Cultured Human Fibroblast Biostimulation Using a 940 nm Diode Laser
Abstract
:1. Introduction
2. Results
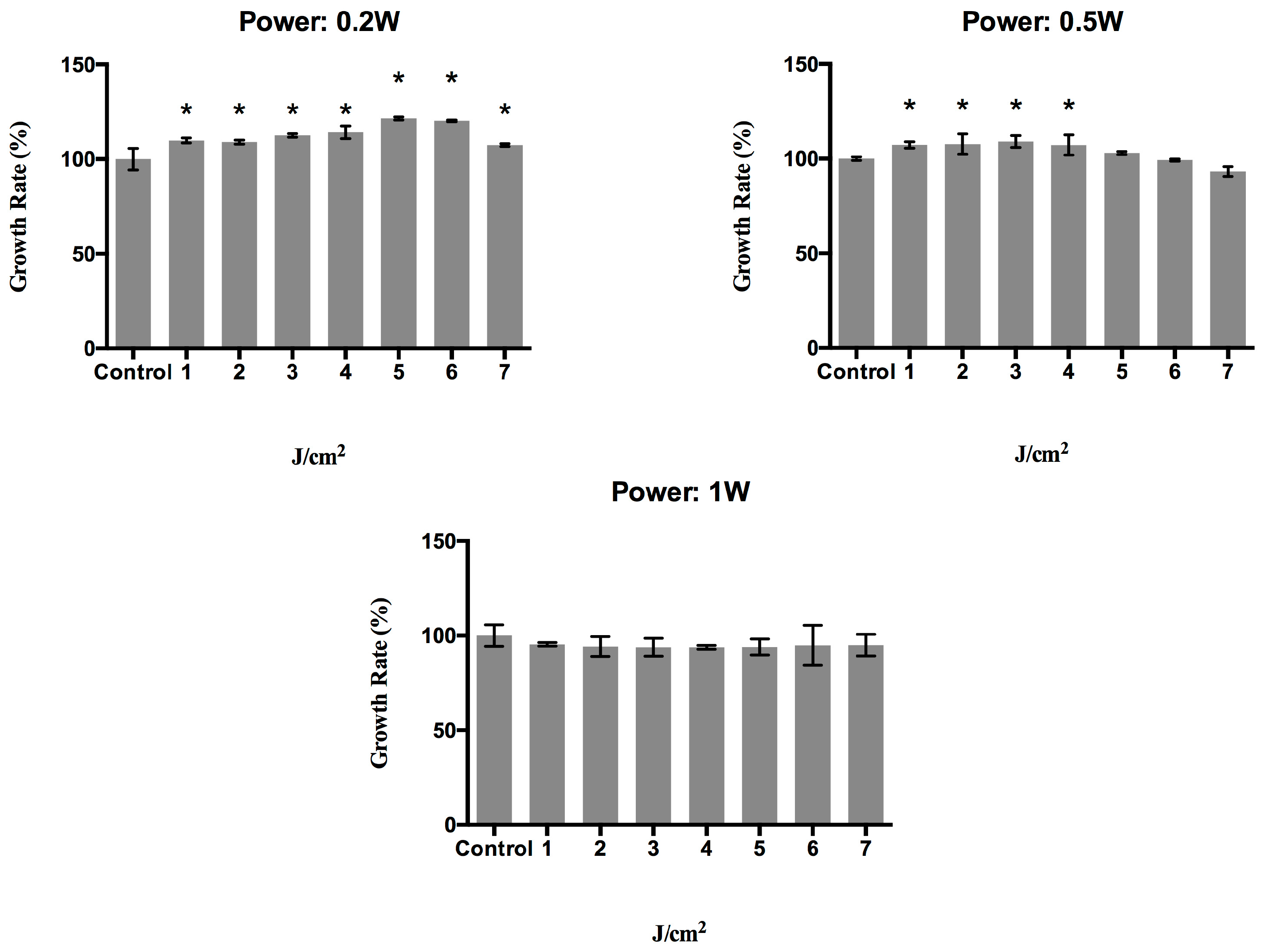
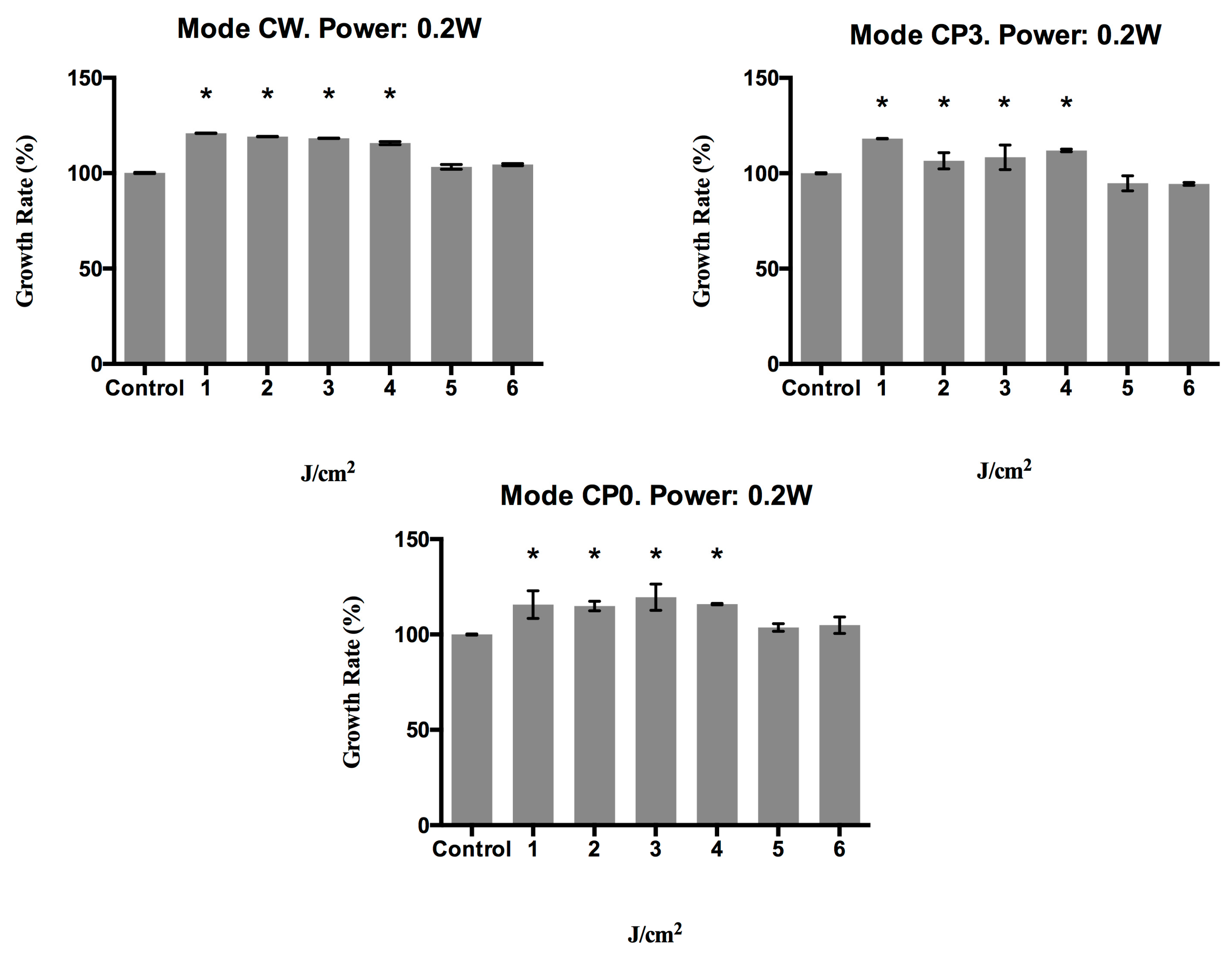
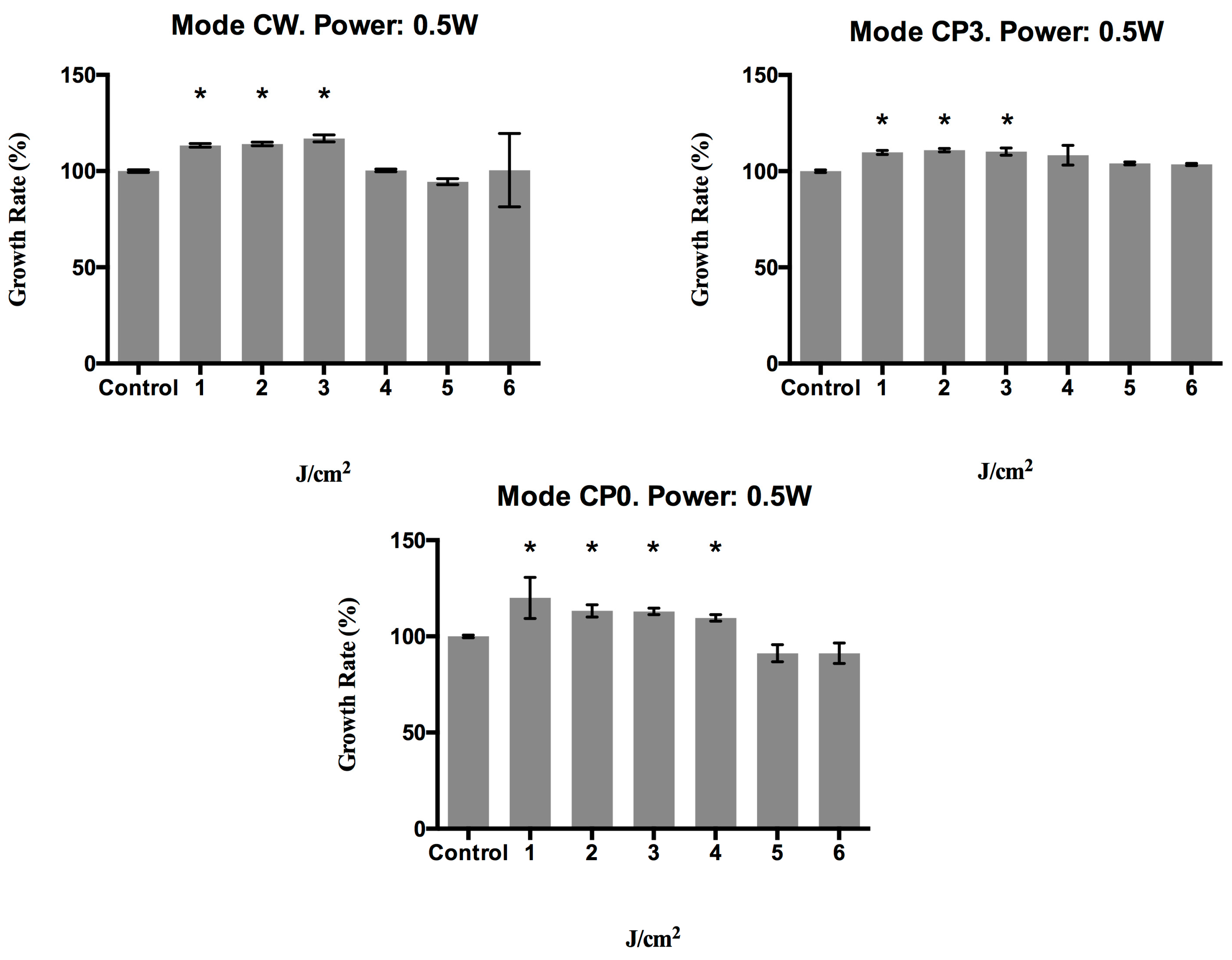
2.1. Effect of a 940 nm Diode Laser on Fibroblast Growth
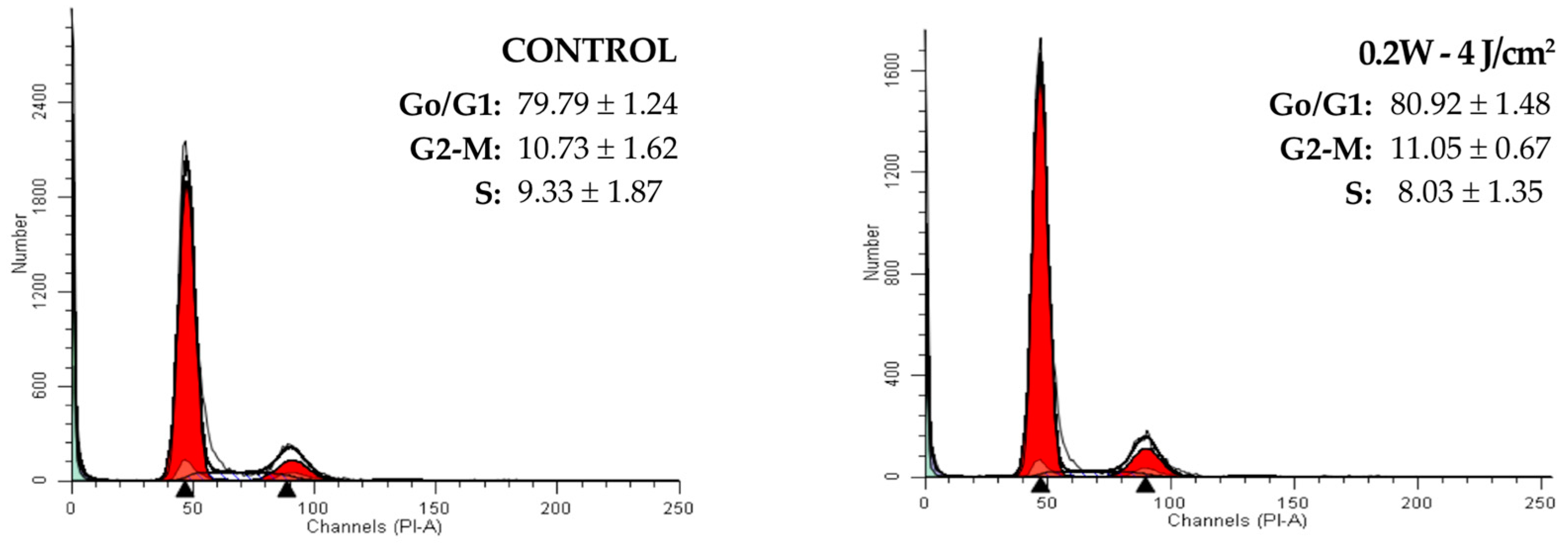
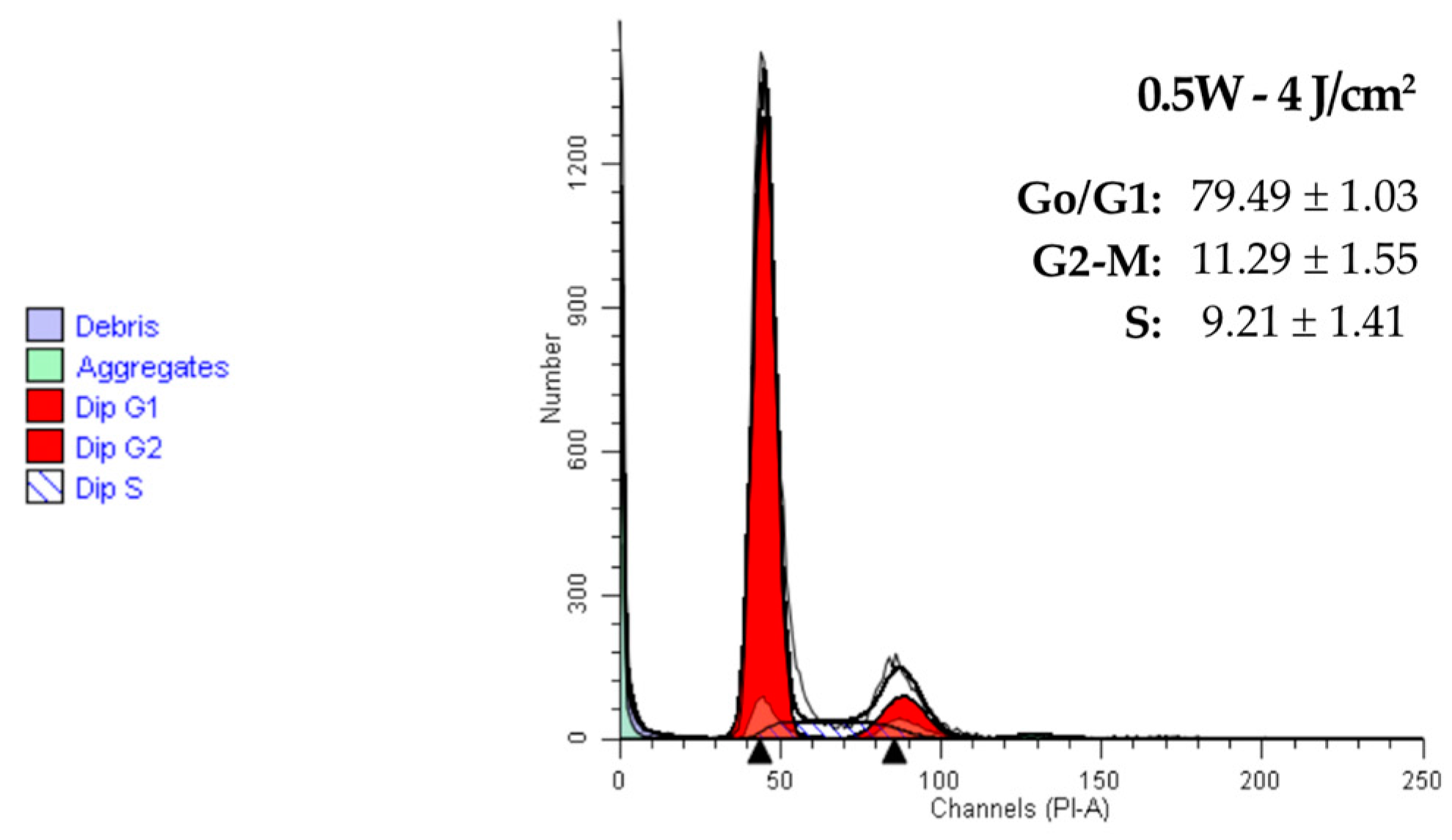
2.2. Effect of a 940 nm Diode Laser on Fibroblast Cell Cycle
2.3. Effect of a 940 nm Diode Laser on Fibronectin and α-Actin Expression in Fibroblasts
3. Discussion
4. Methods
4.1. Cell Culture
4.2. Laser Irradiation
4.3. Cell Proliferation Assay
4.4. Cell Cycle Assay
4.5. Immunofluorescence
4.6. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Babilas, P.; Landthaler, M. New developments in laser therapy. Hautarzt Z. Für Dermatol. Venerol. Verwandte Geb. 2012, 63 (Suppl. 1), 59–66. [Google Scholar] [CrossRef] [PubMed]
- Mignon, C.; Botchkareva, N.V.; Uzunbajakava, N.E.; Tobin, D.J. Photobiomodulation devices for hair regrowth and wound healing: A therapy full of promise but a literature full of confusion. Exp. Dermatol. 2016, 25, 745–749. [Google Scholar] [CrossRef] [PubMed]
- Bjordal, J.M.; Johnson, M.I.; Iversen, V.; Aimbire, F.; Lopes-Martins, R.A.B. Low-level laser therapy in acute pain: A systematic review of possible mechanisms of action and clinical effects in randomized placebo-controlled trials. Photomed. Laser Surg. 2006, 24, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Carroll, J.D.; Milward, M.R.; Cooper, P.R.; Hadis, M.; Palin, W.M. Developments in low level light therapy (LLLT) for dentistry. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2014, 30, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Oltra-Arimon, D.; España-Tost, A.J.; Berini-Aytés, L.; Gay-Escoda, C. Aplicaciones del láser de baja potencia en Odontología. RCOE 2004, 9, 517–524. [Google Scholar] [CrossRef]
- Dörtbudak, O.; Haas, R.; Mallath-Pokorny, G. Biostimulation of bone marrow cells with a diode soft laser. Clin. Oral Implant. Res. 2000, 11, 540–545. [Google Scholar] [CrossRef]
- Saracino, S.; Mozzati, M.; Martinasso, G.; Pol, R.; Canuto, R.A.; Muzio, G. Superpulsed laser irradiation increases osteoblast activity via modulation of bone morphogenetic factors. Lasers Surg. Med. 2009, 41, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Aoki, A.; Sculean, A.; Becker, J. The impact of laser application on periodontal and peri-implant wound healing. Periodontology 2000 2009, 51, 79–108. [Google Scholar] [CrossRef] [PubMed]
- AlGhamdi, K.M.; Kumar, A.; Moussa, N.A. Low-level laser therapy: A useful technique for enhancing the proliferation of various cultured cells. Lasers Med. Sci. 2012, 27, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.C.A.; de Carvalho, P.D.T.C.; Parente, M.; Xavier, M.; Frigo, L.; Aimbire, F.; Leal Junior, E.C.P.; Albertini, R. Low-level laser therapy in different stages of rheumatoid arthritis: A histological study. Lasers Med. Sci. 2013, 28, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, A.P.R.B.; Petri, A.D.; Crippa, G.E.; Stuani, A.S.; Stuani, A.S.; Rosa, A.L.; Stuani, M.B.S. Effect of low-level laser therapy after rapid maxillary expansion on proliferation and differentiation of osteoblastic cells. Lasers Med. Sci. 2012, 27, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Hendudari, F.; Piryaei, A.; Hassani, S.-N.; Darbandi, H.; Bayat, M. Combined effects of low-level laser therapy and human bone marrow mesenchymal stem cell conditioned medium on viability of human dermal fibroblasts cultured in a high-glucose medium. Lasers Med. Sci. 2016, 31, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.H.; Abrahamse, H. Efficacy of three different laser wavelengths for in vitro wound healing. Photodermatol. Photoimmunol. Photomed. 2008, 24, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, R.V.; Novaes, R.D.; Matta, S.L.P.; Benevides, G.P.; Faria, F.R.; Pinto, M.V.M. Comparative study of the effects of gallium-aluminum-arsenide laser photobiomodulation and healing oil on skin wounds in wistar rats: A histomorphometric study. Photomed. Laser Surg. 2010, 28, 597–602. [Google Scholar] [CrossRef] [PubMed]
- De Lima, F.J.C.; de Oliveira Neto, O.B.; Barbosa, F.T.; do Nascimento Galvão, A.M.; Ramos, F.W.S.; de Lima, C.C.F.; de Sousa Rodrigues, C.F. Is there a protocol in experimental skin wounds in rats using low-level diode laser therapy (LLDLT) combining or not red and infrared wavelengths? Systematic review. Lasers Med. Sci. 2016, 31, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Desmoulière, A.; Chaponnier, C.; Gabbiani, G. Tissue repair, contraction, and the myofibroblast. Wound Repair Regen. 2005, 13, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Schuurman, J.-P.; Schoonhoven, L.; Defloor, T.; van Engelshoven, I.; van Ramshorst, B.; Buskens, E. Economic evaluation of pressure ulcer care: A cost minimization analysis of preventive strategies. Nurs. Econ. 2009, 27, 390–400, 415. [Google Scholar] [PubMed]
- Medina Huertas, R.; De Luna-Bertos, E.; Ramos-Torrecillas, J.; Medina Leyva, F.; Ruiz, C.; Garcia-Martinez, O. Effect and Clinical Implications of the Low-Energy Diode Laser on Bone Cell Proliferation. Biol. Res. Nurs. 2014, 16, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Manzano-Moreno, F.J.; Medina-Huertas, R.; Ramos-Torrecillas, J.; Garcia-Martinez, O.; Ruiz, C. The effect of low-level diode laser therapy on early differentiation of osteoblast via BMP-2/TGF-beta 1 and its receptors. J. Cranio-Maxillofac. Surg. 2015, 43, 1926–1932. [Google Scholar] [CrossRef] [PubMed]
- Medina-Huertas, R.; Javier Manzano-Moreno, F.; De Luna-Bertos, E.; Ramos-Torrecillas, J.; Garcia-Martinez, O.; Ruiz, C. The effects of low-level diode laser irradiation on differentiation, antigenic profile, and phagocytic capacity of osteoblast-like cells (MG-63). Lasers Med. Sci. 2014, 29, 1479–1484. [Google Scholar] [CrossRef] [PubMed]
- Darby, I.A.; Laverdet, B.; Bonté, F.; Desmoulière, A. Fibroblasts and myofibroblasts in wound healing. Clin. Cosmet. Investig. Dermatol. 2014, 7, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Torrecillas, J.; De Luna-Bertos, E.; Garcia-Martinez, O.; Diaz-Rodriguez, L.; Ruiz, C. Use of Platelet-Rich Plasma to Treat Pressure Ulcers A Case Study. J. Wound. Ostomy Cont. Nurs. 2013, 40, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Torrecillas, J.; De Luna-Bertos, E.; Garcia-Martinez, O.; Ruiz, C. Clinical utility of growth factors and platelet-rich plasma in tissue regeneration: A review. Wounds 2014, 26, 207–213. [Google Scholar] [PubMed]
- Ramos-Torrecillas, J.; Garcia-Martinez, O.; De Luna-Bertos, E.; Manuel Ocana-Peinado, F.; Ruiz, C. Effectiveness of Platelet-Rich Plasma and Hyaluronic Acid for the Treatment and Care of Pressure Ulcers. Biol. Res. Nurs. 2015, 17, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Torrecillas, J.; de Luna-Bertos, E.; Manzano-Moreno, F.J.; García-Martínez, O.; Ruiz, C. Human Fibroblast–Like Cultures in the Presence of Platelet-Rich Plasma as a Single Growth Factor Source: Clinical Implications. Adv. Skin Wound Care 2014, 27, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; McGrath, C.; Jin, L.; Zhang, C.; Yang, Y. Effect of diode low-level lasers on fibroblasts derived from human periodontal tissue: A systematic review of in vitro studies. Lasers Med. Sci. 2016, 31, 1493–1510. [Google Scholar] [CrossRef] [PubMed]
- Hakki, S.S.; Bozkurt, S.B. Effects of different setting of diode laser on the mRNA expression of growth factors and type I collagen of human gingival fibroblasts. Lasers Med. Sci. 2012, 27, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Basso, F.G.; Soares, D.G.; Pansani, T.N.; Cardoso, L.M.; Scheffel, D.L.; de Souza Costa, C.A.; Hebling, J. Proliferation, migration, and expression of oral-mucosal-healing-related genes by oral fibroblasts receiving low-level laser therapy after inflammatory cytokines challenge. Lasers Surg. Med. 2016, 48, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- Manzano-Moreno, F.J.; Ramos-Torrecillas, J.; De Luna-Bertos, E.; Ruiz, C.; Garcia-Martinez, O. High doses of bisphosphonates reduce osteoblast-like cell proliferation by arresting the cell cycle and inducing apoptosis. J. Cranio-Maxillofac. Surg. 2015, 43, 396–401. [Google Scholar] [CrossRef] [PubMed]

| mAbs | Fluorochrome | Supplier |
|---|---|---|
| Anti-human fibronectin fluorescein | FITC | R&D Systems (Minneapolis, MN, USA) |
| Anti-human α-actin | PE | R&D Systems (Minneapolis, MN, USA) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Illescas-Montes, R.; Melguizo-Rodríguez, L.; Manzano-Moreno, F.J.; García-Martínez, O.; Ruiz, C.; Ramos-Torrecillas, J. Cultured Human Fibroblast Biostimulation Using a 940 nm Diode Laser. Materials 2017, 10, 793. https://doi.org/10.3390/ma10070793
Illescas-Montes R, Melguizo-Rodríguez L, Manzano-Moreno FJ, García-Martínez O, Ruiz C, Ramos-Torrecillas J. Cultured Human Fibroblast Biostimulation Using a 940 nm Diode Laser. Materials. 2017; 10(7):793. https://doi.org/10.3390/ma10070793
Chicago/Turabian StyleIllescas-Montes, Rebeca, Lucía Melguizo-Rodríguez, Francisco Javier Manzano-Moreno, Olga García-Martínez, Concepción Ruiz, and Javier Ramos-Torrecillas. 2017. "Cultured Human Fibroblast Biostimulation Using a 940 nm Diode Laser" Materials 10, no. 7: 793. https://doi.org/10.3390/ma10070793
APA StyleIllescas-Montes, R., Melguizo-Rodríguez, L., Manzano-Moreno, F. J., García-Martínez, O., Ruiz, C., & Ramos-Torrecillas, J. (2017). Cultured Human Fibroblast Biostimulation Using a 940 nm Diode Laser. Materials, 10(7), 793. https://doi.org/10.3390/ma10070793







