Esterification Mechanism of Bagasse Modified with Glutaric Anhydride in 1-Allyl-3-methylimidazolium Chloride
Abstract
:1. Introduction
2. Results and Discussion
2.1. Esterification of Bagasse
2.2. PS of Bagasse
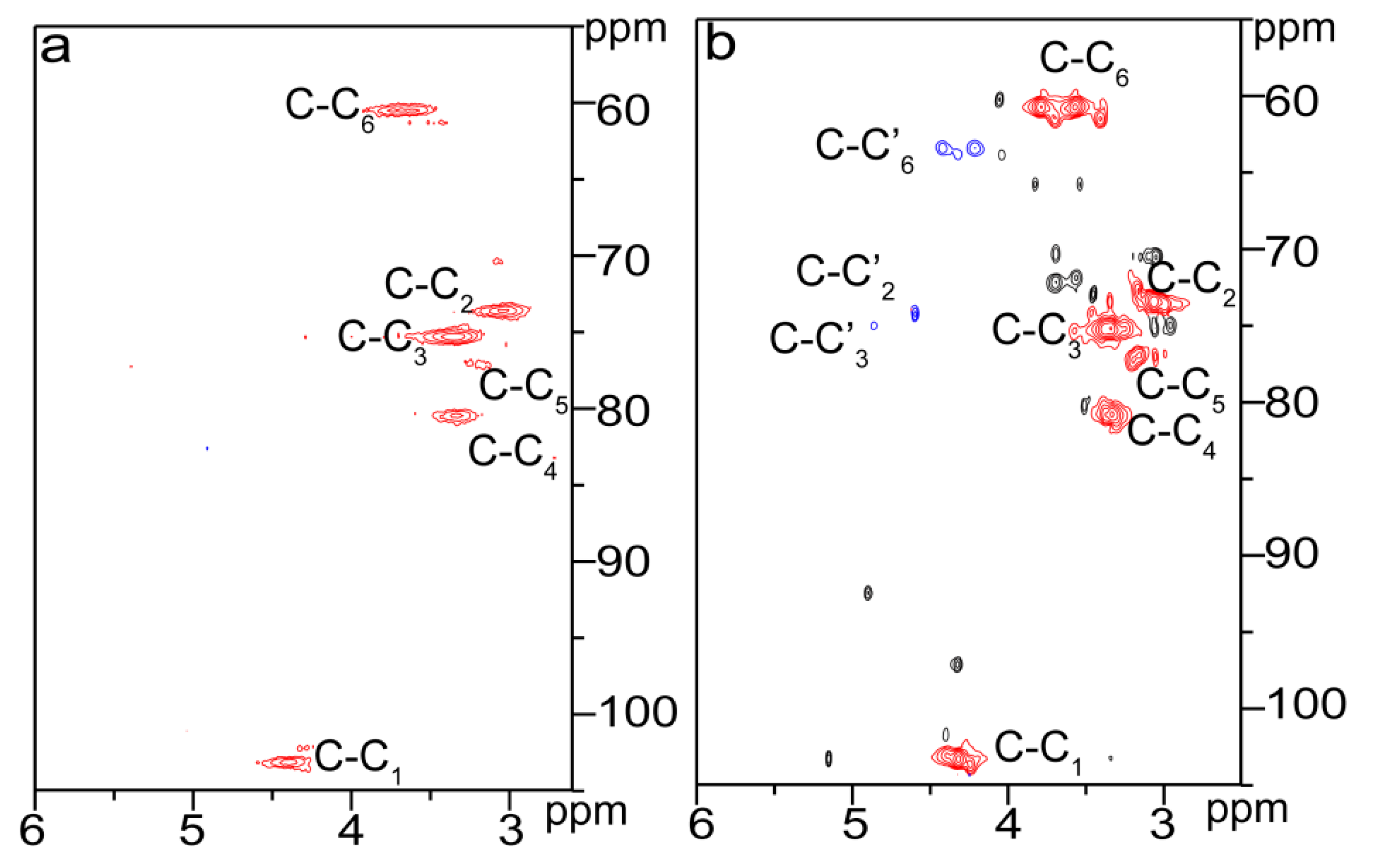
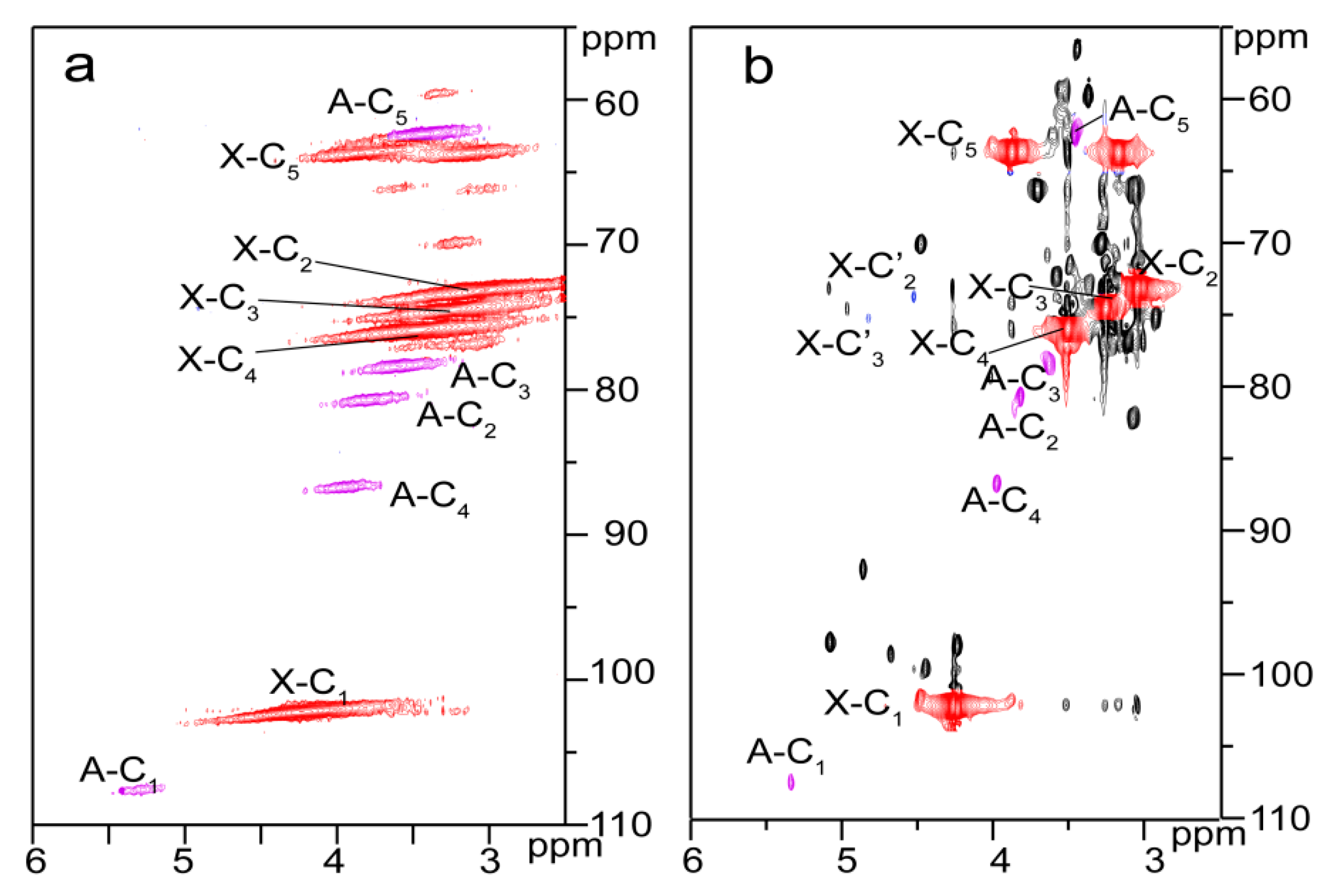
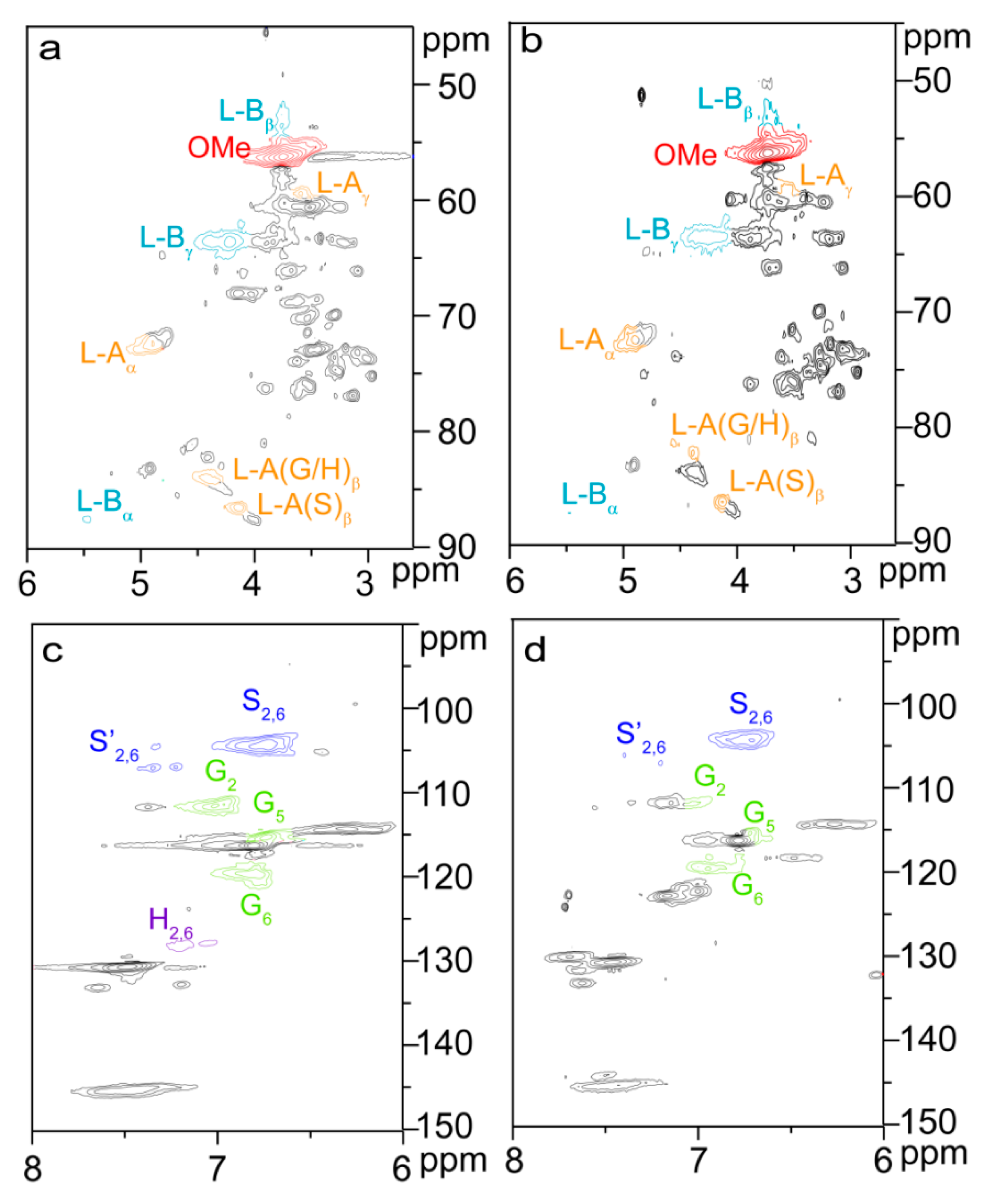
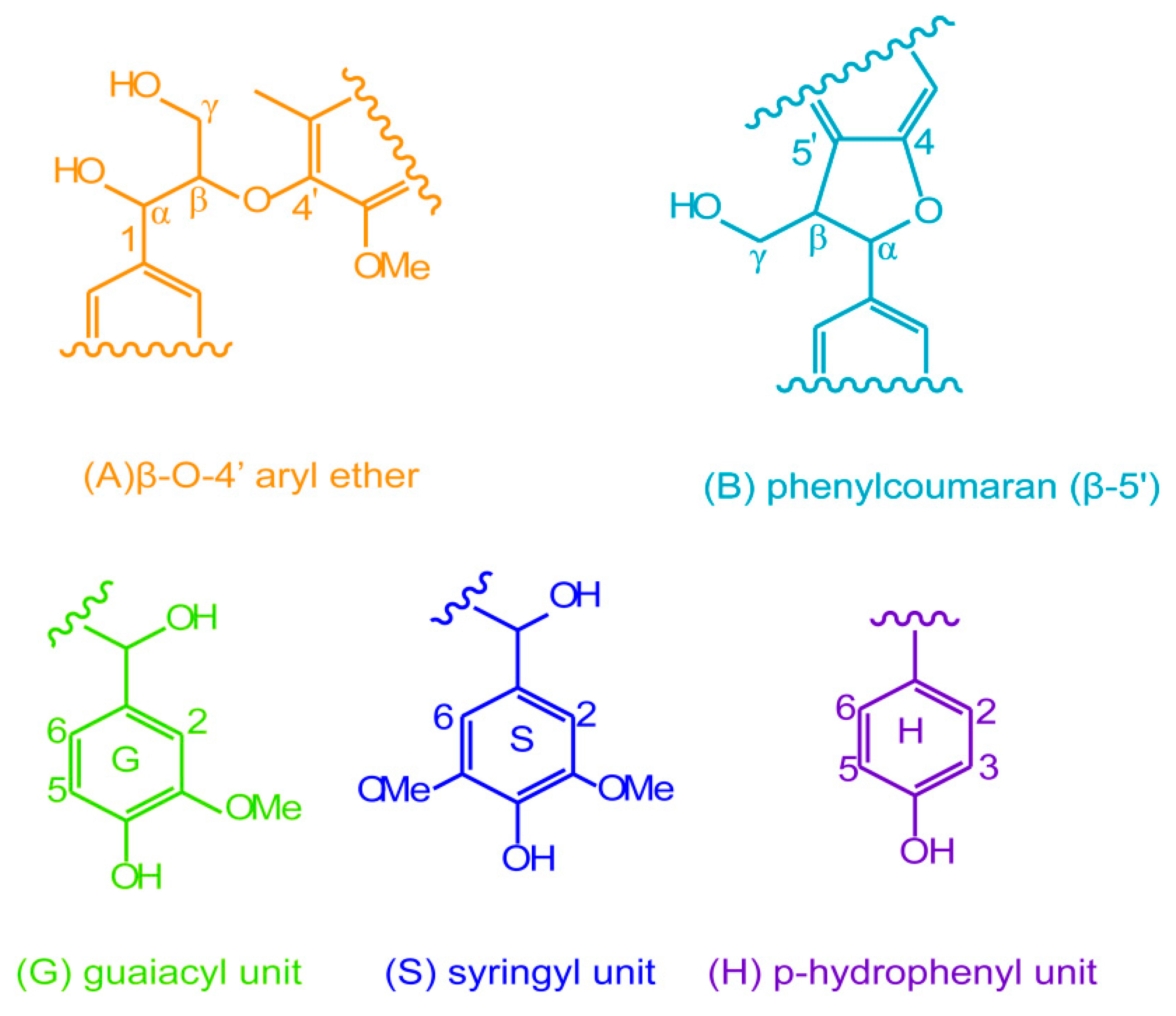
2.3. Reactive Sites of Bagasse
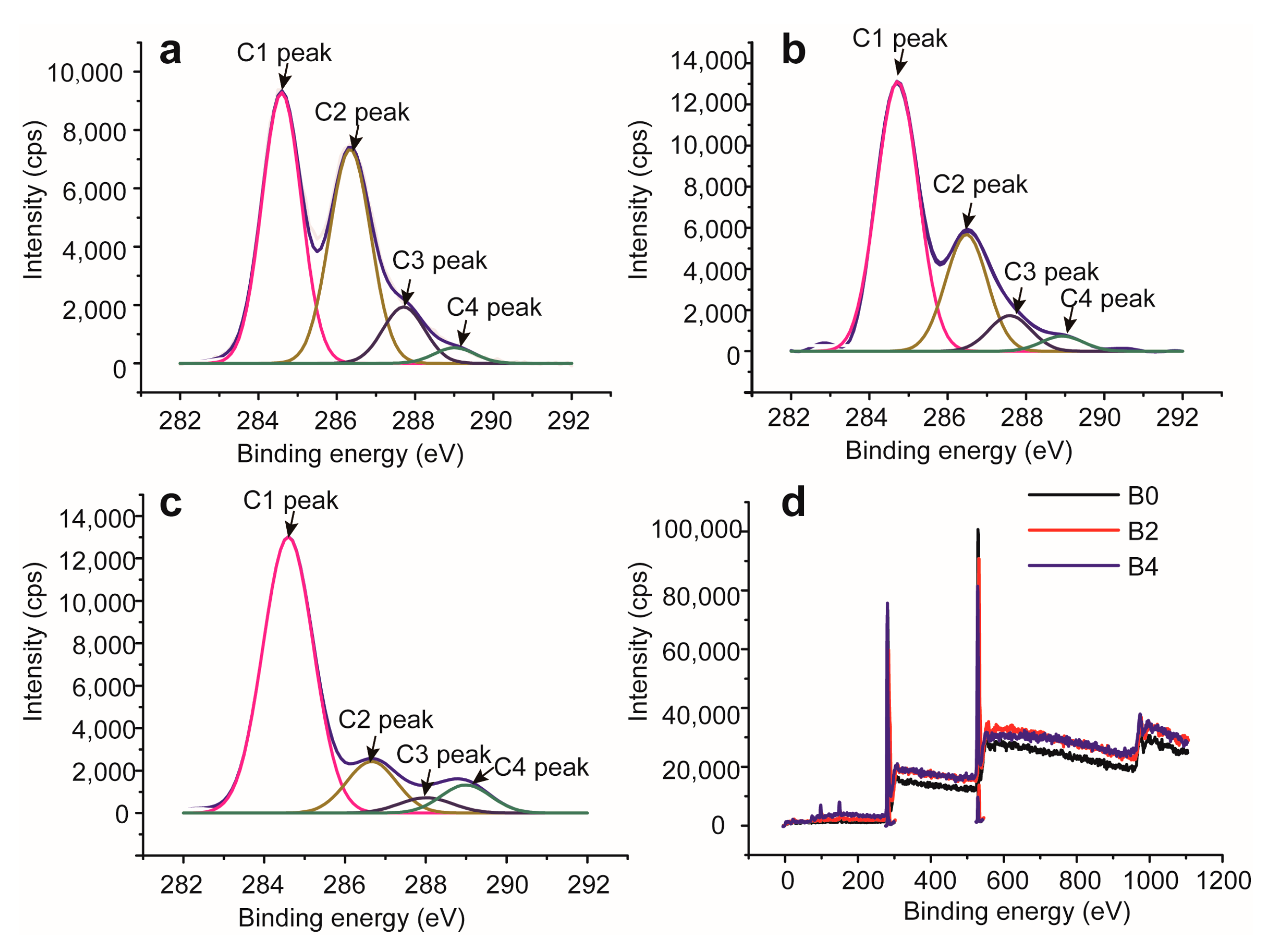
2.4. XPS Analysis of Bagasse Samples
2.5. Degradation of Esterified Bagasse
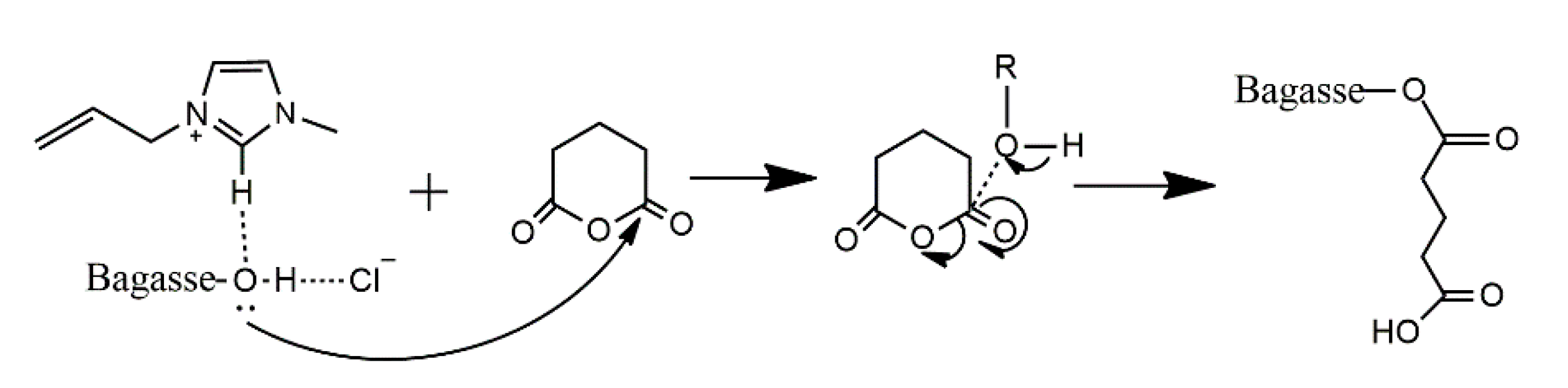
2.6. Possible Esterification Mechanism of Bagasse Modified with Glutairc Anhydride
3. Materials and Methods
3.1. Materials
3.2. Isolation of Cellulose, Hemicelluloses, and Lignin from Bagasse
3.3. Homogeneous Esterification with Glutaric Anhydride in AmimCl
3.4. Determination of PS
3.5. Solubility of Bagasse Samples
3.6. Characterization
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mbotchak, L.; Le Morvan, C.; Duong, K.L.; Rousseau, B.; Tessier, M.; Fradet, A. Purification, structural characterization, and modification of organosolv wheat straw lignin. J. Agric. Food Chem. 2015, 63, 5178–5188. [Google Scholar] [CrossRef] [PubMed]
- Peleteiro, S.; Rivas, S.; Alonso, J.L.; Santos, V.; Parajó, J.C. Utilization of ionic liquids in lignocellulose biorefineries as agents for separation, derivatization, fractionation, or pretreatment. J. Agric. Food Chem. 2015, 63, 8093–8102. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.; Chatterjee, S.G.; Scott, G.M.; Amidon, T.E. Modeling xylan solubilization during autohydrolysis of sugar maple and aspen wood chips: Reaction kinetics and mass transfer. Chem. Eng. Sci. 2009, 64, 3031–3041. [Google Scholar] [CrossRef]
- Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P.; Belgacem, M.N.; Gandini, A. Controlled heterogeneous modification of cellulose fibers with fatty acids: Effect of reaction conditions on the extent of esterification and fiber properties. J. Appl. Polym. Sci. 2006, 100, 1093–1102. [Google Scholar] [CrossRef]
- Jogunola, O.; Eta, V.; Hedenström, M.; Sundman, O.; Salmi, T.; Mikkola, J.P. Ionic liquid mediated technology for synthesis of cellulose acetates using different co-solvents. Carbohydr. Polym. 2016, 135, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Luan, Y.H.; Zhang, J.M.; Zhan, M.S.; Wu, J.; Zhang, J.; He, J.S. Highly efficient propionylation and butyralation of cellulose in an ionic liquid catalyzed by 4-dimethylminopyridine. Carbohydr. Polym. 2013, 92, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.M.; Chen, W.W.; Feng, Y.; Wu, J.; Yu, J.; He, J.S.; Zhang, J. Homogeneous esterification of cellulose in room temperature ionic liquids. Polym. Int. 2015, 64, 963–970. [Google Scholar] [CrossRef]
- Lu, F.C.; Ralph, J. Non-degradative dissolution and acetylation of ball-milled plant cell walls: High-resolution solution-state NMR. Plant J. 2003, 35, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Fasching, M.; Schröder, P.; Wollboldt, R.P.; Weber, H.K.; Sixta, H. A new and facile method for isolation of lignin from wood based on complete wood dissolution. Holzforschung 2008, 62, 15–23. [Google Scholar] [CrossRef]
- Xu, F.; Yuan, T.Q.; He, J.; Sun, R.C. A new vision in the research of biomass resources: Complete-lignocellulose-dissolution system. Prog. Chem. 2010, 22, 472–481. [Google Scholar]
- Zhu, S.D.; Wu, Y.X.; Chen, Q.M.; Yu, Z.N.; Wang, C.W.; Jin, S.W.; Ding, Y.G.; Wu, G. Dissolution of cellulose with ionic liquids and its application: A mini-review. Green Chem. 2006, 8, 325–327. [Google Scholar] [CrossRef]
- Zavrel, M.; Bross, D.; Funke, M.; Büchs, J.; Spiess, A.C. High-throughput screening for ionic liquids dissolving (ligno-) cellulose. Bioresour. Technol. 2009, 100, 2580–2587. [Google Scholar] [CrossRef] [PubMed]
- Swatloski, R.P.; Spear, S.K.; Holbrey, J.D.; Rogers, R.D. Dissolution of cellulose with ionic liquids. J. Am. Chem. Soc. 2002, 124, 4974–4975. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; King, A.; Kilpelainen, I.; Granstrom, M.; Argyropoulos, D.S. Thorough chemical modification of wood-based lignocellulosic materials in ionic liquids. Biomacromolecules 2007, 8, 3740–3748. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.Q.; Sun, S.N.; Xu, F.; Sun, R.C. Homogeneous esterification of poplar wood in an ionic liquid under mild conditions: Characterization and properties. J. Agric. Food Chem. 2010, 58, 11302–11310. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.F.; Sun, R.C.; Zhang, A.P.; Qin, M.H.; Ren, J.L.; Wang, X.A. Preparation and characterization of phthalated cellulose derivatives in room-temperature ionic liquid without catalysts. J. Agric. Food Chem. 2007, 55, 2399–2406. [Google Scholar] [CrossRef] [PubMed]
- Badawy, M.E.I.; Rabea, E.I. Characterization and antimicrobial activity of water-soluble N-(4-carboxybutyroyl) chitosans against some plant pathogenic bacteria and fungi. Carbohydr. Polym. 2012, 87, 250–256. [Google Scholar] [CrossRef]
- Ba, O.M.; Hindie, M.; Marmey, P.; Gallet, O.; Anselme, K.; Ponche, A.; Duncan, A.C. Protein covalent immobilization via its scarce thiol versus abundant amine groups: Effect on orientation, cell binding domain exposure and conformational lability. Colloids Surf. B 2015, 134, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Shigemasa, Y.; Usui, H.; Morimoto, M.; Saimoto, H.; Okamoto, Y.; Minami, S.; Sashiwa, H. Chemical modification of chitin and chitosan 1: Preparation of partially deacetylated chitin derivatives via a ring-opening reaction with cyclic acid anhydrides in lithium chloride/N,N-dimethylacetamide. Carbohydr. Polym. 1999, 39, 237–243. [Google Scholar] [CrossRef]
- Nagaoka, S.; Tobata, H.; Takiguchi, Y.; Satoh, T.; Sakurai, T.; Takafuji, M.; Ihara, H. Characterization of cellulose microbeads prepared by a viscose phase-separation method and their chemical modification with acid anhydride. J. Appl. Polym. Sci. 2005, 97, 149–157. [Google Scholar] [CrossRef]
- Damasio, A.R.L.; Braga, C.M.P.; Brenelli, L.B.; Citadini, A.P.; Mandelli, F.; Cota, J.; de Almeida, R.F.; Salvador, V.H.; Paixao, D.A.A.; Segato, F.; et al. Biomass-to-bio-products application of feruloyl esterase from aspergillus clavatus. Appl. Microbiol. Biotechnol. 2013, 97, 6759–6767. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.J.; Shi, Q.S. Transforming sugarcane bagasse into bioplastics via homogeneous modification with phthalic anhydride in ionic liquid. ACS Sustain. Chem. Eng. 2015, 3, 2510–2515. [Google Scholar] [CrossRef]
- Cachet, N.; Camy, S.; Benjelloun-Mlayah, B.; Condoret, J.S.; Delmas, M. Esterification of organosolv lignin under supercritical conditions. Ind. Crops Prod. 2014, 58, 287–297. [Google Scholar] [CrossRef]
- Hedenström, M.; Wiklund Lindström, S.; Öman, T.; Lu, F.; Gerber, L.; Schatz, P.; Sundberg, B.; Ralph, J. Identification of lignin and polysaccharide modifications in Populus wood by chemometric analysis of 2D NMR spectra from dissolved cell walls. Mol. Plant 2009, 2, 933–942. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.H.; Zhang, X.Q.; Long, P.; Zhang, A.P.; Liu, C.F.; Sun, R.C. Reaction behavior of cellulose in the homogeneous esterification of bagasse modified with phthalic anhydride in ionic liquid 1-allyl-3-methylimidazium chloride. Int. J. Polym. Sci. 2016, 2016. [Google Scholar] [CrossRef]
- Liu, C.F.; Zhang, A.P.; Li, W.Y.; Yue, F.X.; Sun, R.C. Succinoylation of cellulose catalyzed with iodine in ionic liquid. Ind. Crops Prod. 2010, 31, 363–369. [Google Scholar] [CrossRef]
- Peng, F.; Ren, J.L.; Xu, F.; Bian, J.; Peng, P.; Sun, R.C. Comparative study of hemicelluloses obtained by graded ethanol precipitation from sugarcane bagasse. J. Agric. Food Chem. 2009, 57, 6305–6317. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Bian, J.; Peng, P.; Xiao, H.; Ren, J.L.; Xu, F.; Sun, R.C. Separation and characterization of acetyl and non-acetyl hemicelluloses of arundo donax by ammonium sulfate precipitation. J. Agric. Food Chem. 2012, 60, 4039–4047. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.L.; Xue, B.L.; Xu, F.; Sun, R.C.; Pinkert, A. Unmasking the structural features and property of lignin from bamboo. Ind. Crops Prod. 2013, 42, 332–343. [Google Scholar] [CrossRef]
- Wen, J.L.; Yuan, T.Q.; Sun, S.L.; Xu, F.; Sun, R.C. Understanding the chemical transformations of lignin during ionic liquid pretreatment. Green Chem. 2014, 16, 181–190. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, Q.; Chen, J.H.; Wang, K.; Yuan, S.G.; Sun, R.C. Morphological variation of lignin biomacromolecules during acid-pretreatment and biorefinery-based fractionation. Ind. Crops Prod. 2015, 77, 527–534. [Google Scholar] [CrossRef]
- Wang, S.; Jämsä, S.; Mahlberg, R.; Ihalainen, P.; Nikkola, J.; Mannila, J.; Ritschkoff, A.C.; Peltonen, J. Treatments of paper surfaces with sol-gel coatings for laminated plywood. Appl. Surf. Sci. 2014, 288, 295–303. [Google Scholar] [CrossRef]
- Fundador, N.G.V.; Enomoto-Rogers, Y.; Takemura, A.; Iwata, T. Synthesis and characterization of xylan esters. Polymer 2012, 53, 3885–3893. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Wang, H.H.; Wen, X.X.; Zhang, A.P.; Wang, X.Y.; Zhong, L.X.; Liu, C.F.; Sun, R.C. Synthesis and characterization of xylan grafted with polyethylene glycol in ionic liquid and their use as moisture-absorption/retention biomaterials. Macromol. Mater. Eng. 2016, 301, 287–295. [Google Scholar] [CrossRef]
- Chen, C.Y.; Chen, M.J.; Zhang, X.Q.; Liu, C.F.; Sun, R.C. Per-O-acetylation of cellulose in dimethyl sulfoxide with catalyzed transesterification. J. Agric. Food Chem. 2014, 62, 3446–3452. [Google Scholar] [CrossRef] [PubMed]
- Tosh, B.; Saikia, C.N.; Dass, N.N. Homogeneous esterification of cellulose in the lithium chloride-N,N-dimethylacetamide solvent system: Effect of temperature and catalyst. Carbohydr. Res. 2000, 327, 345–352. [Google Scholar] [CrossRef]
- Wang, H.H.; Zhang, X.Q.; Wei, Y.; Liu, C.F. Reaction behaviors of bagasse modified with phthalic anhydride in 1-allyl-3-methylimidazolium chloride with catalyst 4-dimethylaminopyridine. In Biomass Volume Estimation and Valorization for Energy; Tumuluru, J.S., Ed.; INTECH: Rijeka, Croatia, 2017. [Google Scholar]
- Wang, H.H.; Chen, Y.T.; Wei, Y.; Zhang, A.P.; Liu, C.F. Homogeneous esterification mechanism of bagasse modified with phthalic anhydride in ionic liquid. Part 2: Reactive behavior of hemicelluloses. Carbohydr. Polym. 2017, 157, 1365–1373. [Google Scholar] [CrossRef] [PubMed]
- Ramos, L.A.; Frollini, E.; Koschella, A.; Heinze, T. Benzylation of cellulose in the solvent dimethylsulfoxide/tetrabutylammonium fluoride trihydrate. Cellulose 2005, 12, 607–619. [Google Scholar] [CrossRef]
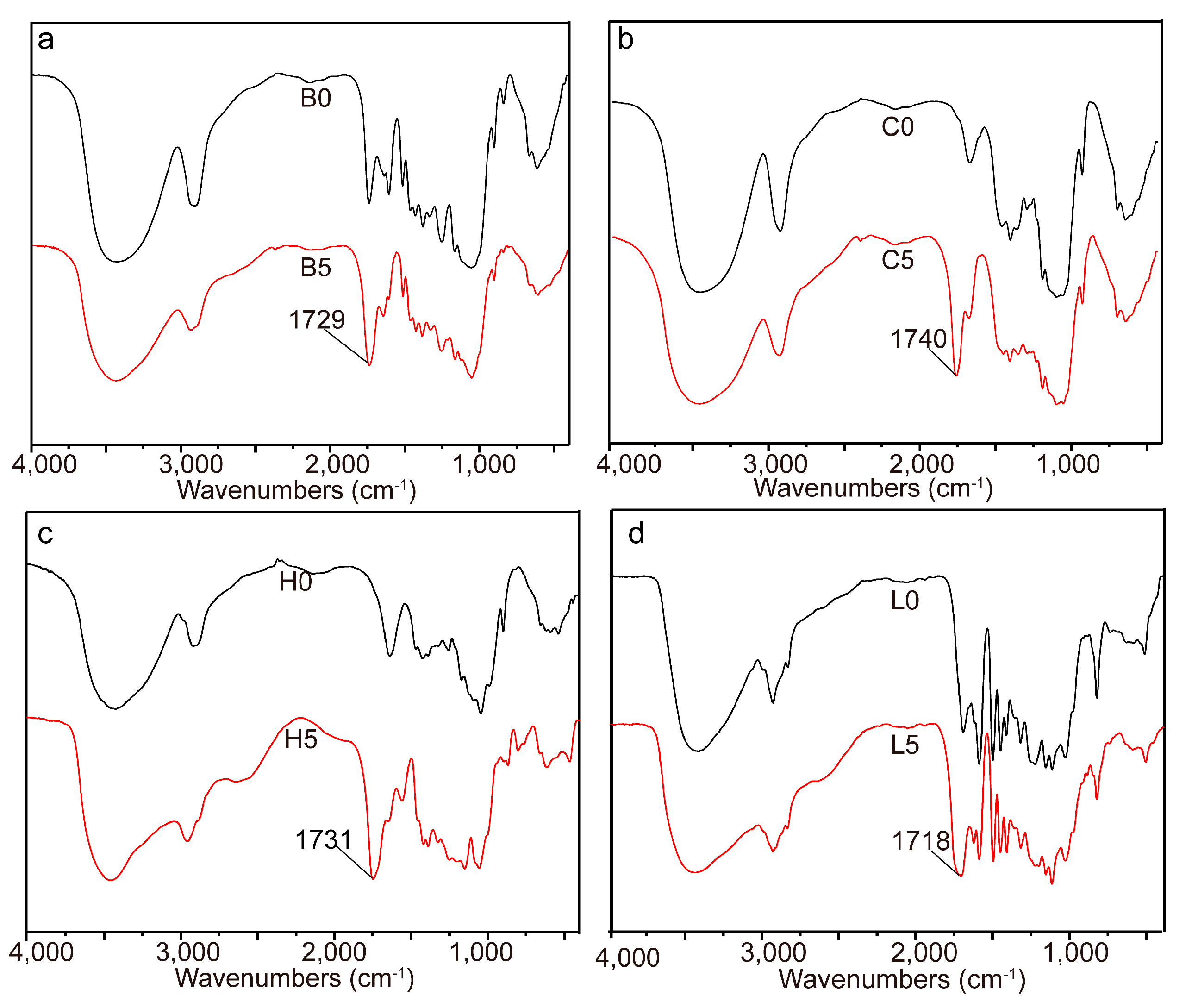
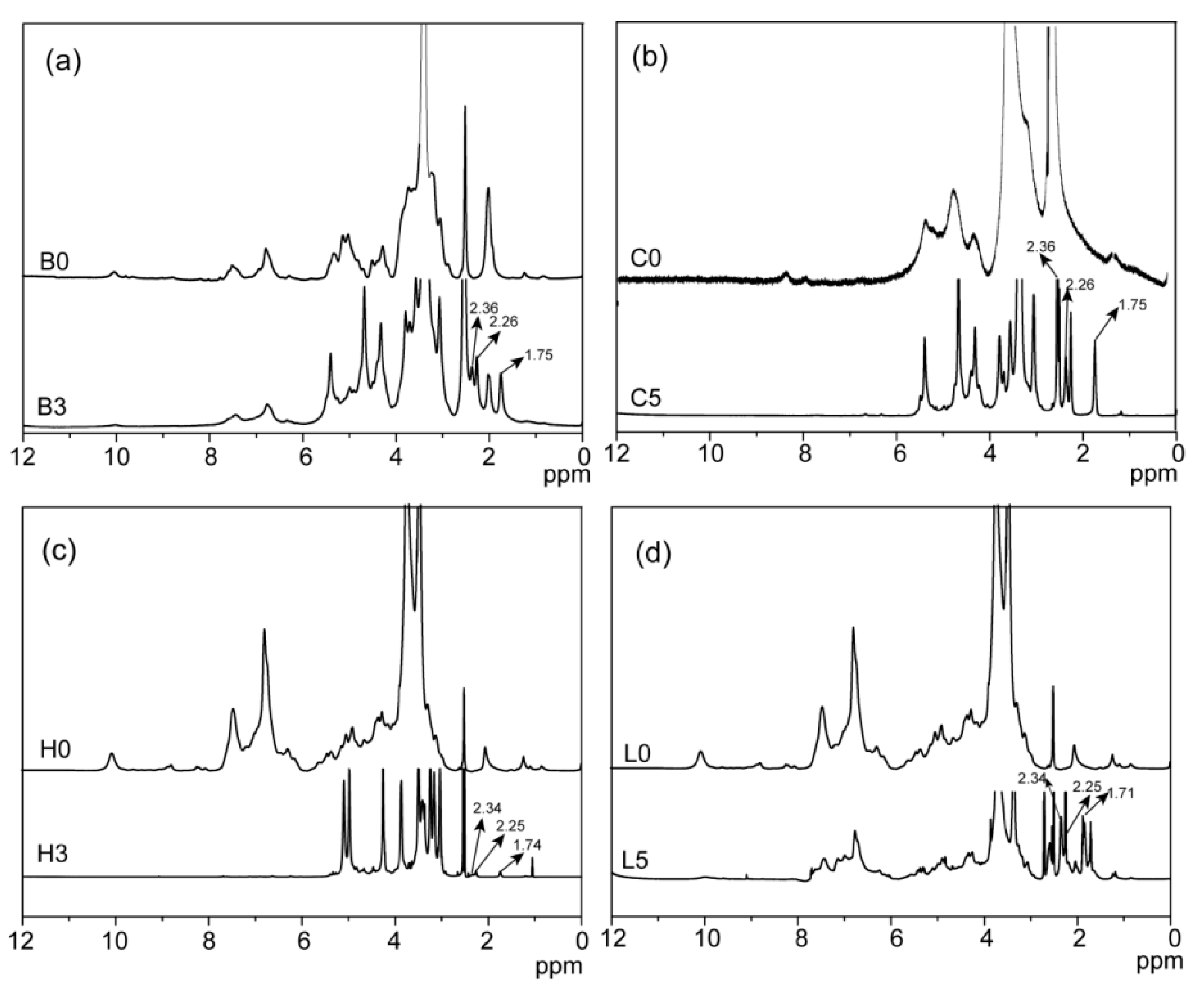
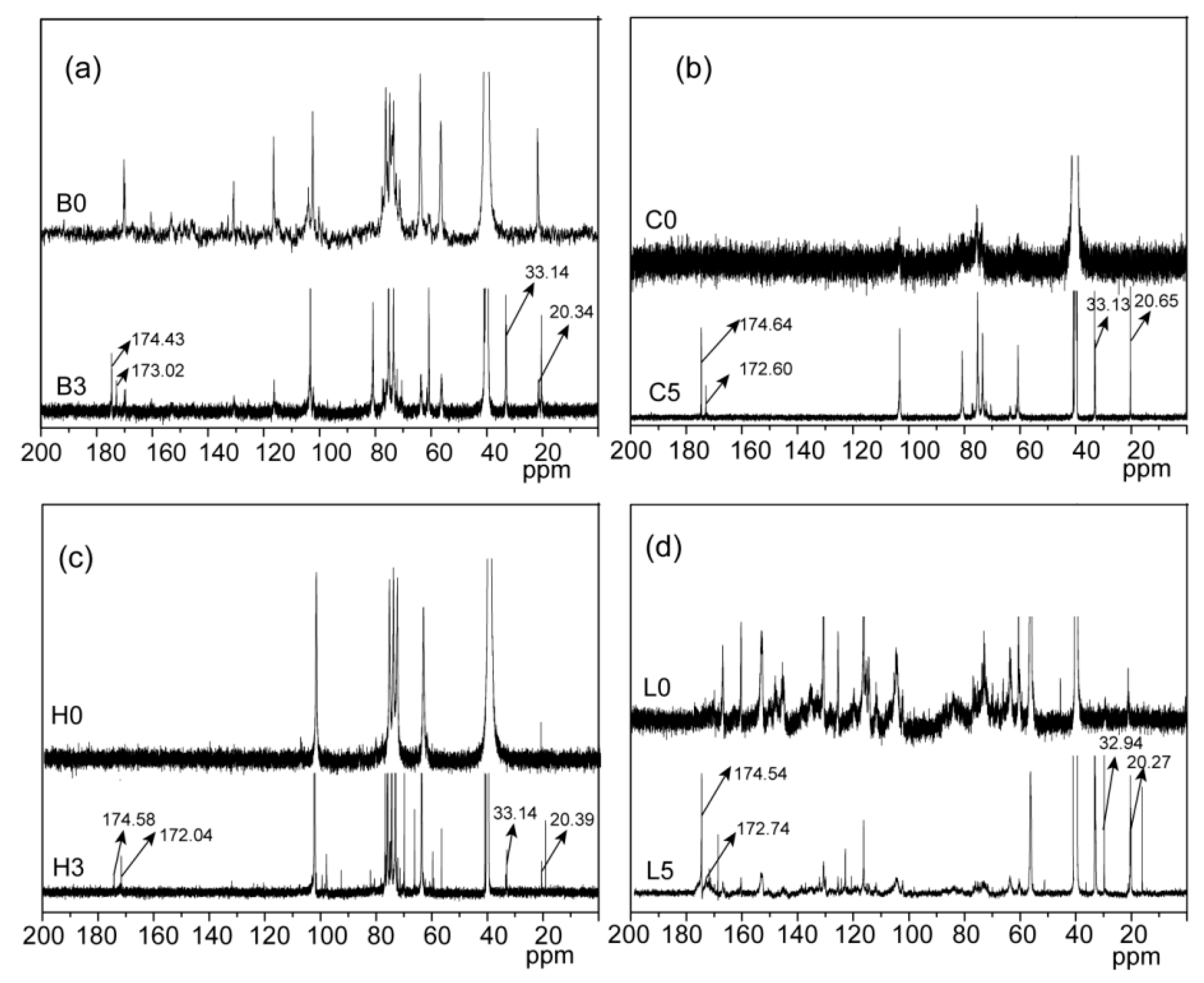
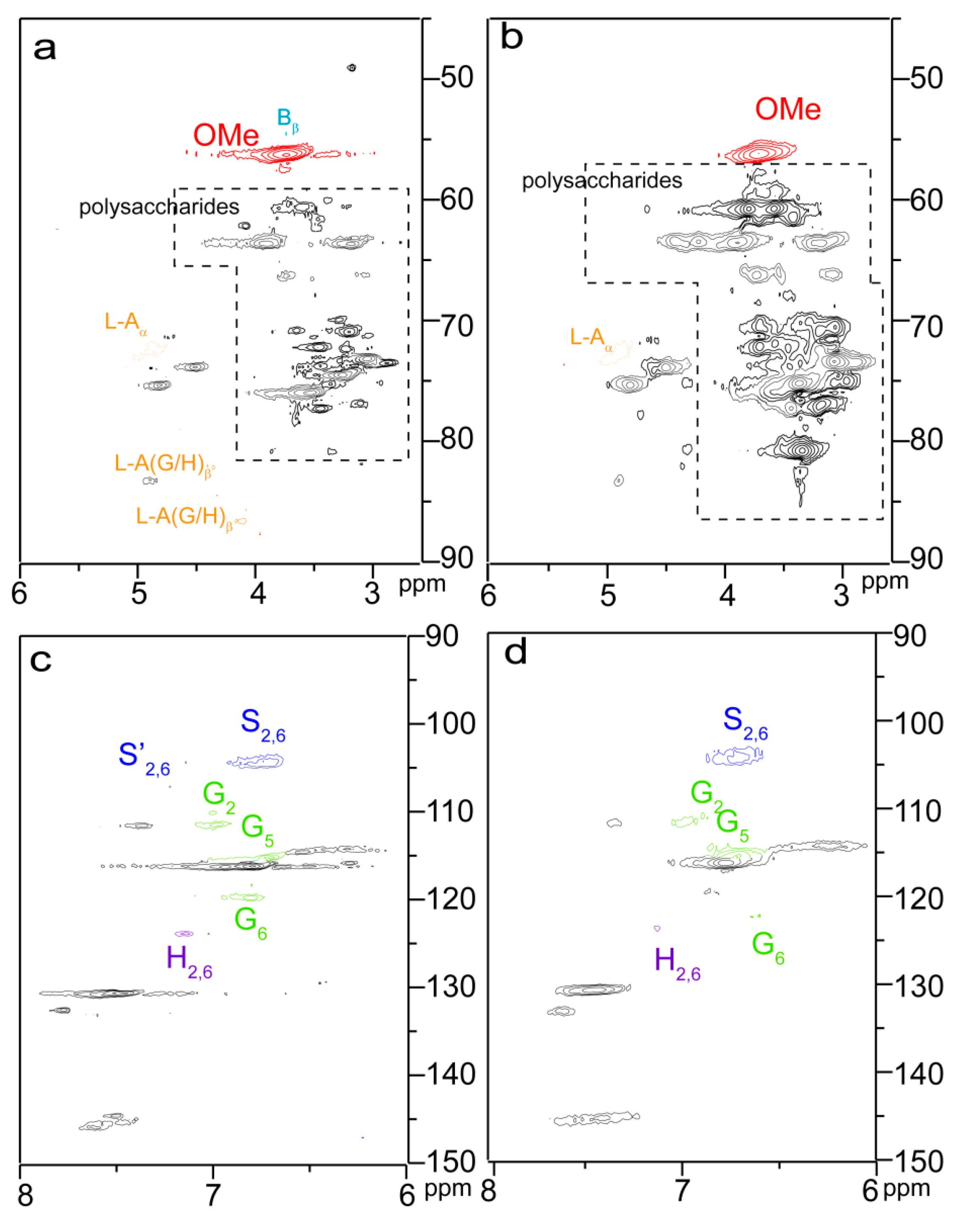
| Samples | Temperature (°C) | Time (Min) | Dosage (mmol/g) | a n’OH (mmol/g) | b PS (%) |
|---|---|---|---|---|---|
| B1 | 90 | 90 | 10 | 1.64 | 11.47 |
| B2 | 90 | 90 | 20 | 2.11 | 14.76 |
| B3 | 90 | 90 | 30 | 3.21 | 22.45 |
| B4 | 90 | 90 | 40 | 4.48 | 31.33 |
| B5 | 90 | 90 | 50 | 3.96 | 27.69 |
| C1 | 90 | 90 | 10 | 1.30 | 7.02 |
| C2 | 90 | 90 | 20 | 1.43 | 7.72 |
| C3 | 90 | 90 | 30 | 1.64 | 8.86 |
| C4 | 90 | 90 | 40 | 1.61 | 8.69 |
| C5 | 90 | 90 | 50 | 4.52 | 24.41 |
| H1 | 90 | 90 | 10 | 1.13 | 7.46 |
| H2 | 90 | 90 | 20 | 1.49 | 9.83 |
| H3 | 90 | 90 | 30 | 1.63 | 10.76 |
| H4 | 90 | 90 | 40 | 2.60 | 17.16 |
| H5 | 90 | 90 | 50 | 4.54 | 29.97 |
| L1 | 90 | 90 | 10 | 2.41 | 47.53 |
| L2 | 90 | 90 | 20 | 2.27 | 44.77 |
| L3 | 90 | 90 | 30 | 2.48 | 48.92 |
| L4 | 90 | 90 | 40 | 3.15 | 62.13 |
| L5 | 90 | 90 | 50 | 2.62 | 51.68 |
| Samples | B0 | Glutaic Anhydride | B1 | B2 | B3 | B4 | B5 |
|---|---|---|---|---|---|---|---|
| Water | - | ++ | + | + | - | - | + |
| DMSO | - | ++ | ++ | ++ | ++ | ++ | ++ |
| DMF | - | ++ | + | + | + | + | ++ |
| Acetone | - | ++ | + | + | + | + | + |
| Samples | L0 | L1 | L2 | L3 | L4 | L5 |
|---|---|---|---|---|---|---|
| Aliphatic OH (mmol/g) | 3.96 | 1.91 | 2.08 | 1.94 | 1.48 | 2.05 |
| Phenolic S–OH (mmol/g) | 0.09 | 0.05 | 0.04 | 0.04 | 0.01 | 0.09 |
| Phenolic G–OH (mmol/g) | 0.26 | 0.14 | 0.12 | 0.10 | 0.06 | 0.15 |
| Phenolic H–OH (mmol/g) | 0.76 | 0.56 | 0.56 | 0.51 | 0.37 | 0.16 |
| Total phenolic hydroxyls (mmol/g) | 1.11 | 0.75 | 0.72 | 0.65 | 0.44 | 0.40 |
| aliphatic OH/Phenolic OH | 3.57 | 2.55 | 2.89 | 2.98 | 3.36 | 5.13 |
| COOH (mmol/g) | 0.11 | 0.17 | 0.18 | 0.41 | 0.31 | 1.03 |
| Samples | a Ara (%) | b Gal(%) | c Glc (%) | d Xyl (%) | e Glua (%) | f Total Side-Chain (%) |
|---|---|---|---|---|---|---|
| H0 | 4.20 | 0.52 | 6.01 | 87.83 | 1.44 | 12.17 |
| H1 | 0 | 0.84 | 4.43 | 93.83 | 0.89 | 6.17 |
| H2 | 0.13 | 0.79 | 4.81 | 93.34 | 0.94 | 6.66 |
| H3 | 1.11 | 0.76 | 4.97 | 92.30 | 0.86 | 7.70 |
| H4 | 1.32 | 0.96 | 5.48 | 91.37 | 0.86 | 8.63 |
| H5 | 4.20 | 0 | 1.52 | 94.27 | 0 | 5.73 |
| Samples | L0 | L5 |
|---|---|---|
| Aryl ether (A) | 45.6/100Ar | 44.4/100Ar |
| Phenylcoumaran (B) | 3.5/100Ar | 3.2/100Ar |
| S/G | 1.11 | 1.16 |
| Samples | B0 | B2 | B4 |
|---|---|---|---|
| O (%) | 34.63 | 28.87 | 25.35 |
| C (%) | 65.37 | 71.13 | 74.65 |
| C/O ratio | 1.88 | 2.53 | 2.94 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Chen, W.; Zhang, X.; Liu, C.; Sun, R. Esterification Mechanism of Bagasse Modified with Glutaric Anhydride in 1-Allyl-3-methylimidazolium Chloride. Materials 2017, 10, 966. https://doi.org/10.3390/ma10080966
Wang H, Chen W, Zhang X, Liu C, Sun R. Esterification Mechanism of Bagasse Modified with Glutaric Anhydride in 1-Allyl-3-methylimidazolium Chloride. Materials. 2017; 10(8):966. https://doi.org/10.3390/ma10080966
Chicago/Turabian StyleWang, Huihui, Wei Chen, Xueqin Zhang, Chuanfu Liu, and Runcang Sun. 2017. "Esterification Mechanism of Bagasse Modified with Glutaric Anhydride in 1-Allyl-3-methylimidazolium Chloride" Materials 10, no. 8: 966. https://doi.org/10.3390/ma10080966
APA StyleWang, H., Chen, W., Zhang, X., Liu, C., & Sun, R. (2017). Esterification Mechanism of Bagasse Modified with Glutaric Anhydride in 1-Allyl-3-methylimidazolium Chloride. Materials, 10(8), 966. https://doi.org/10.3390/ma10080966







