Study of Al-Si Alloy Oxygen Saturation on Its Microstructure and Mechanical Properties
Abstract
:1. Introduction
2. Materials and Methods
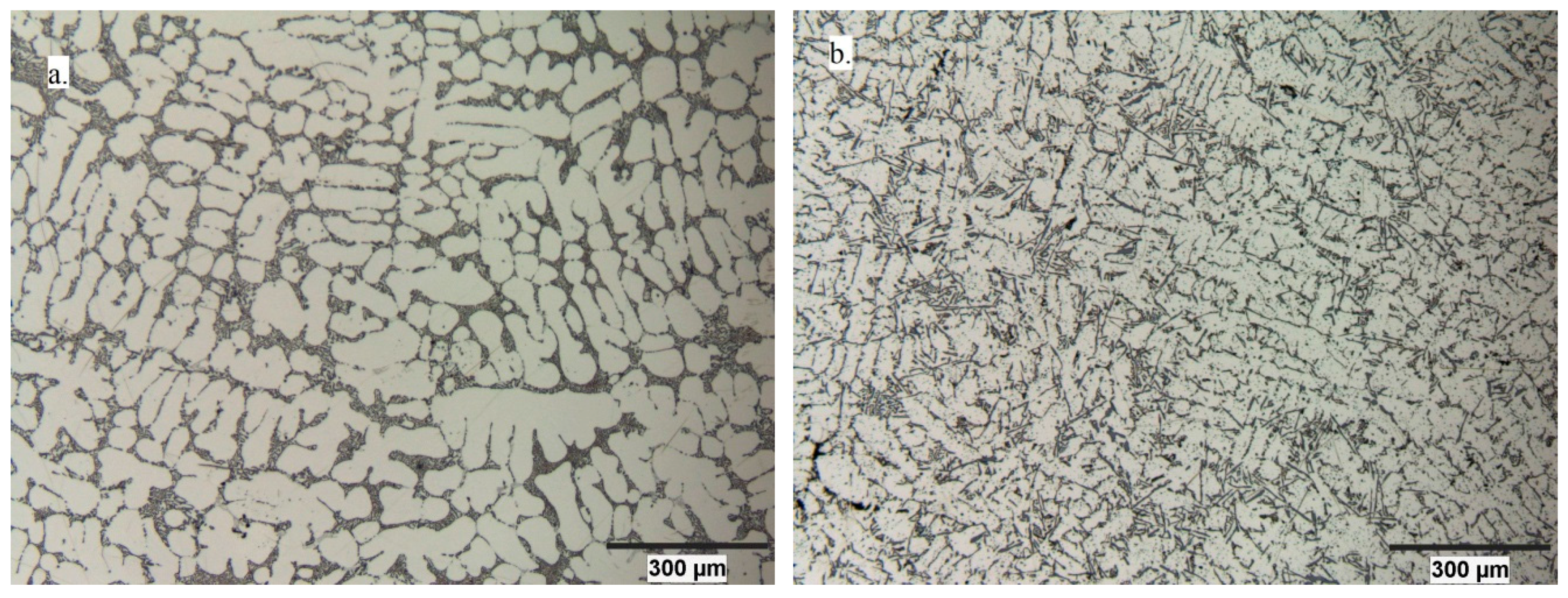
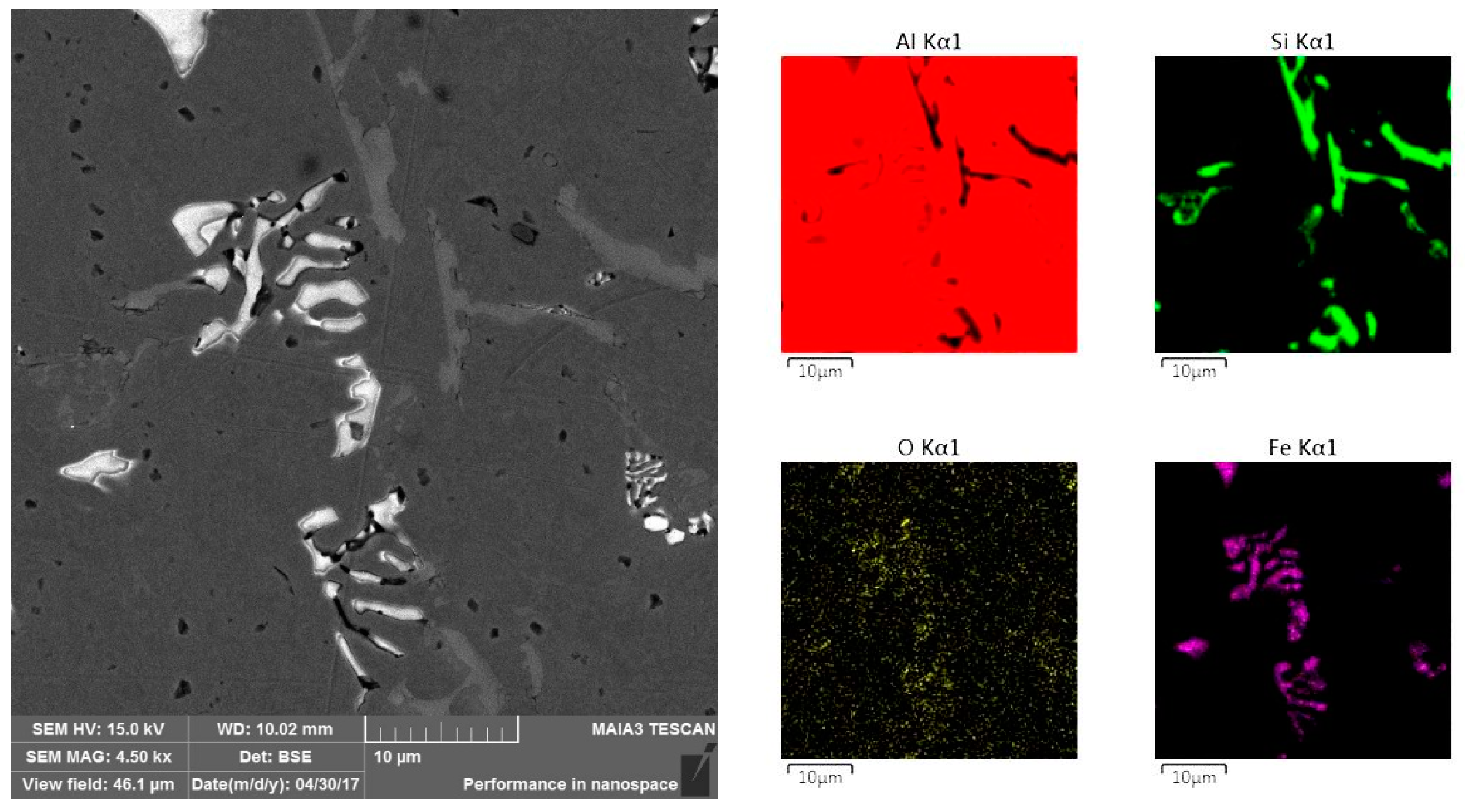
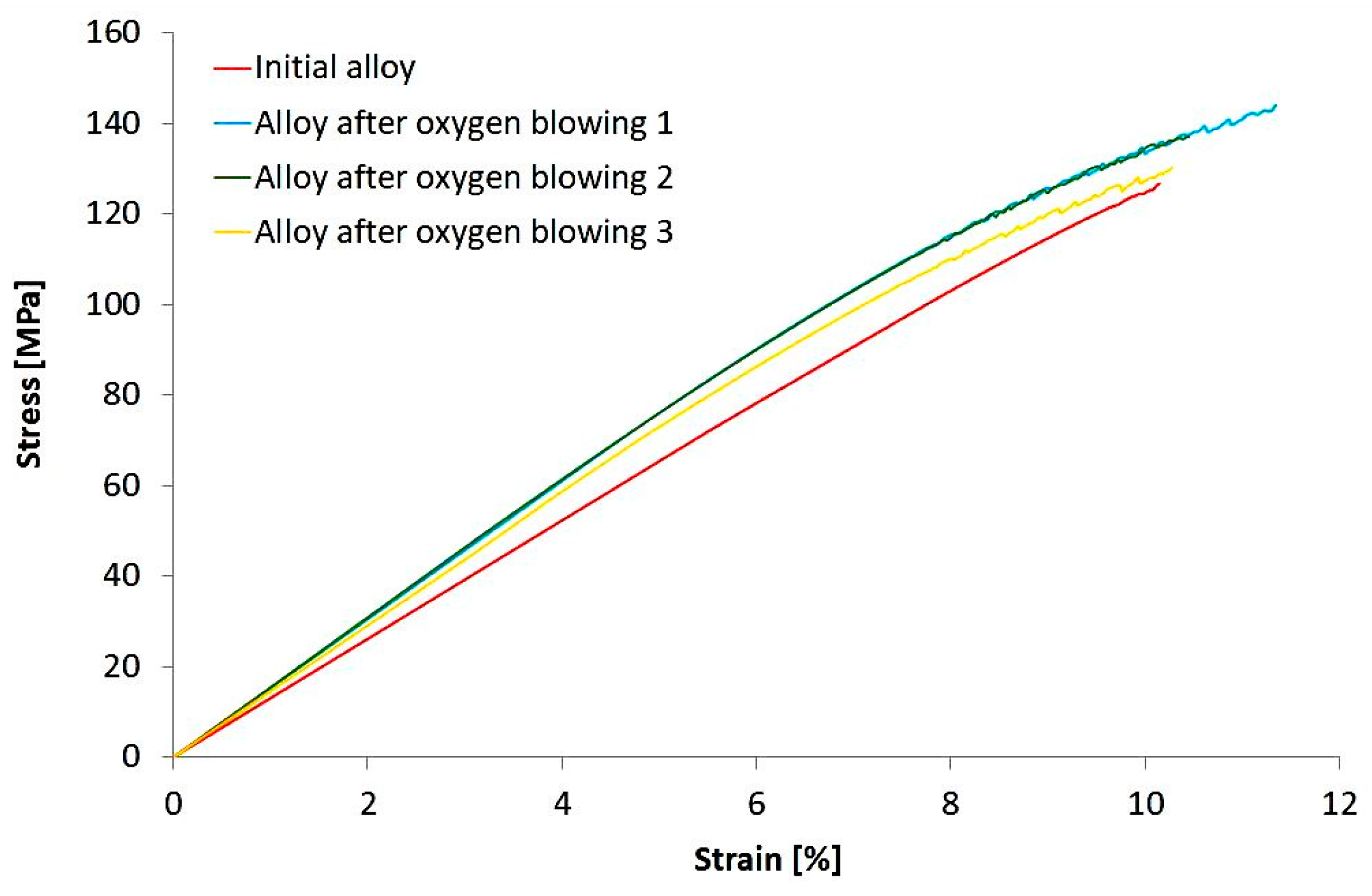
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Callister, W.D. Materials Science and Engineering, 7th ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2007. [Google Scholar]
- Schaffer, P.L.; Dahle, A.K. Settling behavior of different grain refiners in aluminum. Mater. Sci. Eng. A 2005, 413–414, 373–378. [Google Scholar] [CrossRef]
- Birol, Y. AlB3 master alloy to grain refine AlSi10Mg and AlSi12Cu aluminum foundry alloys. J. Alloys Compd. 2012, 513, 150–153. [Google Scholar] [CrossRef]
- Mohanty, P.S.; Gruzleski, J.E. Mechanism of grain refinement in aluminum. Acta Metall. Mater. 1995, 43, 2001–2012. [Google Scholar] [CrossRef]
- Wang, C.; Wang, M.; Yu, B.; Chen, D.; Qin, P.; Feng, M.; Dai, Q. The grain refinement behavior of TiB2 particles prepared with in situ technology. Mater. Sci. Eng. A 2007, 459, 238–243. [Google Scholar] [CrossRef]
- Daoud, A.; Abo-Elkhar, M. Influence of Al2O3 or ZrO2 particulate addition on the microstructure aspects of AlNi and AlSi alloys. J. Mater. Process. Technol. 2002, 120, 296–302. [Google Scholar] [CrossRef]
- Shivaprasad, C.G.; Aithal, K.; Narendranath, S.; Desai, V.; Mukunda, P.G. Effect of combined grain refinement and modification and microstructure and mechanical properties of hypoeutectic, eutectic and hypereutectic Al-Si alloys. Int. J. Microstruct. Mater. Prop. 2010, 10, 274–284. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, Y.; Cheng, X.; Chen, G.; Dai, Q. High-energy ultrasonic field effects on the microstructure and mechanical behaviors of A356 alloy. J. Alloys Compd. 2009, 470, 168–172. [Google Scholar] [CrossRef]
- Han, Y.; Le, K.; Wang, J.; Shu, D.; Sun, B. Influence of high-intensity ultrasound on grain refining performance of Al-5Ti-1B master alloy on aluminum. Mater. Sci. Eng. A 2005, 405, 306–312. [Google Scholar] [CrossRef]
- Das, A.; Kotadia, H.R. Effect of high-intensity ultrasonic irradiation on the modification of solidification microstructure in a Si-rich hypoeutectic Al-Si alloy. Mater. Chem. Phys. 2011, 125, 853–859. [Google Scholar] [CrossRef]
- Wu, B.; Reddy, R.G. In-situ formation of SiC-reinforced Al-Si alloys composites using methane gas mixture. Metall. Mater. Trans. B 2002, 33, 543–550. [Google Scholar] [CrossRef]
- Zheng, Q.; Wu, B.; Reddy, R.G. In-situ processing of Al alloy composites. Adv. Eng. Mater. 2003, 5, 167–172. [Google Scholar] [CrossRef]
- Babcsán, N.; Leitlmeier, D.; Degischer, H.P.; Banhart, J. The role of oxidation in blowing particle stabilized aluminum foams. Adv. Eng. Mater. 2004, 6, 421–428. [Google Scholar] [CrossRef]
- Babcsán, N.; Leitlmeier, D.; Degischer, H.P. Foamability of particle reinforced aluminum melt. Mater. Werkst. 2003, 34, 22–29. [Google Scholar] [CrossRef]
- Elliott, J.C. Method of Producing Metal Foam. U.S. Patent 2751289A, 19 June 1956. [Google Scholar]
- Finkelstein, A.B.; Schaefer, A.; Chikova, O.A. Microstructures, mechanical properties ingot AlSi7Fe1 after blowing oxygen of melt. Acta Metall. Slovaca 2017, 23, 4–11. [Google Scholar] [CrossRef]
- Standard Test Method for Tension Testing of Metallic Materials, ASTM E8M Standard; ASTM International: West Conshohocken, PA, USA, 2016.
- Babcsán, N.; Garcia-Moreno, F.; Banhart, J. Role of oxidation during blowing of aluminium foams by external gas injection. Proceeding of the Met’Foam, Kyoto, Japan, 21–23 September 2005. [Google Scholar]
- Brewer, L.; Searcy, A.W. The gaseous species of the Al-Al2O3 system. JACS 1951, 73, 5308–5314. [Google Scholar] [CrossRef]
- Hoch, M.; Johnston, H.L. Formation, stability and crystal structure of the solid aluminum suboxides: Al2O and AlO. JACS 1954, 76, 2560–2561. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, R.; Zhou, X.; Cai, M.; Sun, X. Selective growth of α-Al2O3 nanowires and nanobelts. J. Nanomater. 2008, 2008, 1–8. [Google Scholar]
- Mirza, F.A.; Chen, D.L. A unified model of the prediction of yield strength in particulate reinforced metal matrix nanocomposites. Materials 2015, 8, 5138–5153. [Google Scholar] [CrossRef]
- Hall, E.O. The deformation and ageing of mild steel: III discussion of results. Proc. Phys. Soc. B 1951, 64, 747–753. [Google Scholar] [CrossRef]
- Cho, Y.H.; Lee, H.C.; Oh, K.H.; Dahle, A.K. Effect of strontium and phosphorus on eutectic Al-Si nucleation and formation of β-Al5FeSi in hypoeutectic Al-Si foundry alloys. Metall. Mater. Trans. A 2008, 39, 2435–2448. [Google Scholar] [CrossRef]
- Borodianskiy, K.; Zinigrad, M. Modification performance of WC nanoparticles in aluminum and an Al-Si casting alloy. Metall. Mater. Trans. B 2016, 47, 1302–1308. [Google Scholar] [CrossRef]
- Kato, Y.; Hisayuki, K.; Sakaguchi, M.; Higashi, K. Effect of alloy elements on microstructures and mechanical properties in Al-Mg-Si alloys. In Proceedings of the 13th International Conference on Aluminum Alloys (ICAA 13), Pittsburgh, PA, USA, 3–7 June 2012; pp. 1521–1526. [Google Scholar]
- Çetin, A.; Kalkanli, A. Investigation of microporosity formation mechanisms in A356 aluminium alloy castings. Int. J. Microstruct. Mater. Prop. 2009, 4, 377–385. [Google Scholar]
- Jackowski, J.; Suchora, M.; Szweycer, M. Relationship between the reinforcement properties and the crystallization of MMC. Arch. Found. Eng. 2008, 8, 59–64. [Google Scholar]
- Wang, J.; He, S.; Sun, B.; Guo, Q.; Nishio, M. Grain refinement of Al-Si alloy (A356) by melt thermal treatment. J. Mater. Process. Technol. 2003, 141, 29–34. [Google Scholar] [CrossRef]


| Si | Mg | Fe | Cu | Mn | Al |
|---|---|---|---|---|---|
| 7.42 | 0.23 | 1.06 | 0.15 | 0.23 | Balance |
| Alloy | Length α-Al (µm) | Eutectic Phase Composition (%) |
|---|---|---|
| Initial alloy | 50.56 | 24.80 |
| Treated alloy | 32.69 | 29.18 |
| Mechanical Property | Initial Alloy | Treated Alloy |
|---|---|---|
| Tensile strength (MPa) | 127 | 134 |
| Yield strength (MPa) | 120 | 96 |
| Ductility (%) | 10.1 | 10.7 |
| Calculated Parameter | Initial Casting Process | Oxygen Blowing Treatment Process |
|---|---|---|
| Mass change (%) | +0.12 | −1.56 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Finkelstein, A.; Schaefer, A.; Chikova, О.; Borodianskiy, K. Study of Al-Si Alloy Oxygen Saturation on Its Microstructure and Mechanical Properties. Materials 2017, 10, 786. https://doi.org/10.3390/ma10070786
Finkelstein A, Schaefer A, Chikova О, Borodianskiy K. Study of Al-Si Alloy Oxygen Saturation on Its Microstructure and Mechanical Properties. Materials. 2017; 10(7):786. https://doi.org/10.3390/ma10070786
Chicago/Turabian StyleFinkelstein, Arkady, Arseny Schaefer, Оlga Chikova, and Konstantin Borodianskiy. 2017. "Study of Al-Si Alloy Oxygen Saturation on Its Microstructure and Mechanical Properties" Materials 10, no. 7: 786. https://doi.org/10.3390/ma10070786
APA StyleFinkelstein, A., Schaefer, A., Chikova, О., & Borodianskiy, K. (2017). Study of Al-Si Alloy Oxygen Saturation on Its Microstructure and Mechanical Properties. Materials, 10(7), 786. https://doi.org/10.3390/ma10070786







