Electrochemical Corrosion and In Vitro Bioactivity of Nano-Grained Biomedical Ti-20Nb-13Zr Alloy in a Simulated Body Fluid
Abstract
:1. Introduction
2. Experimental Results and Discussion
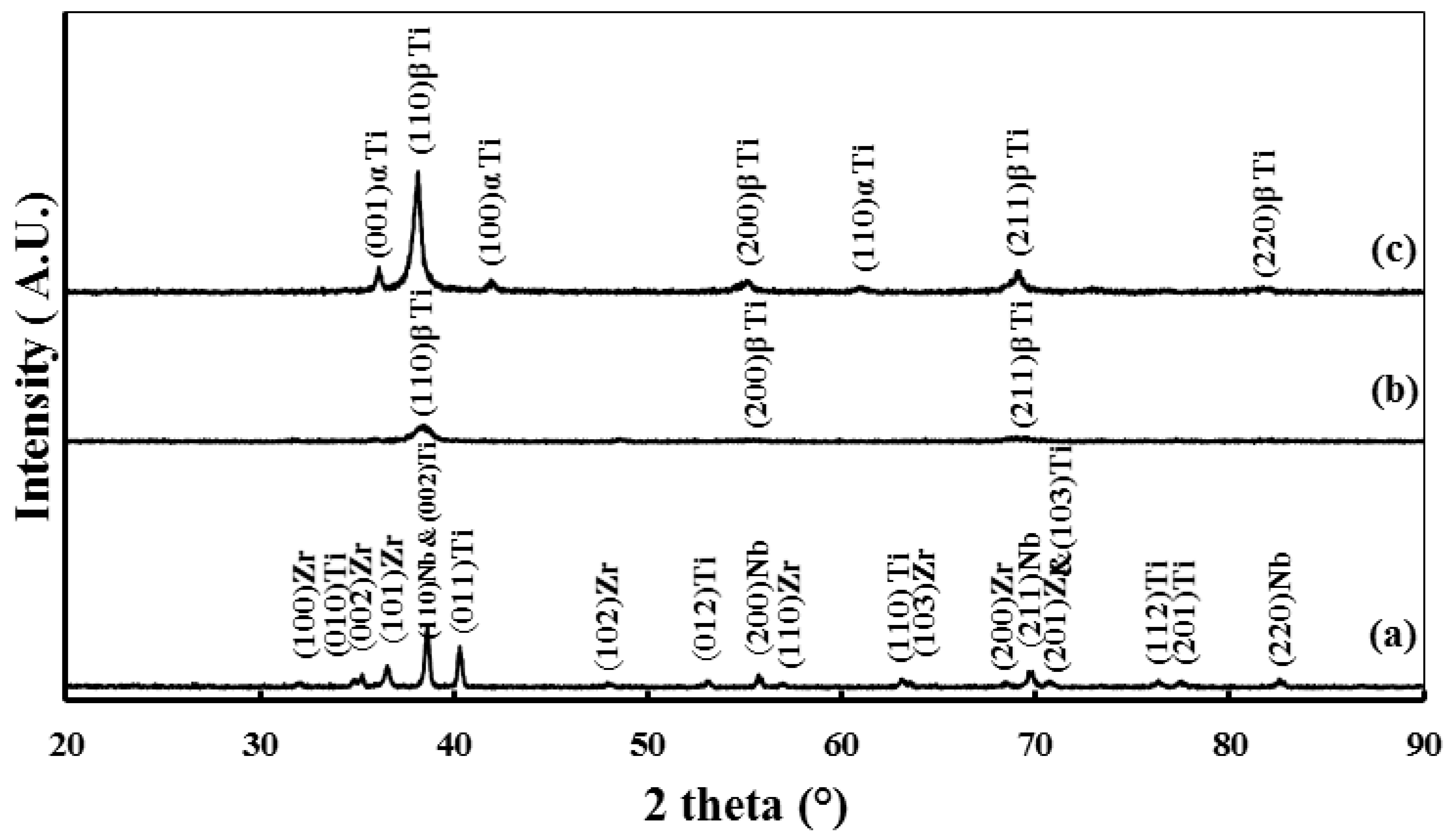
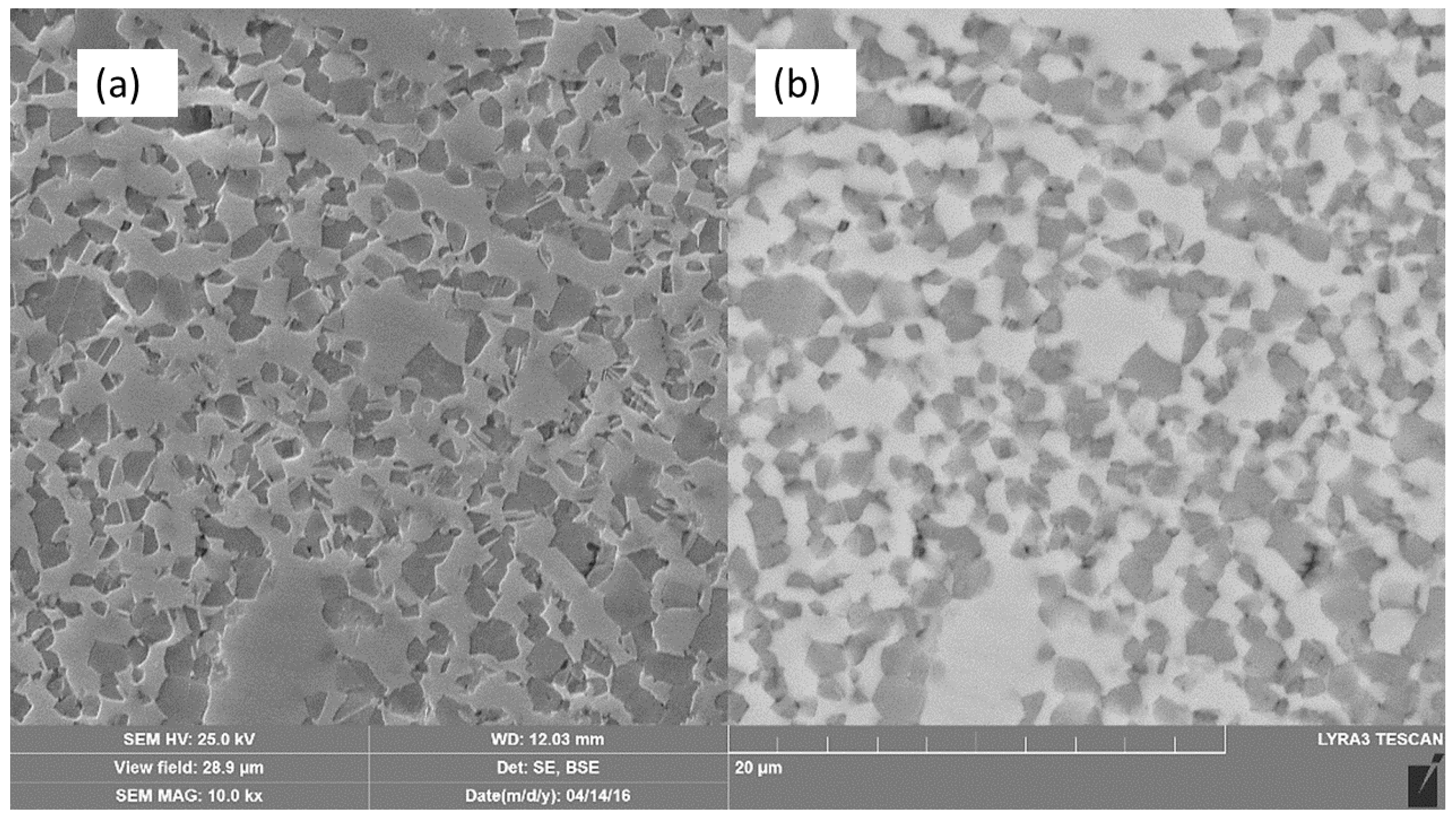
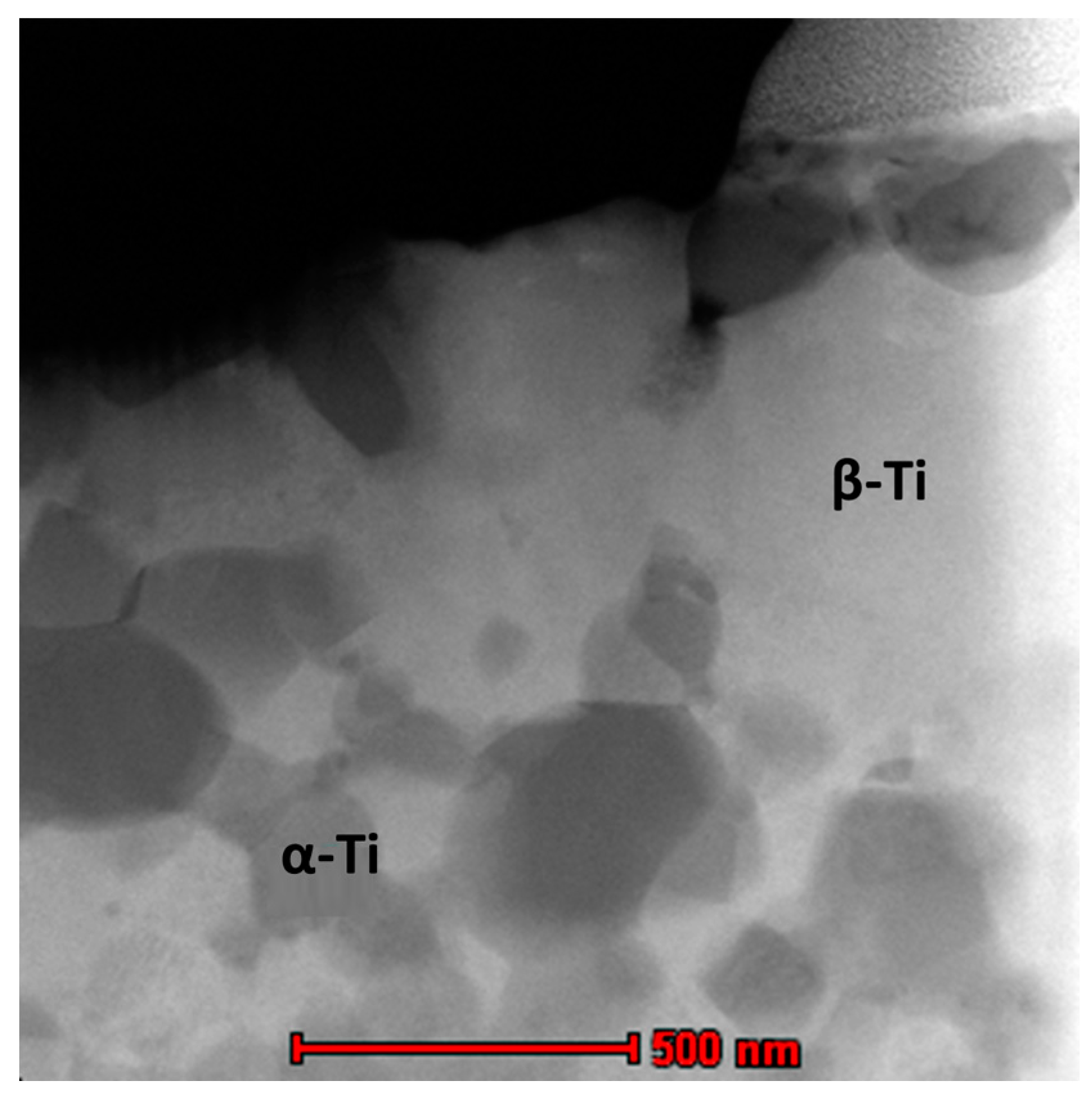
2.1. Microstructure and Phase Constitutions
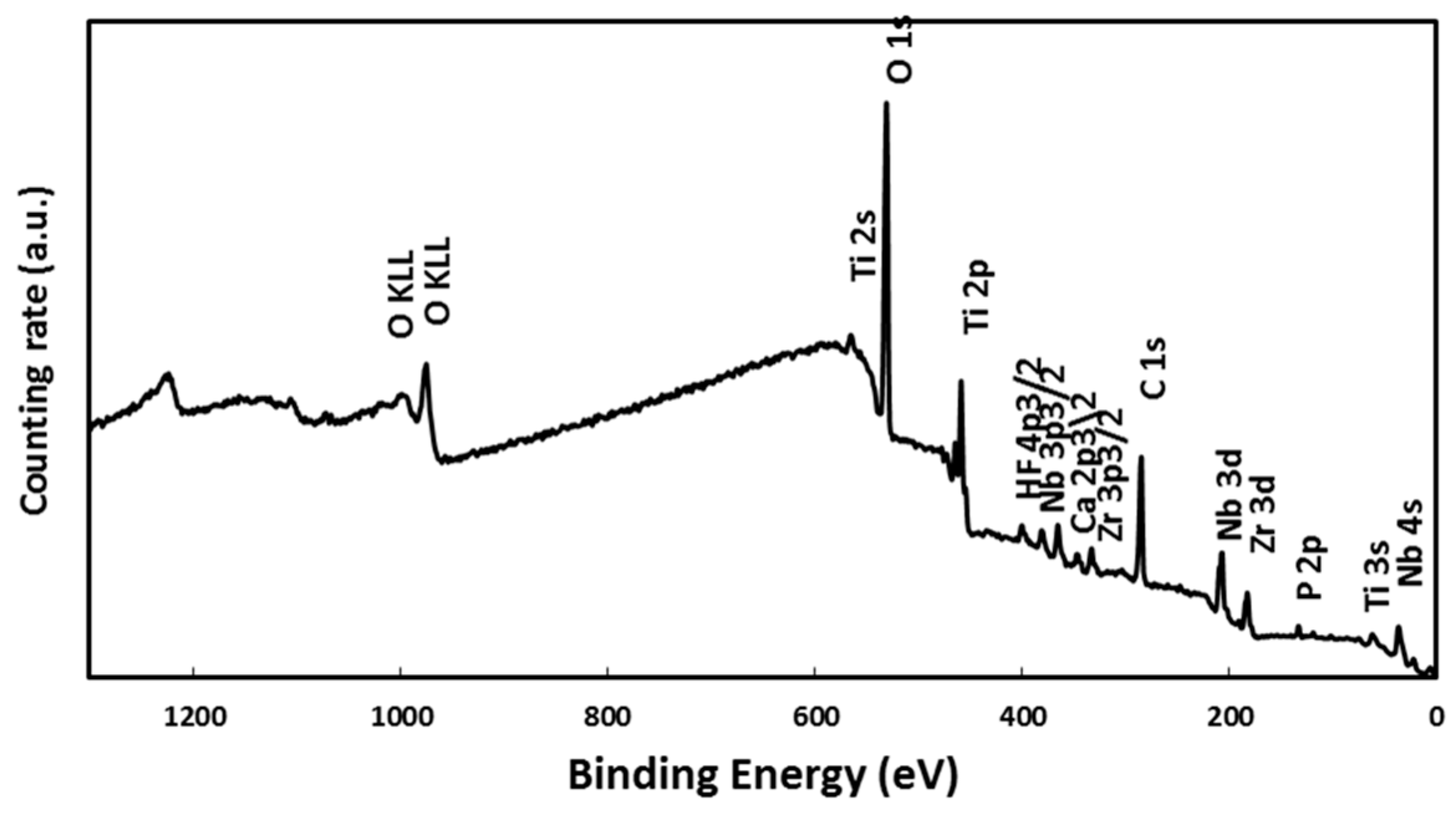
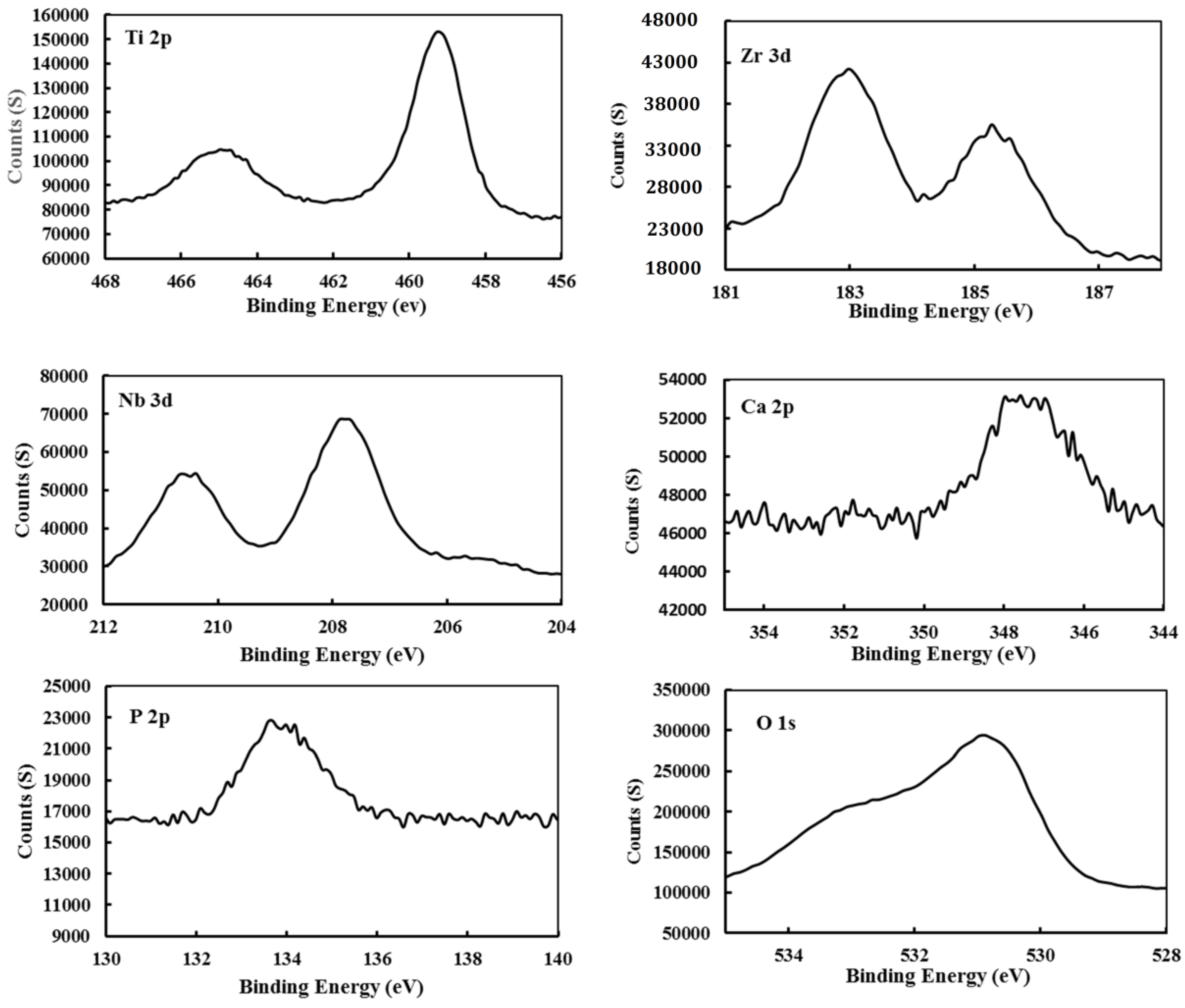
2.2. Alloy Surface Analysis after Immersion in a SBF
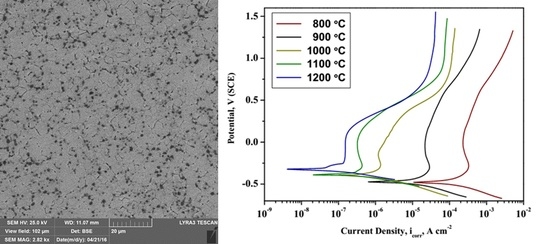
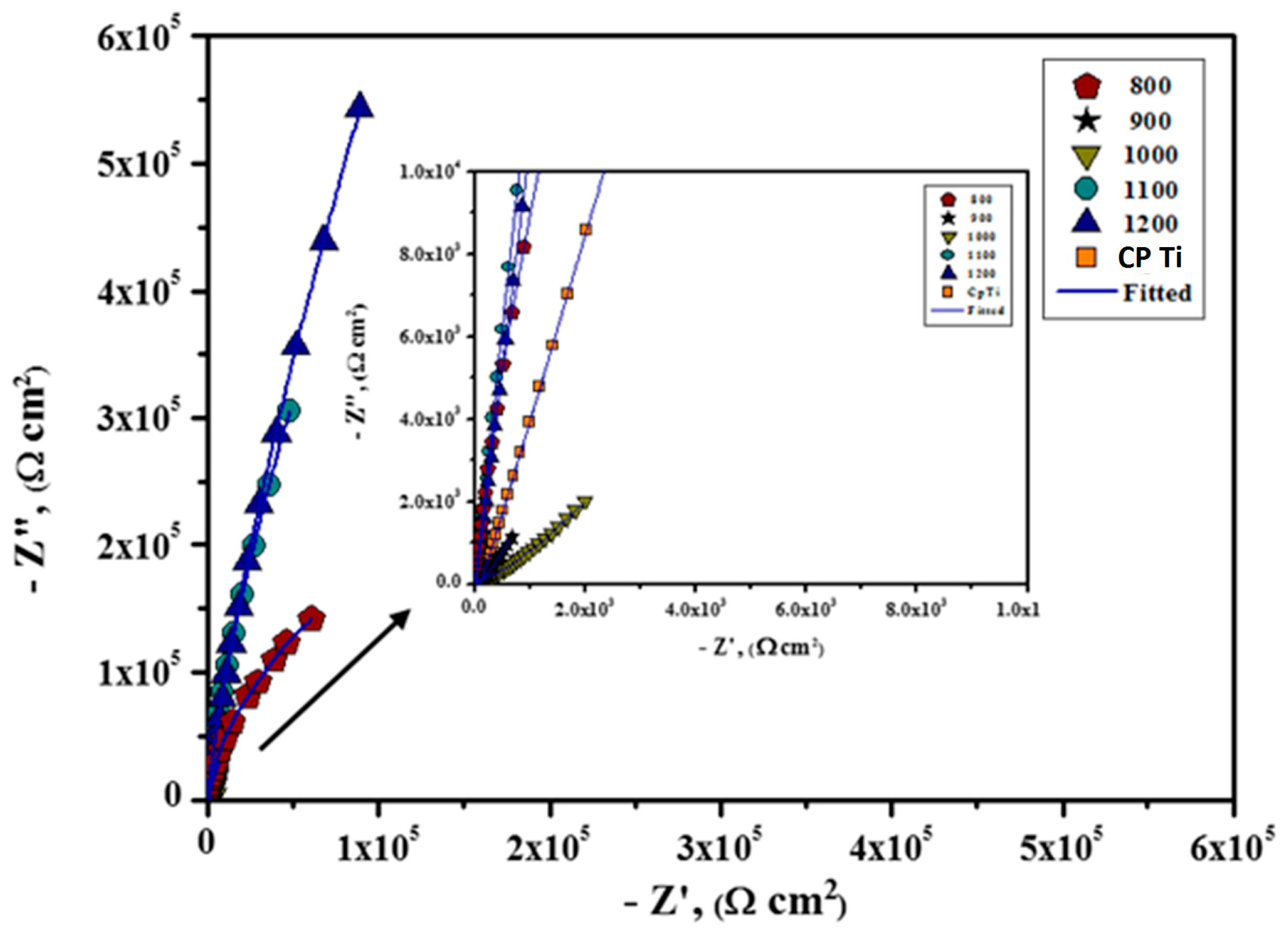
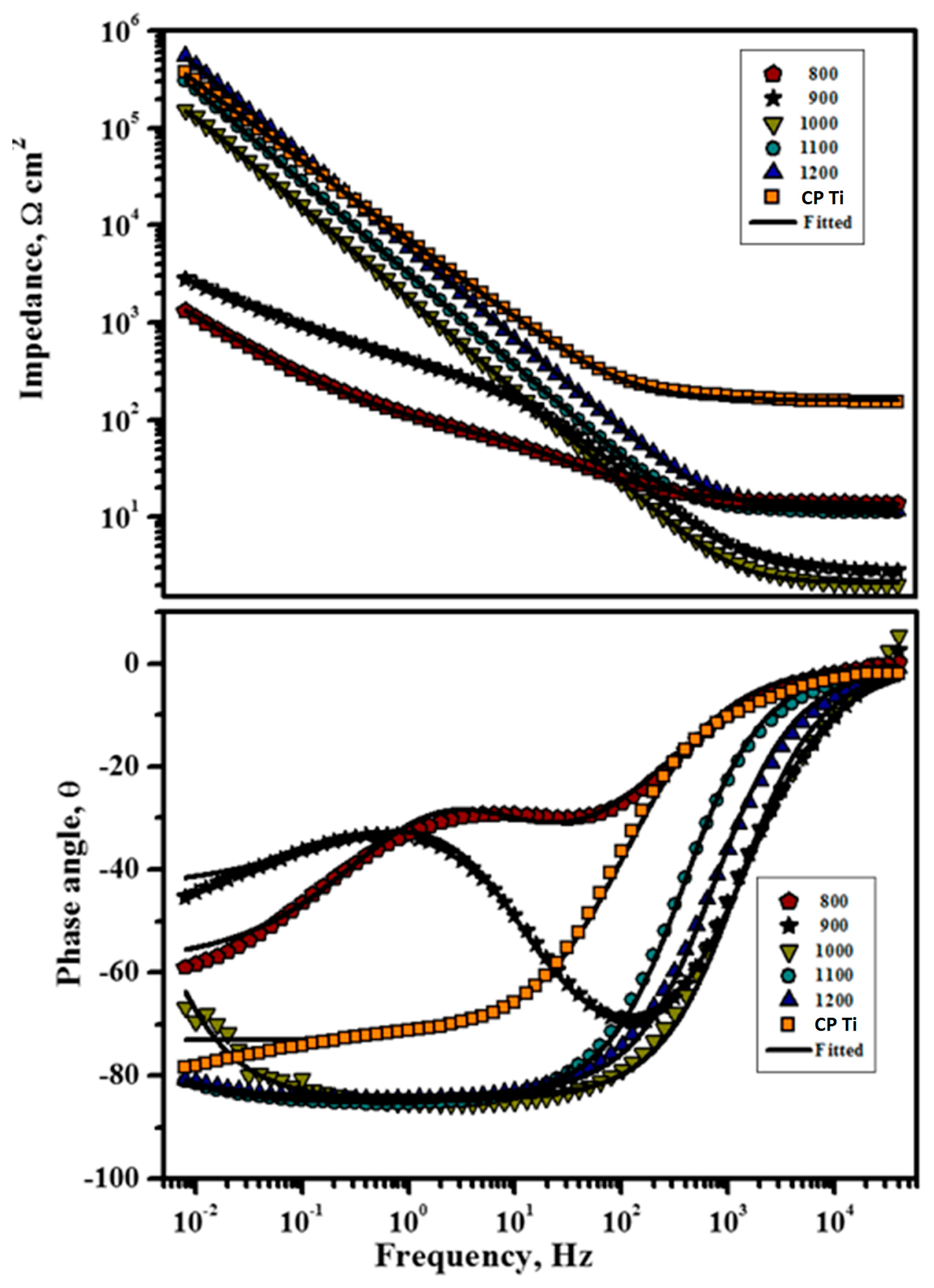
2.3. Electrochemical Analysis
3. Materials and Experimental Work
3.1. Synthesis of Alloy
3.2. In Vitro Bioactivity Test in a SBF
3.3. Surface Analysis of the Alloy by XPS
3.4. Electrochemical Analysis
4. Conclusions
- Structure and microstructure analysis confirmed the formation of the nano-grained alpha phase.
- An analysis of the XPS spectra revealed the existence of protective oxides of the alloy (TiO2, ZrO2 and Nb2O5), which enhance the corrosion protection of the presented alloy.
- The deposition of a Ca3(PO4)2 compound (a precursor of hydroxyapatite) on the surface of the developed alloy indicates that the presented alloy can stimulate bone formation.
- From electrochemical corrosion studies, it was concluded that the newly developed Ti-20Nb-13Zr alloy substrates exhibited higher corrosion protection for sintering temperatures at 1200 °C.
- The bioactivity and corrosion resistance of the developed nanostructured alloy in a SBF medium renders the nanostructured Ti-20Nb-13Zr alloy a promising candidate as an implant material.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hussein, M.A.; Mohammed, A.S.; Al-Aqeeli, N. Wear characteristics of metallic biomaterials: A review. Materials 2015, 8, 2749–2768. [Google Scholar] [CrossRef]
- Niinomi, M.D.D. Metallic biomaterials. J. Artif. Organs 2008, 11, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Karanjai, M.; Sundaresan, R.; Rao, G.V.; Mohan, T.R.; Kashyap, B.P. Development of titanium based biocomposite by powder metallurgy processing with in situ forming of Ca–P phases. Mater. Sci. Eng. A 2007, 447, 19–26. [Google Scholar] [CrossRef]
- Uwais, Z.A.; Hussein, M.A.; Samad, M.A.; Al-Aqeeli, N. Surface Modification of Metallic Biomaterials for Better Tribological Properties: A Review. Arab. J. Sci. Eng. 2017, 42, 4493–4512. [Google Scholar] [CrossRef]
- Ribeiro, A.L.; Junior, R.C.; Cardoso, F.F.; Fernandes Filho, R.B.; Vaz, L.G. Mechanical, physical, and chemical characterization of Ti–35Nb–5Zr and Ti–35Nb–10Zr casting alloys. J. Mater. Sci. Mater. Med. 2009, 20, 1629–1636. [Google Scholar] [CrossRef] [PubMed]
- Niespodziana, K.; Jurczyk, K.; Jurczyk, M. The synthesis of titanium alloys for biomedical applications. Rev. Adv. Mater. Sci. 2008, 18, 236–240. [Google Scholar]
- Henriques, V.A.; Galvani, E.T.; Petroni, S.L.; Paula, M.S.; Lemos, T.G. Production of Ti–13Nb–13Zr alloy for surgical implants by powder metallurgy. J. Mater. Sci. 2010, 45, 5844–5850. [Google Scholar] [CrossRef]
- Wang, K. The use of titanium for medical applications in the USA. Mater. Sci. Eng. A 1996, 213, 134–137. [Google Scholar] [CrossRef]
- Biesiekierski, A.; Wang, J.; Gepreel, M.A.; Wen, C. A new look at biomedical Ti-based shape memory alloys. Acta Biomater. 2012, 8, 1661–1669. [Google Scholar] [CrossRef] [PubMed]
- Eisenbarth, E.; Velten, D.; Müller, M.; Thull, R.; Breme, J. Biocompatibility of β-stabilizing elements of titanium alloys. Biomaterials 2004, 25, 5705–5713. [Google Scholar] [CrossRef] [PubMed]
- Asri, R.I.; Harun, W.S.; Samykano, M.; Lah, N.A.; Ghani, S.A.; Tarlochan, F.; Raza, M.R. Corrosion and surface modification on biocompatible metals: A review. Mater. Sci. Eng. C 2017, 77, 1261–1274. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.; Bazaka, O.; Chua, M.; Rochford, M.; Fedrick, L.; Spoor, J.; Symes, R.; Tieppo, M.; Collins, C.; Cao, A.; et al. Metallic biomaterials: Current challenges and opportunities. Materials 2017, 10, 884. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.Y.; Scully, J.R. Corrosion and passivity of Ti-13% Nb-13% Zr in comparison to other biomedical implant alloys. Corrosion 1997, 53, 965–976. [Google Scholar] [CrossRef]
- Geetha, M.; Mudali, U.K.; Gogia, A.K.; Asokamani, R.; Raj, B. Influence of microstructure and alloying elements on corrosion behavior of Ti–13Nb–13Zr alloy. Corros. Sci. 2004, 46, 877–892. [Google Scholar] [CrossRef]
- Assis, S.L.; Costa, I.S. Electrochemical evaluation of Ti-13Nb-13Zr, Ti-6Al-4V and Ti-6Al-7Nb alloys for biomedical application by long-term immersion tests. Mater. Corros. 2007, 58, 329–333. [Google Scholar] [CrossRef]
- Moreno, J.C.; Vasilescu, E.; Drob, P.; Osiceanu, P.; Vasilescu, C.; Drob, S.I.; Popa, M. Surface and electrochemical characterization of a new ternary titanium-based alloy behavior in electrolytes of varying pH. Corros. Sci. 2013, 77, 52–63. [Google Scholar] [CrossRef]
- Cvijović-Alagić, I.; Cvijović, Z.; Mitrović, S.; Panić, V.; Rakin, M. Wear and corrosion behavior of Ti–13Nb–13Zr and Ti–6Al–4V alloys in simulated physiological solution. Corros. Sci. 2011, 53, 796–808. [Google Scholar] [CrossRef]
- Majumdar, P.; Singh, S.B.; Chatterjee, U.K.; Chakraborty, M. Corrosion behavior of heat treated boron free and boron containing Ti–13Zr–13Nb (wt %) alloy in simulated body fluid. J. Mater. Sci. Mater. Med. 2011, 22, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Long, M.; Rack, H.J. Titanium alloys in total joint replacement—A materials science perspective. Biomaterials 1998, 18, 1621–1639. [Google Scholar] [CrossRef]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Hussein, M.A.; Suryanarayana, C.; Al-Aqeeli, N. Fabrication of nano-grained Ti–Nb–Zr biomaterials using spark plasma sintering. Mater. Des. 2015, 87, 693–700. [Google Scholar] [CrossRef]
- Nouri, A.; Chen, X.; Li, Y.; Yamada, Y.; Hodgson, P.D.; Wen, C.E. Synthesis of Ti–Sn–Nb alloy by powder metallurgy. Mater. Sci. Eng. A 2008, 485, 562–570. [Google Scholar] [CrossRef]
- Bania, P.J. Beta titanium alloys and their role in the titanium industry. J. Miner. Met. Mater. Soc. 1994, 46, 16–19. [Google Scholar] [CrossRef]
- Manda, P.; Pathak, A.; Mukhopadhyay, A.; Chakkingal, U.; Singh, A.K. Ti-5Al-5Mo-5V-3Cr and similar Mo equivalent alloys: First principles calculations and experimental investigations. J. Appl. Res. Technol. 2017, 15, 21–26. [Google Scholar] [CrossRef]
- Guo, S.; Meng, Q.; Zhao, X.; Wei, Q.; Xu, H. Design and fabrication of a metastable β-type titanium alloy with ultralow elastic modulus and high strength. Nat. Sci. Rep. 2015, 5, 14688. [Google Scholar] [CrossRef] [PubMed]
- Geetha, M.; Singh, A.K.; Gogia, A.K.; Asokamani, R. Effect of thermomechanical processing on evolution of various phases in Ti–Nb–Zr alloys. J. Alloys Compd. 2004, 384, 131–144. [Google Scholar] [CrossRef]
- Tanigawa, H.; Asoh, H.; Ohno, T.; Kubota, M.; Ono, S. Electrochemical corrosion and bioactivity of titanium–hydroxyapatite composites prepared by spark plasma sintering. Corros. Sci. 2013, 70, 212–220. [Google Scholar]
- Wang, H.; Lee, J.K.; Moursi, A.; Lannutti, J.J. Ca/P ratio effects on the degradation of hydroxyapatite in vitro. J. Biomed. Mater. Res. Part A 2003, 67, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; USA Inc.: Chamhassen, MN, USA, 1995. [Google Scholar]
- Kraut-Vass, A.; Powell, C.J.; Gaarenstroom, S.W. NIST X-ray photoelectron spectroscopy database. In NIST Standard Reference Database 20; version 4.1; US Secretary of Commerce on behalf of the United States of America: Gaithersburg, MD, USA, 2012. [Google Scholar]
- Calderon-Moreno, J.M.; Vasilescu, C.; Drob, S.I.; Ivanescu, S.; Osiceanu, P.; Drob, P.; Popa, M.; Preda, S.; Vasilescu, E. Microstructural and mechanical properties, surface and electrochemical characterization of a new Ti–Zr–Nb alloy for implant applications. J. Alloys Compd. 2014, 612, 398–410. [Google Scholar] [CrossRef]
- Tanaka, Y.; Nakai, M.; Akahori, T.; Niinomi, M.; Tsutsumi, Y.; Doi, H.; Hanawa, T. Characterization of air-formed surface oxide film on Ti–29Nb–13Ta–4.6Zr alloy surface using XPS and AES. Corros. Sci. 2008, 50, 2111–2166. [Google Scholar] [CrossRef]
- Park, I.S.; Choi, U.J.; Yi, H.K.; Park, B.K.; Lee, M.H.; Bae, T.S. Biomimetic apatite formation and biocompatibility on chemically treated Ti-6Al-7Nb alloy. Surf. Interface Anal. 2008, 40, 37–42. [Google Scholar] [CrossRef]
- Milošev, I.; Kosec, T.; Strehblow, H.H. XPS and EIS study of the passive film formed on orthopaedic Ti–6Al–7Nb alloy in Hank’s physiological solution. Electrochim. Acta 2008, 53, 3547–3558. [Google Scholar] [CrossRef]
- Simka, W. Preliminary investigations on the anodic oxidation of Ti–13Nb–13Zr alloy in a solution containing calcium and phosphorus. Electrochim. Acta 2011, 56, 9831–9837. [Google Scholar] [CrossRef]
- Milošev, I.; Metikoš-Huković, M.; Strehblow, H.H. Passive film on orthopaedic TiAlV alloy formed in physiological solution investigated by X-ray photoelectron spectroscopy. Biomaterials 2000, 21, 2103–2113. [Google Scholar] [CrossRef]
- Crist, B.V. Handbook of Monochromatic XPS Spectra, the Elements, and Native Oxides; John Wiley & Sons, Ltd.: Mountain View, CA, USA, 2000. [Google Scholar]
- Hryniewicz, T.; Rokosz, K.; Rokicki, R.; Prima, F. Nanoindentation and XPS studies of titanium TNZ alloy after electrochemical polishing in a magnetic field. Materials 2015, 8, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.J.; Ong, C.K. Epitaxial Y-stabilized ZrO2 films on silicon: Dynamic growth process and interface structure. Appl. Phys. Lett. 2002, 80, 2541–2543. [Google Scholar] [CrossRef]
- Simka, W.; Krząkała, A.; Masełbas, M.; Dercz, G.; Szade, J.; Winiarski, A.; Michalska, J. Formation of bioactive coatings on Ti–13Nb–13Zr alloy for hard tissue implants. RSC Adv. 2013, 28, 11195–11204. [Google Scholar] [CrossRef]
- Khan, M.A.; Williams, R.L.; Williams, D.F. In-vitro corrosion and wear of titanium alloys in the biological environment. Biomaterials 1996, 22, 2117–2126. [Google Scholar] [CrossRef]
- Hussein, M.A.; Kumar, A.M.; Yilbas, B.S.; Al-Aqeeli, N. Laser Nitriding of the Newly Developed Ti-20Nb-13Zr at.% Biomaterial Alloy to Enhance Its Mechanical and Corrosion Properties in Simulated Body Fluid. J. Mater. Eng. Perform. 2017, 26, 5553–5562. [Google Scholar] [CrossRef]
- De Almeida, L.H.; Bastos, I.N.; Santos, I.D.; Dutra, A.J.; Nunes, C.A.; Gabriel, S.B. Corrosion resistance of aged Ti–Mo–Nb alloys for biomedical applications. J. Alloys Compd. 2014, 615, S666–S669. [Google Scholar] [CrossRef]
- Kumar, A.M.; Sudhagar, P.; Ramakrishna, S.; Kang, Y.S.; Kim, H.; Gasem, Z.M.; Rajendran, N. Evaluation of chemically modified Ti–5Mo–3Fe alloy surface: Electrochemical aspects and in vitro bioactivity on MG63 cells. Appl. Surf. Sci. 2014, 307, 52–61. [Google Scholar] [CrossRef]
- Schultze, J.W.; Lohrengel, M.M. Stability, reactivity and breakdown of passive films. Problems of recent and future research. Electrochim. Acta 2000, 45, 2499–2513. [Google Scholar] [CrossRef]
- Xue, P.; Li, Y.; Li, K.; Zhang, D.; Zhou, C. Superelasticity, corrosion resistance and biocompatibility of the Ti–19Zr–10Nb–1Fe alloy. Mater. Sci. Eng. C 2015, 50, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Dhandapani, V.S.; Subbiah, R.; Thangavel, E.; Arumugam, M.; Park, K.; Gasem, Z.M.; Veeraragavan, V.; Kim, D.E. Tribological properties, corrosion resistance and biocompatibility of magnetron sputtered titanium-amorphous carbon coatings. Appl. Surf. Sci. 2016, 371, 262–274. [Google Scholar] [CrossRef]
- Dalmau, A.; Pina, V.G.; Devesa, F.; Amigo, V.; Muñoz, A.I. Electrochemical behavior of near-beta titanium biomedical alloys in phosphate buffer saline solution. Mater. Sci. Eng. C 2015, 48, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Madhankumar, A.; Rajendran, N. Poly (m-phenylendiamine-co-o-aminophenol) coatings on mild steel: effect of comonomers feed ratio on surface and corrosion protection aspects. Prog. Org. Coat. 2017, 76, 1445–1453. [Google Scholar] [CrossRef]
- Tamilselvi, S.; Raman, V.; Rajendran, N. Corrosion behaviour of Ti–6Al–7Nb and Ti–6Al–4V ELI alloys in the simulated body fluid solution by electrochemical impedance spectroscopy. Electrochem. Acta 2006, 52, 839–846. [Google Scholar] [CrossRef]
- Chelariu, R.; Bolat, G.; Izquierdo, J.; Mareci, D.; Gordin, D.M.; Gloriant, T.; Souto, R.M. Metastable beta Ti-Nb-Mo alloys with improved corrosion resistance in saline solution. Electrochim. Acta 2014, 137, 280–289. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Mhaede, M.; Wollmann, M.; Wagner, L. Effect of micro shot peening on the mechanical properties and corrosion behavior of two microstructure Ti–6Al–4V alloy. Appl. Surf. Sci. 2016, 363, 50–58. [Google Scholar] [CrossRef]
- Balakrishnan, A.; Lee, B.C.; Kim, T.N.; Panigrahi, B.B. Corrosion behavior of ultra-fine grained titanium in simulated body fluid for implant application. Trends Biomater. Artif. Organs 2008, 22, 194–208. [Google Scholar]
- Madhankumar, A.; Nagarajan, S.; Rajendran, N.; Nishimura, T. EIS evaluation of protective performance and surface characterization of epoxy coating with aluminum nanoparticles after wet and dry corrosion test. J. Solid State Electrochem. 2012, 16, 2085–2093. [Google Scholar] [CrossRef]
- Guo, S.; Chu, A.; Wu, H.; Cai, C.; Qu, X. Effect of sintering processing on microstructure, mechanical properties and corrosion resistance of Ti–24Nb–4Zr–7.9Sn alloy for biomedical applications. J. Alloys Compd. 2014, 597, 211–216. [Google Scholar] [CrossRef]
- Hussein, M.A.; Suryanarayana, C.; Arumugam, M.K.; Al-Aqeeli, N. Effect of sintering parameters on microstructure, mechanical properties and electrochemical behavior of Nb–Zr alloy for biomedical applications. Mater. Des. 2015, 83, 344–351. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity. Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
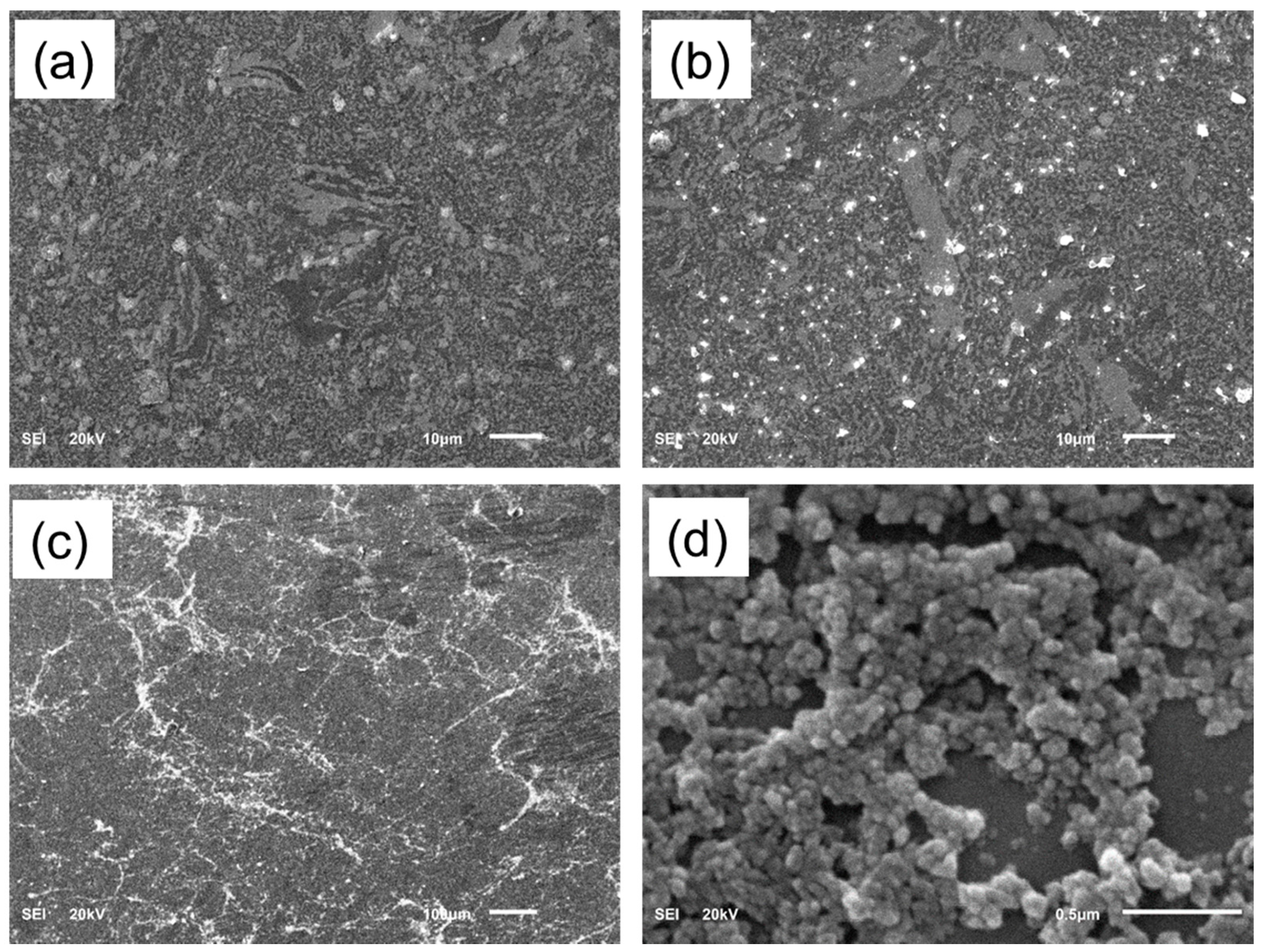
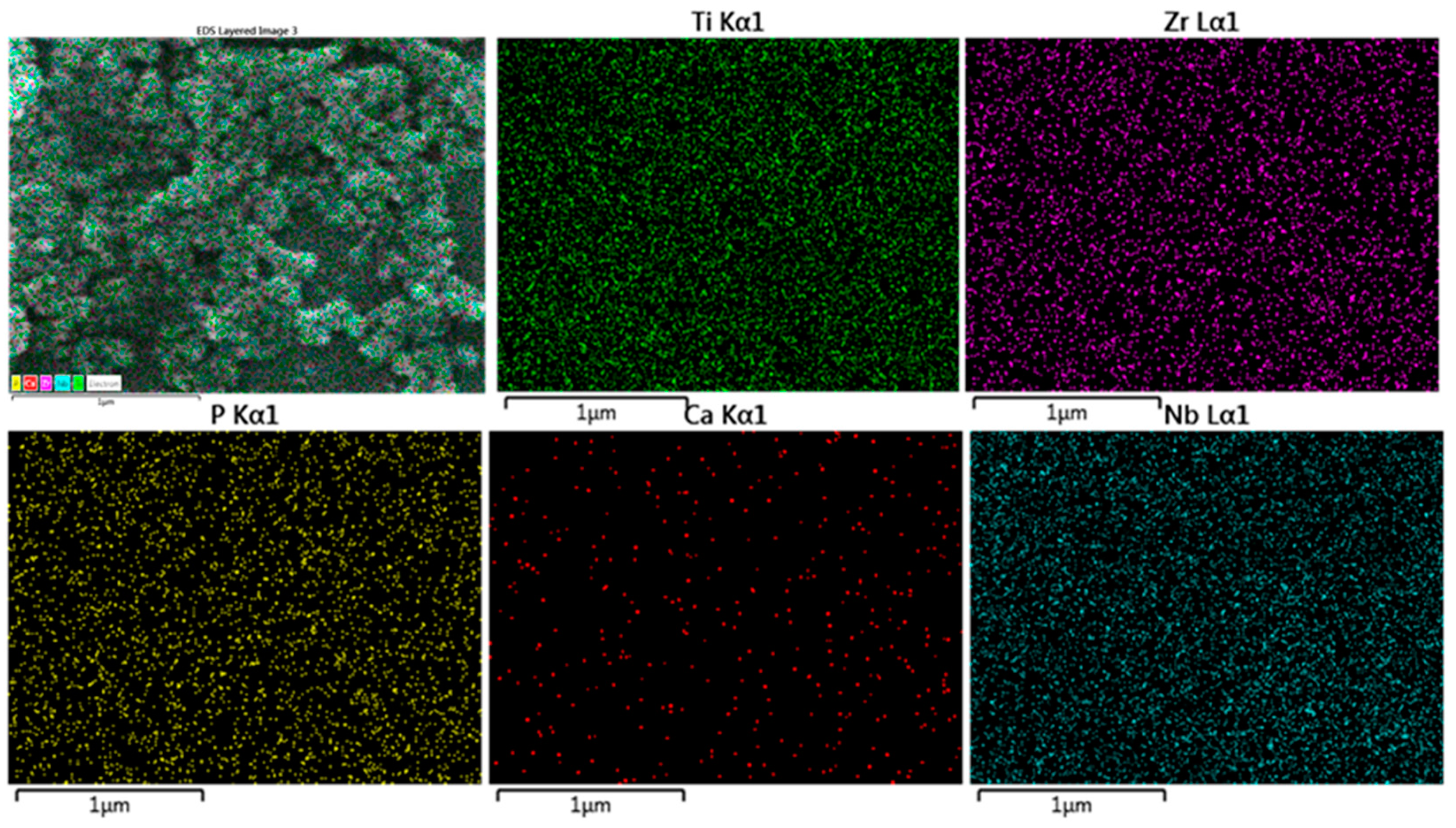
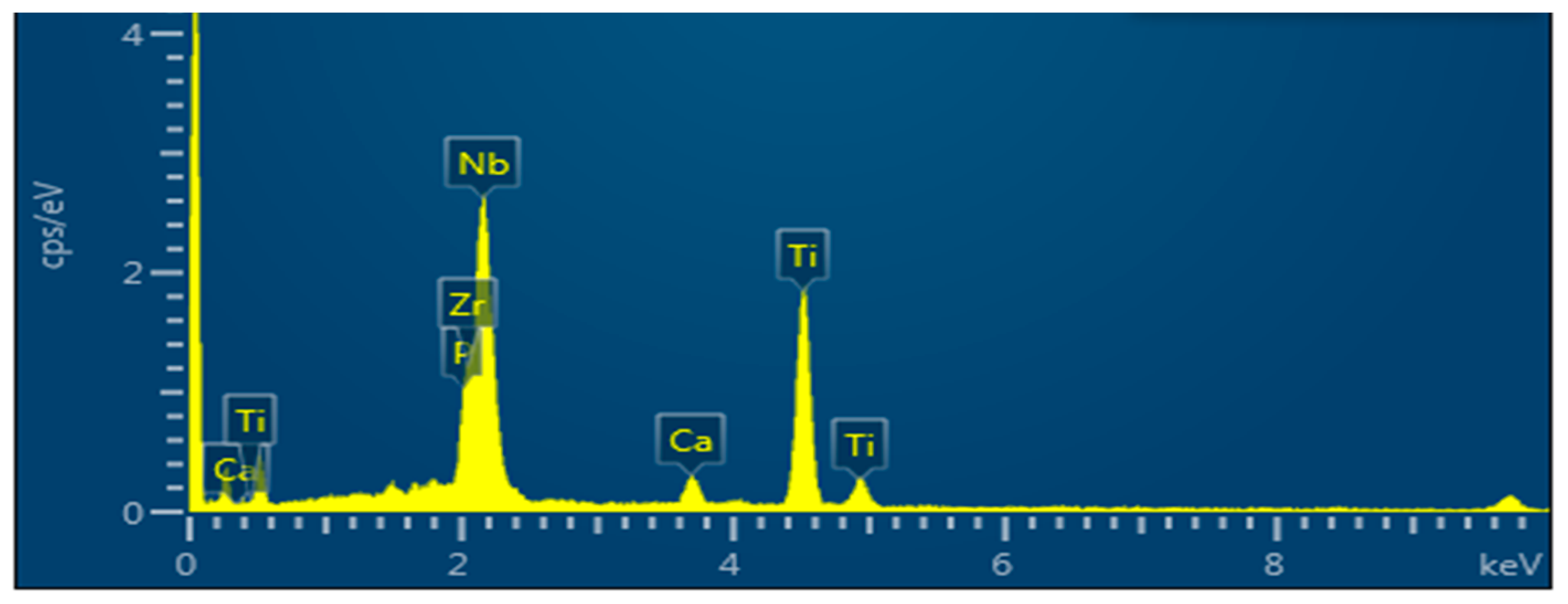
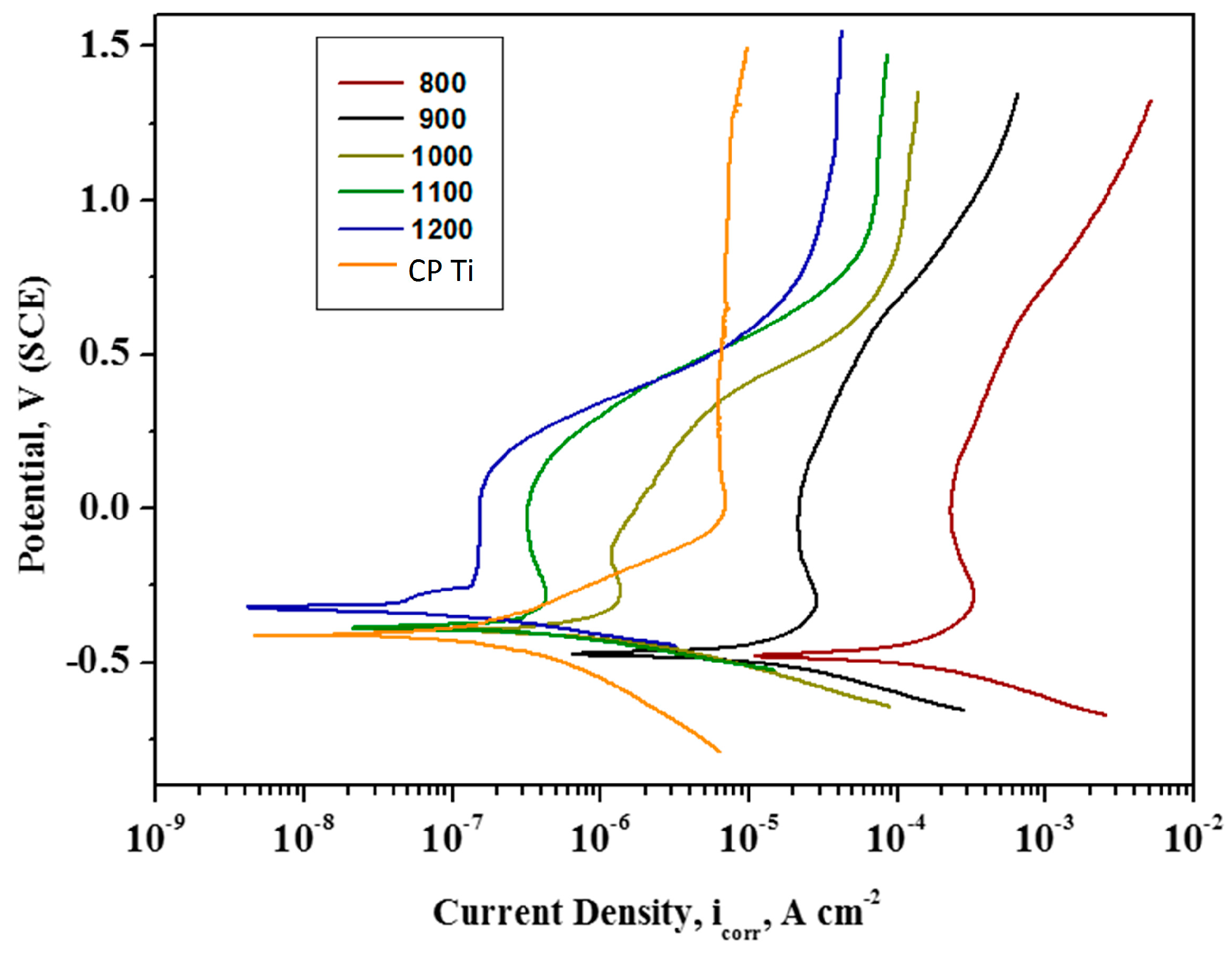

| Sintered Temperature (°C) | Ecorr (mV) | icorr (µA cm2) | βa (mV/dec) | βb (mV/dec) | Corr. Rate (mpy) × 10−3 |
|---|---|---|---|---|---|
| CP Ti | −418 | 50.90 | 84 | 69 | 44.90 |
| 800 | −478 | 149.285 | 94 | 79 | 63.840 |
| 900 | −474 | 18.232 | 65 | 77 | 8.336 |
| 1000 | −397 | 1.240 | 94 | 74 | 0.565 |
| 1100 | −388 | 0.438 | 85 | 69 | 0.200 |
| 1200 | −328 | 0.060 | 72 | 83 | 0.0275 |
| Sintered Temperature (°C) | Rs (cm2) | Rct (kΩ·cm2) | Qdl (µA·cm2) | N |
|---|---|---|---|---|
| CP Ti | 25.98 | 397 | 41.82 | 93.27 |
| 800 | 12.73 | 1.226 | 3879 | 92.49 |
| 900 | 11.98 | 2.837 | 624.71 | 91.53 |
| 1000 | 15.36 | 154 | 93.72 | 94.32 |
| 1100 | 14.86 | 306 | 54.44 | 94.67 |
| 1200 | 15.32 | 530 | 30.03 | 95.12 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussein, M.A.; Kumar, M.; Drew, R.; Al-Aqeeli, N. Electrochemical Corrosion and In Vitro Bioactivity of Nano-Grained Biomedical Ti-20Nb-13Zr Alloy in a Simulated Body Fluid. Materials 2018, 11, 26. https://doi.org/10.3390/ma11010026
Hussein MA, Kumar M, Drew R, Al-Aqeeli N. Electrochemical Corrosion and In Vitro Bioactivity of Nano-Grained Biomedical Ti-20Nb-13Zr Alloy in a Simulated Body Fluid. Materials. 2018; 11(1):26. https://doi.org/10.3390/ma11010026
Chicago/Turabian StyleHussein, Mohamed A., Madhan Kumar, Robin Drew, and Nasser Al-Aqeeli. 2018. "Electrochemical Corrosion and In Vitro Bioactivity of Nano-Grained Biomedical Ti-20Nb-13Zr Alloy in a Simulated Body Fluid" Materials 11, no. 1: 26. https://doi.org/10.3390/ma11010026
APA StyleHussein, M. A., Kumar, M., Drew, R., & Al-Aqeeli, N. (2018). Electrochemical Corrosion and In Vitro Bioactivity of Nano-Grained Biomedical Ti-20Nb-13Zr Alloy in a Simulated Body Fluid. Materials, 11(1), 26. https://doi.org/10.3390/ma11010026