-Ceramics: Influence of Network Carbon Versus Segregated Carbon. J. Am. Ceram. Soc. 2014, 97, 3935–3942. [Google Scholar] [CrossRef]
- Roth, F.; Schmerbauch, C.; Ionescu, E.; Raj, R.; Nicoloso, N.; Guillon, O. High-temperature piezoresistive C/SiOC sensors. J. Sens. Sens. Syst. 2015, 4, 133–136. [Google Scholar] [CrossRef]
- Riedel, R.; Toma, L.; Janssen, E.; Hanselka, H.; Nuffer, J.; Melz, T. Piezoresistive Effect in SiOC Ceramics for Integrated Pressure Sensors. J. Am. Ceram. Soc. 2010, 93, 920–924. [Google Scholar] [CrossRef]
- Graczyk-Zajac, M.; Reinold, L.M.; Kaspar, J.; Sasikumar, P.V.; Soraru, G.D.; Riedel, R. New Insights into Understanding Irreversible and Reversible Lithium Storage within SiOC and SiCN Ceramics. Nanomaterials 2015, 5, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Roth, F.; Waleska, P.; Hess, C.; Ionescu, E.; Nicoloso, N. UV Raman spectroscopy of segregated carbon in silicon oxycarbides. J. Ceram. Soc. Jpn. 2016, 124, 1042–1045. [Google Scholar] [CrossRef]
- Cordelair, J.; Greil, P. Electrical conductivity measurements as a microprobe for structure transitions in polysiloxane derived Si–O–C ceramics. J. Eur. Ceram. Soc. 2000, 20, 1947–1957. [Google Scholar] [CrossRef]
- Montagnac, G.; Caracas, R.; Bobocioiu, E.; Vittozb, F.; Reynarda, B. Anharmonicity of graphite from UV Raman spectroscopy to 2700 K. Carbon 2013, 54, 68–75. [Google Scholar] [CrossRef]
- Linas, S.; Magnin, Y.; Poinsot, B.; Boisron, O.; Förster, G.D.; Martinez, V.; Fulcrand, R.; Tournus, F.; Dupuis, V.; Rabilloud, F.; et al. Interplay between Raman shift and thermal expansion in graphene: Temperature-dependent measurements and analysis of substrate corrections. Phys. Rev. B 2015, 91, 075426. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, F.; Zhao, D.; Cai, L.; Luan, P.; Zhang, Q.; Zhou, W.; Zhang, N.; Fan, Q.X.; Wang, Y.; et al. Temperature dependent Raman spectra of isolated suspended single-walled carbon nanotubes. Nanoscale 2014, 6, 3949–3953. [Google Scholar] [CrossRef] [PubMed]
- Ci, L.; Zhou, Z.; Song, L.; Yan, X.; Liu, D.; Yuan, H.; Gao, Y.; Wang, J.; Liu, L.; Zhou, W.; et al. Temperature dependence of resonant Raman scattering in double-wall carbon nanotubes. Appl. Phys. Lett. 2003, 82, 3098–3100. [Google Scholar] [CrossRef]
- Koukaras, E.N.; Kalosakas, G.; Galiotis, C.; Papagelis, K. Phonon properties of graphene derived from molecular dynamics simulations. Sci. Rep. 2015, 5, 12923. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, L.; Broido, D.A. Optimized Tersoff and Brenner empirical potential parameters for lattice dynamics and phonon thermal transport in carbon nanotubes and graphene. Phys. Rev. B 2010, 81, 205441. [Google Scholar] [CrossRef]
- Calizo, I.; Miao, F.; Bao, W.; Lau, C.N. Variable temperature Raman microscopy as a nanometrology tool for graphene layers and graphene-based devices. Appl. Phys. Lett. 2007, 91, 071913. [Google Scholar] [CrossRef]
- Tan, P.; Deng, Y.; Zhao, Q. Temperature-dependent Raman spectra and anomalous Raman phenomenon of highly oriented pyrolytic graphite. Phys. Rev. B 1998, 58, 5435–5439. [Google Scholar] [CrossRef]
- Raravikar, N.R.; Keblinski, P.; Rao, A.M.; Dresselhaus, M.S.; Schadler, L.S.; Ajayan, P.M. Temperature dependence of radial breathing mode Raman frequency of single-walled carbon nanotubes. Phys. Rev. B 2002, 66, 235424. [Google Scholar] [CrossRef]
- Bassil, A.; Puech, P.; Tubery, L.; Bacsa, W. Controlled laser heating of carbon nanotubes. Appl. Phys. Lett. 2006, 88, 173113. [Google Scholar] [CrossRef]
- Osswald, S.; Flahaut, E.; Gogotsi, Y. In Situ Raman Spectroscopy Study of Oxidation of Double- and Single-Wall Carbon Nanotubes. Chem. Mater. 2006, 18, 1525–1533. [Google Scholar] [CrossRef]
- Baranov, A.V.; Bekhterev, A.N.; Bobovich, Y.S.; Petrov, V.I. Interpretation of certain characteristics in Raman spectra of graphite and glassy carbon. Opt. Spectrosc. 1987, 62, 612–616. [Google Scholar]
- Thomsen, C.; Reich, S. Double resonant raman scattering in graphite. Phys. Rev. Lett. 2000, 85, 5214–5217. [Google Scholar] [CrossRef] [PubMed]
- Vidano, R.; Fischbach, D.B. New Lines in the Raman Spectra of Carbons and Graphite. J. Am. Ceram. Soc. 1978, 61, 13–17. [Google Scholar] [CrossRef]
- Nemanich, R.J.; Solin, S.A. First- and second-order Raman scattering from finite-size crystals of graphite. Phys. Rev. B 1979, 20, 392–401. [Google Scholar] [CrossRef]
- Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S.; Cançado, L.G.; Jorio, A.; Saito, R. Studying disorder in graphite-based systems by Raman spectroscopy. Phys. Chem. Chem. Phys. 2007, 9, 1276–1291. [Google Scholar] [CrossRef] [PubMed]
- Cançado, L.G.; Takai, K.; Enoki, T.; Endo, M.; Kim, Y.A.; Mizusaki, H. General equation for the determination of the crystallite size La of nanographite by Raman spectroscopy. Appl. Phys. Lett. 2006, 88, 163106. [Google Scholar] [CrossRef]
- Ni, Z.H.; Fan, H.M.; Fan, X.F.; Wang, H.M.; Zheng, Z.; Feng, Y.P.; Wu, Y.H.; Shen, Z.X. High temperature Raman spectroscopy studies of carbon nanowalls. J. Raman Spectrosc. 2007, 38, 1449–1453. [Google Scholar] [CrossRef]
- Tan, P.; Deng, Y.; Zhao, Q.; Cheng, W. The intrinsic temperature effect of the Raman spectra of graphite. Appl. Phys. Lett. 1999, 74, 1818–1820. [Google Scholar] [CrossRef]
- Huang, F.; Yue, K.T.; Tan, P.; Zhang, S.-L. Temperature dependence of the Raman spectra of carbon nanotubes. J. Appl. Phys. 1998, 84, 4022. [Google Scholar] [CrossRef]
- Bonini, N.; Lazzeri, M.; Marzari, N.; Mauri, F. Phonon anharmonicities in graphite and graphene. Phys. Rev. Lett. 2007, 99, 176802. [Google Scholar] [CrossRef] [PubMed]
- Eckmann, A.; Felten, A.; Mishchenko, A.; Britnell, L.; Krupke, R.; Novoselov, K.S.; Casiraghi, C. Probing the nature of defects in graphene by Raman spectroscopy. Nano Lett. 2012, 12, 3925–3930. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosenburg, F.; Ionescu, E.; Nicoloso, N.; Riedel, R. High-Temperature Raman Spectroscopy of Nano-Crystalline Carbon in Silicon Oxycarbide. Materials 2018, 11, 93. https://doi.org/10.3390/ma11010093
Rosenburg F, Ionescu E, Nicoloso N, Riedel R. High-Temperature Raman Spectroscopy of Nano-Crystalline Carbon in Silicon Oxycarbide. Materials. 2018; 11(1):93. https://doi.org/10.3390/ma11010093
Chicago/Turabian StyleRosenburg, Felix, Emanuel Ionescu, Norbert Nicoloso, and Ralf Riedel. 2018. "High-Temperature Raman Spectroscopy of Nano-Crystalline Carbon in Silicon Oxycarbide" Materials 11, no. 1: 93. https://doi.org/10.3390/ma11010093
APA StyleRosenburg, F., Ionescu, E., Nicoloso, N., & Riedel, R. (2018). High-Temperature Raman Spectroscopy of Nano-Crystalline Carbon in Silicon Oxycarbide. Materials, 11(1), 93. https://doi.org/10.3390/ma11010093






