The Impact of Metal Ion Exposure on the Cellular Behavior of Human Osteoblasts and PBMCs: In Vitro Analyses of Osteolytic Processes
Abstract
:1. Introduction
2. Results
2.1. Effects of CoCr29Mo6 Ions in Single Cultures of Human Osteoblasts and PBMCs
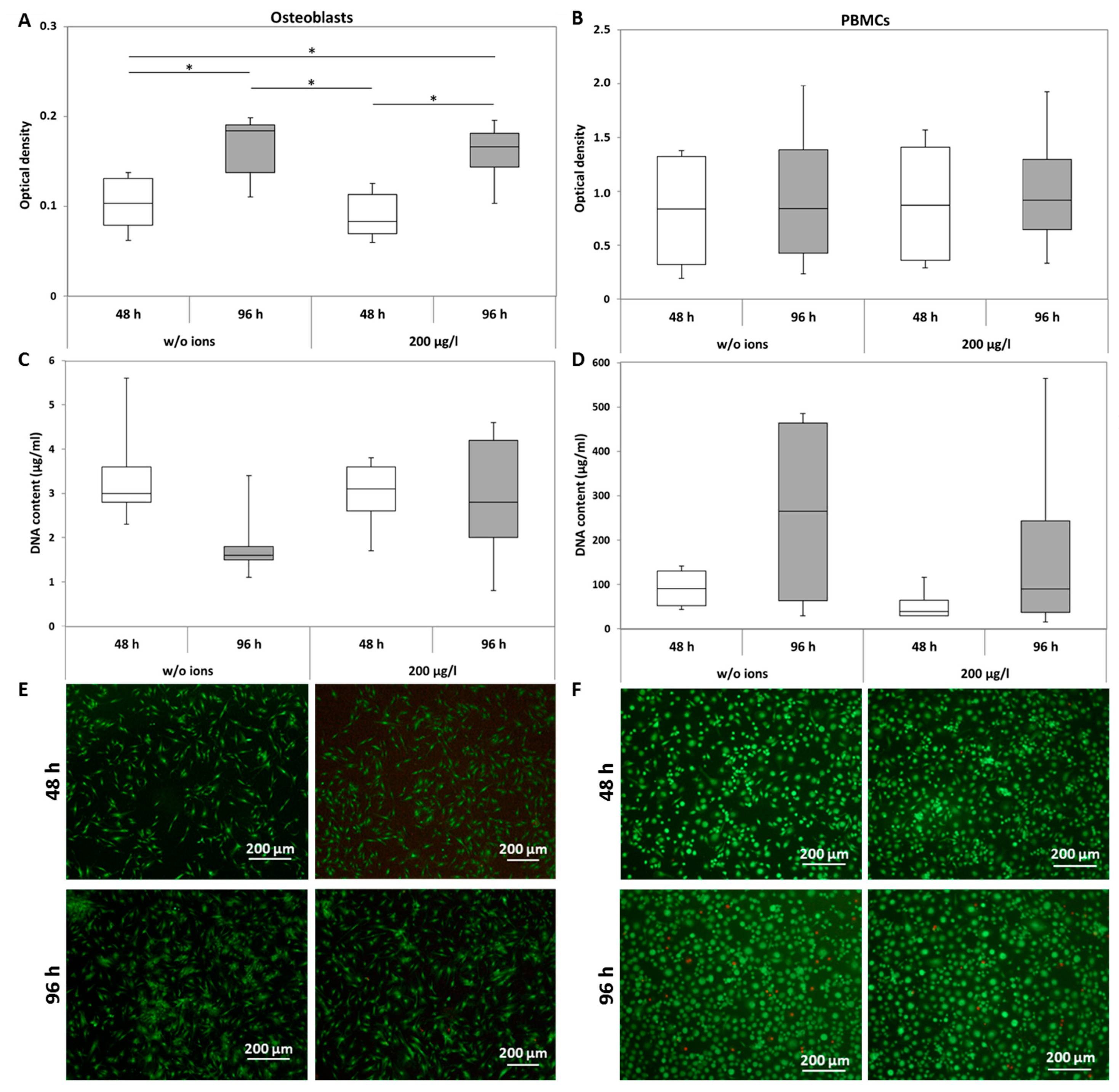
2.1.1. Viability
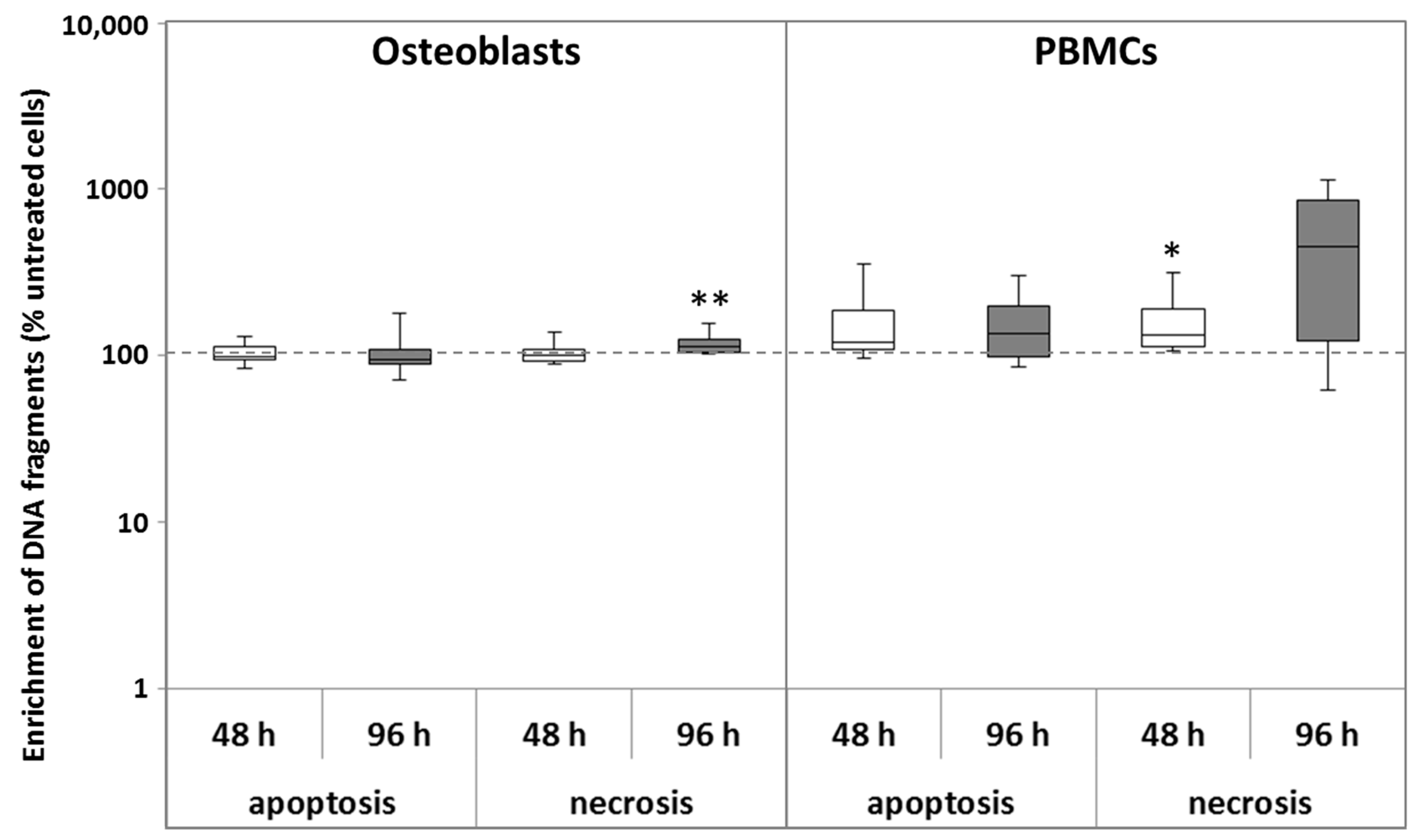
2.1.2. Apoptosis and Necrosis
2.1.3. Osteoblastic Differentiation and Induction of Osteoclastic Differentiation Markers
2.1.4. Gene Expression of Pro-Osteolytic Mediators
2.2. Influence of CoCr29Mo6 Ions in Co-Cultures
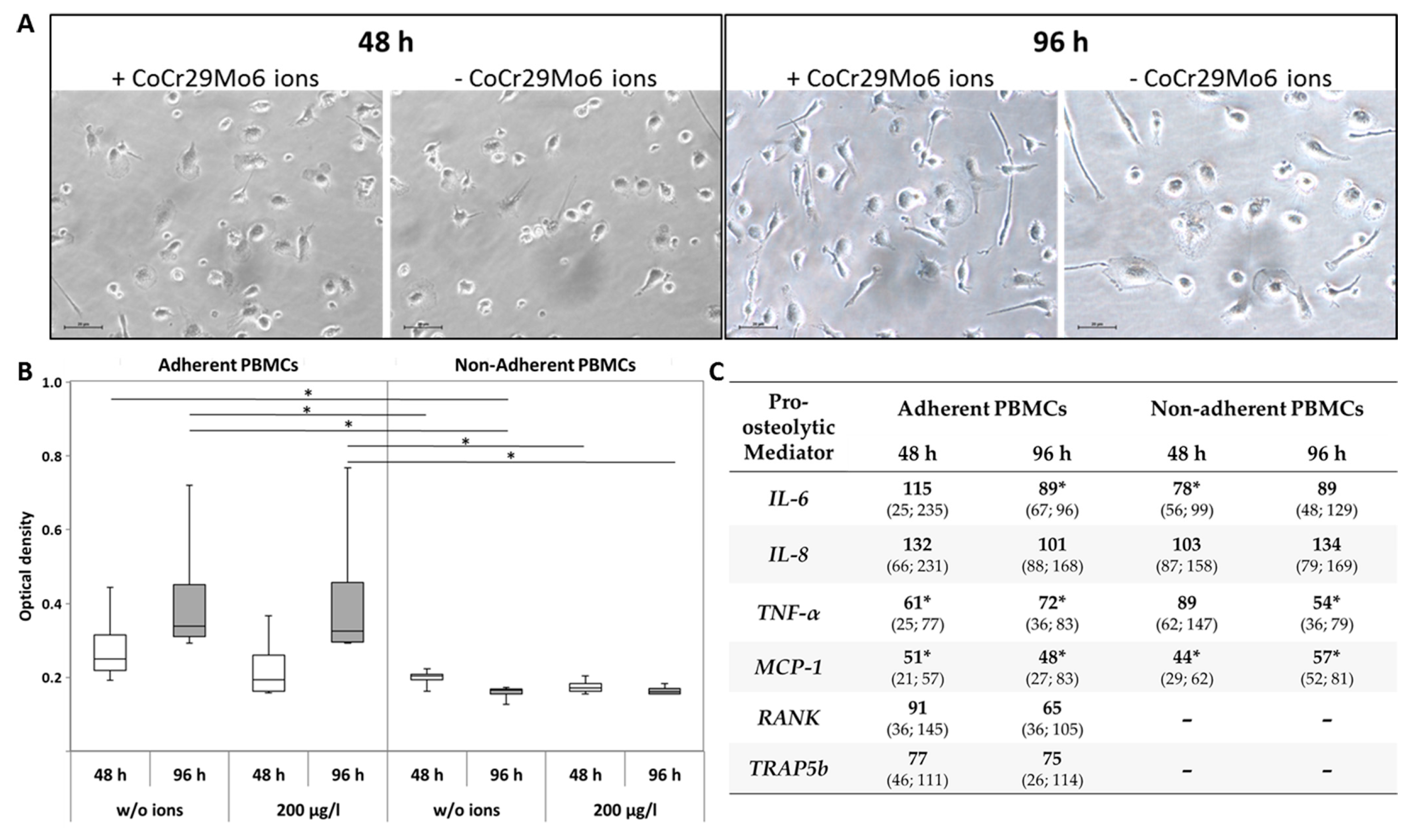
2.2.1. Adherent and Non-Adherent PBMCs
2.2.2. PBMCs and Osteoblasts
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Isolation of Human Osteoblastic Cells and PBMCs
5.2. Cell Viability
5.3. Determination of Apoptosis and Necrosis
5.4. Gene Expression Analyses
5.5. Pro-Collagen Type 1 Synthesis Rate
5.6. Experimental Design
5.7. Data Illustration and Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bitar, D.; Parvizi, J. Biological response to prosthetic debris. World J. Orthop. 2015, 6, 172–189. [Google Scholar] [CrossRef] [PubMed]
- Lenz, R.; Mittelmeier, W.; Hansmann, D.; Brem, R.; Diehl, P.; Fritsche, A.; Bader, R. Response of human osteoblasts exposed to wear particles generated at the interface of total hip stems and bone cement. J. Biomed. Mater. Res. A 2009, 89, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Lochner, K.; Fritsche, A.; Jonitz, A.; Hansmann, D.; Mueller, P.; Mueller-Hilke, B.; Bader, R. The potential role of human osteoblasts for periprosthetic osteolysis following exposure to wear particles. Int. J. Mol. Med. 2011, 28, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
- Jonitz-Heincke, A.; Lochner, K.; Schulze, C.; Pohle, D.; Pustlauk, W.; Hansmann, D.; Bader, R. Contribution of human osteoblasts and macrophages to bone matrix degradation and proinflammatory cytokine release after exposure to abrasive endoprosthetic wear particles. Mol. Med. Rep. 2016, 14, 1491–1500. [Google Scholar] [CrossRef] [PubMed]
- Schulze, C.; Lochner, K.; Jonitz, A.; Lenz, R.; Duettmann, O.; Hansmann, D.; Bader, R. Cell viability, collagen synthesis and cytokine expression in human osteoblasts following incubation with generated wear particles using different bone cements. Int. J. Mol. Med. 2013, 32, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Matusiewicz, H. Potential release of in vivo trace metals from metallic medical implants in the human body: From ions to nanoparticles—A systematic analytical review. Acta Biomater. 2014, 10, 2379–2403. [Google Scholar] [CrossRef] [PubMed]
- Cadosch, D.; Schlett, C.L.; Gautschi, O.P.; Frei, H.C.; Filgueira, L. Metal ions: Important co-players in aseptic loosening. Z ORTHOP UNFALLCHIR 2010, 148, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Magone, K.; Luckenbill, D.; Goswami, T. Metal ions as inflammatory initiators of osteolysis. Arch. Orthop. Trauma Surg. 2015, 135, 683–695. [Google Scholar] [CrossRef] [PubMed]
- Scharf, B.; Clement, C.C.; Zolla, V.; Perino, G.; Yan, B.; Elci, S.G.; Purdue, E.; Goldring, S.; Macaluso, F.; Cobelli, N.; et al. Molecular analysis of chromium and cobalt-related toxicity. Sci. Rep. 2014, 4, 5729. [Google Scholar] [CrossRef] [PubMed]
- Gibon, E.; Amanatullah, D.F.; Loi, F.; Pajarinen, J.; Nabeshima, A.; Yao, Z.; Hamadouche, M.; Goodman, S.B. The biological response to orthopaedic implants for joint replacement: Part I: Metals. J. Biomed. Mater. Res. B Appl. Biomater. 2016. [Google Scholar] [CrossRef] [PubMed]
- Catelas, I.; Petit, A.; Zukor, D.J.; Antoniou, J.; Huk, O.L. TNF-alpha secretion and macrophage mortality induced by cobalt and chromium ions in vitro-qualitative analysis of apoptosis. Biomaterials 2003, 24, 383–391. [Google Scholar] [CrossRef]
- Caicedo, M.S.; Desai, R.; McAllister, K.; Reddy, A.; Jacobs, J.J.; Hallab, N.J. Soluble and particulate Co-Cr-Mo alloy implant metals activate the inflammasome danger signaling pathway in human macrophages: A novel mechanism for implant debris reactivity. J. Orthop. Res. 2009, 27, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Delaunay, C.; Petit, I.; Learmonth, I.D.; Oger, P.; Vendittoli, P.A. Metal-on-metal bearings total hip arthroplasty: The cobalt and chromium ions release concern. Orthop. Traumatol. Surg. Res. 2010, 96, 894–904. [Google Scholar] [CrossRef] [PubMed]
- De Smet, K.; De Haan, R.; Calistri, A.; Campbell, P.A.; Ebramzadeh, E.; Pattyn, C.; Gill, H.S. Metal ion measurement as a diagnostic tool to identify problems with metal-on-metal hip resurfacing. J. Bone Jt. Surg. Am. 2008, 90, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Madl, A.K.; Kovochich, M.; Liong, M.; Finley, B.L.; Paustenbach, D.J.; Oberdorster, G. Toxicology of wear particles of cobalt-chromium alloy metal-on-metal hip implants Part II: Importance of physicochemical properties and dose in animal and in vitro studies as a basis for risk assessment. Nanomedicine 2015, 11, 1285–1298. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Shi, Q.; Yang, H. The role of TLR and chemokine in wear particle-induced aseptic loosening. J. Biomed. Biotechnol. 2012, 2012, 596870. [Google Scholar] [CrossRef] [PubMed]
- Granchi, D.; Ciapetti, G.; Amato, I.; Pagani, S.; Cenni, E.; Savarino, L.; Avnet, S.; Peris, J.L.; Pellacani, A.; Baldini, N.; et al. The influence of alumina and ultra-high molecular weight polyethylene particles on osteoblast-osteoclast cooperation. Biomaterials 2004, 25, 4037–4045. [Google Scholar] [CrossRef] [PubMed]
- Jablonski, H.; Rekasi, H.; Jager, M. The influence of calcitonin gene-related peptide on markers of bone metabolism in MG-63 osteoblast-like cells co-cultured with THP-1 macrophage-like cells under virtually osteolytic conditions. BMC Musculoskelet. Disord. 2016, 17, 199. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.H.; Kao, S.; Sato, T.; Pajarinen, J.; Zhang, R.; Loi, F.; Goodman, S.B.; Yao, Z. Exposure of polyethylene particles induces interferon-gamma expression in a natural killer T lymphocyte and dendritic cell coculture system in vitro: A preliminary study. J. Biomed. Mater. Res. A 2015, 103, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Schofer, M.D.; Fuchs-Winkelmann, S.; Kessler-Thones, A.; Rudisile, M.M.; Wack, C.; Paletta, J.R.; Boudriot, U. The role of mesenchymal stem cells in the pathogenesis of Co-Cr-Mo particle induced aseptic loosening: An in vitro study. Biomed. Mater. Eng. 2008, 18, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Sommer, B.; Felix, R.; Sprecher, C.; Leunig, M.; Ganz, R.; Hofstetter, W. Wear particles and surface topographies are modulators of osteoclastogenesis in vitro. J. Biomed. Mater. Res. A 2005, 72, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Sansone, V.; Pagani, D.; Melato, M. The effects on bone cells of metal ions released from orthopaedic implants. A review. Clin. Cases Miner. Bone Metab. 2013, 10, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Jämsen, E.; Kouri, V.P.; Olkkonen, J.; Cör, A.; Goodman, S.B.; Konttinen, Y.T.; Pajarinen, J. Charakterization of macrophage polarizing cytokines in the aseptic loosening of total hip replacement. J. Orthop. Res. 2014, 32, 1241–1246. [Google Scholar] [CrossRef] [PubMed]
- Jämsen, E.; Kouri, V.P.; Ainola, M.; Goodman, S.B.; Nordström, D.C.; Eklund, K.K.; Pajarinen, J. Correlations between macrophage polarizing cytokines, inflammatory mediators, osteoclast activity, and toll-like receptors in tissues around aseptically loosened hip implants. J. Biomed. Mater. Res. A 2017, 105, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Felzmann, T.; Witt, V.; Wimmer, D.; Ressmann, G.; Wagner, D.; Paul, P.; Hüttner, K.; Fritsch, G. Monocyte enrichment from leukapharesis products for the generation of DCs by plastic adherence, or by positive or negative selection. Cytotherapy 2003, 5, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; Wicklund, B.H.; Gustilo, R.B.; Tsukayama, D.T. Prosthetic metals impair murine immune response and cytokine release in vivo and in vitro. J. Orthop. Res. 1997, 15, 688–699. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; Tsukayama, D.T.; Wicklund, B.H.; Gustilo, R.B. Inhibition of T and B cell proliferation by titanium, cobalt, and chromium: Role of IL-2 and IL-6. J. Biomed. Mater. Res. 1996, 32, 655–661. [Google Scholar] [CrossRef]
- Jonitz-Heincke, A.; Schröder, M.; Hansmann, D.; Utzschneider, S.; Kretzer, J.P.; Bader, R. The influence of metallic ions from CoCr28Mo6 on the osteogenic differentiation and cytokine release of human osteoblasts. Curr. Direct. Biomed. Eng. 2015, 1, 498–502. [Google Scholar] [CrossRef]
- Kudo, O.; Sabokbar, A.; Pocock, A.; Itonaga, I.; Fujikawa, Y.; Athanasou, N.A. Interleukin-6 and interleukin-11 support human osteoclast formation by a RANKL-independent mechanism. Bone 2003, 32, 1–7. [Google Scholar] [CrossRef]
- Anissian, L.; Stark, A.; Dahlstrand, H.; Granberg, B.; Good, V.; Bucht, E. Cobalt ions influence proliferation and function of human osteoblast-like cells. Acta Orthop. Scand. 2002, 73, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Fleury, C.; Petit, A.; Mwale, F.; Antoniou, J.; Zukor, D.J.; Tabrizian, M.; Huk, O.L. Effect of cobalt and chromium ions on human MG-63 osteoblasts in vitro: Morphology, cytotoxicity, and oxidative stress. Biomaterials 2006, 27, 3351–3360. [Google Scholar] [CrossRef] [PubMed]

| Differentiation Marker | Osteoblasts | Adherent PBMCs | ||
|---|---|---|---|---|
| 48 h | 96 h | 48 h | 96 h | |
| Col1A1 | 95 (57; 119) | 214 ** (104; 411) | - | - |
| C1CP (protein) | 80 (66; 102) | 136 (85; 179) | - | - |
| ALP | 91 (42; 124) | 116 (76; 141) | - | - |
| OC | 110 (82; 248) | 90 (72; 193) | - | - |
| OPG | 95 (36; 162) | 107 (19; 139) | - | - |
| RANKL | 286 (5; 2026) | 46 (14; 837) | - | - |
| RANK | - | - | 56 *,# (50; 76) | 36 *,# (35; 49) |
| TRAP5b | - | - | 5 * (2; 9) | 7 * (2; 11) |
| Pro-Osteolytic Mediators | Osteoblasts | Adherent PBMCs | ||
|---|---|---|---|---|
| 48 h | 96 h | 48 h | 96 h | |
| MMP1 | 95 (36; 162) | 52 (36; 144) | 234 (92; 338) | 161 (23; 778) |
| TIMP1 | 140 * (110; 299) | 110 (66; 269) | 91 (30; 337) | 122 (89; 675) |
| IL-6 | 276 ** (117; 546) | 99 (75; 204) | 120 (14; 659) | 94 (56; 166) |
| IL-8 | 143 (48; 247) | 58 * (50; 73) | 404 * (127; 1594) | 246 (34; 4104) |
| TNF-α | 104 (51; 165) | 198 (51; 435) | 129 (96; 312) | 83 (76; 130) |
| MCP-1 | 120 (42; 149) | 101 (68; 130) | 29 * (19; 41) | 703 (13; 1628) |
| Differentiaton or Pro-Osteolytic Mediators | Osteoblasts | Adherent PBMCs |
|---|---|---|
| Col1A1 | 31 * (24; 49) | - |
| RANKL | 2 (1; 5) | - |
| OPG | 155 * (118; 206) | - |
| RANK | - | 85 (47; 108) |
| TRAP5b | - | 10,649 * (9263; 12,099) |
| MMP1 | 128 (111; 307) | 285 * (212; 361) |
| TIMP1 | 2950 * (307; 8706) | 2573 * (978; 3490) |
| IL-6 | 5,306,604 * (3,684,037; 5,701,287) | 1093 * (700; 1315) |
| IL-8 | 4,089,451 * (1,769,434; 5,611,116) | 31,637 * (21,602; 46,144) |
| TNF-α | 489,124 * (194,021; 741,895) | 603 * (258; 887) |
| MCP-1 | 207,580 * (169,374; 267,626) | 654 (98; 1277) |
| Primer | Forward (Sequence 5′-3′) | Reverse (Sequence 5′-3′) |
|---|---|---|
| Alkaline phosphatase (ALP) | cattgtgaccaccacgagag | ccatgatcacgtcaatgtcc |
| Collagen 1 (Col1A1) | acgaagacatcccaccaatc | agatcacgtcatcgcacaac |
| Hypoxanthine-guanine phosphoribosyltransferase (HPRT) | ccctggcgtcgtgattagtg | tcgagcaagacgttcagtcc |
| Interleukin 6 (IL-6) | tggattcaatgaggagacttgcc | ctggcatttgtggttgggtc |
| Interleukin 8 (IL-8) | tctgtgtgaaggtgcagttttg | atttctgtgttggcgcagtg |
| Matrix metalloproteinase 1 (MMP1) | agagcagatgtggacatgc | tcccgatgatctcccctgac |
| Monocyte chemotactic protein 1 (MCP-1) | ccgagaggctgagactaacc | ggcattgattgcatctggctg |
| Osteocalcin (OC) | ggtgcagcctttgtgtcc | tcagccaactcgtcacagtc |
| Osteoprotegerin (OPG) | tggattcaatgaggagacttgcc | ctggcatttgtggttgggtc |
| Receptor activator of nuclear κ-b (RANK) | agaaaaccaccaaatgaacccc | gccacaagcctcattgatcc |
| Receptor activator of nuclear κ-b-Ligand (RANKL) | agaagccaccaaagaattgcag | accatcgctttctctgctctg |
| Tartrate-resistant acid phosphatase 5b (TRAP5b) | gggagatctgtgagccagtg | gtccacatgtccatccaggg |
| Tissue inhibitor of metallo-proteinase 1 (TIMP1) | attgctggaaaactgcaggatg | gtccacaagcaatgagtgcc |
| Tumour necrosis factor α (TNF-α) | gttgtagcaaaccctcaagctg | gaggtacaggccctctgatg |
| Content in | Co | Cr | Mo | Ni |
|---|---|---|---|---|
| stock solution | 12.0 ± 2.4 mg/L | 3.9 ± 0.6 mg/L | 0.9 ± 0.1 mg/L | 1.3 ± 0.6 mg/L |
| experimental solution | 120 ± 24 µg/L | 39 ± 5.7 µg/L | 8.8 ± 1.1 µg/L | 12.8 ± 6.0 µg/L |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jonitz-Heincke, A.; Tillmann, J.; Klinder, A.; Krueger, S.; Kretzer, J.P.; Høl, P.J.; Paulus, A.C.; Bader, R. The Impact of Metal Ion Exposure on the Cellular Behavior of Human Osteoblasts and PBMCs: In Vitro Analyses of Osteolytic Processes. Materials 2017, 10, 734. https://doi.org/10.3390/ma10070734
Jonitz-Heincke A, Tillmann J, Klinder A, Krueger S, Kretzer JP, Høl PJ, Paulus AC, Bader R. The Impact of Metal Ion Exposure on the Cellular Behavior of Human Osteoblasts and PBMCs: In Vitro Analyses of Osteolytic Processes. Materials. 2017; 10(7):734. https://doi.org/10.3390/ma10070734
Chicago/Turabian StyleJonitz-Heincke, Anika, Jenny Tillmann, Annett Klinder, Simone Krueger, Jan Philippe Kretzer, Paul Johan Høl, Alexander C. Paulus, and Rainer Bader. 2017. "The Impact of Metal Ion Exposure on the Cellular Behavior of Human Osteoblasts and PBMCs: In Vitro Analyses of Osteolytic Processes" Materials 10, no. 7: 734. https://doi.org/10.3390/ma10070734
APA StyleJonitz-Heincke, A., Tillmann, J., Klinder, A., Krueger, S., Kretzer, J. P., Høl, P. J., Paulus, A. C., & Bader, R. (2017). The Impact of Metal Ion Exposure on the Cellular Behavior of Human Osteoblasts and PBMCs: In Vitro Analyses of Osteolytic Processes. Materials, 10(7), 734. https://doi.org/10.3390/ma10070734




