Study on Temper Embrittlement and Hydrogen Embrittlement of a Hydrogenation Reactor by Small Punch Test
Abstract
:1. Introduction
2. Sampling and Testing
2.1. Sampling
2.2. SPT and Charpy Impact Testing
2.3. Hydrogen Charging Test
3. Results
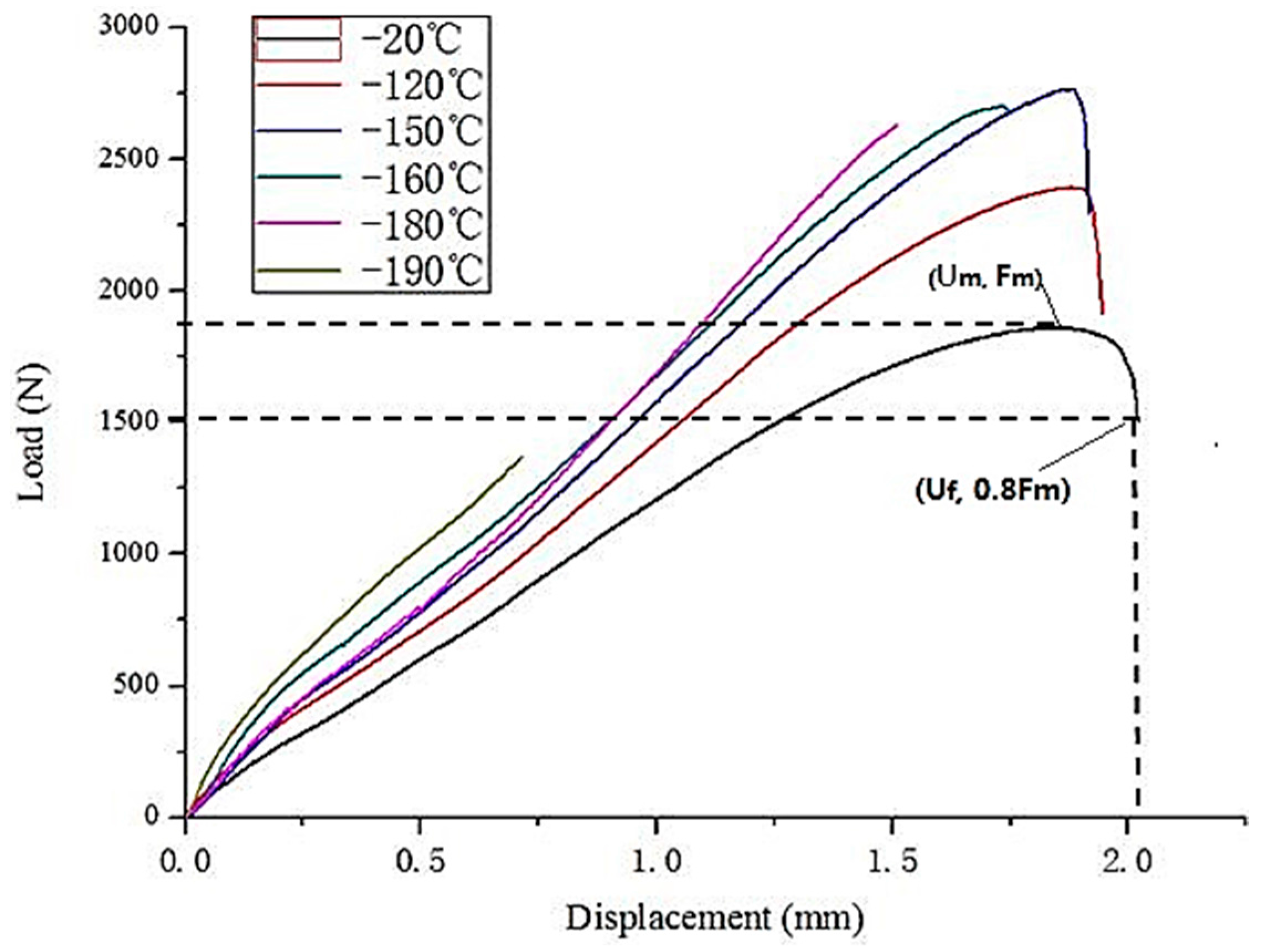
3.1. Load-Displacement Curve of SPT in Different Temperatures
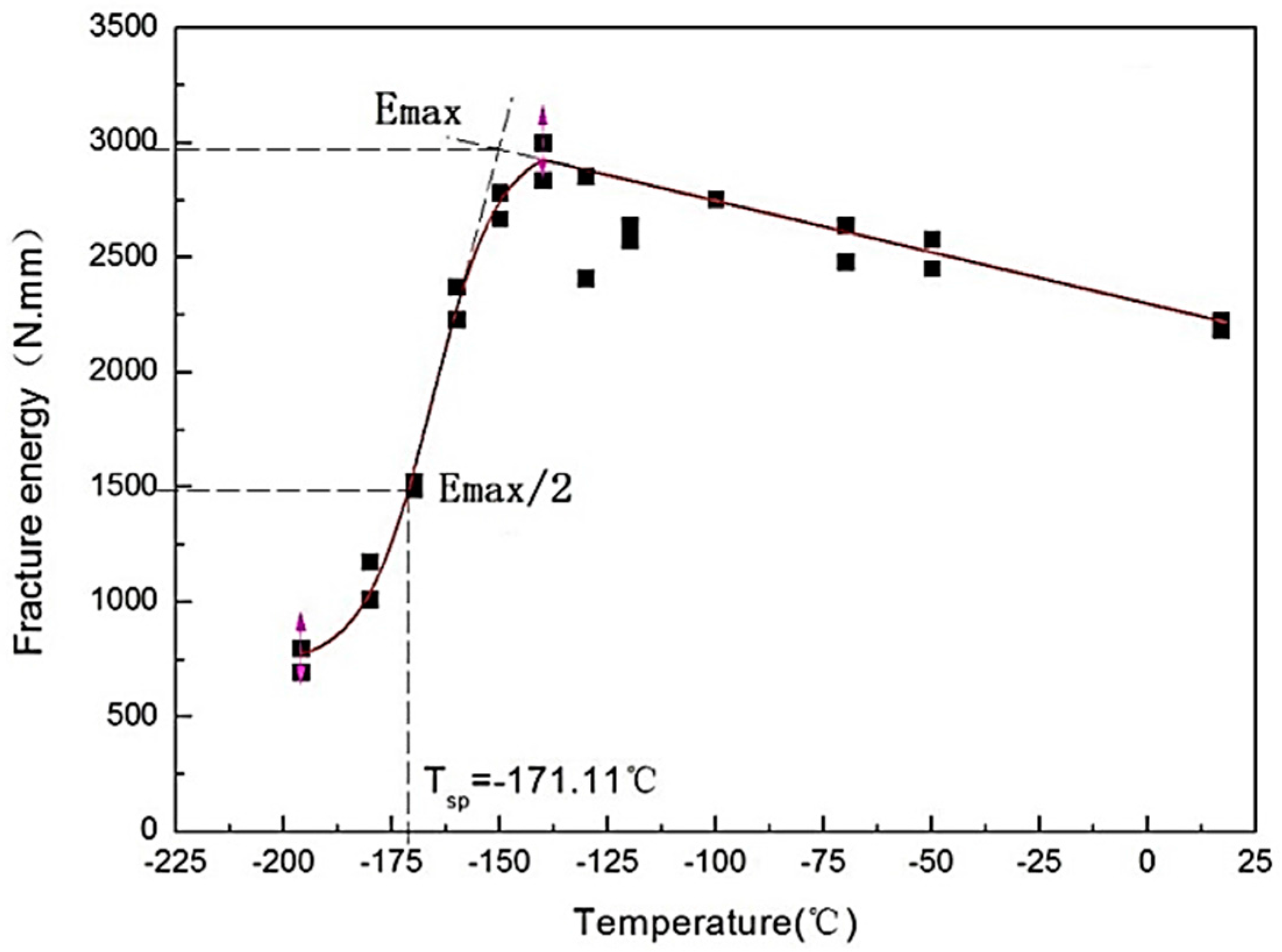
3.2. Energy Transition Temperature of SPT
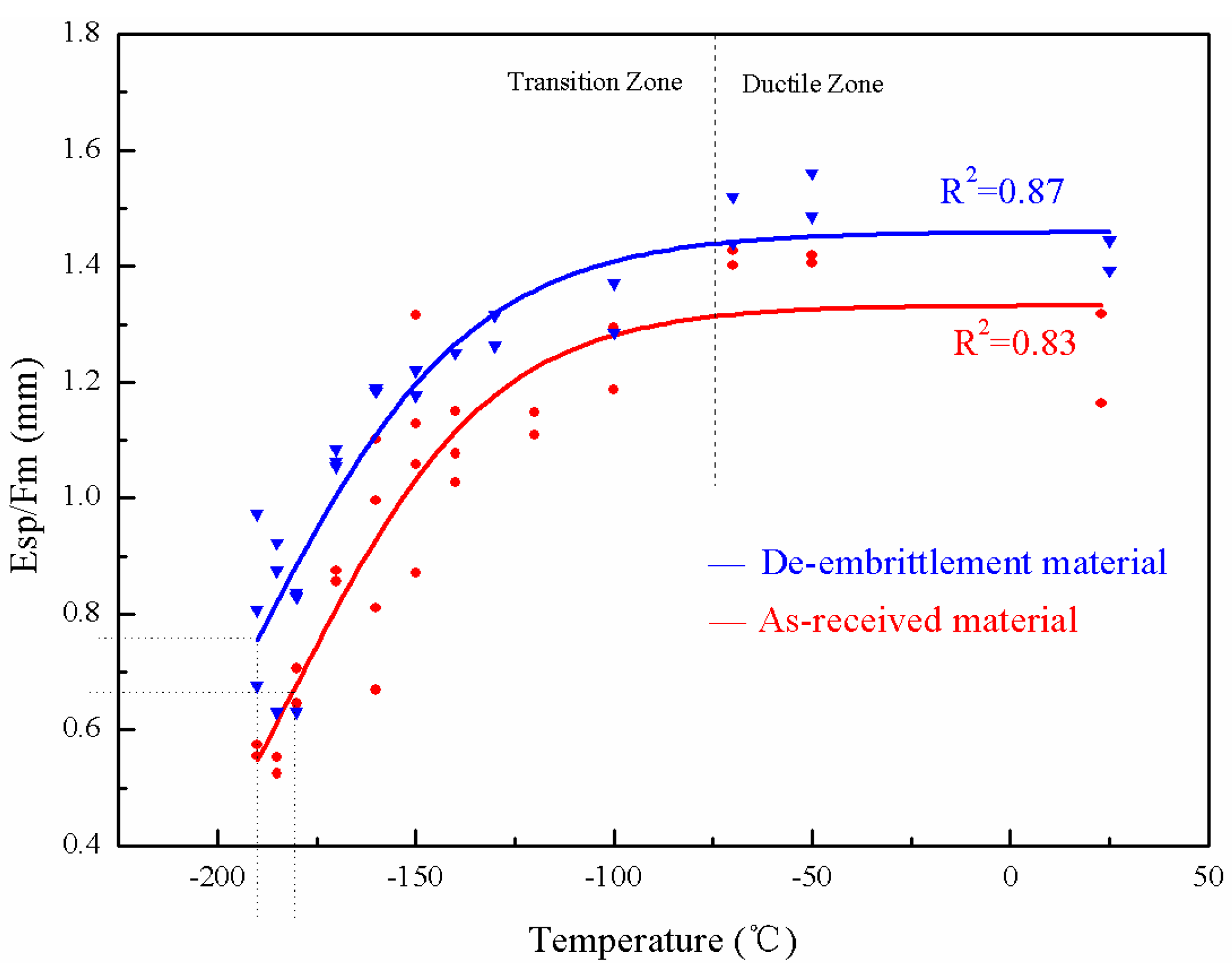
3.3. Temper Embrittlement in the Base Metal and the Weld Metal
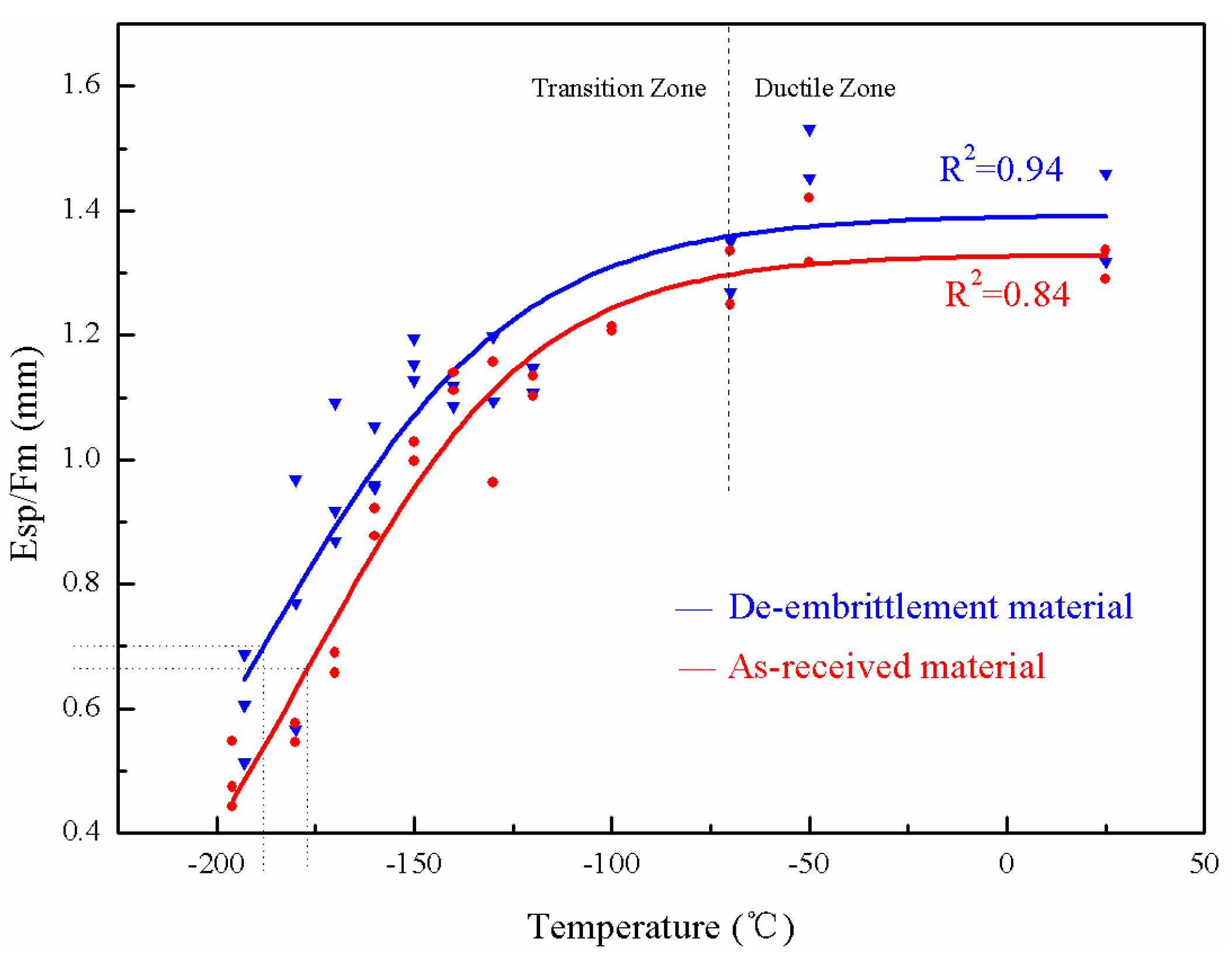
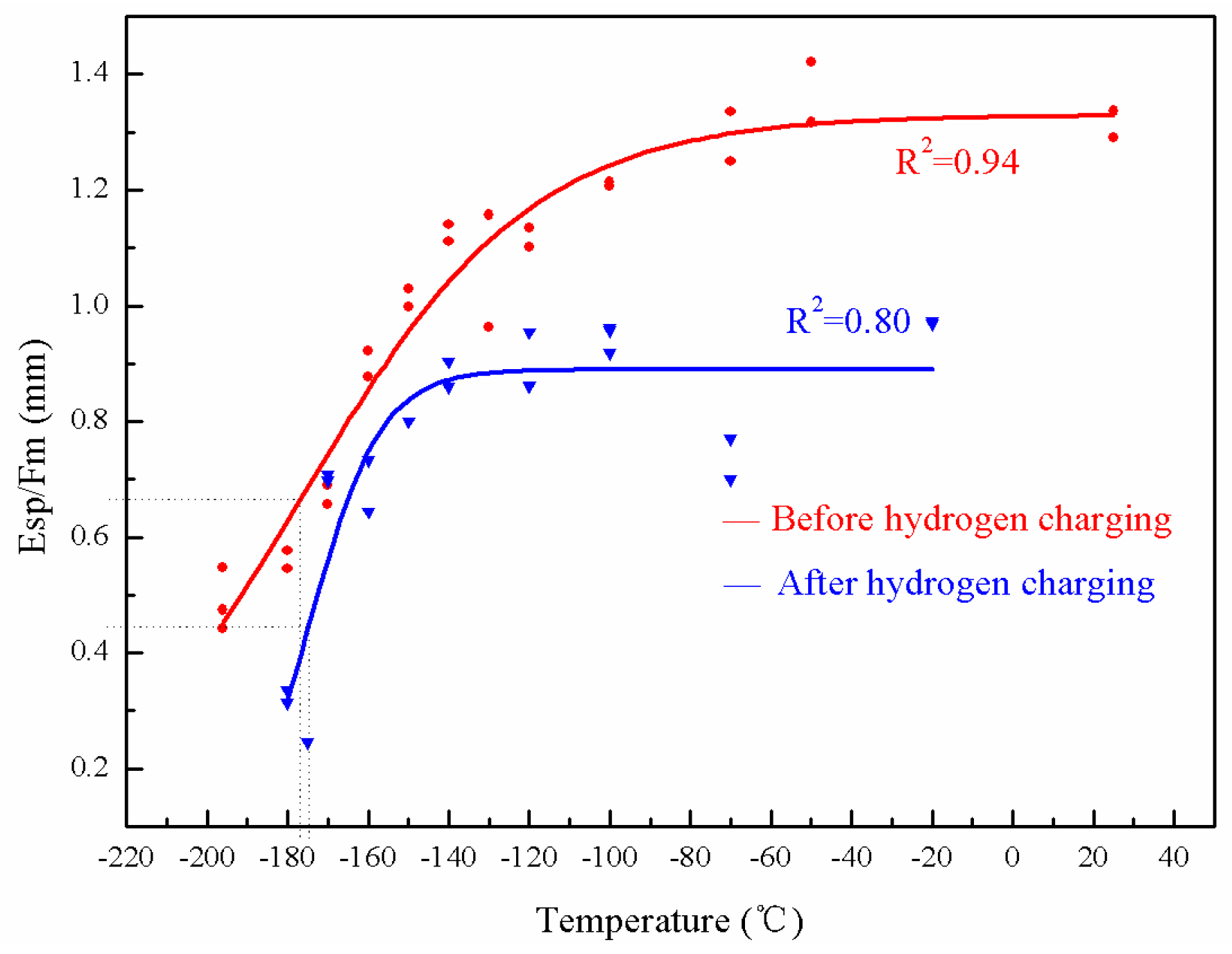
3.4. Tsp: Hydrogen Embrittlement in the As-Received and Brittle State of the Weld Metal
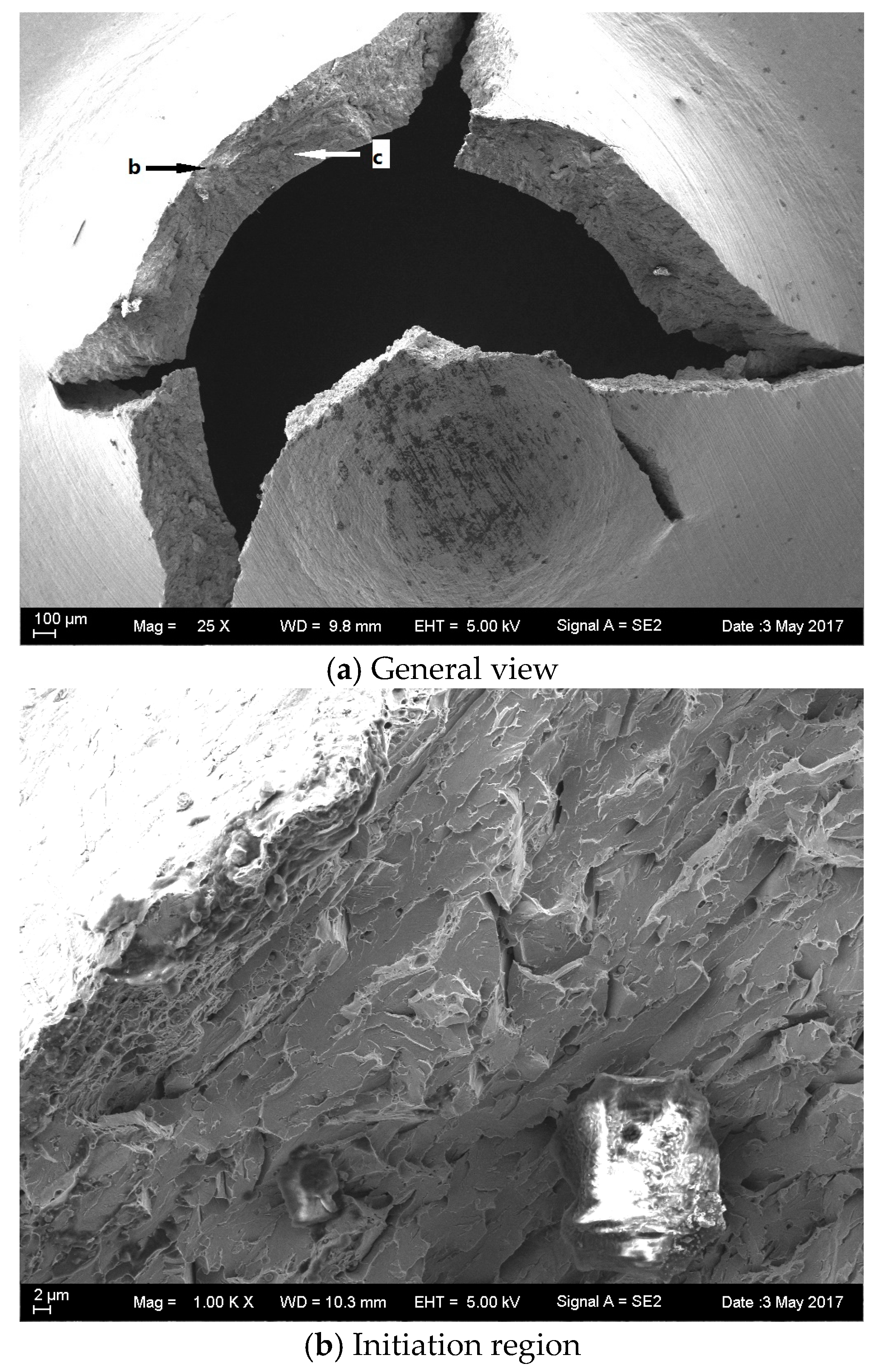
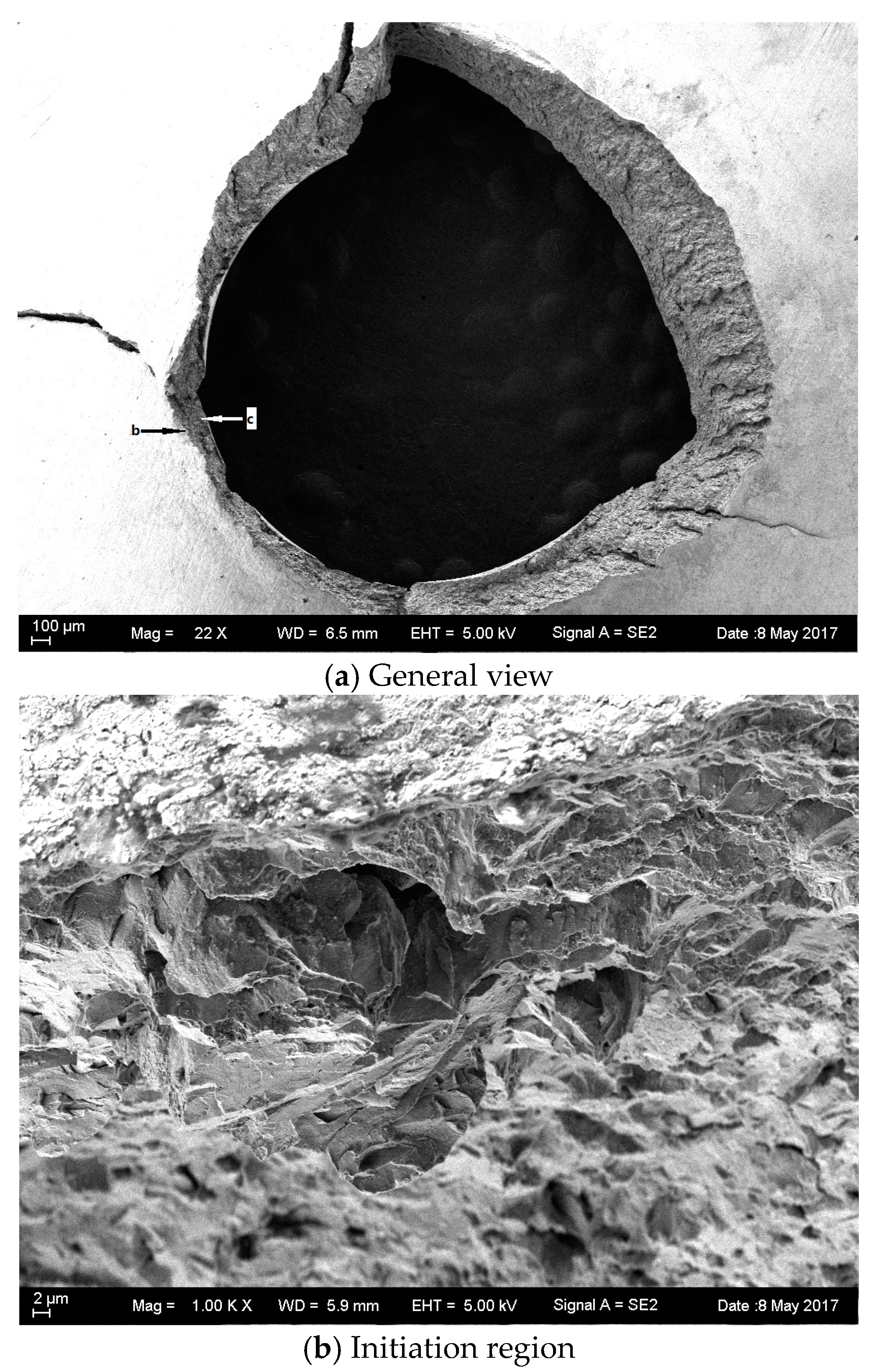
3.5. Fractography of SPT
4. Discussion
4.1. Determination of Tsp
4.2. Temper Embrittlement of 3Cr1Mo1/4V
4.3. Hydrogen Embrittlement of 3Cr1Mo1/4V
5. Conclusions
- (1)
- Esp decreases with temperature increase at the upper shelf. However, it becomes constant at the upper shelf when Esp is divided by the maximum load. Esp/Fm-Temperature is able to be used to better characterize the SPT energy transition.
- (2)
- The SPT energy transition temperature (Tsp) of the base metal and the weld metal increased 8.9 °C and 11.2 °C, respectively, in the de-embrittlement and as-received states before hydrogen charging, indicating that there is a slight extent of temper embrittlement for the 3Cr1Mo1/4V steel serviced at 390 °C for ten years.
- (3)
- The specimen becomes slightly more brittle after hydrogen charging. The synergetic effect of temper embrittlement and hydrogen embrittlement has not been found in the 3Cr1Mo1/4V steel.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pillot, S.; Chauvy, C.; Corre, S.; Coudreuse, L.; Gingell, A.; Héritier, D. Effect of temper and hydrogen embrittlement on mechanical properties of 2.25Cr–1Mo steel grades—Application to minimum pressurizing temperature (mpt) issues. Part I: General considerations & materials’ properties. Int. J. Press. Vessel. Pip. 2013, 110, 17–23. [Google Scholar]
- Zabil’Skii, V.V. Temper embrittlement of structural alloy steels (review). Met. Sci. Heat Treat. 1987, 29, 32–42. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, C. Study on the Hydrogen Effect on the Temper Embrittlement of 2.25Cr-1Mo Steel. In Proceedings of the International Conference on Nuclear Engineering, Xi’an, China, 17–21 May 2010; pp. 179–184. [Google Scholar]
- Bulloch, J.H.; Crowe, D. Embrittlement observed in Cr–Mo turbine bolts after service. Theor. Appl. Fract. Mech. 1998, 29, 59–66. [Google Scholar] [CrossRef]
- Pillot, S.; Chauvy, C.; Corre, S.; Coudreuse, L.; Gingell, A.; Héritier, D. Effect of temper and hydrogen embrittlement on mechanical properties of 2.25Cr–1Mo steel grades—Application to minimum pressurizing temperature (MPT) issues. Part II: Vintage reactors & MPT determination. Int. J. Press. Vessel. Pip. 2013, 110, 24–31. [Google Scholar]
- Guan, K.S.; Wang, Z.W. Effects of material microdefects on results of small punch test. Mater. Mech. Eng. 2011, 35, 25–27. [Google Scholar]
- Manahan, M.P.; Argon, A.S.; Harling, O.K. The development of a miniaturized disk bend test for the determination of postirradiation mechanical properties. J. Nucl. Mater. 1981, 104, 1545–1550. [Google Scholar] [CrossRef]
- Chang, Y.S.; Kim, J.M.; Choi, J.B.; Kim, Y.J.; Kim, M.C.; Lee, B.S. Derivation of ductile fracture resistance by use of small punch specimens. Eng. Fract. Mech. 2008, 75, 3413–3427. [Google Scholar] [CrossRef]
- Mang, A.I.; Zhen, Y.; ZhiWen, W. Origination, development and application of small punch test method. J. Mech. Strength 2002, 22, 279–282. [Google Scholar]
- Matocha, K.; Filip, M.; Karthik, V.; Kumar, R.V.; Lacalle, R.; Tonti, A. Results of the Round Robin Test for Determination of TSP of P22 Steel by Small Punch Tests. In Determination of Mechanical Properties by Small Punch and Other Miniature Testing Techniques, Proceedings of the 2nd International Conference on SSTT, Ostrava, Czech Republic, October 2012; Matocha, K., Hurst, R, Sun, W., Eds.; Springer: Berlin, Germany, 2012; pp. 227–232. [Google Scholar]
- Prescott, G.R. Operating Issues of Aging Reactors; Joint Industry Project Aging Hydroprocessing Reactors, Tech.: Edinburgh, UK, 1998. [Google Scholar]
- Hurst, R.; Matocha, K. Where are we now with the European Code of Practice for Small Punch Testing. In Determination of Mechanical Properties of Materials by Small Punch and Other Miniature Testing Techniques; Ocelot: Ostrava, Czech Republic, 2012; pp. 4–18. [Google Scholar]
- GB/T 29459-2012. Small Punch Test Methods of Metallic Materials for In-Service Pressure Equipments. Part 1 and Part 2; Standardization Administration of China: Beijing, China, 2012.
- Guan, K.; Hua, L.; Wang, Q.; Zou, X.; Song, M. Assessment of toughness in long term service CrMo low alloy steel by fracture toughness and small punch test. Nucl. Eng. Des. 2011, 241, 1407–1413. [Google Scholar] [CrossRef]
- Metallic Materials-Charpy Pendulum Impact Test Method; GB/T 229-2007; Standardization Administration of China: Beijing, China, 2008.
- Impact Test Methods on Welded Joints; GB/T 2650-2008; Standardization Administration of China: Beijing, China, 2008.
- Yang, J.; Guan, K. Evaluation of Hydrogen Embrittlement Susceptibility of 2205 Duplex Stainless Weld Joint; WIT Transactions on Engineering Sciences: Shanghai, China, 2014; pp. 563–570. [Google Scholar]
- Albert, S.K.; Padhy, G.K. A Brief Review on Methods for Diffusible Hydrogen Measurement in Welds. Indian Weld. J. 2014, 47, 101–113. [Google Scholar]
- Finarelli, D.; Carsughi, F.; Jung, P. The small ball punch test at FZJ. J. Nucl. Mater. 2008, 377, 65–71. [Google Scholar] [CrossRef]
- Zhang, E.Y.; Yu, C.; Xu, Y.-F.; Guan, K.S. Effects of temper embrittlement on mechanical properties of hydrogenation reactor steel weld determined by small punch test. Mater. Mech. Eng. 2014, 38, 16–20. [Google Scholar] [CrossRef]
- Matocha, K. The evaluation of materials properties of inservice components by small punch tests. J. KONES 2009, 16, 315–321. [Google Scholar]
- Matocha, K. The Use of Small Punch Tests for Determination of Fracture behaviour of Ferritic Steels. Procedia Eng. 2014, 86, 885–891. [Google Scholar] [CrossRef]
- API 934-F. Guidance on Heavy Wall Reactor Startup and Shutdown; American Petroleum Institute: Washington, DC, USA, 2007.
- Taniguchi, G.; Yamashita, K.; Otsu, M.; Nako, H.; Sakata, M. A study on the development of creep rupture and temper embrittlement properties in 21/4Cr-1Mo-V steel weld metal. Weld. World 2015, 59, 785–796. [Google Scholar] [CrossRef]
- Eliaz, N.; Shachar, A.; Tal, B.; Eliezer, D. Characteristics of hydrogen embrittlement, stress corrosion cracking and tempered martensite embrittlement in high-strength steels. Eng. Fail. Anal. 2002, 9, 167–184. [Google Scholar] [CrossRef]
- Shimazu, H.; Konosu, S.; Tanaka, Y.; Yuga, M.; Yamamoto, H.; Ohtsuka, N. Combined Effect of Temper and Hydrogen Embrittlement on Threshold for Hydrogen–Induced Fracture in Cr-Mo Steels. In Proceedings of the ASME 2012 Pressure Vessels and Piping Conference, Toronto, ON, Canada, 15–19 July 2012; pp. 825–833. [Google Scholar]
- Woodtli, J.; Kieselbach, R. Damage due to hydrogen embrittlement and stress corrosion cracking. Eng. Fail. Anal. 2000, 7, 427–450. [Google Scholar] [CrossRef]
- Masaoka, I.; Kinoshita, K.; Chiba, R. Hydrogen Attack Limit of 2.25Cr-1Mo Steel; Welding Research Council, Bulletins: New York, NY, USA, 1985; Volume 305, pp. 1–8. [Google Scholar]
- JPVRC. Embrittlement of Pressure Vessel Steels in High Temperature, High Pressure Hydrogen Environment; Welding Research Council, Bulletins: New York, NY, USA, 1985; Volume 305, pp. 9–21. [Google Scholar]
- JPVRC. Hydrogen Embrittlement of Bond Structure between Stainless Steel Overlay and Base Metal; Welding Research Council, Bulletins: New York, NY, USA, 1985; Volume 305, p. 22. [Google Scholar]




| Yield Strength Rel (MPa) | Maximum Strength Rm (MPa) | Percentage of Elongation after Fracture δ4 (%) | Ductile-Brittle Transition Temperature vTr54 (°C) |
|---|---|---|---|
| 466 | 590 | 28 | −71.1 |
| Elements | C | Si | Mn | P | S | Cr | Mo | Ni | Cu | Sb | Sn | As |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Base material | 0.115 | 0.07 | 0.52 | 0.006 | 0.006 | 3.03 | 0.93 | 0.1 | 0.082 | 0.008 | 0.0078 | 0.0067 |
| Weld material | 0.074 | 0.155 | 1.057 | 0.006 | 0.005 | 2.862 | 0.904 | 0.045 | 0.09 | 0.0073 | 0.0039 | 0.0051 |
| Temperature (°C) | Fm (N) | Uf (mm) |
|---|---|---|
| −20 | 1861.08 | 2.023 |
| −120 | 2392.38 | 1.945 |
| −150 | 2765.74 | 1.917 |
| −160 | 2700.46 | 1.747 |
| −180 | 2628.22 | 1.509 |
| −190 | 1369.10 | 0.713 |
| Material | As-Received State vTr54 | De-Embrittlement vTr54 | As-Received State Tsp | De-Embrittlement Tsp | ΔvTr54 | ΔTsp |
|---|---|---|---|---|---|---|
| Base metal (°C) | −60.3 | −71 | −181 | −189.9 | 10.7 | 8.9 |
| Weld metal (°C) | −17.3 | −31 | −176.9 | −188.1 | 13.7 | 11.2 |
| State | Before Hydrogen Charging | After Hydrogen Charging | ΔTsp |
|---|---|---|---|
| Tsp of As-received state (°C) | −176.9 | −174.8 | 2.1 |
| Tsp of De-embrittlement (°C) | −188.1 | −186.4 | 1.7 |
| State | Tsp | vTr54 by Equations (3) and (4) | vTr54 (Tests) | ΔvTr54 |
|---|---|---|---|---|
| As-received (°C) | −181.0 | −22.7 | −60.3 | 37.6 |
| De-embrittlement (°C) | −189.9 | −36.7 | −71.0 | 34.3 |
| State | Tsp | vTr54 by Equations (3) and (4) | vTr54 (Tests) | ΔvTr54 |
|---|---|---|---|---|
| As-received (°C) | −176.9 | −16.2 | −17.3 | 1.1 |
| De-embrittlement (°C) | −188.1 | −33.9 | −31 | −2.9 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, K.; Szpunar, J.A.; Matocha, K.; Wang, D. Study on Temper Embrittlement and Hydrogen Embrittlement of a Hydrogenation Reactor by Small Punch Test. Materials 2017, 10, 671. https://doi.org/10.3390/ma10060671
Guan K, Szpunar JA, Matocha K, Wang D. Study on Temper Embrittlement and Hydrogen Embrittlement of a Hydrogenation Reactor by Small Punch Test. Materials. 2017; 10(6):671. https://doi.org/10.3390/ma10060671
Chicago/Turabian StyleGuan, Kaishu, Jerzy A. Szpunar, Karel Matocha, and Duwei Wang. 2017. "Study on Temper Embrittlement and Hydrogen Embrittlement of a Hydrogenation Reactor by Small Punch Test" Materials 10, no. 6: 671. https://doi.org/10.3390/ma10060671
APA StyleGuan, K., Szpunar, J. A., Matocha, K., & Wang, D. (2017). Study on Temper Embrittlement and Hydrogen Embrittlement of a Hydrogenation Reactor by Small Punch Test. Materials, 10(6), 671. https://doi.org/10.3390/ma10060671






