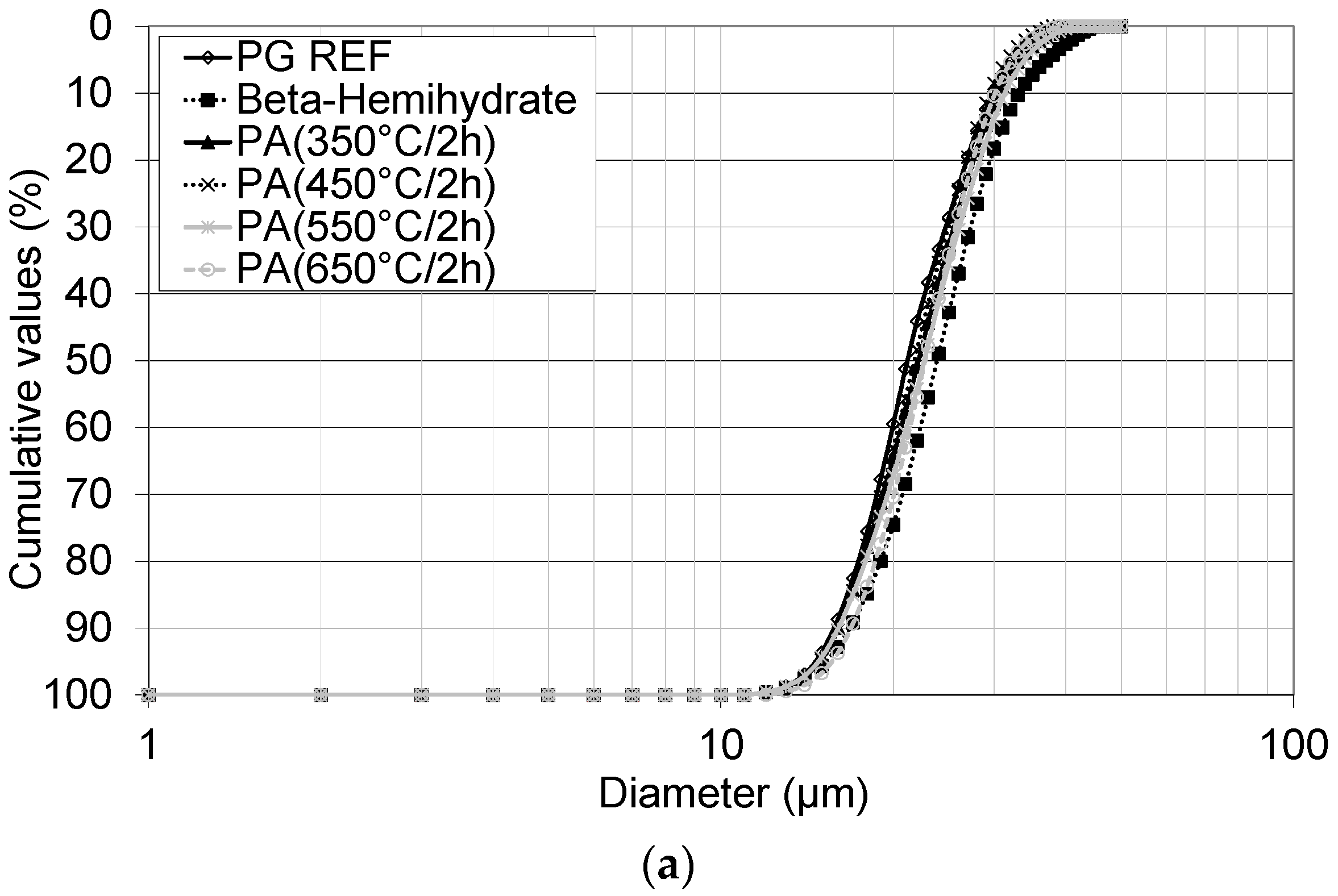
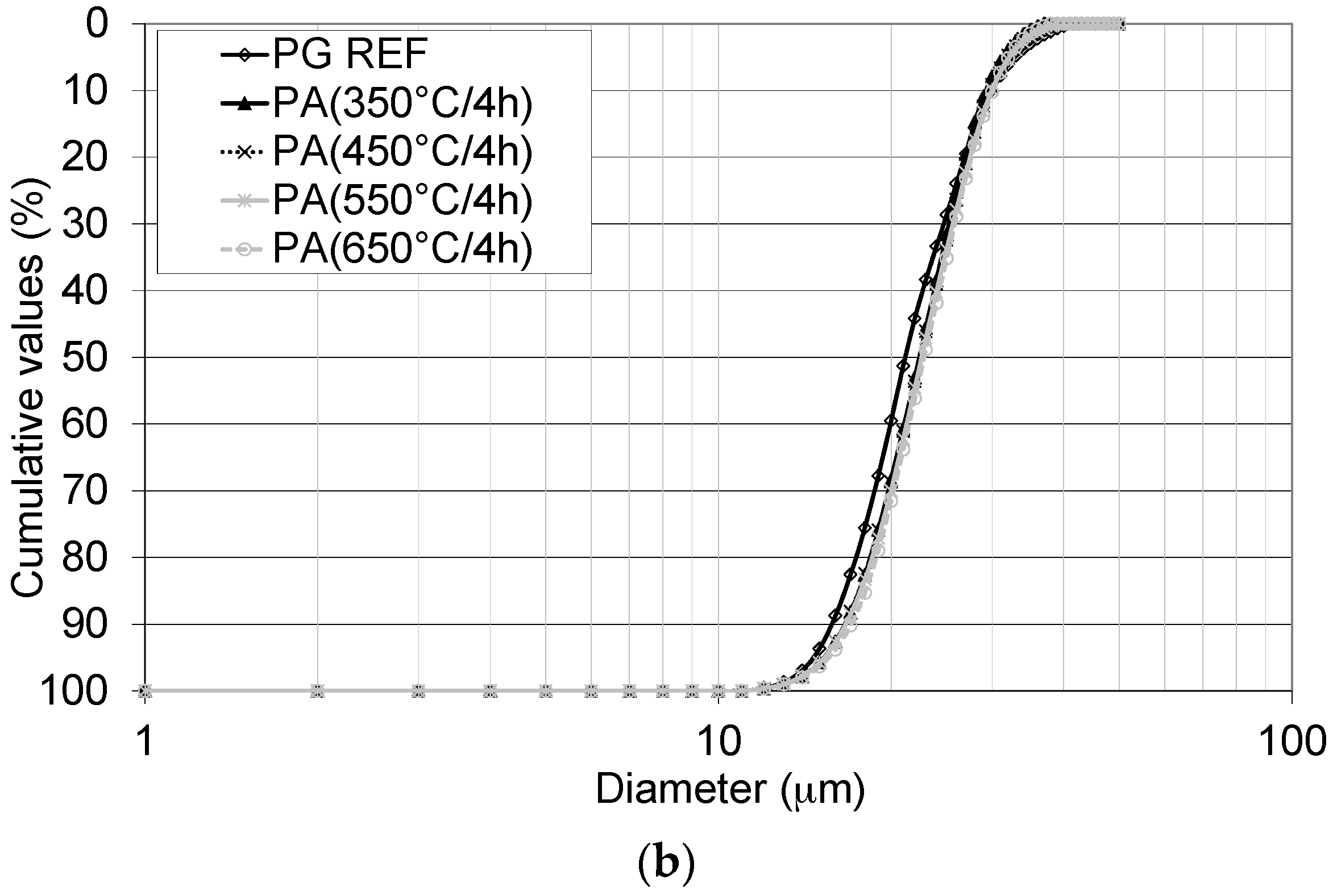
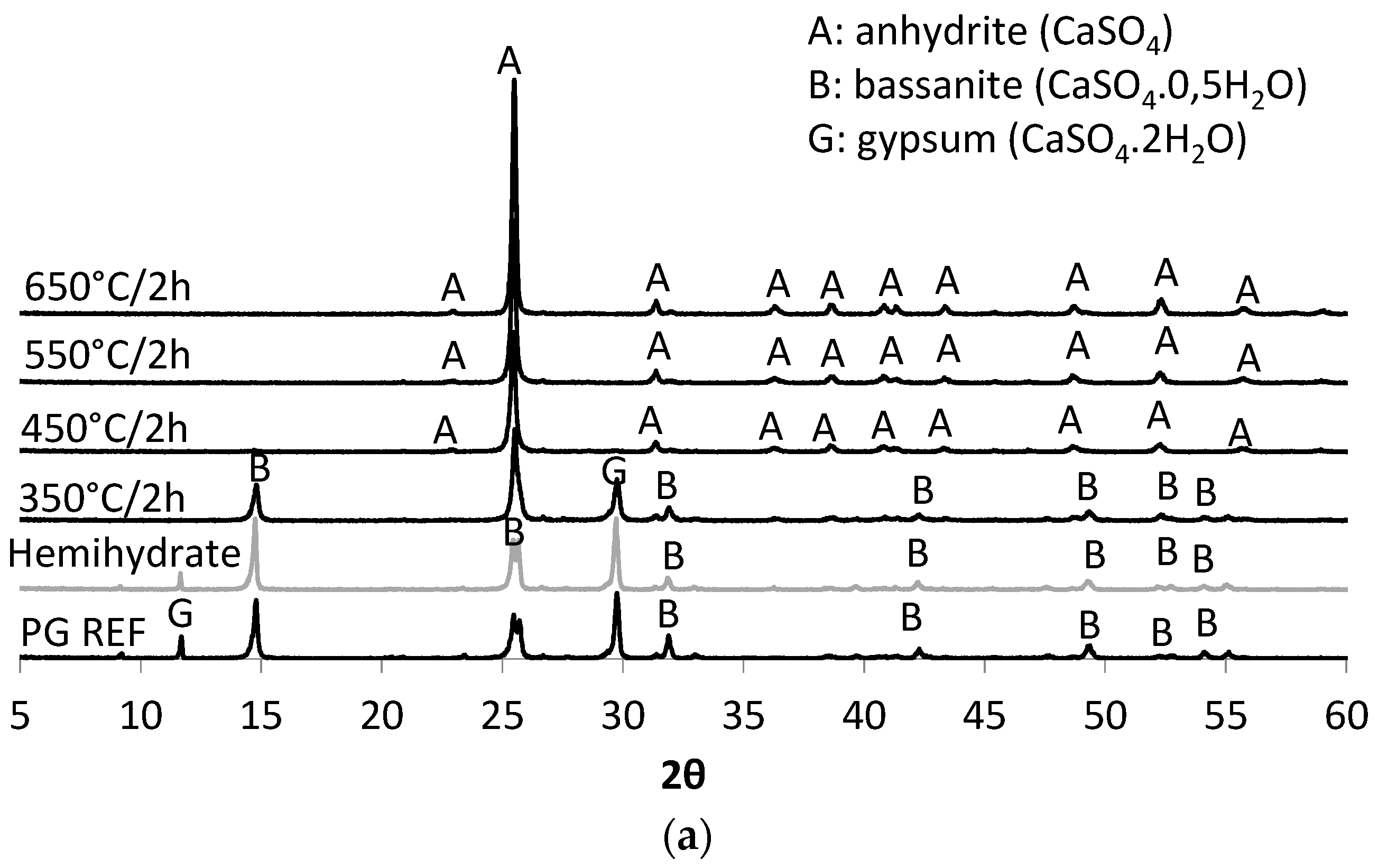
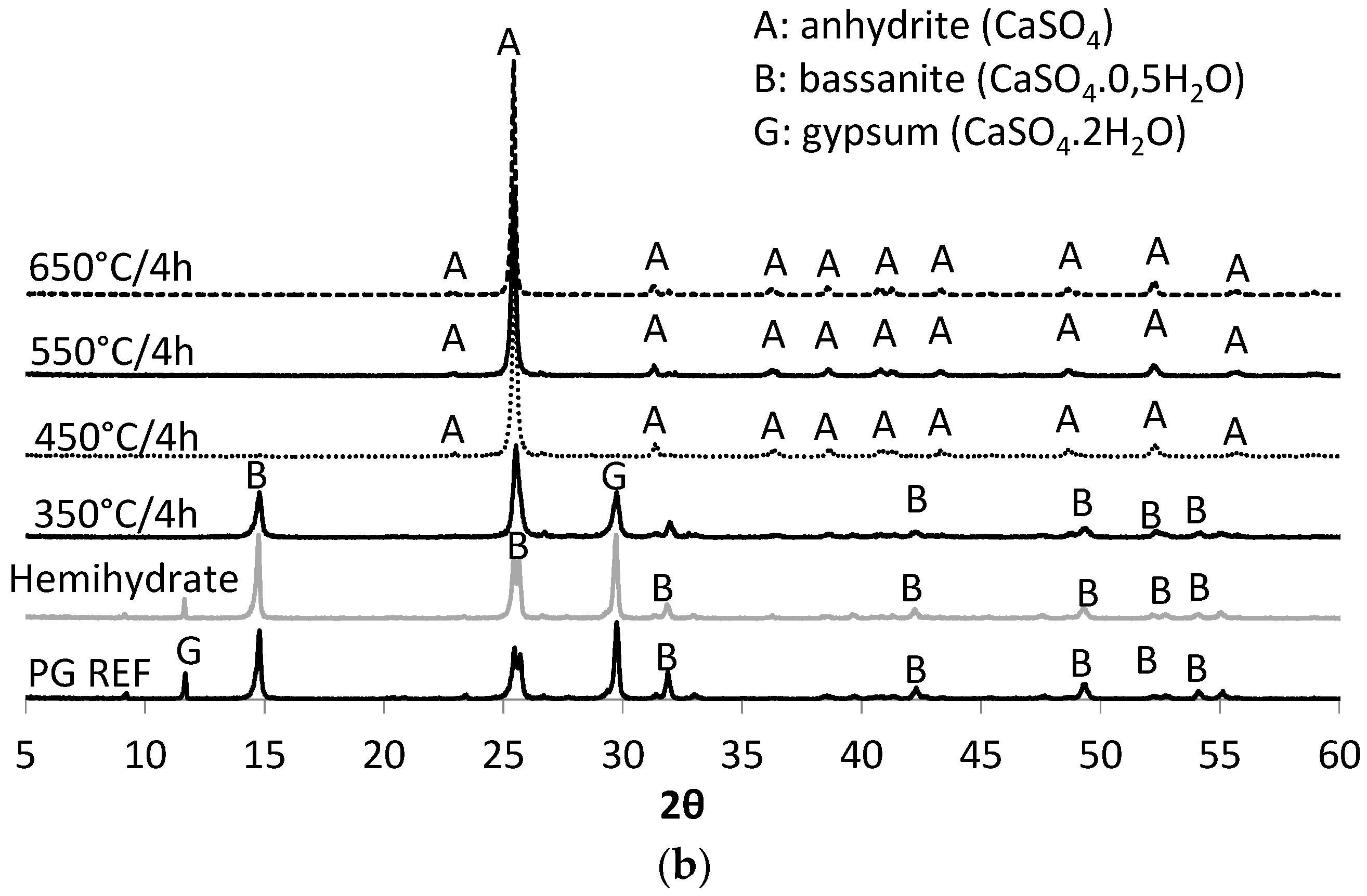
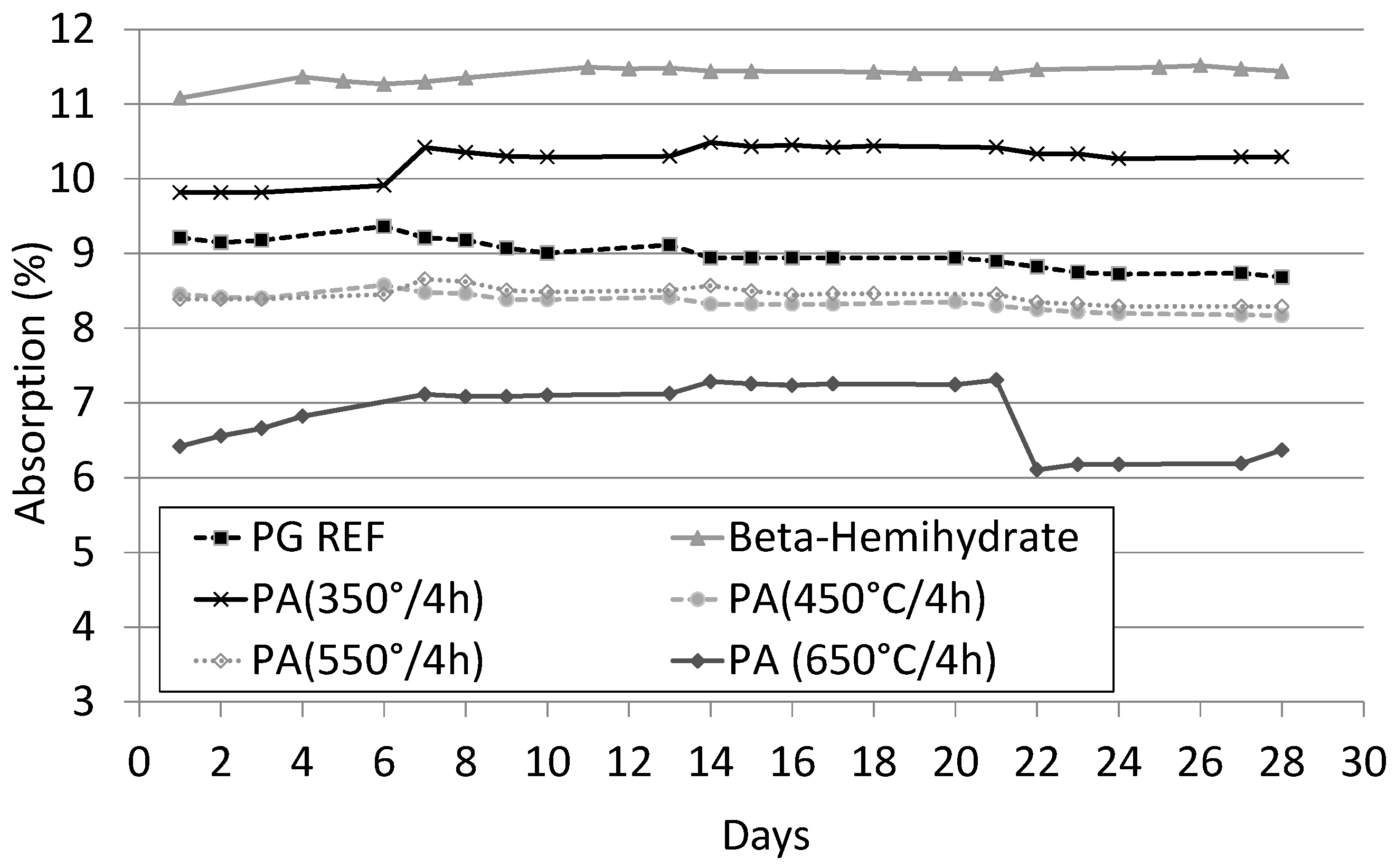
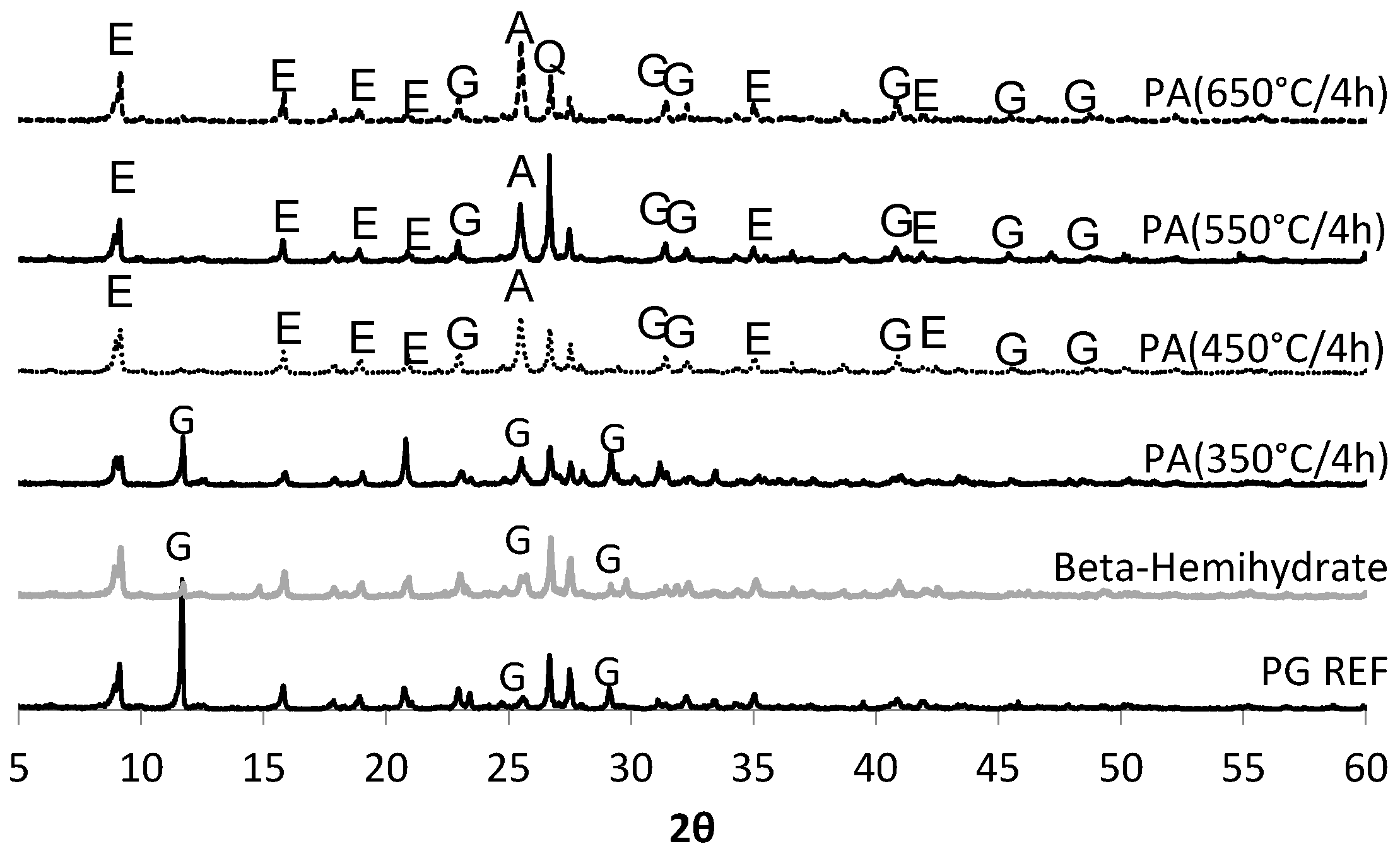
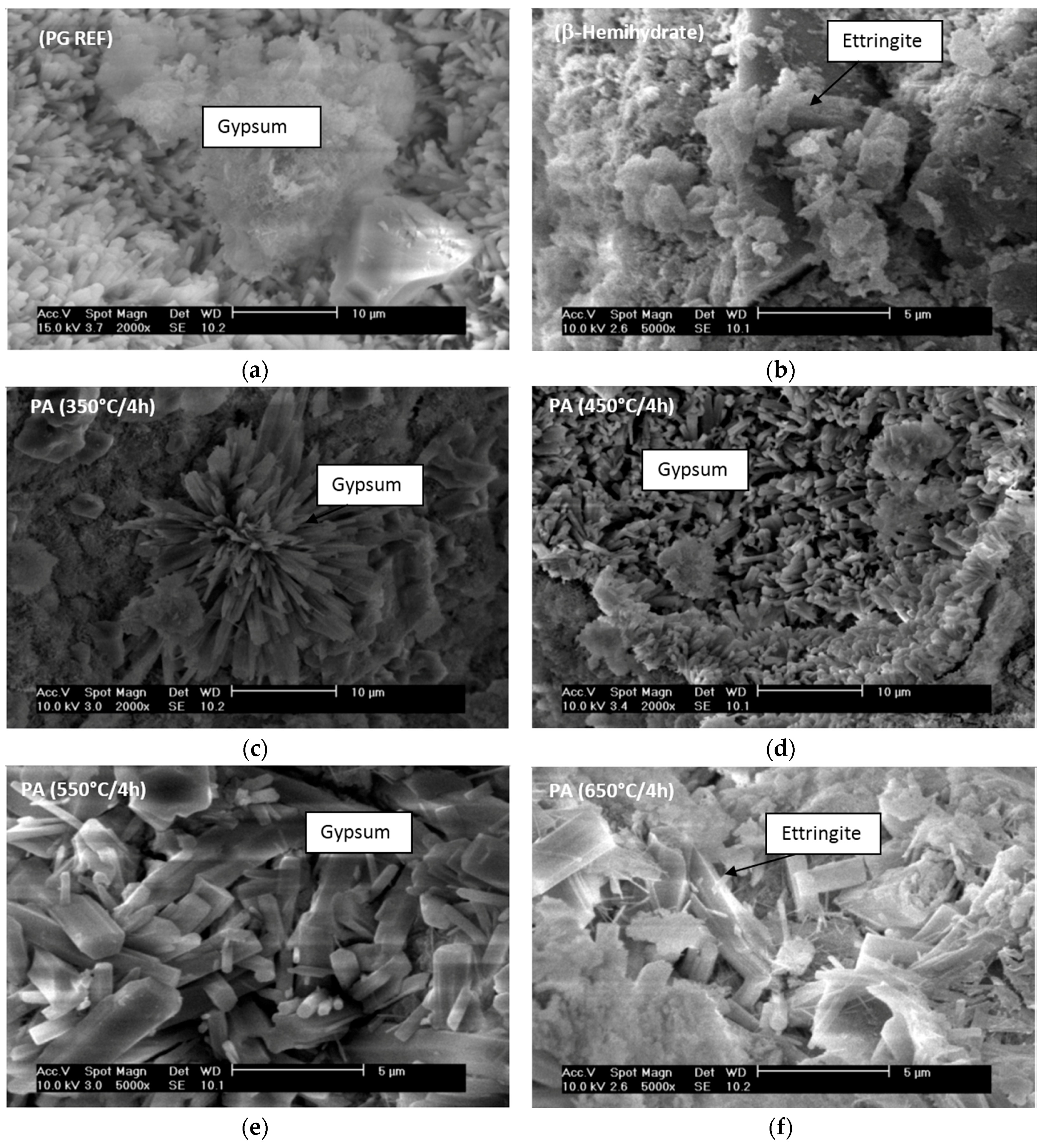
1. Introduction
A self-leveling underlayment (SLU) is a fluid product comprised of a binder, sand, water, and additives, and its main characteristics are low viscosity and high fluidity [
1,
2]. This building material requires no vibration and leveling, and has a very small thickness of around 30 mm.
Most SLUs are comprised of either Portland and calcium sulfoaluminate cement or calcium sulfate. Cement-based SLUs have better water resistance, higher strength and a shorter curing period than calcium sulfate-based SLUs. The advantages of calcium sulfate-based SLUs are fast setting, low shrinkage, and little cracking and they can be applied to large surfaces without construction joints; however, they may be sensitive to moisture depending on the calcium sulfate used [
3,
4]. One way to minimize water sensitivity is through the use of calcium aluminate cement (CAC). This type of cement, which is rich in alumina, is normally used in self-leveling underlayments in ternary mixtures of CAC, calcium sulfate (C
$), and Portland cement (PC). One use of CAC is as a precursor for the formation of ettringite (C
3A·3C
$·H
32). These systems are known as ettringite-rich products due to the main hydration product formed. When formulated correctly, ettringite-rich products are able to provide rapid drying and hardening and shrinkage compensation and they are not sensitive to the action of water [
5].
The authors [
6] carried out an extensive investigation on the effect of calcium sulfate hemihydrate on the hydration of ternary blends composed of CAC and a limestone-CAC system was also evaluated. They concluded that high levels of calcium sulfate could promote expansion and lead to the destruction of the material.
However, the products of the hydration of Portland cement (PC-based system), such as C–S–H, can favor the interlocking of the crystals in the matrixes, producing predominantly with calcium sulfate [
7,
8].
In addition, the type of calcium sulfate employed affects the expansion or retraction of the SLU. Beta hemihydrate has expansive as well as dihydrate characteristics, although the latter is on a smaller scale. Anhydrate, in general, shows retraction with or without a little expansion [
9]. The formation of a form of ettringite known as massive ettringite is related to expansion and cracking in systems containing beta hemihydrate [
10]. Anhydrite forms non-expansive ettringite, with the typical format of long fine needles [
11]. Expansion is normally attributed to the crystal growth theory. This suggests that ettringite grows around the cement grain, resulting in a crystallization pressure which leads to expansion. This behavior occurs when the crystals interact with each other [
11]. Although some authors have reported the formation and expansion of ettringite, this behavior is still not completely understood. According to literature review, ternary have been blended in CAC-dominate formulations, with CAC/C
$ ratios of (2.5–2.8) [
12,
13], 1.5–1.8 [
14], or in a PC-dominated formulation with a CAC/C
$ ratio of 2.0–2.8 [
7,
13,
15]. There is formation of an ettringite-phase in both systems, but the CAC-dominated formulations react faster and produce more ettringite than PC-dominated formulations [
15].
In recent study, the authors [
13] proposed two main stages in hydration processes. The first involves the dissolution of the anhydrous and sulfate phase (calcium sulfate α-hemihydrate), where the sulfate source is totally consumed within 1 day and the formation of ettringite continues for 28 days and then decreases. An AH3 phase appears in system, and suggesting a delay in the silicate reaction due to a barrier layer covering the surface of unhydrated particles. The second stage is related to the formation of monosulfate through the consumption of ettringite. In view of practical purposes and applications, an overview of the properties of ternary mixtures of CAC-PC-C
$, based on study of a natural anhydrite and alpha-hemihydrate [
16] has been given.
Calcium sulfate (C
$) can be found in anhydrite, hemihydrate, and dehydrate forms, and can be obtained from natural source or byproducts as secondary gypsum. Several papers describe existing forms of calcium sulfate [
17,
18,
19,
20,
21] that are obtained as a function of temperature and are basically dihydrate or gypsum (CaSO
4·2H
2O), hemihydrate or plaster of Paris (CaSO
4·1/2H
2O), and anhydrite (CaSO
4). The hemihydrate is obtained within the temperature range of 140–160 °C and has two forms of crystallization: α and β. The β form is obtained by calcination and α-hemihydrate is derived from the autoclaving process. In the temperature range of 160–250 °C, anhydrite III or soluble anhydrite is formed, an unstable phase which becomes hemihydrate in the presence of moisture. Above 300 °C anhydrite III becomes anhydrite II (CaSO
4) which has a slower hydration rate, but this can be accelerated by means of activators that enhance the rate of dissolution, forming transient hydration products that act as nucleation points and favor the formation of gypsum (CaSO
4·2H
2O) [
19]. Some examples of activators are a carbonate component (Li
2CO
3), a sulfate-base component (Na
2SO
4, NaHSO
4, FeSO
4, K
2SO
4, KHSO
4), lime, Ca(OH)
2 and NaOH, Portland cement clinker, and others [
20]. Anhydrite I (CaSO
4) is obtained only at temperatures of around 1000 °C and may be inert [
22,
23].
Phosphogypsum or chemical gypsum is a byproduct generated during the production of phosphoric acid used in the correction of farmland. Phosphogypsum may provide a source of calcium sulfate for use in the production of self-leveling underlayments. It is possible to obtain anhydrite from the heat treatment of phosphogypsum, as shown in a study by [
23]. According to [
9,
24,
25,
26,
27], the morphology plays an important role in the crystal growth and mechanical properties of a calcium sulfate-based system. The dissolution rate of calcium sulfate is related to the extent and speed of dissolution in aqueous media. It is possible to evaluate the calcium sulfate dissolution rate by estimating the content dissolved (g/L) in an aqueous medium according to the relationships CaSO
42−/H
2O [
27]. When anhydrite comes into contact with water, its dissolution starts immediately and leads to a saturated solution of Ca
2+ and SO
42− ions [
17].
Numerous studies involving the production of phosphogypsum have been carried out, covering phosphogypsum generation and some environmental aspects [
28,
29]. Trace metal contents (arsenic, silver, barium, cadmium, chromium, lead, mercury, and selenium) in some PG sources, also radionuclides naturally present in the phosphate rock. Initially, the research carried out in Brazil and worldwide was focused on the radioactive species present in phosphogypsum [
30,
31,
32,
33] and also, radon exhalation from stockpiles and areas near stacks. The specific activities of the radionuclides
226Ra, U,
232Th,
228Ra, and
210Po have been determined at different production sites, and phosphogypsum can be classified as a TENORM (Technologically Enhanced Naturally Occurring Radioactive Material). Recent approaches have focused on the assessment of radionuclides activity concentration in building materials [
33,
34,
35]. To evaluate the radiological impact associated with the use of phosphogypsum, a pilot study was performed to investigate gamma exposure and radon concentration [
34]. This approach consisted of developing a prototype using building materials based on phosphogypsum as a secondary raw material, simulating the application of new materials. Internal and external exposure was monitored and the activity concentrations of
40K,
226Ra, and
232Th were considered to establish the
I index. In the first step, the index is determined via the following equation:
Due to the variation in the density, this index can be adjusted by an expression, considering the density of the materials and the wall thickness [
33,
35]. With regard to Brazilian phosphogypsum, the authors verified that the external exposure dose should be <1 mSv y
−1, this being the recommended value for the protection of the public against the radiological impact of naturally occurring radioactive materials [
34,
36].
Research efforts to develop a norm are now in progress; however, data on radon emanation from specific types of building materials are scarce in the literature. According to [
35], an additional target must be sought in order to encourage the use of low-radiation construction products as building materials. In a Brazilian study on PG conducted on building dwellers (prototype), it was concluded that the use of phosphogypsum as a building material represents no additional internal exposure risk due to the inhalation of radon and external exposure from gamma emitters [
37]. Application (prototype) and modelling approaches can be considered to evaluate random exhalation from building materials and these represent appropriate areas for further studies.
Thus, the aim of this study was to obtain a synthetic anhydrite (anhydrite II), referred to herein as phosphoanhydrite (PA), from the calcination of phosphogypsum and subsequently to apply it as the main binder in SLUs. The advantages of this type of cement compared to ordinary Portland cement (OPC) are related to reducing the clinker factor and minimizing the amount of Portland cement used, reducing CO2 emissions, and introducing a new binder based on calcium sulfate.