In Vitro Degradation of Pure Magnesium―The Effects of Glucose and/or Amino Acid
Abstract
:1. Introduction
2. Results and Discussion
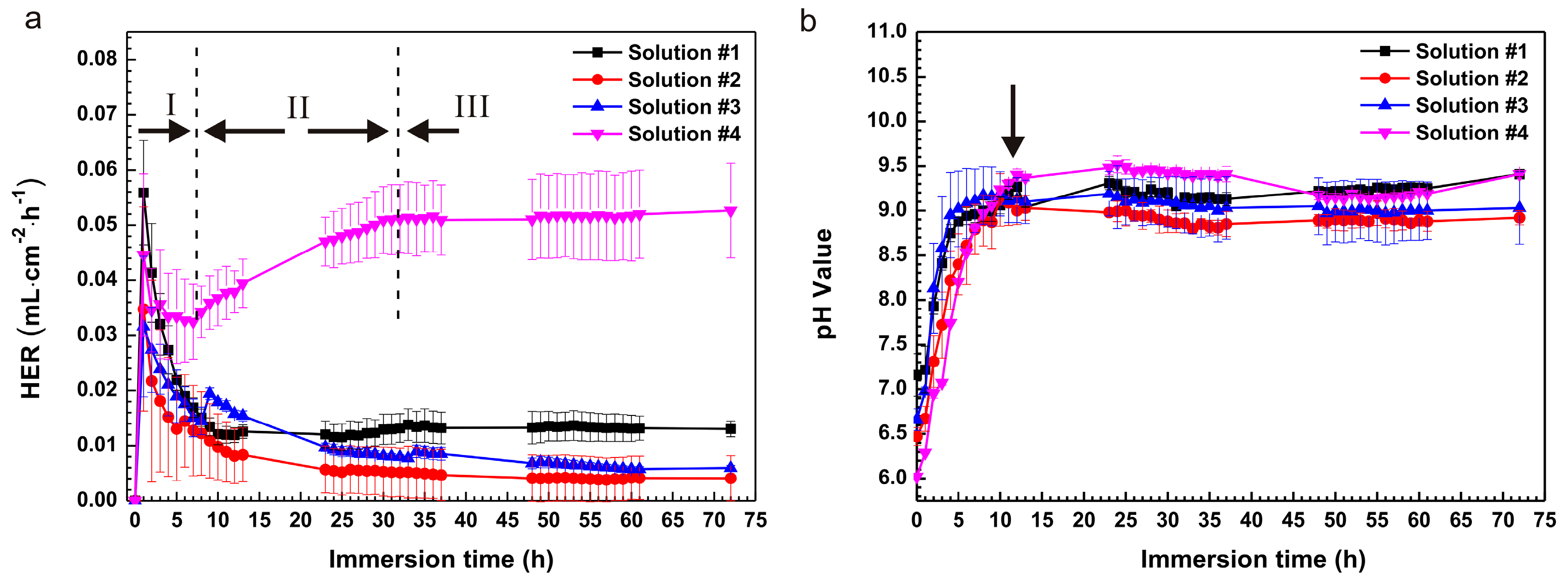
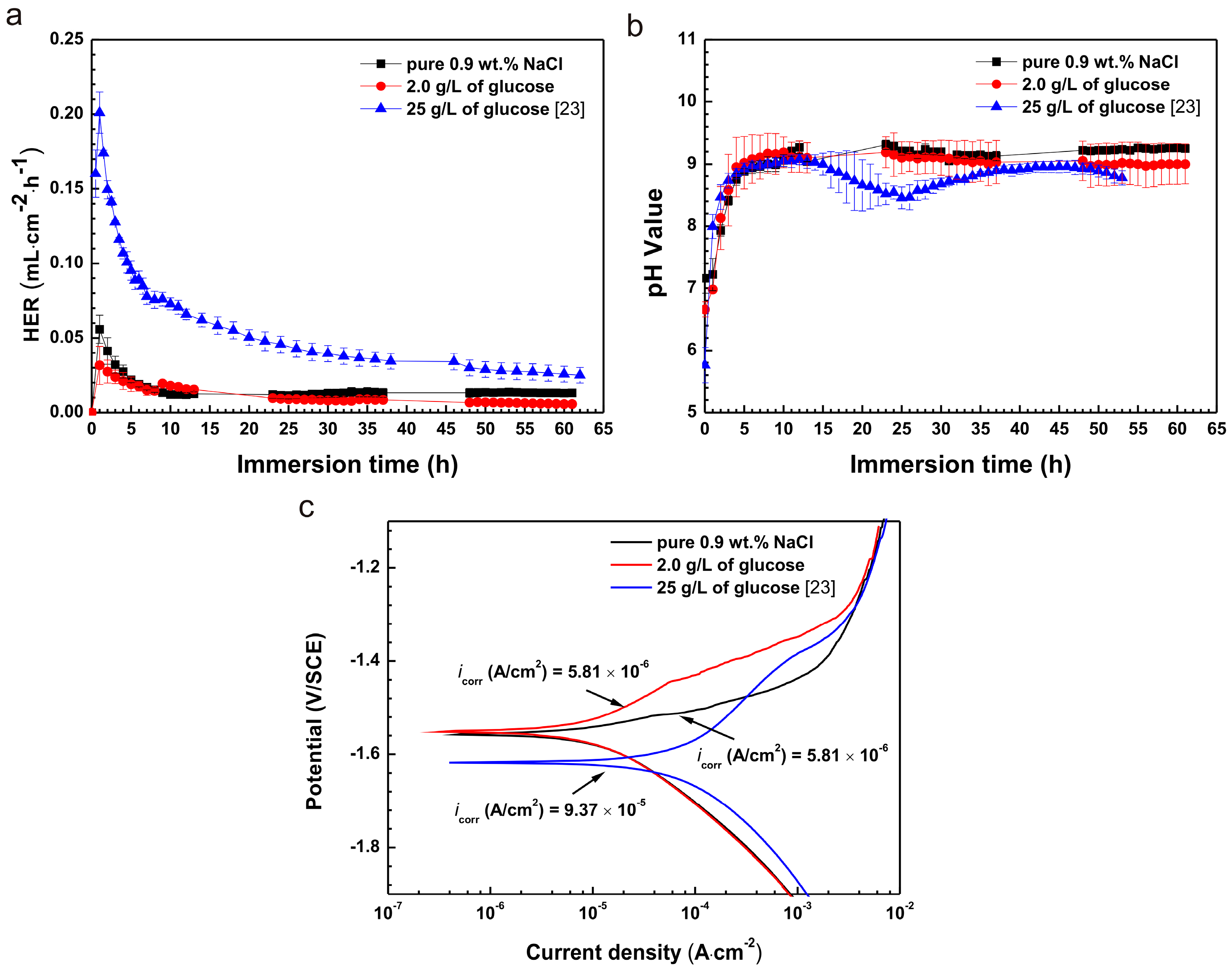
2.1. Immersion Test
2.2. Electrochemical Experiments
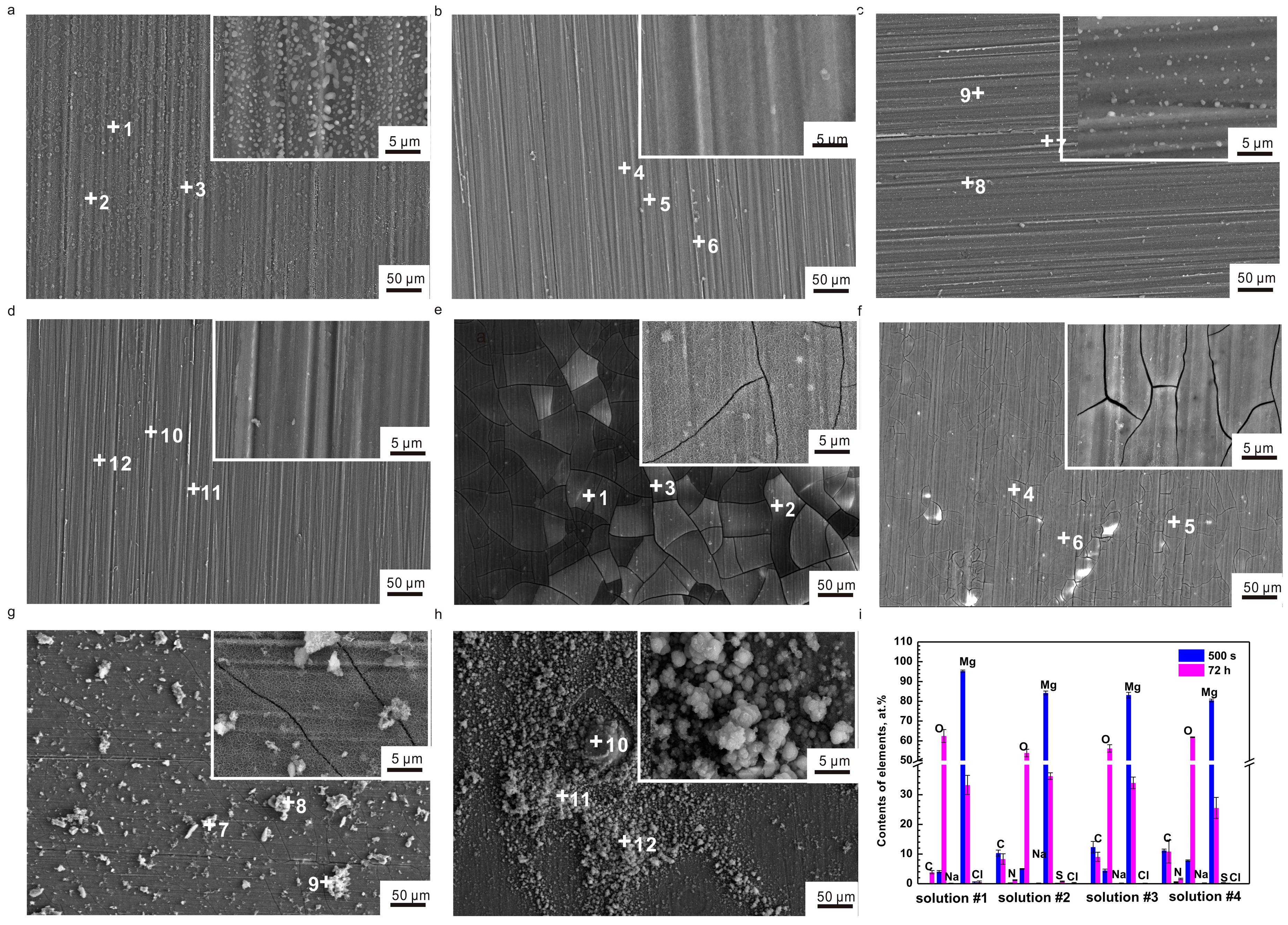
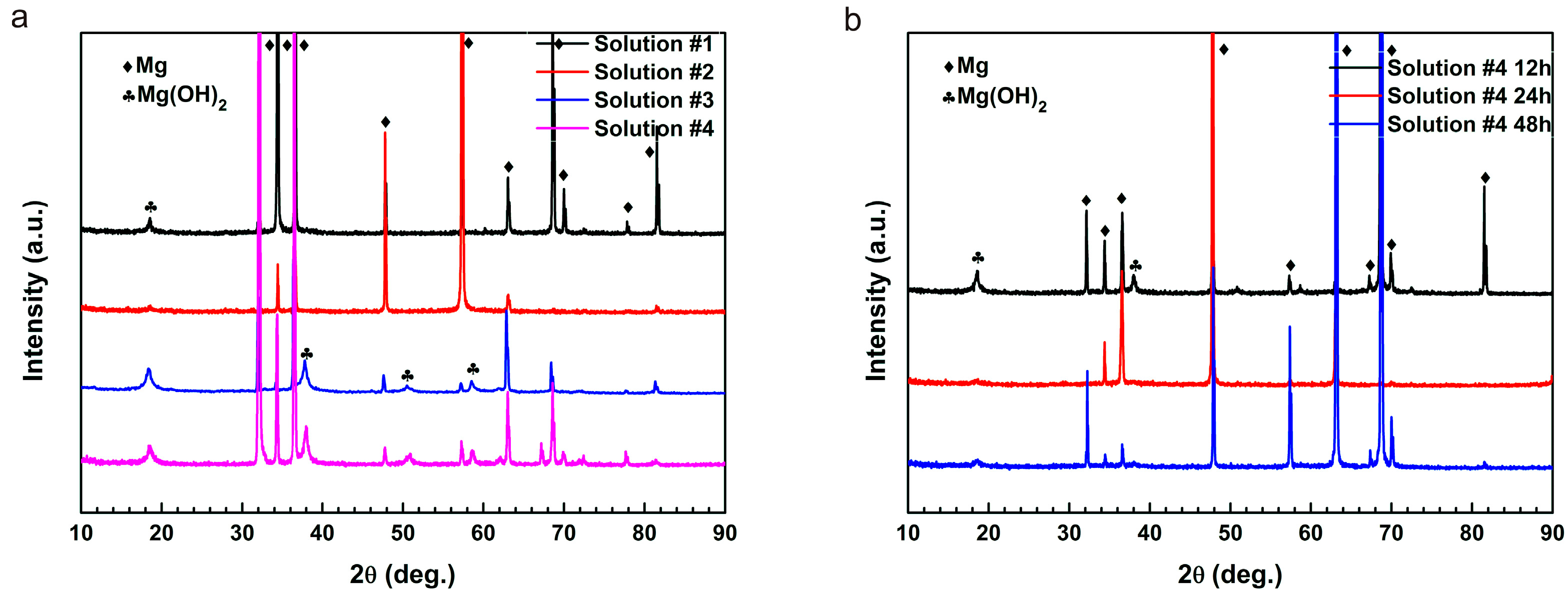
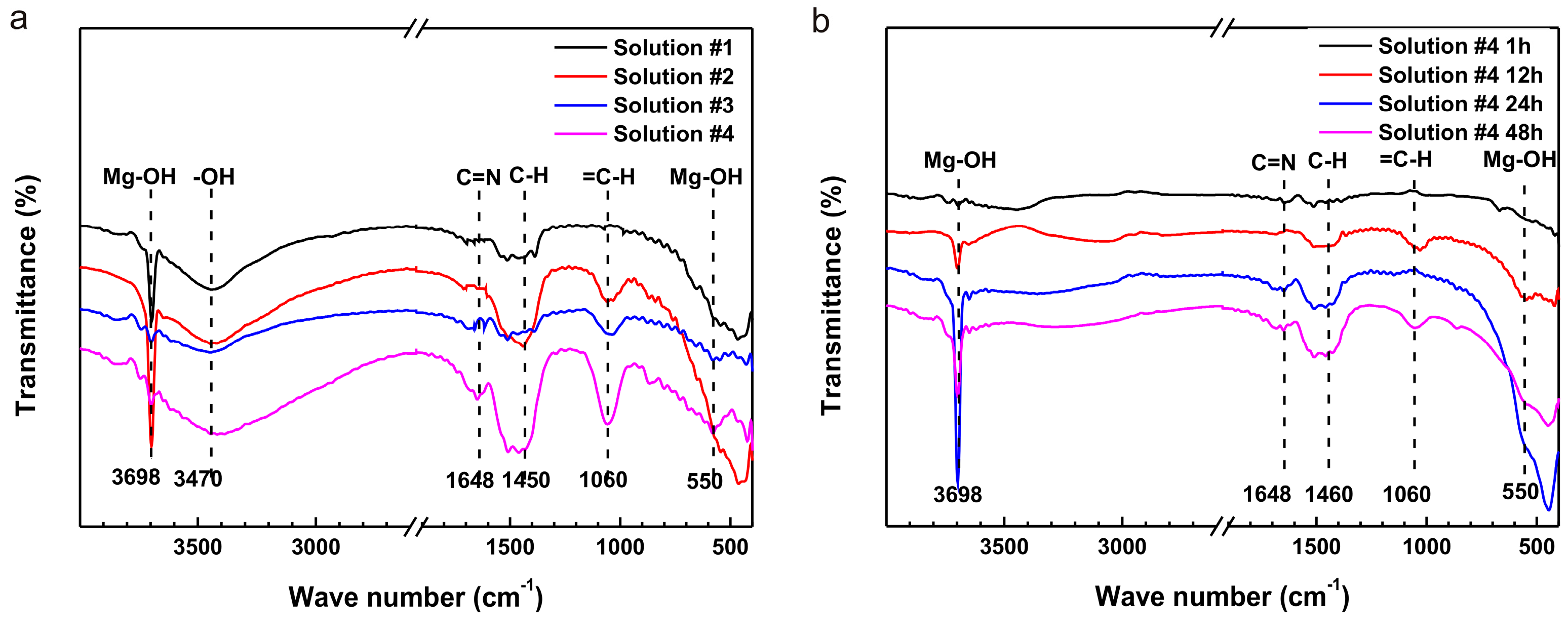
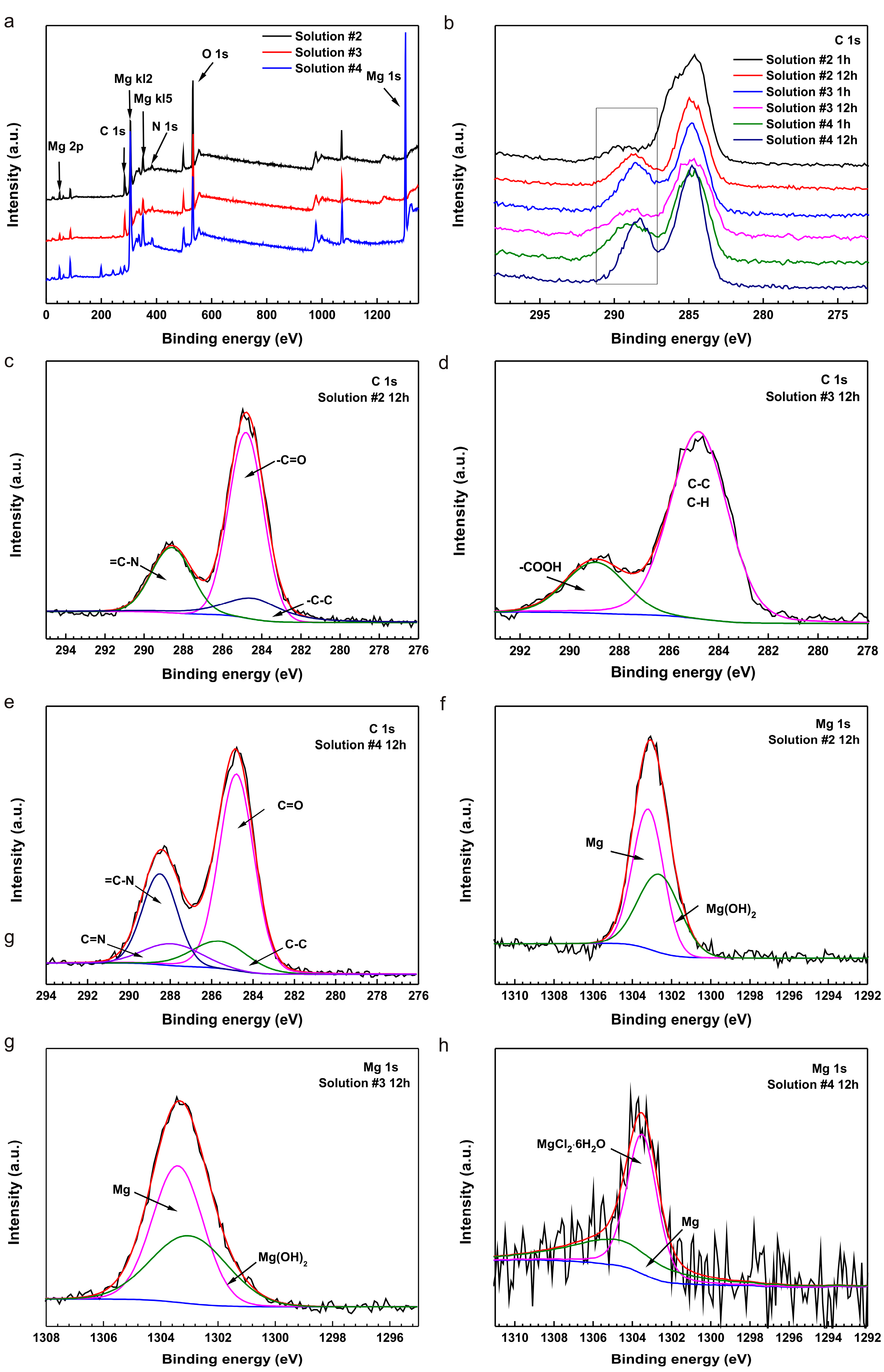
2.3. Surface Analysis
3. Discussion
3.1. Influence of L-Cysteine on Degradation
3.2. Influence of Glucose on Degradation
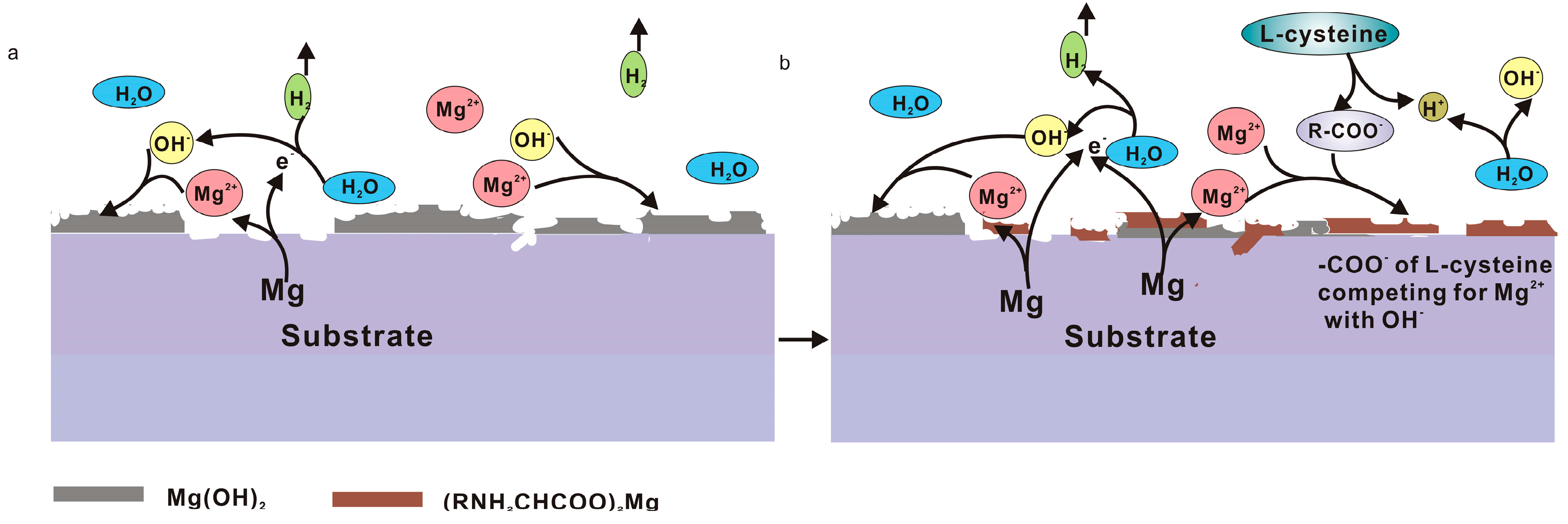
3.3. Degradation Mechanism of the Coupling Effect of Amino Acids and Glucose
4. Materials and Methods
4.1. Materials
4.2. Immersion Testing
4.3. Electrochemical Experiments
4.4. Surface Analysis
5. Conclusions
- (1)
- The polarization, EIS and hydrogen evolution tests indicate that glucose or amino acids (L-cysteine) delay the corrosion of pure Mg in saline solutions, whereas L-cysteine coupled with glucose enhances the corrosion rate of the samples.
- (2)
- The XPS results demonstrate that the coupling effect of L-cysteine and glucose gives rise to the formation of Schiff base (R’C=N-CH-R) due to the alkalinization of the initial acidic solutions with both amino acids and glucose during corrosion of pure Mg.
- (3)
- The results of SEM, EDS and FTIR show that the formation of (RNH2CHCOO)2Mg on the surfaces and the depletion of amino acids prevents the further corrosion of pure Mg; hence, the corrosion rate remains stable.
- (4)
- There might be a critical glucose content regarding its influence on corrosion rate of pure magnesium. Further explorations are needed to understand these effects.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ho, Y.H.; Vora, H.D.; Dahotre, N.B. Laser surface modification of AZ31B Mg alloy for bio-wettability. J. Biomater. Appl. 2015, 29, 915–928. [Google Scholar] [CrossRef] [PubMed]
- Zeng, R.C.; Cui, L.; Ke, J.; Rui, L.; Zhao, B.D.; Zheng, Y.F. In Vitro Corrosion and Cytocompatibility of a Microarc Oxidation Coating and Poly(l-lactic acid) Composite Coating on Mg–1Li–1Ca Alloy for Orthopedic Implants. ACS Appl. Mater. Interfaces 2016, 8, 10014–10028. [Google Scholar] [CrossRef] [PubMed]
- He, Y. Biocompatibility of magnesium-zinc alloy in biodegradable orthopedic implants. Int. J. Mol. Med. 2011, 28, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Zeng, R.C.; Sun, L.; Zheng, Y.F.; Cui, H.Z.; Han, E.H. Corrosion and characterisation of dual phase Mg–Li–Ca alloy in Hank’s solution: The influence of microstructural features. Corros. Sci. 2014, 79, 69–82. [Google Scholar] [CrossRef]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its allys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Esmaily, M.; Svensson, J.E.; Fajardo, S.; Birbilis, N.; Frankel, G.S.; Virtanen, S.; Arrabal, R.; Thomas, S.; Johansson, L.G. Fundamentals and advances in magnesium alloy corrosion. Prog. Mater. Sci. 2017, 89, 92–193. [Google Scholar] [CrossRef]
- Myrissa, A.; Agha, N.A.; Lu, Y.; Martinelli, E.; Eichler, J.; Szakacs, G.; Kleinhans, C.; Willumeit-Romer, R.; Schafer, U.; Weinberg, A.M. In Vitro and In Vivo comparison of binary Mg alloys and pure Mg. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 61, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Shinohara, T.; Zhang, B.-P. Influence of chloride, sulfate and bicarbonate anions on the corrosion behavior of AZ31 magnesium alloy. J. Alloys Compd. 2010, 496, 500–507. [Google Scholar] [CrossRef]
- Xin, Y.; Huo, K.; Tao, H.; Tang, G.; Chu, P.K. Influence of aggressive ions on the degradation behavior of biomedical magnesium alloy in physiological environment. Acta Biomater. 2008, 4, 2008–2015. [Google Scholar] [CrossRef] [PubMed]
- Zeng, R.C.; Guo, X.L.; Liu, C.L.; Cui, H.Z.; Tao, W.; Liu, Y.Y.; Li, B.W. Study on corrosion of medical Mg-Ca and Mg-Li-Ca alloys. Acta Metall. 2011, 47, 1477–1482. [Google Scholar]
- Zeng, R.C.; Qi, W.C.; Zhang, F.; Cui, H.Z.; Zheng, Y.F. In Vitro corrosion of Mg–1.21Li–1.12Ca–1Y alloy. Prog. Nat. Sci. Mater. Int. 2014, 24, 492–499. [Google Scholar] [CrossRef]
- Liu, L.; Yuan, F.; Zhao, M.; Gao, C.; Feng, P.; Yang, Y.; Yang, S.; Shuai, C. Rare Earth Element Yttrium Modified Mg-Al-Zn Alloy: Microstructure, Degradation Properties and Hardness. Materials 2017, 10, 477. [Google Scholar] [CrossRef]
- Feng, H.; Liu, S.; Du, Y.; Lei, T.; Zeng, R.; Yuan, T. Corrosion mechanism was studied by corrosion morphology and the equivalent circuits. Effect of the second phases on corrosion behavior of the Mg-Al-Zn alloys. J. Alloys Compd. 2016. [Google Scholar] [CrossRef]
- Zeng, R.C. In Vitro corrosion of pure magnesium and AZ91 alloy—The influence of thin electrolyte layer thickness. Regen. Biomater. 2016, 3, rbv028. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.W.; Shan, D.; Chen, R.; Han, E.H. Effect of second phases on the corrosion behaviour of wrought Mg–Zn–Y–Zr alloy. Corros. Sci. 2010, 52, 1830–1837. [Google Scholar] [CrossRef]
- Vlček, M.; Lukáč, F.; Kudrnová, H.; Smola, B.; Stulíková, I.; Luczak, M.; Szakács, G.; Hort, N.; Willumeit-Römer, R. Microhardness and In Vitro Corrosion of Heat-Treated Mg–Y–Ag Biodegradable Alloy. Materials 2017, 10, 55. [Google Scholar] [CrossRef]
- Wagener, V.; Killian, M.S.; Turhan, C.M.; Virtanen, S. Albumin coating on magnesium via linker molecules—Comparing different coating mechanisms. Colloids Surf. B Biointerfaces 2013, 103, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Silva-Bermudez, P.; Rodil, S.E. An overview of protein adsorption on metal oxide coatings for biomedical implants. Surf. Coat. Technol. 2013, 233, 147–158. [Google Scholar] [CrossRef]
- Cui, L.Y.; Hu, Y.; Zeng, R.C.; Yang, Y.X.; Sun, D.D.; Li, S.-Q.; Zhang, F.; Han, E.-H. New insights into the effect of Tris-HCl and Tris on corrosion of magnesium alloy in presence of bicarbonate, sulfate, hydrogen phosphate and dihydrogen phosphate ions. J. Mater. Sci. Technol. 2016. [Google Scholar] [CrossRef]
- Yamamoto, A.; Hiromoto, S. Effect of inorganic salts, amino acids and proteins on the degradation of pure magnesium in vitro. Mater. Sci. Eng. C 2009, 29, 1559–1568. [Google Scholar] [CrossRef]
- Li, X.; Weng, Z.; Yuan, W.; Luo, X.; Wong, H.M.; Liu, X.; Wu, S.; Yeung, K.W.K.; Zheng, Y.; Chu, P.K. Corrosion resistance of dicalcium phosphate dihydrate/poly(lactic-co-glycolic acid) hybrid coating on AZ31 magnesium alloy. Corros. Sci. 2016, 102, 209–221. [Google Scholar] [CrossRef]
- Zeng, R.C.; Hu, Y.; Guan, S.K.; Cui, H.Z.; Han, E.H. Corrosion of magnesium alloy AZ31: The influence of bicarbonate, sulphate, hydrogen phosphate and dihydrogen phosphate ions in saline solution. Corros. Sci. 2014, 86, 171–182. [Google Scholar] [CrossRef]
- Zeng, R.C.; Li, X.T.; Li, S.Q.; Zhang, F.; Han, E.H. In vitro degradation of pure Mg in response to glucose. Sci. Rep. 2015, 5, 13026. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.L.; Wang, Y.J.; Zeng, R.C.; Zhang, X.M.; Huang, W.J.; Chu, P.K. In Vitro corrosion degradation behaviour of Mg–Ca alloy in the presence of albumin. Corros. Sci. 2010, 52, 3341–3347. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, Y.; Wang, Q.; Liu, J. Influence of Sulfate-Reducing Bacteria on the Corrosion Residual Strength of an AZ91D Magnesium Alloy. Materials 2014, 7, 7118–7129. [Google Scholar] [CrossRef]
- Hornberger, H.; Witte, F.; Hort, N.; Mueller, W.D. Effect of fetal calf serum on the corrosion behaviour of magnesium alloys. Mater. Sci. Eng. B 2011, 176, 1746–1755. [Google Scholar] [CrossRef]
- Neirinck, B.; Singer, F.; Braem, A.; Virtanen, S.; Vleugels, J. Alternating Current Electrophoretic Deposition of Bovine Serum Albumin onto Magnesium. Key Eng. Mater. 2015, 654, 139–143. [Google Scholar] [CrossRef]
- Wang, Y.; Lim, C.S.; Lim, C.V.; Yong, M.S.; Teo, E.K.; Moh, L.N. In Vitro degradation behavior of M1A magnesium alloy in protein-containing simulated body fluid. Mater. Sci. Eng. C 2011, 31, 579–587. [Google Scholar] [CrossRef]
- Yang, L.; Hort, N.; Willumeit, R.; Feyerabend, F. Effects of corrosion environment and proteins on magnesium corrosion. Corros. Eng. Sci. Technol. 2012, 47, 335–339. [Google Scholar] [CrossRef]
- Thirugnanaselvi, S.; Kuttirani, S.; Emelda, A.R. Effect of Schiff base as corrosion inhibitor on AZ31 magnesium alloy in hydrochloric acid solution. Trans. Nonferrous Met. Soc. China 2014, 24, 1969–1977. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, C.; Wang, F.; Li, W. Electrochemical behavior of anodized Mg alloy AZ91D in chloride containing aqueous solution. Corros. Sci. 2005, 47, 2816–2831. [Google Scholar] [CrossRef]
- Song, Y.W.; Han, E.H.; Shan, D.; Yim, C.D.; You, B.S. The effect of Zn concentration on the corrosion behavior of Mg–xZn alloys. Corros. Sci. 2012, 65, 322–330. [Google Scholar] [CrossRef]
- Zeng, R.C.; Li, X.T.; Liu, Z.G.; Zhang, F.; Li, S.Q.; Cui, H.Z. Corrosion resistance of Zn–Al layered double hydroxide/poly(lactic acid) composite coating on magnesium alloy AZ31. Front. Mater. Sci. 2015, 9, 355–365. [Google Scholar] [CrossRef]
- Zeng, R.C.; Liu, Z.G.; Zhang, F.; Li, S.Q.; He, Q.K.; Cui, H.Z.; Han, E.-H. Corrosion resistance of in-situ Mg–Al hydrotalcite conversion film on AZ31 magnesium alloy by one-step formation. Trans. Nonferrous Met. Soc. China 2015, 25, 1917–1925. [Google Scholar] [CrossRef]
- Anik, M.; Celikten, G. Analysis of the electrochemical reaction behavior of alloy AZ91 by EIS technique in H3PO4/KOH buffered K2SO4 solutions. Corros. Sci. 2007, 49, 1878–1894. [Google Scholar] [CrossRef]
- Song, Y.; Shan, D.; Chen, R.; Han, E.-H. Corrosion characterization of Mg–8Li alloy in NaCl solution. Corros. Sci. 2009, 51, 1087–1094. [Google Scholar] [CrossRef]
- De Araujo, E.L.; Barbosa, H.F.; Dockal, E.R.; Cavalheiro, E.T. Synthesis, characterization and biological activity of Cu(II), Ni(II) and Zn(II) complexes of biopolymeric Schiff bases of salicylaldehydes and chitosan. Int. J. Biol. Macromol. 2016, 95, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Wang, L.; Fan, L.; Xiao, W.; Lin, B.; Xu, Y.; Liang, J.; Cao, B. RGDC Peptide-Induced Biomimetic Calcium Phosphate Coating Formed on AZ31 Magnesium Alloy. Materials 2017, 10, 358. [Google Scholar] [CrossRef]
- Aytaç, A.; Özmen, Ü.; Kabasakaloğlu, M. Investigation of some Schiff bases as acidic corrosion of alloy AA3102. Mater. Chem. Phys. 2005, 89, 176–181. [Google Scholar] [CrossRef]
- Liu, C.L.; Zhang, Y.; Zhang, C.Y.; Wang, W.; Huang, W.J.; Chu, P.K. Synergistic effect of chloride ion and albumin on the corrosion of pure magnesium. Front. Mater. Sci. 2014, 8, 244–255. [Google Scholar] [CrossRef]
- Mueller, W.D.; de Mele, M.F.; Nascimento, M.L.; Zeddies, M. Degradation of magnesium and its alloys: Dependence on the composition of the synthetic biological media. J. Biomed. Mater. Res. Part A 2009, 90, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Zhang, M.; Liu, J.; Jones, G.S. Construction and application of recombinant strain for the production of an alkaline protease from Bacillus licheniformis. J. Biosci. Bioeng. 2015, 119, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Cui, C.H.; Kim, J.K.; Jin, F.X.; Kim, S.C.; Im, W.T. Enzymatic Biotransformation of Ginsenoside Rb1 and Gypenoside XVII into Ginsenosides Rd and F2 by Recombinant beta-glucosidase from Flavobacterium johnsoniae. J. Ginseng Res. 2012, 36, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Şafak, S.; Duran, B.; Yurt, A.; Türkoğlu, G. Schiff bases as corrosion inhibitor for aluminium in HCl solution. Corros. Sci. 2012, 54, 251–259. [Google Scholar] [CrossRef]
- Seifzadeh, D.; Basharnavaz, H. Corrosion protection of AZ91 magnesium alloy in cooling systems. Trans. Nonferrous Met. Soc. China 2013, 23, 2577–2584. [Google Scholar] [CrossRef]
- Rocha, M.; Di Santo, A.; Echeverría, G.A.; Piro, O.E.; Cukiernik, F.D.; Ulic, S.E.; Gil, D.M. Supramolecular self-assembly of a new multi-conformational Schiff base through hydrogen bonds: Crystal structure, spectroscopic and theoretical investigation. J. Mol. Struct. 2017, 1133, 24–36. [Google Scholar] [CrossRef]
- Harding, M.M.; Long, H.A. The Crystal and Molecular Structure of L-Cysteine. Acta Cryst. 1968, B24, 1096–1102. [Google Scholar] [CrossRef]
- Omar, M.M.; Elbashir, A.A.; Schmitz, O.J. Capillary electrophoresis method with UV-detection for analysis of free amino acids concentrations in food. Food Chem. 2017, 214, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Balint, E.; Szabo, P.; Marshall, C.F.; Sprague, S.M. Glucose-induced Inhibition of In Vitro Bone Mineralization. Bone 2001, 28, 21–28. [Google Scholar] [CrossRef]
- Li, H.; Zheng, Y.; Qin, L. Progress of biodegradable metals. Prog. Nat. Sci. Mater. Int. 2014, 24, 414–422. [Google Scholar] [CrossRef]
- Shi, Z.; Atrens, A. An innovative specimen configuration for the study of Mg corrosion. Corros. Sci. 2011, 53, 226–246. [Google Scholar] [CrossRef]
- Cui, L.Y.; Zeng, R.C.; Guan, S.K.; Qi, W.C.; Zhang, F.; Li, S.Q.; Han, E.H. Degradation mechanism of micro-arc oxidation coatings on biodegradable Mg-Ca alloys: The influence of porosity. J. Alloys Compd. 2016. [Google Scholar] [CrossRef]
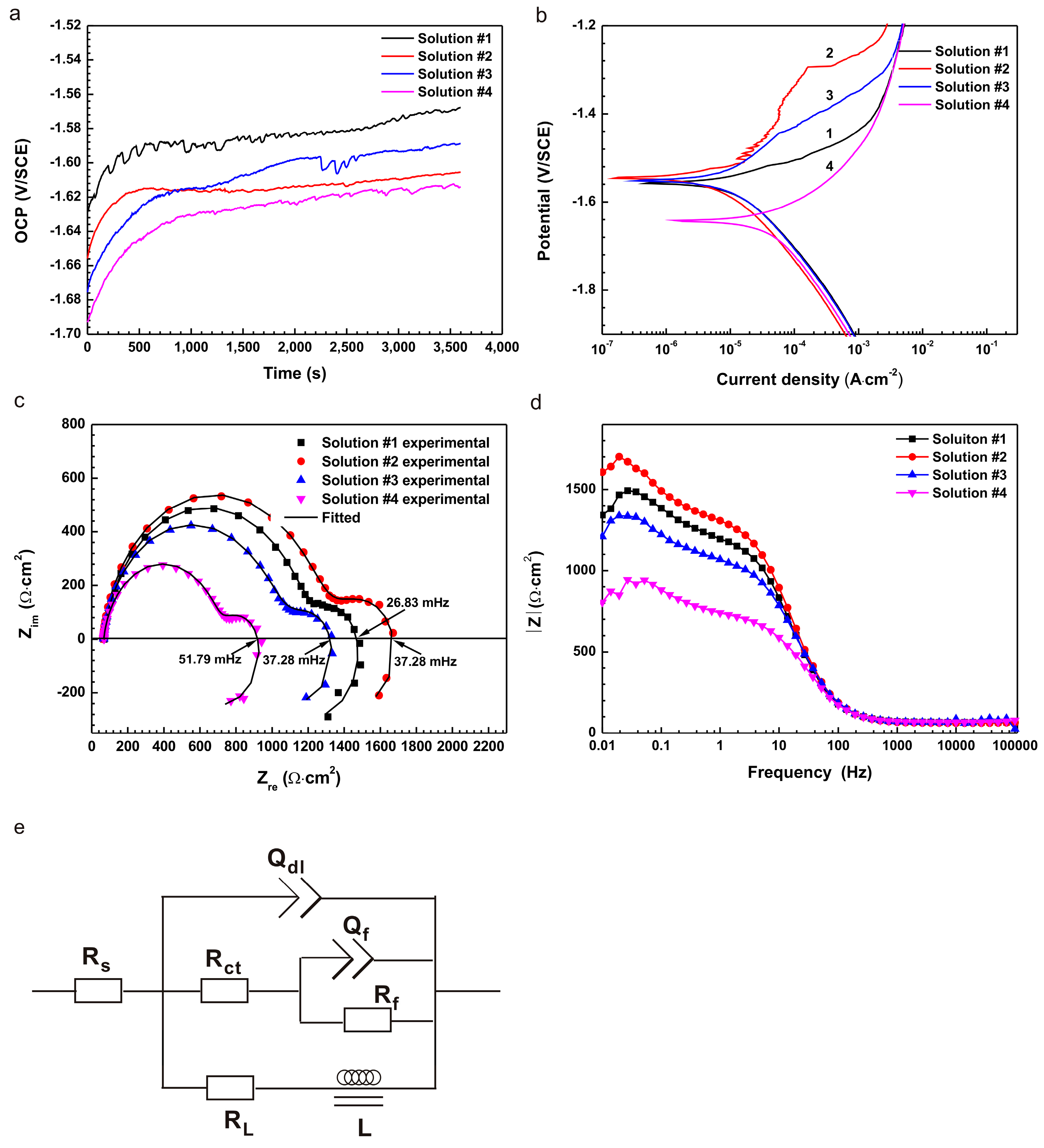

| Solution | Ecorr (V/SCE) | icorr (A/cm2) | ba (mV/Decade) | −bc (mV/Decade) | Rp (Ω·cm2) |
|---|---|---|---|---|---|
| #1 | −1.56 | 6.90 × 10−6 | 81.50 | 245.01 | 7. 70 × 103 |
| #2 | −1.54 | 4.67 × 10−6 | 129.14 | 159.56 | 6.30 × 104 |
| #3 | −1.55 | 5.81 × 10−6 | 112.76 | 187.84 | 2.11 × 104 |
| #4 | −1.64 | 4.89 × 10−5 | 130.11 | 240.77 | 2.29 × 103 |
| Solution | Rs (Ω·cm2) | Y0 (Ω−1·cm−2·s−1) | n | Rct (Ω·cm2) | Qf (F cm−2) | Rf (Ω·cm2) | L (H cm−2) | RL (Ω·cm2) | Chi Square |
|---|---|---|---|---|---|---|---|---|---|
| #1 | 66.25 | 1.84 × 10−5 | 0.909 | 1127 | 3.80 × 10−3 | 407.7 | 6.18 × 104 | 1969 | 1.7 × 10−4 |
| #2 | 66.15 | 1.63 × 10−5 | 0.917 | 1207 | 3.28 × 10−3 | 1078 | 7.04 × 104 | 2584 | 1.5 × 10−4 |
| #3 | 65.81 | 1.62 × 10−5 | 0.921 | 922.70 | 4.28 × 10−3 | 5470 | 3.80 × 104 | 1430 | 2.6 × 10−4 |
| #4 | 66.62 | 1.95 × 10−5 | 0.907 | 644.80 | 3.94 × 10−3 | 292 | 2.59 × 104 | 700.9 | 5.2 × 10−4 |
| Materials | Solutions | icorr (A/cm2) | Rct (Ω cm2) | Refs. |
|---|---|---|---|---|
| Pure Mg | 0.8 wt. % NaCl | 6.20 × 10−4 | 103 | Liu [40] |
| 0.8 wt. % NaCl + 1 g/L albumin | 5.80 × 10−4 | 128 | ||
| 0.8 wt. % NaCl + 10 g/L albumin | 4.50 × 10−4 | 149 | ||
| M1A | SBF | 3.62 × 10−4 | - | Wang [28] |
| SBF + 40 g/L albumin | 2.81 × 10−4 | - | ||
| Pure Mg | PBS + 0.1 albumin | (3.53 ± 3.39) × 10−4 | 202 ± 241 | Mueller [41] |
| PBS + 1 albumin | (0.13 ± 7.56) × 10−5 | 405 ± 469 | ||
| PBS + 10 albumin | (3.73 ± 5.41) × 10−5 | 2117 ± 1509 | ||
| PBS | (7.76 ± 8.81) × 10−6 | 7133 ± 5167 | ||
| Pure Mg | 0.9 wt. % + 0.006 g/L L-cysteine | 4.67 × 10−6 | 1207 | present work |
| Solution | NaCl | L-Cysteine (HSCH2CH(NH2)COOH) | Glucose (C6H12O6) |
|---|---|---|---|
| #1 | 9.0 | - | - |
| #2 | 9.0 | 0.006 | - |
| #3 | 9.0 | - | 2.0 |
| #4 | 9.0 | 0.006 | 2.0 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Cui, L.-Y.; Zeng, R.-C.; Li, S.-Q.; Zou, Y.-H.; Han, E.-H. In Vitro Degradation of Pure Magnesium―The Effects of Glucose and/or Amino Acid. Materials 2017, 10, 725. https://doi.org/10.3390/ma10070725
Wang Y, Cui L-Y, Zeng R-C, Li S-Q, Zou Y-H, Han E-H. In Vitro Degradation of Pure Magnesium―The Effects of Glucose and/or Amino Acid. Materials. 2017; 10(7):725. https://doi.org/10.3390/ma10070725
Chicago/Turabian StyleWang, Yu, Lan-Yue Cui, Rong-Chang Zeng, Shuo-Qi Li, Yu-Hong Zou, and En-Hou Han. 2017. "In Vitro Degradation of Pure Magnesium―The Effects of Glucose and/or Amino Acid" Materials 10, no. 7: 725. https://doi.org/10.3390/ma10070725
APA StyleWang, Y., Cui, L.-Y., Zeng, R.-C., Li, S.-Q., Zou, Y.-H., & Han, E.-H. (2017). In Vitro Degradation of Pure Magnesium―The Effects of Glucose and/or Amino Acid. Materials, 10(7), 725. https://doi.org/10.3390/ma10070725






