Photoswitchable Fluorescent Diarylethene Derivatives with Thiophene 1,1-Dioxide Groups: Effect of Alkyl Substituents at the Reactive Carbons
Abstract
1. Introduction
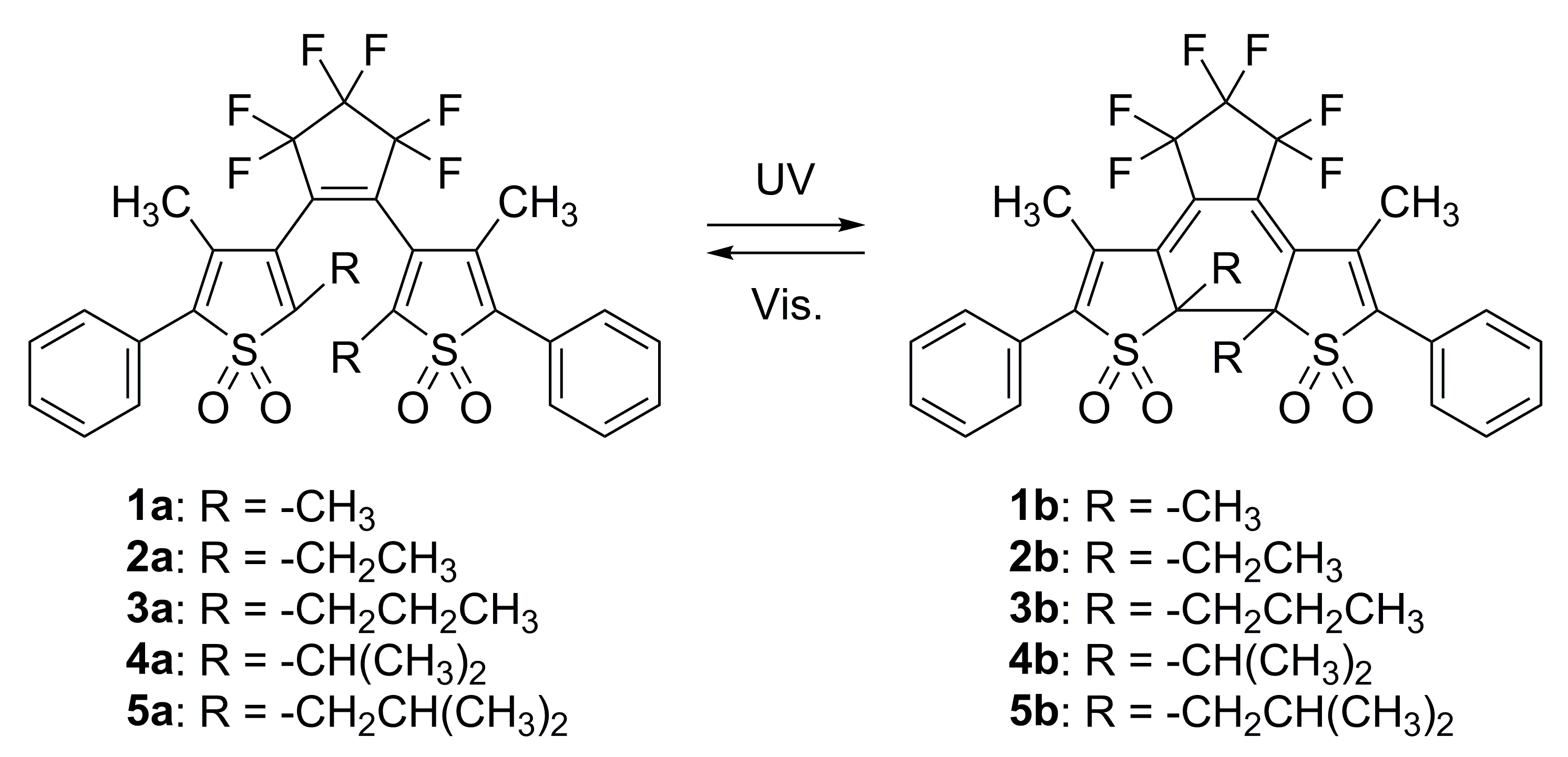
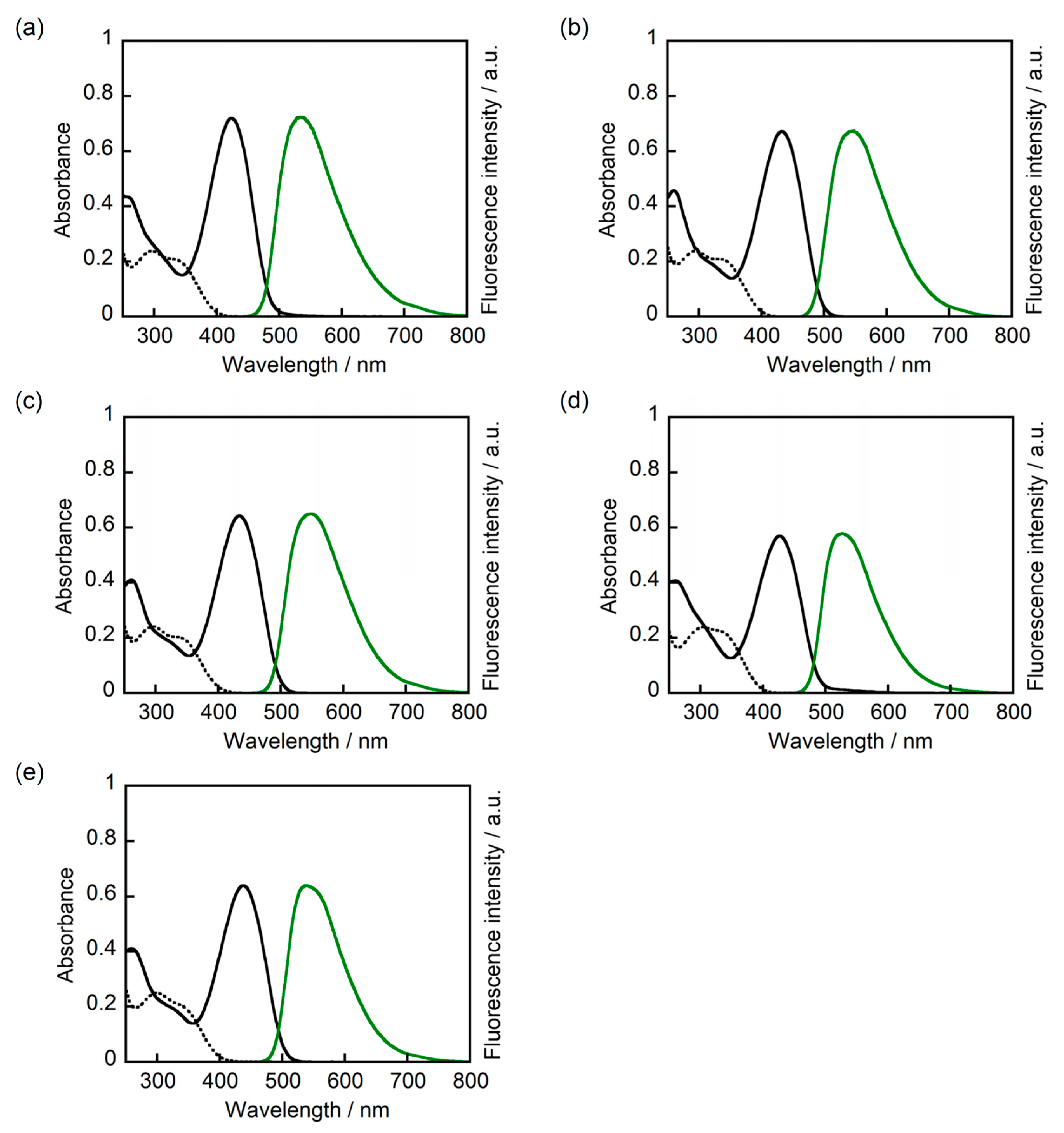
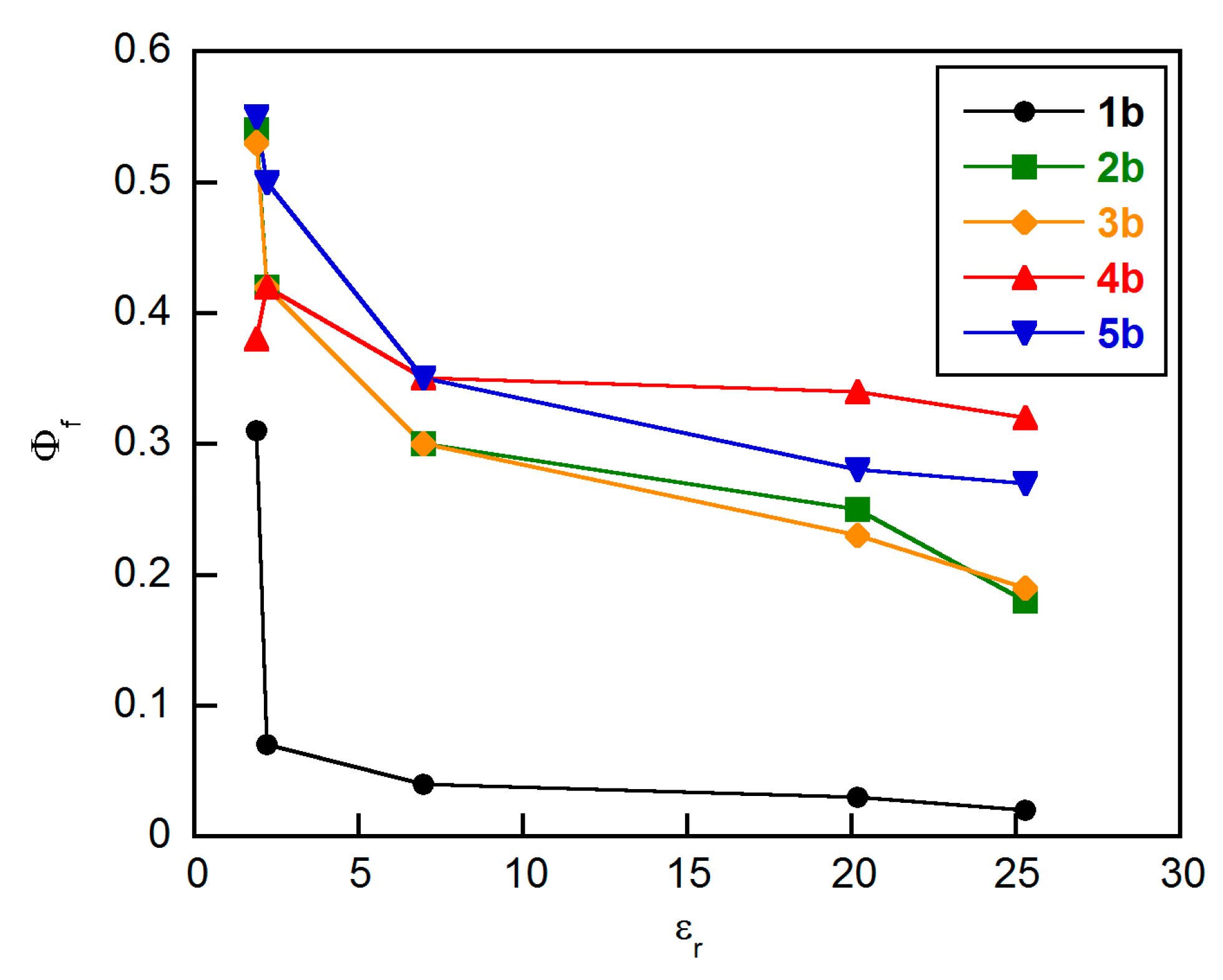
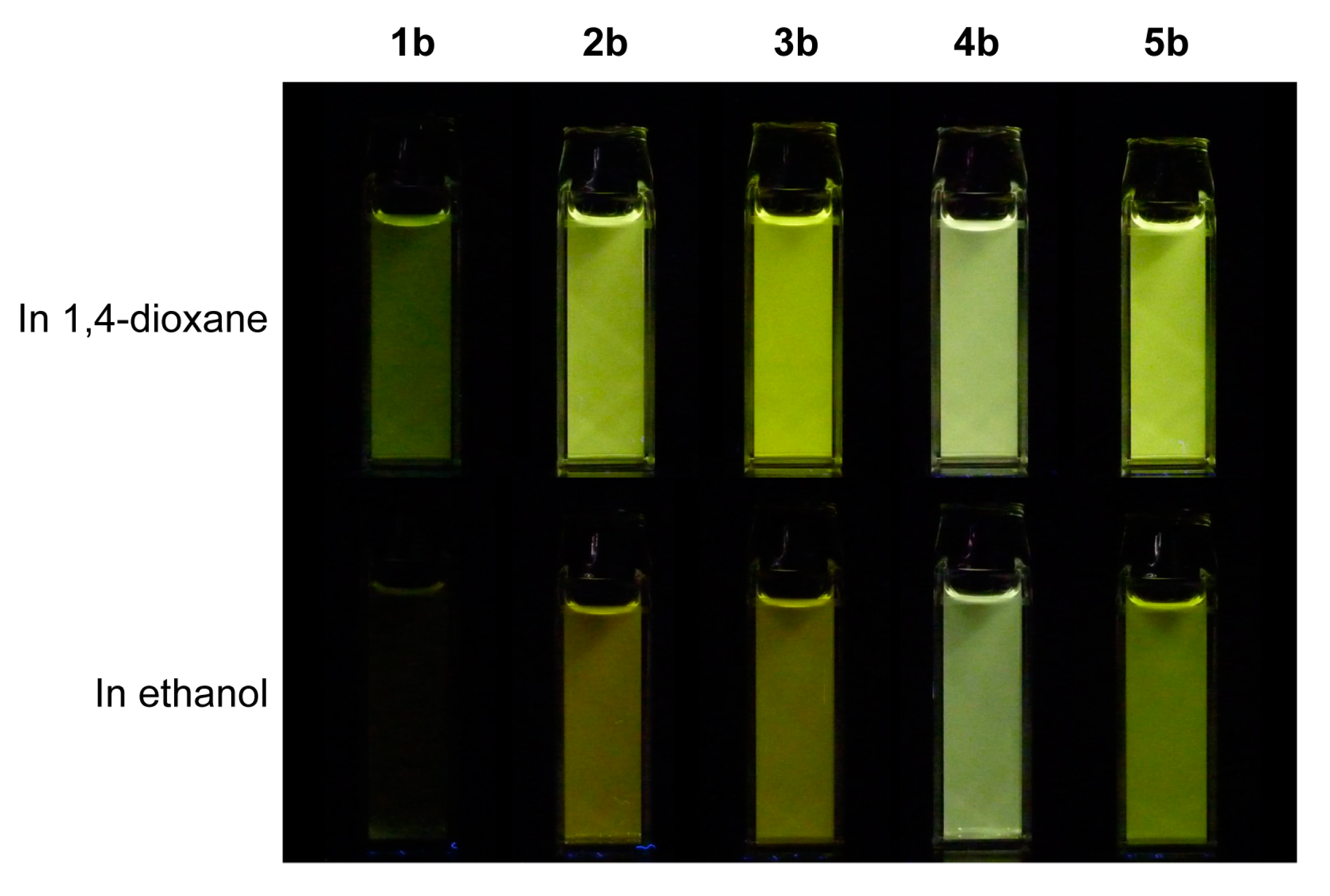
2. Results and Discussion
3. Materials and Methods
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Heilemann, M.; Dedecker, P.; Hofkens, J.; Sauer, M. Photoswitches: Key molecules for subdiffraction-resolution fluorescence imaging and molecular quantification. Laser Photon. Rev. 2009, 3, 180–202. [Google Scholar] [CrossRef]
- Raymo, F.M. Photoactivatable synthetic fluorophores. Phys. Chem. Chem. Phys. 2013, 15, 14840–14850. [Google Scholar] [CrossRef] [PubMed]
- Fürstenberg, A.; Heilemann, M. Single-molecule localization microscopy-near molecular spatial resolution in light microscopy with photoswitchable fluorophores. Phys. Chem. Chem. Phys. 2013, 15, 14919–14930. [Google Scholar] [CrossRef] [PubMed]
- Fukaminato, T. Single-molecule fluorescence photoswitching: Design and synthesis of photoswitchable fluorescent molecules. J. Photochem. Photobiol. C 2011, 12, 177–208. [Google Scholar] [CrossRef]
- Zhang, J.; Qi, Z.; Tian, H. Photochromic materials: More than meets the eye. Adv. Mater. 2013, 25, 378–399. [Google Scholar] [CrossRef] [PubMed]
- Irie, M.; Fukaminato, T.; Sasaki, T.; Tamai, N.; Kawai, T. Organic chemistry: A digital fluorescent molecular photoswitch. Nature 2002, 420, 759–760. [Google Scholar] [CrossRef] [PubMed]
- Fukaminato, T.; Sasaki, T.; Kawai, T.; Tamai, N.; Irie, M. Digital photoswitching of fluorescence based on the photochromism of diarylethene derivatives at a single-molecule level. J. Am. Chem. Soc. 2004, 126, 14843–14849. [Google Scholar] [CrossRef] [PubMed]
- Fukaminato, T.; Umemoto, T.; Iwata, Y.; Yokojima, S.; Yoneyama, M.; Nakamura, S.; Irie, M. Photochromism of diarylethene single molecules in polymer matrices. J. Am. Chem. Soc. 2007, 129, 5932–5938. [Google Scholar] [CrossRef] [PubMed]
- Fukaminato, T.; Tanaka, M.; Doi, T.; Tamaoki, N.; Katayama, T.; Mallick, A.; Ishibashi, Y.; Miyasaka, H.; Irie, M. Fluorescence photoswitching of a diarylethene-perylenebisimide dyad based on intramolecular electron transfer. Photochem. Photobiol. Sci. 2010, 9, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Fukaminato, T.; Doi, T.; Tamaoki, N.; Okuno, K.; Ishibashi, Y.; Miyasaka, H.; Irie, M. Single-molecule fluorescence photoswitching of a diarylethene-perylenebisimide dyad: Non-destructive fluorescence readout. J. Am. Chem. Soc. 2011, 133, 4984–4990. [Google Scholar] [CrossRef] [PubMed]
- Golovkova, T.A.; Kozlov, D.V.; Neckers, D.C. Synthesis and properties of novel fluorescent switches. J. Org. Chem. 2005, 70, 5545–5549. [Google Scholar] [CrossRef] [PubMed]
- Bossi, M.; Belov, V.; Polyakova, S.; Hell, S.W. Reversible red fluorescent molecular switches. Angew. Chem. Int. Ed. 2006, 45, 7462–7465. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.F.; Belov, V.N.; Bossi, M.L.; Hell, S.W. Switchable fluorescent and solvatochromic molecular probes based on 4-amino-n-methylphthalimide and a photochromic diarylethene. Eur. J. Org. Chem. 2008, 2008, 2531–2538. [Google Scholar] [CrossRef]
- Berberich, M.; Krause, A.-M.; Orlandi, M.; Scandola, F.; Würthner, F. Toward fluorescent memories with nondestructive readout: Photoswitching of fluorescence by intramolecular electron transfer in a diaryl ethene-perylene bisimide photochromic system. Angew. Chem. Int. Ed. 2008, 47, 6616–6619. [Google Scholar] [CrossRef] [PubMed]
- Berberich, M.; Natali, M.; Spenst, P.; Chiorboli, C.; Scandola, F.; Würthner, F. Nondestructive photoluminescence read-out by intramolecular electron transfer in a perylene bisimide-diarylethene dyad. Chem.-Eur. J. 2012, 18, 13651–13664. [Google Scholar] [CrossRef] [PubMed]
- Berberich, M.; Würthner, F. Terrylene bisimide-diarylethene photochromic switch. Chem. Sci. 2012, 3, 2771–2777. [Google Scholar] [CrossRef]
- Hell, S.W. Far-field optical nanoscopy. Science 2007, 316, 1153–1158. [Google Scholar] [CrossRef] [PubMed]
- Hell, S.W. Microscopy and its focal switch. Nat. Meth. 2009, 6, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Betzig, E.; Patterson, G.H.; Sougrat, R.; Lindwasser, O.W.; Olenych, S.; Bonifacino, J.S.; Davidson, M.W.; Lippincott-Schwartz, J.; Hess, H.F. Imaging intracellular fluorescent proteins at nanometer resolution. Science 2006, 313, 1642–1645. [Google Scholar] [CrossRef] [PubMed]
- Rust, M.J.; Bates, M.; Zhuang, X. Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM). Nat. Meth. 2006, 3, 793–796. [Google Scholar] [CrossRef] [PubMed]
- Bates, M.; Huang, B.; Dempsey, G.T.; Zhuang, X.W. Multicolor super-resolution imaging with photo-switchable fluorescent probes. Science 2007, 317, 1749–1753. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.-C.; Yang, S.I.; Ahn, K.-H.; Kim, E. Highly fluorescent photochromic diarylethene in the closed-ring form. Chem. Commun. 2005, 2503–2505. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.-C.; Yang, S.I.; Kim, E.; Ahn, K.-H. Development of highly fluorescent photochromic material with high fatigue resistance. Tetrahedron 2006, 62, 5855–5861. [Google Scholar] [CrossRef]
- Jeong, Y.-C.; Yang, S.I.; Kim, E.; Ahn, K.-H. A high-content diarylethene photochromic polymer for an efficient fluorescence modulation. Macromol. Rapid Commun. 2006, 27, 1769–1773. [Google Scholar] [CrossRef]
- Jeong, Y.-C.; Park, D.G.; Lee, I.S.; Yang, S.I.; Ahn, K.-H. Highly fluorescent photochromic diarylethene with an excellent fatigue property. J. Mater. Chem. 2009, 19, 97–103. [Google Scholar] [CrossRef]
- Uno, K.; Niikura, H.; Morimoto, M.; Ishibashi, Y.; Miyasaka, H.; Irie, M. In situ preparation of highly fluorescent dyes upon photoirradiation. J. Am. Chem. Soc. 2011, 133, 13558–13564. [Google Scholar] [CrossRef] [PubMed]
- Takagi, Y.; Kunishi, T.; Katayama, T.; Ishibashi, Y.; Miyasaka, H.; Morimoto, M.; Irie, M. Photoswitchable fluorescent diarylethene derivatives with short alkyl chain substituents. Photochem. Photobiol. Sci. 2012, 11, 1661–1665. [Google Scholar] [CrossRef] [PubMed]
- Gillanders, F.; Giordano, L.; Diaz, S.A.; Jovin, T.M.; Jares-Erijman, E.A. Photoswitchable fluorescent diheteroarylethenes: Substituent effects on photochromic and solvatochromic properties. Photochem. Photobiol. Sci. 2014, 13, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Sumi, T.; Kaburagi, T.; Morimoto, M.; Une, K.; Sotome, H.; Ito, S.; Miyasaka, H.; Irie, M. Fluorescent photochromic diarylethene that turns on with visible light. Org. Lett. 2015, 17, 4802–4805. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Johnsen, B.; Qin, Z.; Morimoto, M.; Baillie, D.; Irie, M.; Branda, N.R. Two-colour fluorescent imaging in organisms using self-assembled nano-systems of upconverting nanoparticles and molecular switches. Nanoscale 2015, 7, 11263–11266. [Google Scholar] [CrossRef] [PubMed]
- Bälter, M.; Li, S.; Morimoto, M.; Tang, S.; Hernando, J.; Guirado, G.; Irie, M.; Raymo, F.M.; Andréasson, J. Emission color tuning and white-light generation based on photochromic control of energy transfer reactions in polymer micelles. Chem. Sci. 2016, 7, 5867–5871. [Google Scholar] [CrossRef]
- Morimoto, M.; Kashihara, R.; Mutoh, K.; Kobayashi, Y.; Abe, J.; Sotome, H.; Ito, S.; Miyasaka, H.; Irie, M. Turn-on mode fluorescence photoswitching of diarylethene single crystals. CrystEngComm 2016, 18, 7241–7248. [Google Scholar] [CrossRef]
- Takagi, Y.; Morimoto, M.; Kashihara, R.; Fujinami, S.; Ito, S.; Miyasaka, H.; Irie, M. Turn-on mode fluorescent diarylethenes: Control of the cycloreversion quantum yield. Tetrahedron 2017, 73, 4918–4924. [Google Scholar] [CrossRef]
- Morimoto, M.; Irie, M. Turn-on mode fluorescent diarylethenes. In Photon-Working Switches; Yokoyama, Y., Nakatani, K., Eds.; Springer Japan KK: Tokyo, Japan, 2017; ISBN 978-4-431-56542-0. [Google Scholar]
- Nevskyi, O.; Sysoiev, D.; Oppermann, A.; Huhn, T.; Wöll, D. Nanoscopic visualization of soft matter using fluorescent diarylethene photoswitches. Angew. Chem. Int. Ed. 2016, 55, 12698–12702. [Google Scholar] [CrossRef] [PubMed]
- Arai, Y.; Ito, S.; Fujita, H.; Yoneda, Y.; Kaji, T.; Takei, S.; Kashihara, R.; Morimoto, M.; Irie, M.; Miyasaka, H. One-colour control of activation, excitation and deactivation of a fluorescent diarylethene derivative in super-resolution microscopy. Chem. Commun. 2017, 53, 4066–4069. [Google Scholar] [CrossRef] [PubMed]
- Roubinet, B.; Weber, M.; Shojaei, H.; Bates, M.; Bossi, M.L.; Belov, V.N.; Irie, M.; Hell, S.W. Fluorescent photoswitchable diarylethenes for biolabeling and single-molecule localization microscopies with optical superresolution. J. Am. Chem. Soc. 2017, 139, 6611–6620. [Google Scholar] [CrossRef] [PubMed]
- Roubinet, B.; Bossi, M.L.; Alt, P.; Leutenegger, M.; Shojaei, H.; Schnorrenberg, S.; Nizamov, S.; Irie, M.; Belov, V.N.; Hell, S.W. Carboxylated photoswitchable diarylethenes for biolabeling and super-resolution RESOLFT microscopy. Angew. Chem. Int. Ed. 2016, 55, 15429–15433. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, M.; Nakagawa, T.; Nakashima, T.; Kawai, T. Photochromic and fluorescence switching properties of oxidized triangle terarylenes in solution and in amorphous solid states. J. Mater. Chem. 2011, 21, 17425–17432. [Google Scholar] [CrossRef]
- Kitagawa, D.; Sasaki, K.; Kobatake, S. Correlation between steric substituent constants and thermal cycloreversion reactivity of diarylethene closed-ring isomers. Bull. Chem. Soc. Jpn. 2011, 84, 141–147. [Google Scholar] [CrossRef]
- Dedecker, P.; Hotta, J.-I.; Flors, C.; Sliwa, M.; Uji-i, H.; Roeffaers, M.B.J.; Ando, R.; Mizuno, H.; Miyawaki, A.; Hofkens, J. Subdiffraction imaging through the selective donut-mode depletion of thermally stable photoswitchable fluorophores: Numerical analysis and application to the fluorescent protein dronpa. J. Am. Chem. Soc. 2007, 129, 16132–16141. [Google Scholar] [CrossRef] [PubMed]
- Shibata, K.; Muto, K.; Kobatake, S.; Irie, M. Photocyclization/cycloreversion quantum yields of diarylethenes in single crystals. J. Phys. Chem. A 2002, 106, 209–214. [Google Scholar] [CrossRef]
| Open-Ring Isomer, a | Closed-Ring Isomer, b | ||||
|---|---|---|---|---|---|
| λmax/nm (ε/104 M−1 cm−1) | Φoc | λmax/nm (ε/104 M−1 cm−1) | Φco | Φf | |
| 1 | 296 (0.79) | 0.14 | 424 (2.4) | 3.5 × 10−5 | 0.07 |
| 2 | 297 (0.79) | 0.09 | 433 (2.2) | 3.8 × 10−5 | 0.42 |
| 3 | 298 (0.80) | 0.10 | 434 (2.1) | 7.1 × 10−5 | 0.42 |
| 4 | 301 (0.79) | 0.21 | 427 (1.8) | 2.6 × 10−5 | 0.42 |
| 5 | 299 (0.83) | 0.12 | 438 (2.1) | 2.1 × 10−3 | 0.50 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morimoto, M.; Sumi, T.; Irie, M. Photoswitchable Fluorescent Diarylethene Derivatives with Thiophene 1,1-Dioxide Groups: Effect of Alkyl Substituents at the Reactive Carbons. Materials 2017, 10, 1021. https://doi.org/10.3390/ma10091021
Morimoto M, Sumi T, Irie M. Photoswitchable Fluorescent Diarylethene Derivatives with Thiophene 1,1-Dioxide Groups: Effect of Alkyl Substituents at the Reactive Carbons. Materials. 2017; 10(9):1021. https://doi.org/10.3390/ma10091021
Chicago/Turabian StyleMorimoto, Masakazu, Takaki Sumi, and Masahiro Irie. 2017. "Photoswitchable Fluorescent Diarylethene Derivatives with Thiophene 1,1-Dioxide Groups: Effect of Alkyl Substituents at the Reactive Carbons" Materials 10, no. 9: 1021. https://doi.org/10.3390/ma10091021
APA StyleMorimoto, M., Sumi, T., & Irie, M. (2017). Photoswitchable Fluorescent Diarylethene Derivatives with Thiophene 1,1-Dioxide Groups: Effect of Alkyl Substituents at the Reactive Carbons. Materials, 10(9), 1021. https://doi.org/10.3390/ma10091021





