Micro-Structures and High-Temperature Friction-Wear Performances of Laser Cladded Cr–Ni Coatings
Abstract
:1. Introduction
2. Experiment
3. Analysis and Discussion
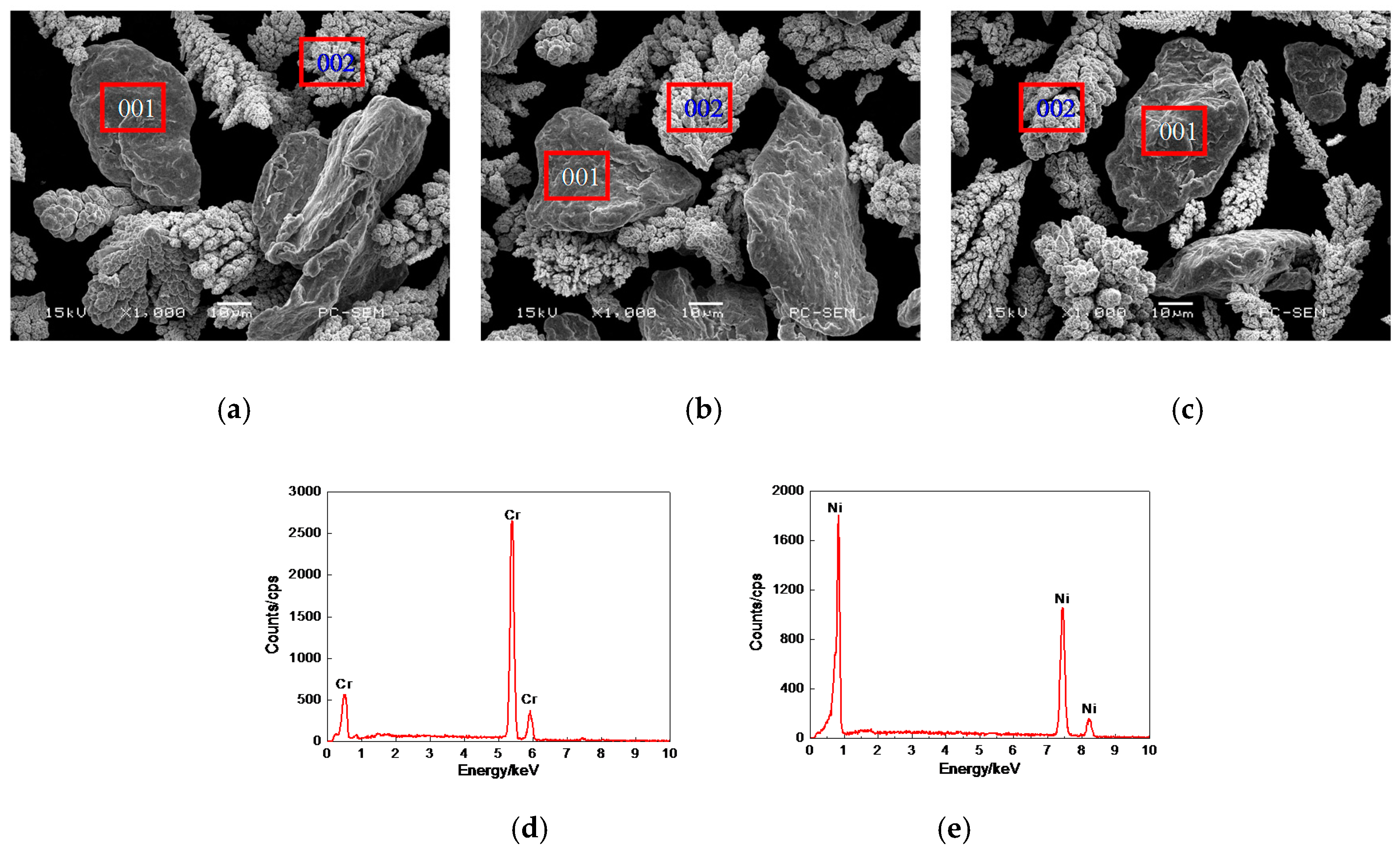
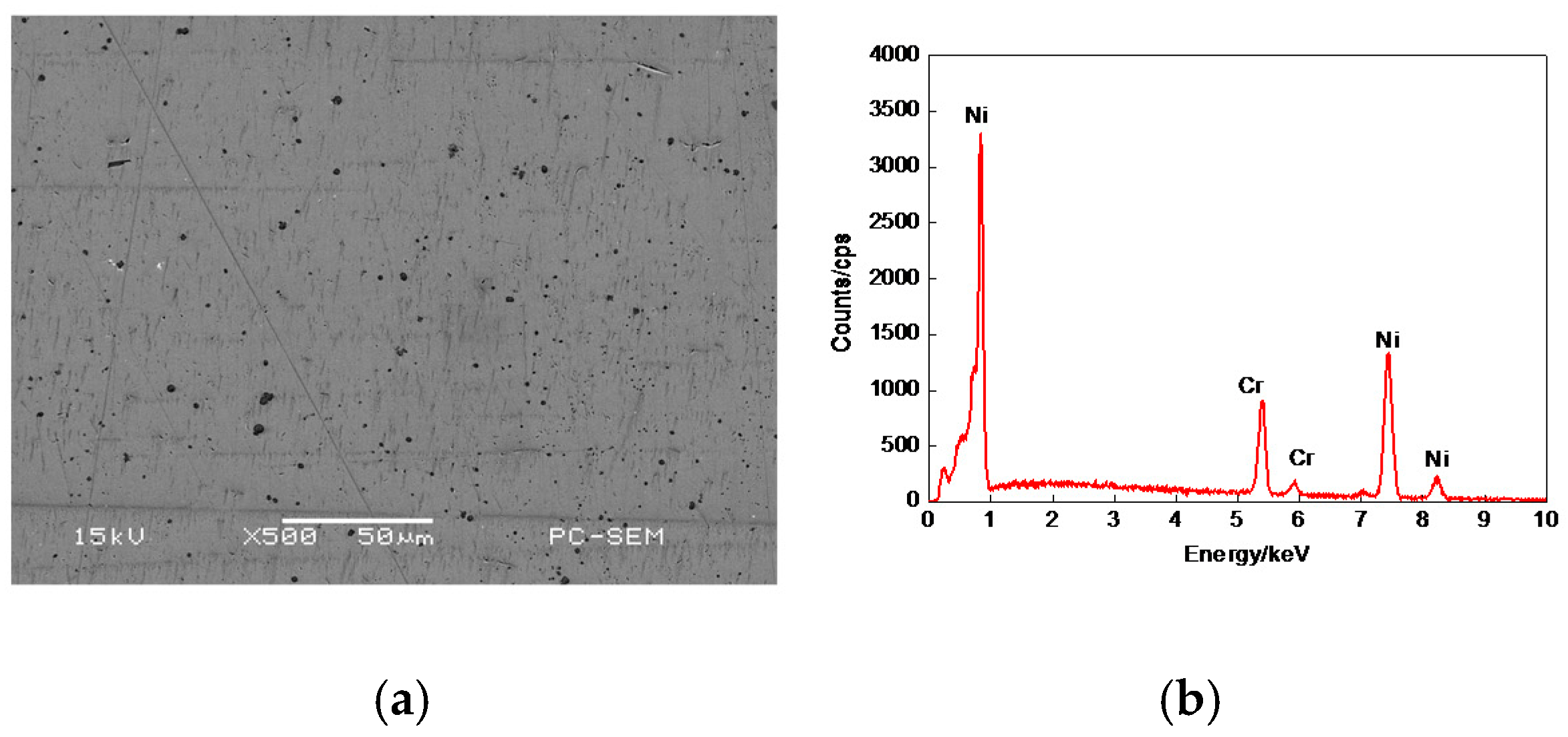
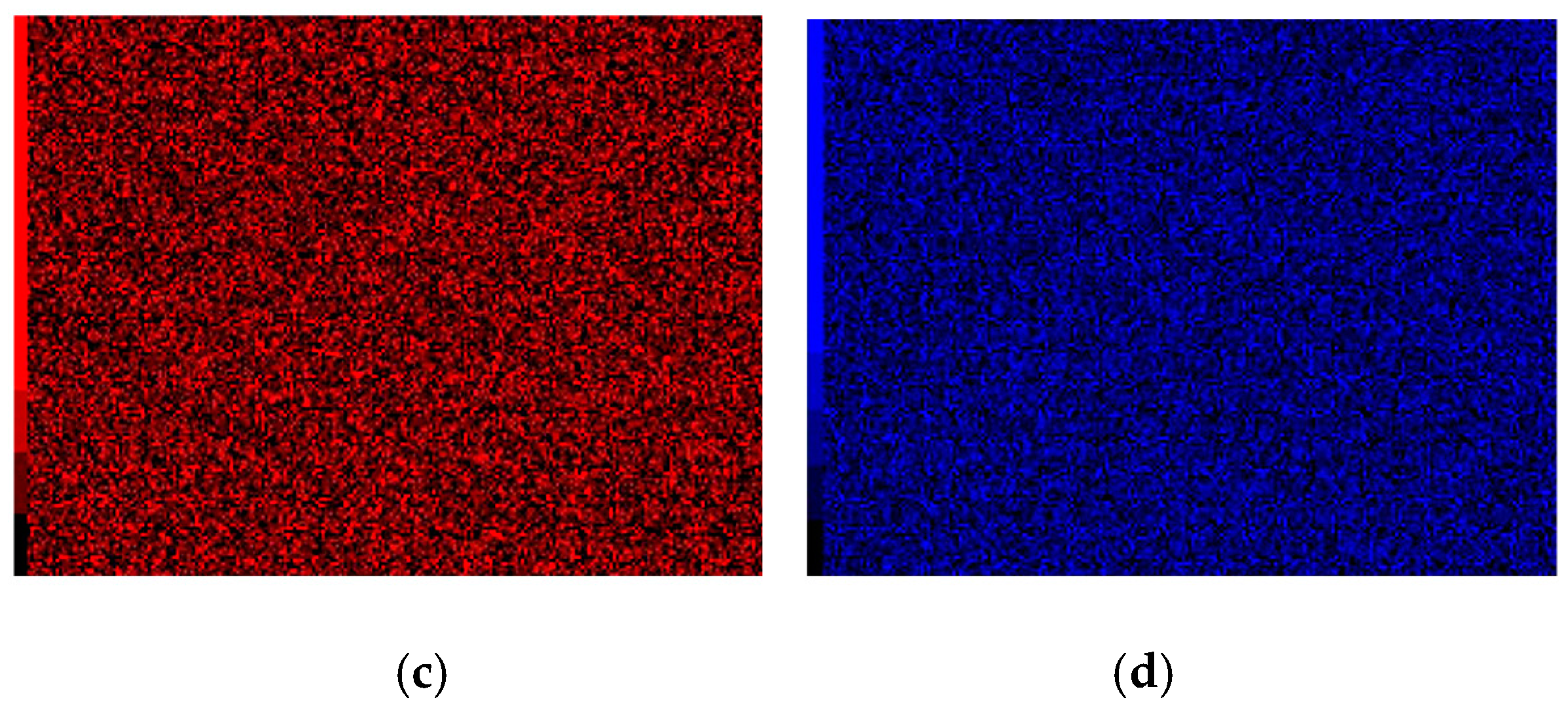
3.1. Morphologies, EDS and XRD Analysis of Cr–Ni Powder
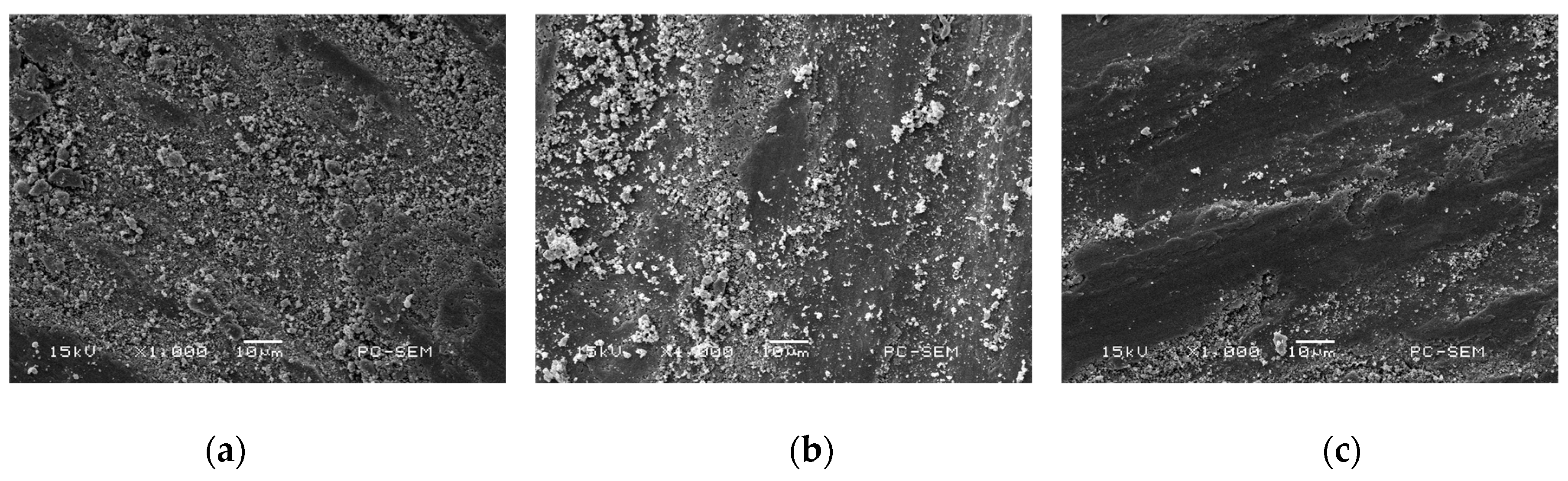
3.2. Morphologies of Cr–Ni Coating Surface
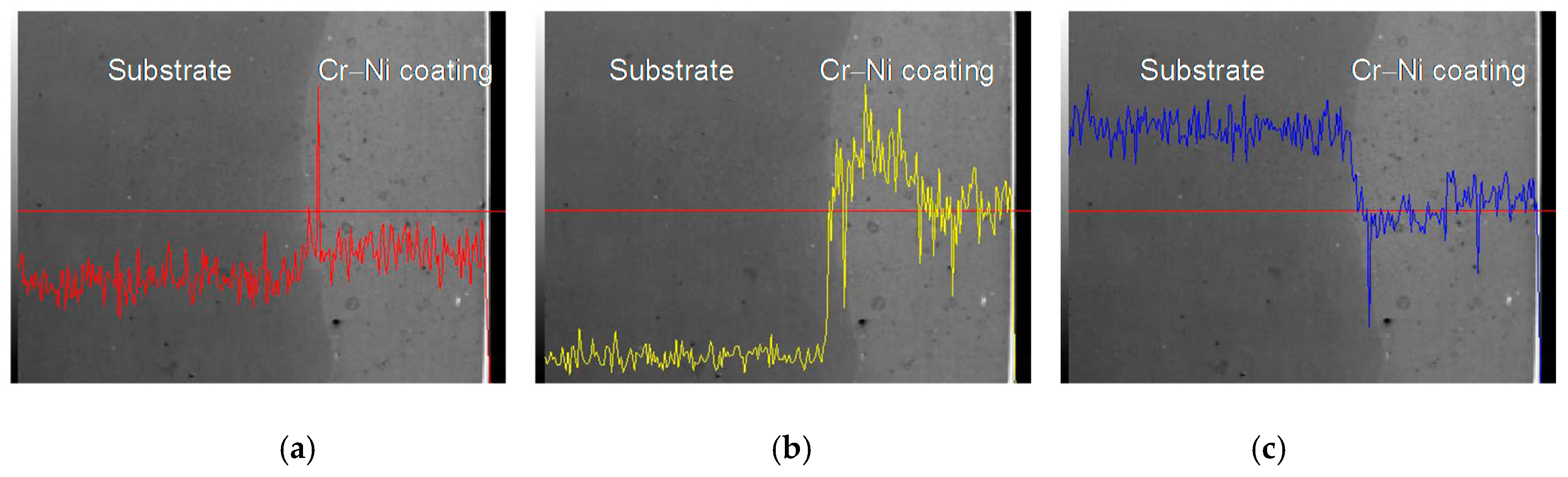
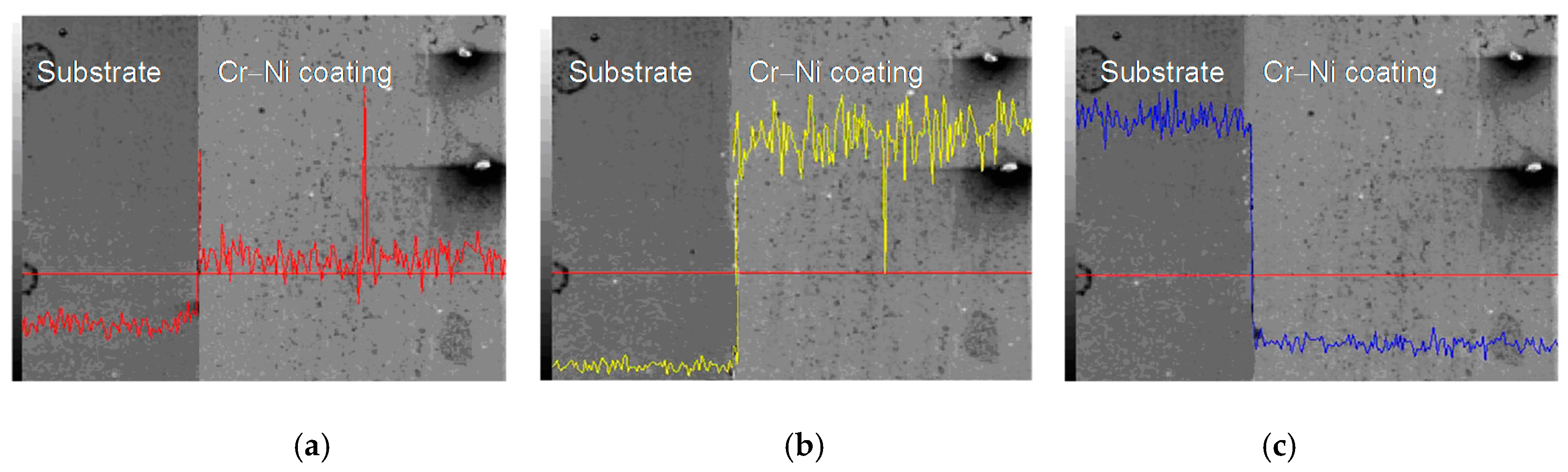
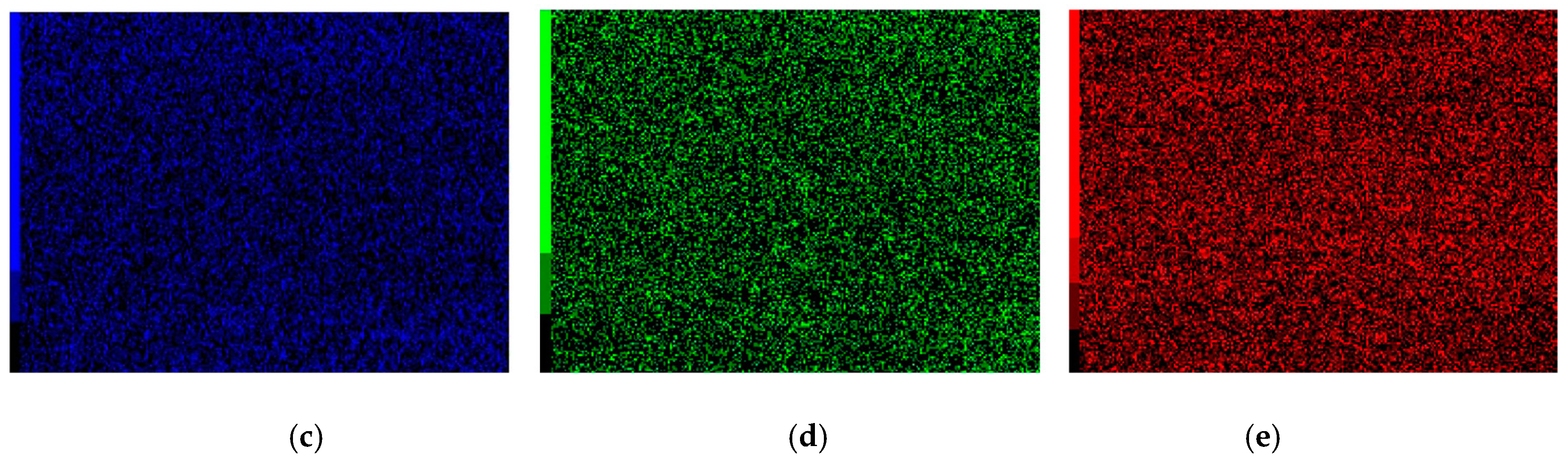
3.3. Line Scan Analysis of Cr–Ni Coating Surface
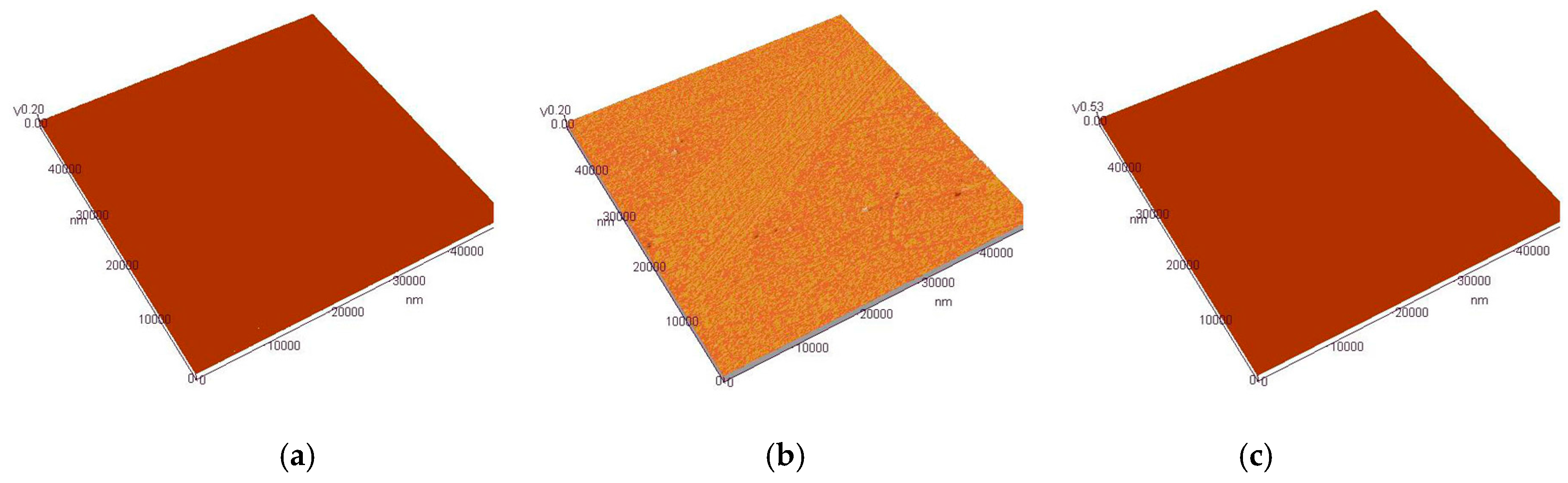
3.4. AFM Analysis of Cr–Ni Coating Surface
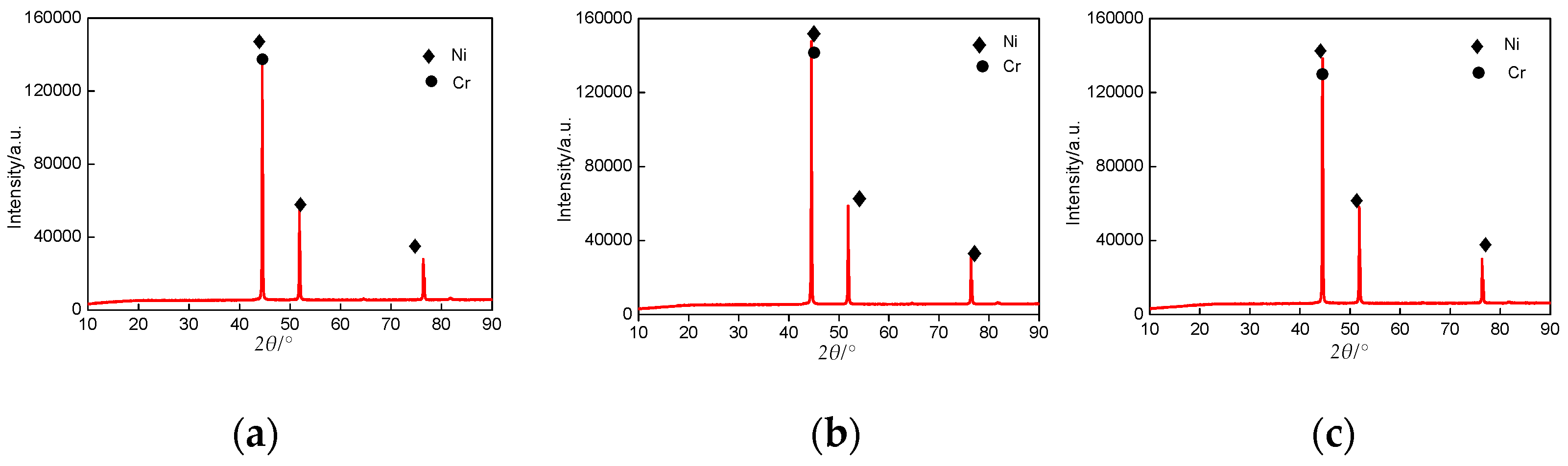
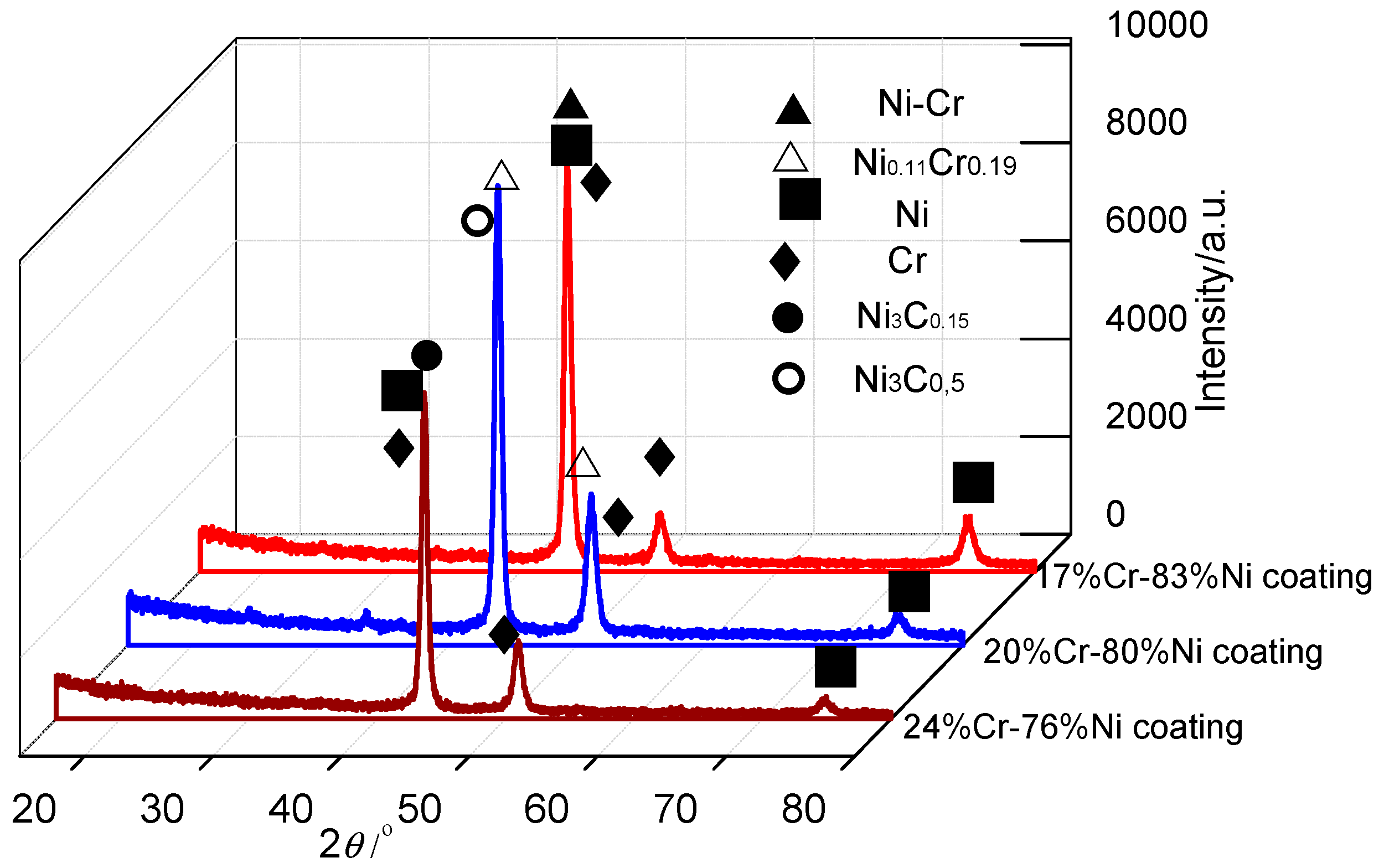
3.5. XRD Analysis of Cr–Ni Coatings
3.6. Friction and Wear Performances
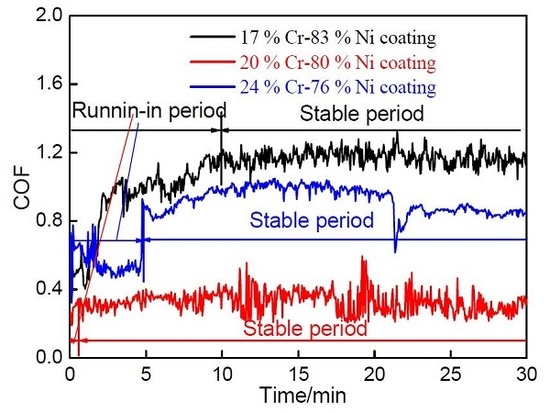
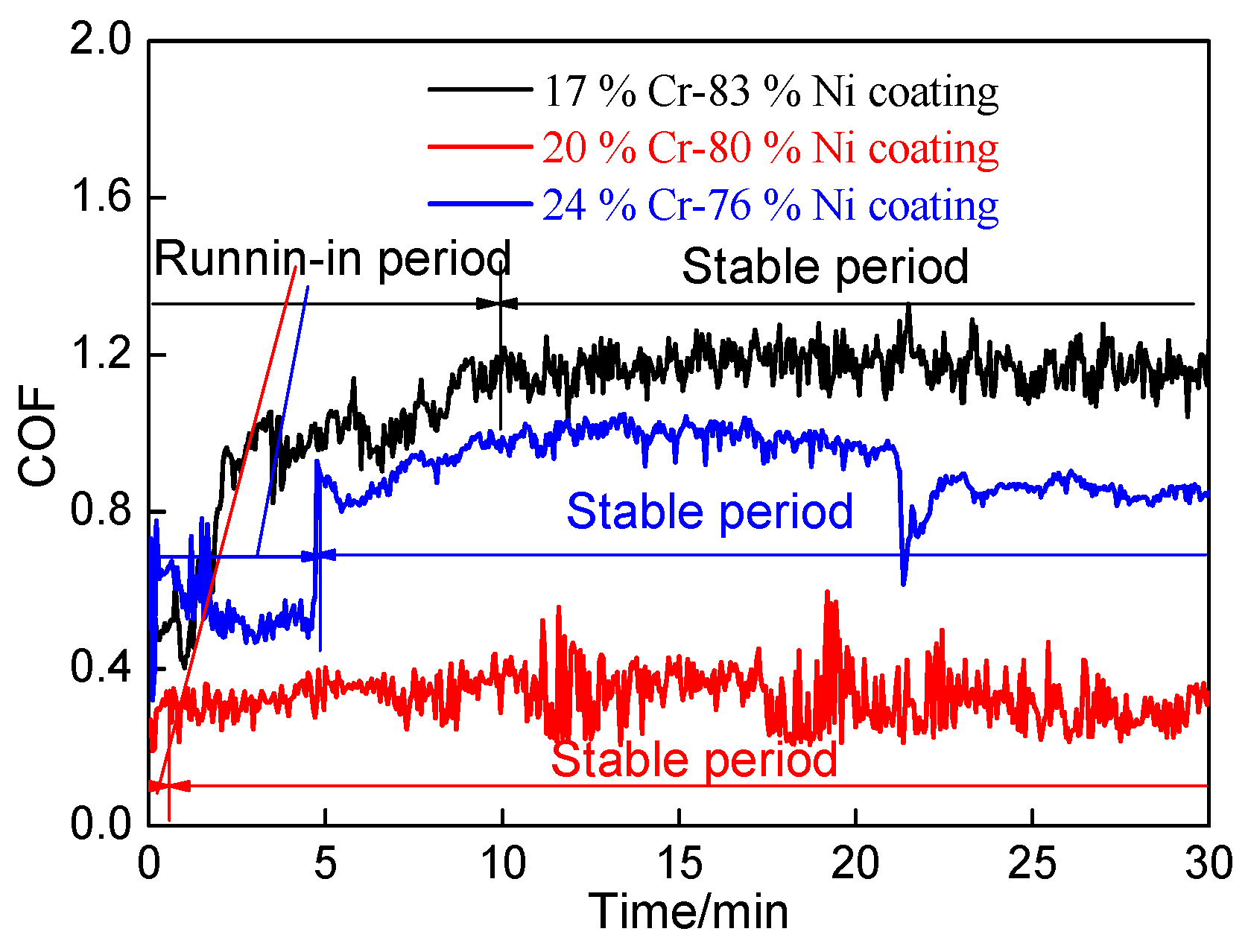
3.6.1. Coefficients of Friction
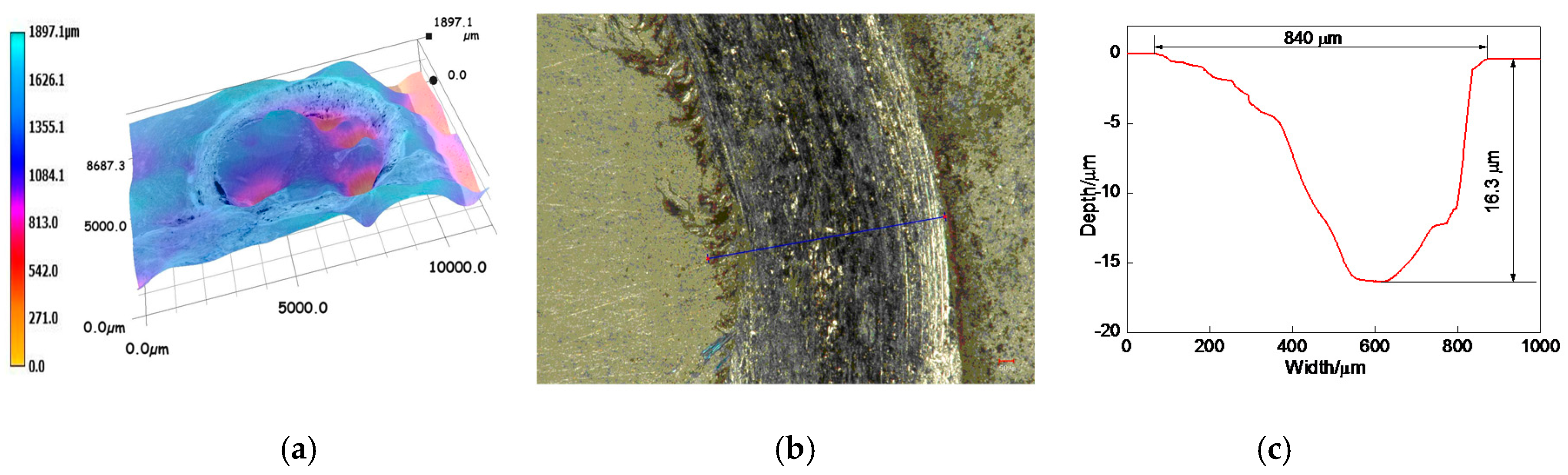
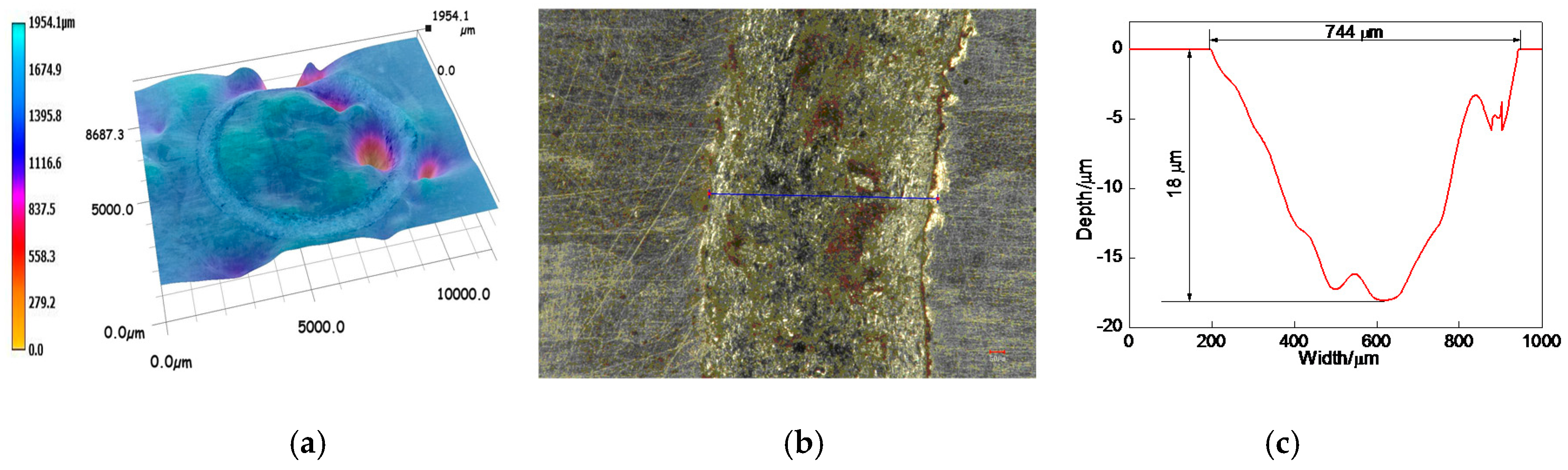
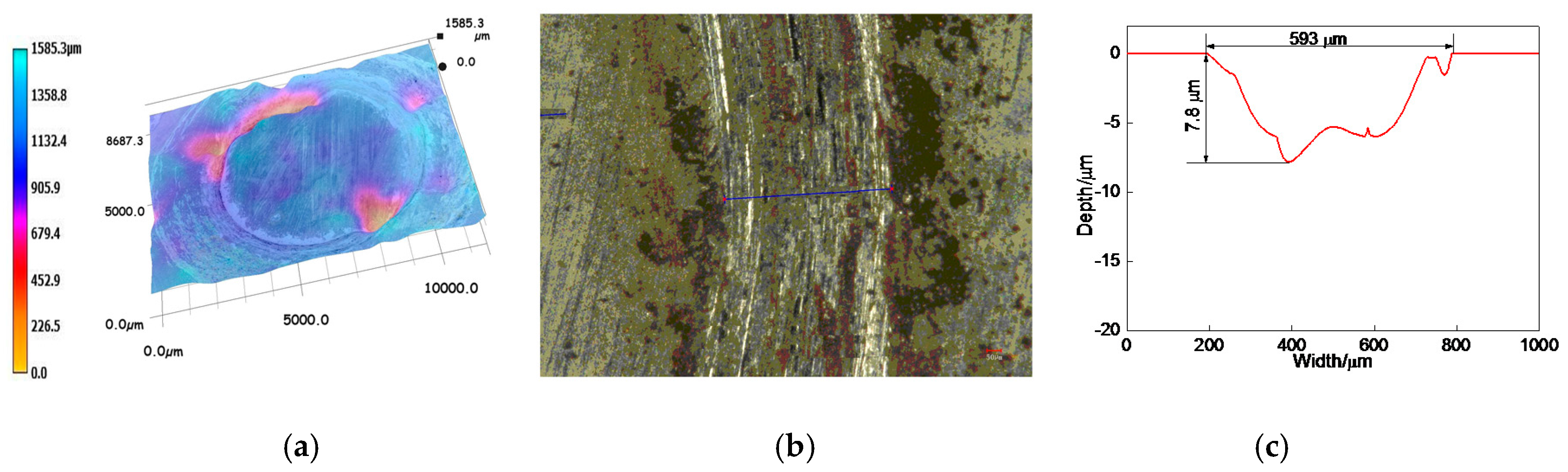
3.6.2. Profiles of Worn Tracks
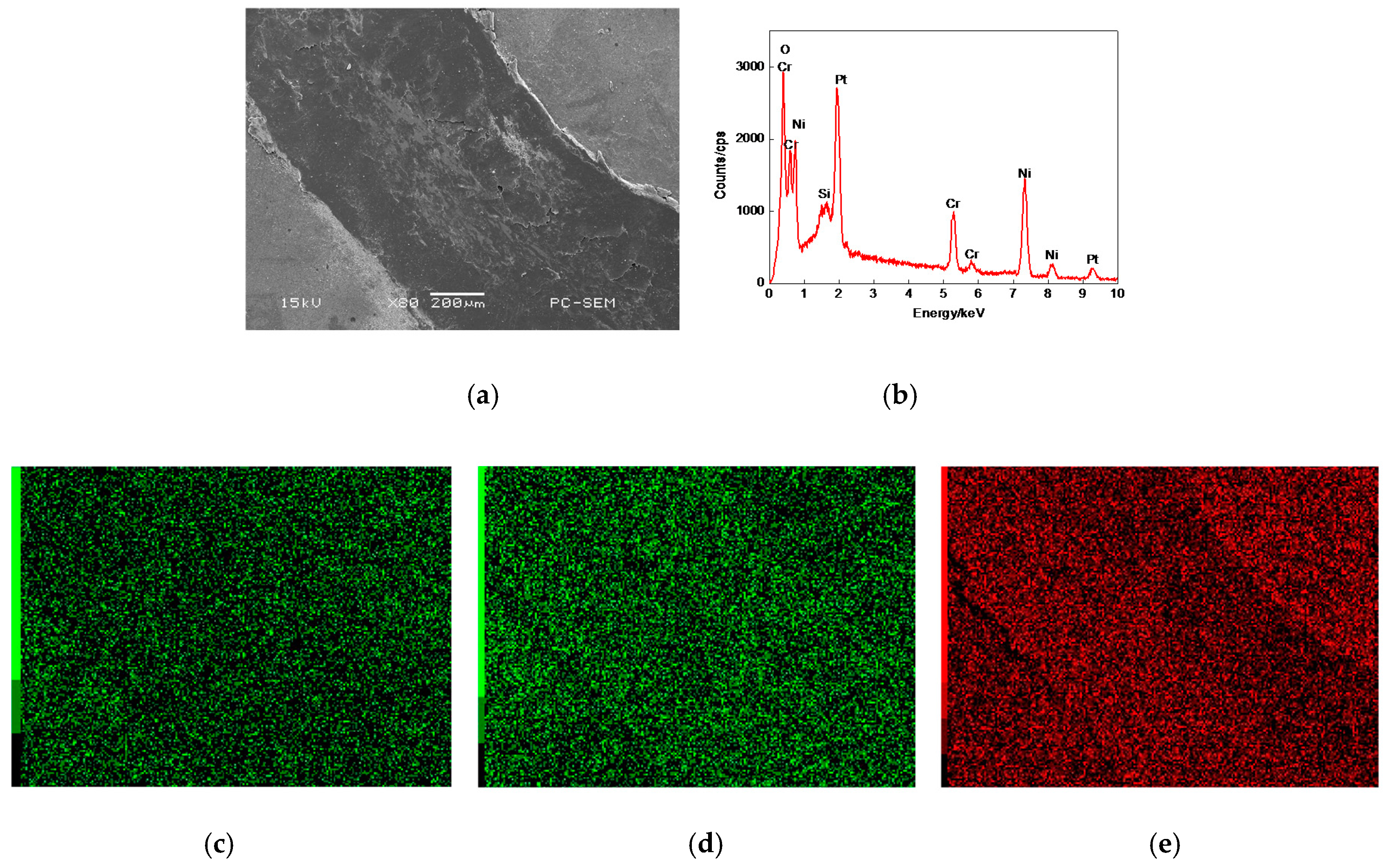
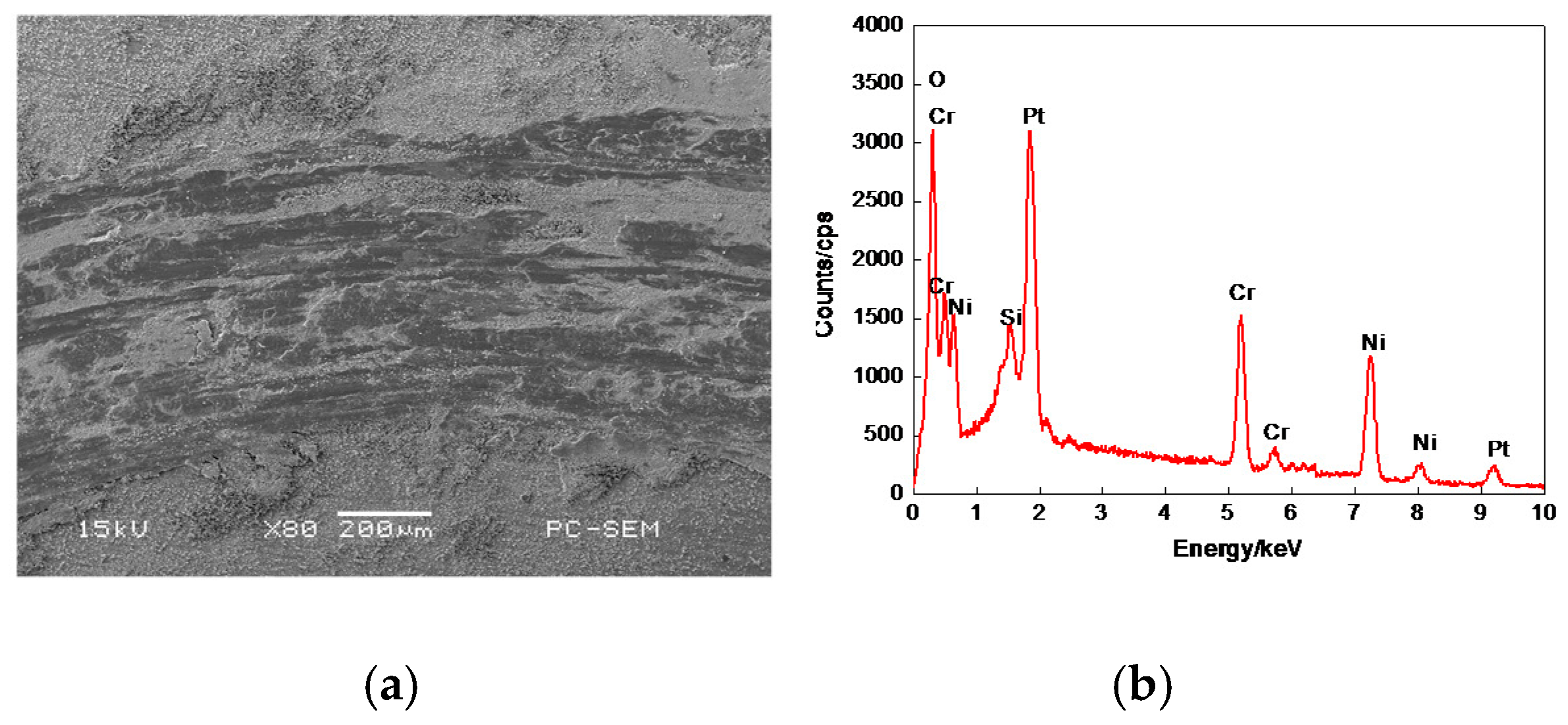
3.6.3. Worn Morphologies of Friction–pairs
3.7. Wear Mechanism
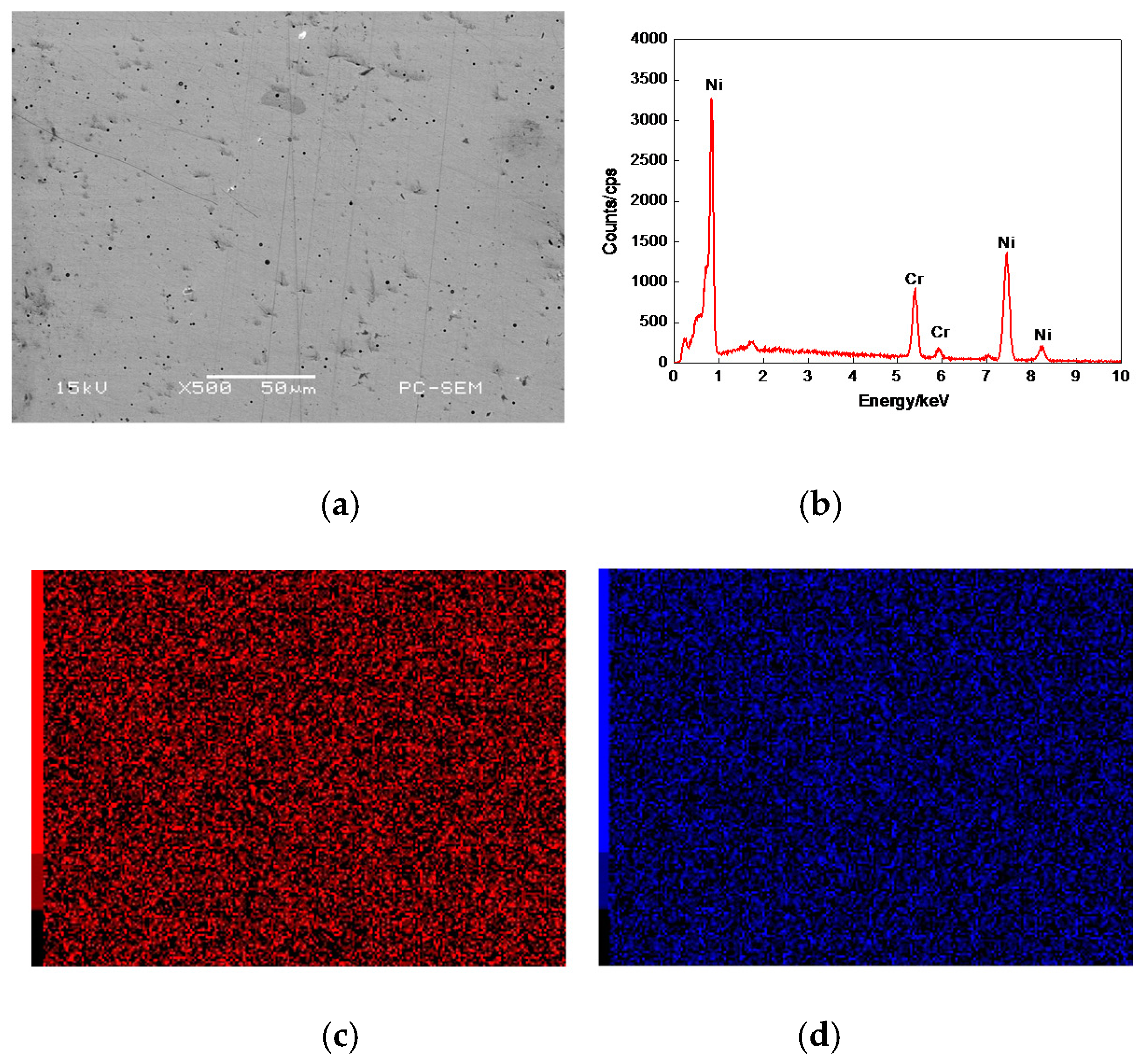
3.7.1. Plane Scan Analysis of Worn Tracks
3.7.2. Worn Morphologies
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Min, Y.G.; Wu, X.C.; Wang, R.; Li, L.; Xu, L.P. Prediction and analysis on oxidation of H13 hot work steel. J. Iron Steel Res. Int. 2006, 13, 44–49. [Google Scholar] [CrossRef]
- Li, J.Y.; Chen, Y.L.; Hu, J.H. Mechanism of improvement on strength and toughness of H13 die steel by nitrogen. Mater. Sci. Eng. A 2015, 640, 16–23. [Google Scholar] [CrossRef]
- Bailey, N.S.; Katinas, C.; Shin, Y.C. Laser direct deposition of AISI H13 tool steel powder with numerical modeling of solid phase transformation, hardness, and residual stresses. J. Mater. Process. Technol. 2017, 247, 223–233. [Google Scholar] [CrossRef]
- Liu, J.H.; Wang, G.X.; Bao, Y.P.; Yang, Y.; Yao, W.; Cui, X.N. Inclusion Variations of hot working die steel H13 in refining process. J. Iron Steel Res. Int. 2012, 19, 1–7. [Google Scholar] [CrossRef]
- Tazegul, O.; Dylmishi, V.; Cimenoglu, H. Copper matrix composite coatings produced by cold spraying process for electrical applications. Arch. Civ. Mech. Eng. 2016, 163, 344–350. [Google Scholar] [CrossRef]
- Chen, S.; Liang, J.; Liu, C.; Sun, K.; Mazumder, J. Preparation of a novel Ni/Co-based alloy gradient coating on surface of the crystallizer copper alloy by laser. Appl. Surf. Sci. 2011, 258, 1443–1450. [Google Scholar] [CrossRef]
- Xu, X.; Mi, G.Y.; Chen, L.; Xiong, L.D.; Jiang, P.; Shao, X.Y.; Wang, C.M. Research on microstructures and properties of Inconel 625 coatings obtained by laser cladding with wire. J. Alloys Compd. 2017, 715, 362–373. [Google Scholar] [CrossRef]
- Comesana, R.; Quintero, F.; Lusquinos, F.; Pascual, M.J.; Boutinguiza, M.; Duran, A.; Pou, J. Laser cladding of bioactive glass coatings. Acta Biomater. 2010, 6, 953–961. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.F.; Lei, J.B.; Dai, X.Q.; Guo, J.B.; Gu, Z.J.; Pan, H.B. Comparative study of the structure and wear resistance of NiCrBSi/50 wt % WC composite coatings by laser cladding and laser induction hybrid cladding. Int. J. Refract. Met. Hard Mater. 2016, 60, 17–27. [Google Scholar] [CrossRef]
- Tao, Y.F.; Li, J.; Lv, Y.H.; Hu, L.F. Effect of heat treatment on residual stress and wear behaviors of the TiNi/Ti2Ni based laser cladding composite coatings. Opt. Laser Technol. 2017, 97, 379–389. [Google Scholar] [CrossRef]
- Lei, J.B.; Shi, C.; Zhou, S.F.; Gu, Z.J.; Zhang, L.C. Enhanced corrosion and wear resistance properties of carbon fiber reinforced Ni-based composite coating by laser cladding. Opt. Laser Technol. 2017, 97, 379–389. [Google Scholar] [CrossRef]
- Weng, F.; Yu, H.J.; Chen, C.Z.; Liu, J.L.; Zhao, L.J.; Dai, J.J.; Zhao, Z.H. Effect of process parameters on the microstructure evolution and wear property of the laser cladding coatings on Ti-6Al-4V alloy. J Alloys Compd. 2017, 692, 989–996. [Google Scholar] [CrossRef]
- He, A.P.; Zeng, J.M. Direct preparation of low Ni-Cr alloy cast iron from red mud and laterite nickel ore. Mater. Des. 2017, 115, 433–440. [Google Scholar] [CrossRef]
- Zhang, P.R.; Liu, Z.Q. On sustainable manufacturing of Cr-Ni alloy coatings by laser cladding and high-efficiency turning process chain and consequent corrosion resistance. J. Clean. Prod. 2017, 161, 676–687. [Google Scholar] [CrossRef]
- Bala, N.; Singh, H.; Prakash, S. High-temperature oxidation studies of cold-sprayed Ni-20Cr and Ni-50Cr coatings on SAE 213-T22 boiler steel. Appl. Surf. Sci. 2009, 255, 6862–6869. [Google Scholar] [CrossRef]
- Zhang, Y.; Peng, X.; Wang, F. Development and oxidation at 800 °C of a novel electrodeposited Ni-Cr nanocomposite film. Mater. Lett. 2004, 58, 1134–1138. [Google Scholar] [CrossRef]
- Weng, Z.K.; Wang, A.H.; Wu, X.H.; Wang, Y.Y.; Yang, Z.X. Wear resistance of diode laser-clad Ni/WC composite coatings at different temperatures. Surf. Coat. Technol. 2016, 304, 283–292. [Google Scholar] [CrossRef]
- Da Silva, M.R.; Gargarella, P.; Gustmann, T.; Botta Filho, W.J.; Kiminami, C.S.; Eckert, J.; Pauly, S.; Bolfarini, C. Laser surface remelting of a Cu-Al-Ni-Mn shape memory alloy. Mater. Sci. Eng. 2016, A661, 61–67. [Google Scholar] [CrossRef]
- Zhou, C.Y.; Zhao, S.S.; Wang, Y.B.; Liu, F.L.; Gao, W.Y.; Lin, X.H. Mitigation of pores generation at overlapping zone during laser cladding. J. Mater. Process. Technol. 2015, 216, 369–374. [Google Scholar] [CrossRef]
- Zhao, G.G.; Zhou, Y.B.; Zhang, H.J. Sliding wear behaviors of electrodeposited Ni composite coatings containing micrometer and nanometer Cr particles. Trans. Nonferrous Met. Soc. China 2009, 19, 319–323. [Google Scholar] [CrossRef]
- Nath, L.; Saha, G.C. Synthesis and characterization of nanocrystalline Al2O3-Ni(Cr) particles using high-energy mechanical alloying process. Surf. Coat. Technol. 2017, 318, 262–269. [Google Scholar] [CrossRef]
- Quan, C.; He, Y.D.; Zhang, J. High temperature oxidation behavior of a novel Ni-Cr binary alloy coating prepared by cathode plasma electrolytic deposition. Surf. Coat. Technol. 2016, 292, 11–19. [Google Scholar] [CrossRef]
- Conceição, L.D.; D’Oliveira, A.S.C.M. The effect of oxidation on the tribolayer and sliding wear of a Co-based coating. Surf. Coat. Technol. 2016, 288, 69–78. [Google Scholar] [CrossRef]
- Wei, D.B.; Zhang, P.Z.; Yao, Z.J.; Wei, X.F.; Zhou, J.T.; Chen, X.H. Preparation and high-temperature oxidation behavior of plasma Cr-Ni alloying on Ti6Al4V alloy based on double glow plasma surface metallurgy technology. Appl. Surf. Sci. 2016, 388, 571–578. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Chen, K.M.; Wang, L.; Cui, X.H.; Wang, S.Q. Characteristics of oxidative wear and oxidative mild wear. Tribol. Int. 2013, 61, 214–223. [Google Scholar] [CrossRef]
- Feng, K.; Chen, Y.; Deng, P.S.; Li, Y.Y.; Zhao, H.X.; Lu, F.G.; Li, R.F.; Huang, J.; Li, Z.G. Improved high-temperature hardness and wear resistance of Inconel 625 coatings fabricated by laser cladding. J. Mater. Process. Technol. 2017, 243, 82–91. [Google Scholar] [CrossRef]
- Yin, J.; Wang, D.Z.; Meng, L.; Ke, L.D.; Hu, Q.W.; Xiao, Y.Z. High-temperature slide wear of Ni-Cr-Si metal silicide based composite coatings on copper substrate by laser-induction hybrid cladding. Surf. Coat. Technol. 2017, 325, 120–126. [Google Scholar] [CrossRef]






| C | Si | Mn | Cr | Mo | V | P | S | Fe |
|---|---|---|---|---|---|---|---|---|
| 0.32–0.45 | 0.80–1.20 | 0.20–0.50 | 4.75–5.50 | 1.10–1.75 | 0.80–1.20 | ≤0.03 | ≤0.03 | other |
| Coating Kinds | Average Roughness Sa/nm | Root mean Square Sq/nm | Surface Skewness Ssk | Surface kurtosis Sku | Ten Point Height Sz/nm |
|---|---|---|---|---|---|
| 17% Cr–83% Ni | 3.96 | 6.82 | −2.18 | 24.80 | 126 |
| 20% Cr–80% Ni | 4.97 | 9.55 | 3.62 | 34.30 | 166 |
| 24% Cr–76% Ni | 4.07 | 7.44 | −3.35 | 32.1 | 131 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiahong, L.; Dejun, K. Micro-Structures and High-Temperature Friction-Wear Performances of Laser Cladded Cr–Ni Coatings. Materials 2018, 11, 137. https://doi.org/10.3390/ma11010137
Jiahong L, Dejun K. Micro-Structures and High-Temperature Friction-Wear Performances of Laser Cladded Cr–Ni Coatings. Materials. 2018; 11(1):137. https://doi.org/10.3390/ma11010137
Chicago/Turabian StyleJiahong, Li, and Kong Dejun. 2018. "Micro-Structures and High-Temperature Friction-Wear Performances of Laser Cladded Cr–Ni Coatings" Materials 11, no. 1: 137. https://doi.org/10.3390/ma11010137
APA StyleJiahong, L., & Dejun, K. (2018). Micro-Structures and High-Temperature Friction-Wear Performances of Laser Cladded Cr–Ni Coatings. Materials, 11(1), 137. https://doi.org/10.3390/ma11010137