Advances in Photocatalytic CO2 Reduction with Water: A Review
Abstract
:1. Introduction
2. Photocatalysis and Photocatalytic Reduction of CO2 with H2O
2.1. Theoretical Approach
- (i)
- Absorption of photons with suitable energy and generation of electron–hole pairs;
- (ii)
- Separation and transportation of electron–hole pairs (charge carriers); and
- (iii)
2.2. Measures of Photocatalytic Efficiency
3. Recent Photocatalysts for CO2 Reduction with H2O
3.1. TiO2 and Modified TiO2
3.2. Ag co-Catalyst Loaded ALa4Ti4O5 (A = Ca, Sr, and Ba)
3.3. Ferroelectric LiNbO3
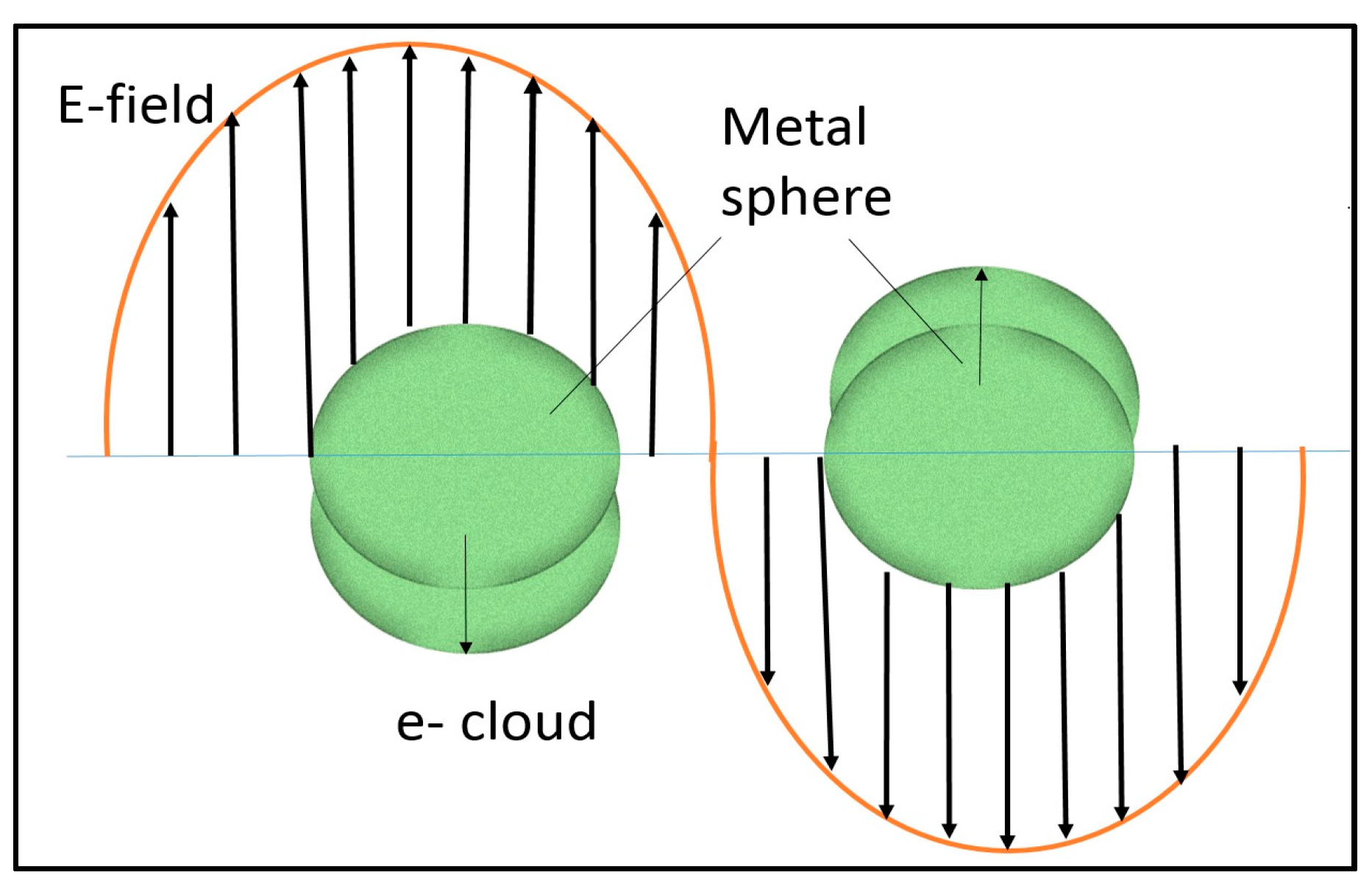
4. Plasmonic Photocatalyst
4.1. Fundamental of Plasmonic Photocatalyst
4.2. Reduction of CO2 with H2O by Plasmonic Photocatalyst
4.2.1. Au Deposited TiO2
4.2.2. Ag Supported on AgIO3
4.2.3. Ag Supported on Ag2SO3
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Roy, S.C.; Varghese, O.K.; Paulose, M.; Grimes, C.A. Toward solar fuels: Photocatalytic conversion of carbon dioxide to hydrocarbons. ACS Nano 2010, 4, 1259–1278. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.J.; Cho, D.H. Research activities on the utilization of carbon dioxide in korea. CLEAN–Soil Air Water 2008, 36, 426–432. [Google Scholar] [CrossRef]
- Houghton, J.T.; Ding, Y.; Griggs, D.J.; Noguer, M.; van der Linden, P.J.; Dai, X.; Maskell, K.; Johnson, C. Climate Change 2001: The Scientific Basis; The Press Syndicate of the University of Cambridge: Cambridge, UK, 2001. [Google Scholar]
- Khatib, H. Iea world energy outlook 2011—A comment. Energy Policy 2012, 48, 737–743. [Google Scholar] [CrossRef]
- Windle, C.D.; Perutz, R.N. Advances in molecular photocatalytic and electrocatalytic CO2 reduction. Coord. Chem. Rev. 2012, 256, 2562–2570. [Google Scholar] [CrossRef]
- Qu, Y.; Duan, X. One-dimensional homogeneous and heterogeneous nanowires for solar energy conversion. J. Mater. Chem. 2012, 22, 16171–16181. [Google Scholar] [CrossRef]
- Hurst, T.F.; Cockerill, T.T.; Florin, N.H. Life cycle greenhouse gas assessment of a coal-fired power station with calcium looping CO2 capture and offshore geological storage. Energy Environ. Sci. 2012, 5, 7132–7150. [Google Scholar] [CrossRef]
- Sato, S.; Arai, T.; Morikawa, T. Toward solar-driven photocatalytic CO2 reduction using water as an electron donor. Inorg. Chem. 2015, 54, 5105–5113. [Google Scholar] [CrossRef] [PubMed]
- Indrakanti, V.P.; Kubicki, J.D.; Schobert, H.H. Photoinduced activation of CO2 on TiO2 surfaces: Quantum chemical modeling of CO2 adsorption on oxygen vacancies. Fuel Process. Technol. 2011, 92, 805–811. [Google Scholar] [CrossRef]
- Habisreutinger, S.N.; Schmidt-Mende, L.; Stolarczyk, J.K. Photocatalytic reduction of CO2 on TiO2 and other semiconductors. Angew. Chem. Int. Ed. 2013, 52, 7372–7408. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Rajeshwar, K.; de Tacconi, N.R.; Chenthamarakshan, C. Semiconductor-based composite materials: Preparation, properties, and performance. Chem. Mater. 2001, 13, 2765–2782. [Google Scholar] [CrossRef]
- Tong, H.; Ouyang, S.; Bi, Y.; Umezawa, N.; Oshikiri, M.; Ye, J. Nano-photocatalytic materials: Possibilities and challenges. Adv. Mater. 2012, 24, 229–251. [Google Scholar] [CrossRef] [PubMed]
- An, C.; Wang, J.; Qin, C.; Jiang, W.; Wang, S.; Li, Y.; Zhang, Q. Synthesis of Ag@AgBr/AgCl heterostructured nanocashews with enhanced photocatalytic performance via anion exchange. J. Mater. Chem. 2012, 22, 13153–13158. [Google Scholar] [CrossRef]
- An, C.; Wang, J.; Jiang, W.; Zhang, M.; Ming, X.; Wang, S.; Zhang, Q. Strongly visible-light responsive plasmonic shaped AgX:Ag (X = Cl, Br) nanoparticles for reduction of CO2 to methanol. Nanoscale 2012, 4, 5646–5650. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhuang, Z.; Li, W.; Pan, H. Photocatalytic reduction of CO2 over noble metal-loaded and nitrogen-doped mesoporous TiO2. Appl. Catal. A Gen. 2012, 429, 31–38. [Google Scholar] [CrossRef]
- Sayama, K. Solar hydrogen production on photocatalysis-electrolysis hybrid system using redox mediator and porous oxide photoelectrodes. In Solar to Chemical Energy Conversion; Springer: Berlin/Heidelberg, Germany, 2016; pp. 345–365. [Google Scholar]
- Li, K.; An, X.; Park, K.H.; Khraisheh, M.; Tang, J. A critical review of CO2 photoconversion: Catalysts and reactors. Catal. Today 2014, 224, 3–12. [Google Scholar] [CrossRef]
- Mao, J.; Li, K.; Peng, T. Recent advances in the photocatalytic CO2 reduction over semiconductors. Catal. Sci. Technol. 2013, 3, 2481–2498. [Google Scholar] [CrossRef]
- Izumi, Y. Recent advances in the photocatalytic conversion of carbon dioxide to fuels with water and/or hydrogen using solar energy and beyond. Coord. Chem. Rev. 2013, 257, 171–186. [Google Scholar] [CrossRef]
- White, J.L.; Baruch, M.F.; Pander, J.E., III; Hu, Y.; Fortmeyer, I.C.; Park, J.E.; Zhang, T.; Liao, K.; Gu, J.; Yan, Y. Light-driven heterogeneous reduction of carbon dioxide: Photocatalysts and photoelectrodes. Chem. Rev. 2015, 115, 12888–12935. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Zhang, Q.; Liu, G.; Wang, Y. Photocatalytic and photoelectrocatalytic reduction of CO2 using heterogeneous catalysts with controlled nanostructures. Chem. Commun. 2016, 52, 35–59. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, D.; Han, H.; Li, C. Roles of co-catalysts in photocatalysis and photoelectrocatalysis. Acc. Chem. Res. 2013, 46, 1900–1909. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Zhang, W.; Ren, J.; Xu, R. Photocatalytic reduction of CO2: A brief review on product analysis and systematic methods. Anal. Methods 2013, 5, 1086–1097. [Google Scholar] [CrossRef]
- Neațu, Ș.; Maciá-Agulló, J.A.; Garcia, H. Solar light photocatalytic CO2 reduction: General considerations and selected bench-mark photocatalysts. Int. J. Mol. Sci. 2014, 15, 5246–5262. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Xu, Y.-J. Photocatalytic conversion of CO2 into value-added and renewable fuels. Appl. Surf. Sci. 2015, 342, 154–167. [Google Scholar] [CrossRef]
- Kumar, S.G.; Devi, L.G. Review on modified TiO2 photocatalysis under UV/visible light: Selected results and related mechanisms on interfacial charge carrier transfer dynamics. J. Phys. Chem. A 2011, 115, 13211–13241. [Google Scholar] [CrossRef] [PubMed]
- Dhakshinamoorthy, A.; Navalon, S.; Corma, A.; Garcia, H. Photocatalytic CO2 reduction by TiO2 and related titanium containing solids. Energy Environ. Sci. 2012, 5, 9217–9233. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y. Understanding the reaction mechanism of photocatalytic reduction of CO2 with H2O on TiO2-based photocatalysts: A review. Aerosol. Air Qual. Res. 2014, 14, 453–469. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Y.L.; Liu, R.-S.; Tsai, D.P. Plasmonic photocatalysis. Rep. Prog. Phys. 2013, 76, 046401. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Cronin, S.B. A review of surface plasmon resonance-enhanced photocatalysis. Adv. Funct. Mater. 2013, 23, 1612–1619. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, G.; Yu, J.; Fan, W. Surface plasmon resonance-mediated photocatalysis by noble metal-based composites under visible light. J. Mater. Chem. 2012, 22, 21337–21354. [Google Scholar] [CrossRef]
- Shrestha, S.R.; Oanh, N.T.K.; Xu, Q.; Rupakheti, M.; Lawrence, M.G. Analysis of the vehicle fleet in the kathmandu valley for estimation of environment and climate co-benefits of technology intrusions. Atmos. Environ. 2013, 81, 579–590. [Google Scholar] [CrossRef]
- Parmon, V. Photocatalysis as a phenomenon: Aspects of terminology. Catal. Today 1997, 39, 137–144. [Google Scholar] [CrossRef]
- Sánchez, A.; Artola, A.; Font, X.; Gea, T.; Barrena, R.; Gabriel, D.; Sánchez-Monedero, M.Á.; Roig, A.; Cayuela, M.L.; Mondini, C. Greenhouse gas from organic waste composting: Emissions and measurement. In CO2 Sequestration, Biofuels and Depollution; Springer: Berlin/Heidelberg, Germany, 2015; pp. 33–70. [Google Scholar]
- Li, Y.; Wang, W.-N.; Zhan, Z.; Woo, M.-H.; Wu, C.-Y.; Biswas, P. Photocatalytic reduction of CO2 with H2O on mesoporous silica supported Cu/TiO2 catalysts. Appl. Catal. B Environ. 2010, 100, 386–392. [Google Scholar] [CrossRef]
- Yan, S.C.; Ouyang, S.X.; Gao, J.; Yang, M.; Feng, J.Y.; Fan, X.X.; Wan, L.J.; Li, Z.S.; Ye, J.H.; Zhou, Y. A room-temperature reactive-template route to mesoporous ZnGa2O4 with improved photocatalytic activity in reduction of CO2. Angew. Chem. 2010, 122, 6544–6548. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, Y.; Kou, J.; Chen, X.; Tian, Z.; Gao, J.; Yan, S.; Zou, Z. High-yield synthesis of ultralong and ultrathin Zn2GeO4 nanoribbons toward improved photocatalytic reduction of CO2 into renewable hydrocarbon fuel. J. Am. Chem. Soc. 2010, 132, 14385–14387. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, K.; Wato, T.; Miseki, Y.; Saito, K.; Kudo, A. Photocatalytic reduction of carbon dioxide over Ag co-catalyst-loaded ALa4Ti4O15 (A = Ca, Sr, and Ba) using water as a reducing reagent. J. Am. Chem. Soc. 2011, 133, 20863–20868. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, Y.; Ackerman, E.A.; Gajdardziska-Josifovska, M.; Li, H. Visible light responsive iodine-doped TiO2 for photocatalytic reduction of CO2 to fuels. Appl. Catal. A Gen. 2011, 400, 195–202. [Google Scholar] [CrossRef]
- Stock, M.; Dunn, S. LiNbO3—A polar material for solid-gas artificial photosynthesis. Ferroelectrics 2011, 419, 9–13. [Google Scholar] [CrossRef]
- Tu, W.; Zhou, Y.; Liu, Q.; Tian, Z.; Gao, J.; Chen, X.; Zhang, H.; Liu, J.; Zou, Z. Robust hollow spheres consisting of alternating titania nanosheets and graphene nanosheets with high photocatalytic activity for CO2 conversion into renewable fuels. Adv. Funct. Mater. 2012, 22, 1215–1221. [Google Scholar] [CrossRef]
- Hsu, H.-C.; Shown, I.; Wei, H.-Y.; Chang, Y.-C.; Du, H.-Y.; Lin, Y.-G.; Tseng, C.-A.; Wang, C.-H.; Chen, L.-C.; Lin, Y.-C. Graphene oxide as a promising photocatalyst for CO2 to methanol conversion. Nanoscale 2013, 5, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Xi, G.; Ouyang, S.; Li, P.; Ye, J.; Ma, Q.; Su, N.; Bai, H.; Wang, C. Ultrathin W18O49 nanowires with diameters below 1 nm: Synthesis, near-infrared absorption, photoluminescence, and photochemical reduction of carbon dioxide. Angew. Chem. Int. Ed. 2012, 51, 2395–2399. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhou, Y.; Tian, Z.; Chen, X.; Gao, J.; Zou, Z. Zn2GeO4 crystal splitting toward sheaf-like, hyperbranched nanostructures and photocatalytic reduction of CO2 into CH4 under visible light after nitridation. J. Mater. Chem. 2012, 22, 2033–2038. [Google Scholar] [CrossRef]
- Yan, S.; Yu, H.; Wang, N.; Li, Z.; Zou, Z. Efficient conversion of CO2 and H2O into hydrocarbon fuel over ZnAl2O4-modified mesoporous ZnGaNO under visible light irradiation. Chem. Commun. 2012, 48, 1048–1050. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-A.; Choi, J.H.; Choi, K.M.; Lee, D.K.; Kang, J.K. Highly porous gallium oxide with a high CO2 affinity for the photocatalytic conversion of carbon dioxide into methane. J. Mater. Chem. 2012, 22, 5304–5307. [Google Scholar] [CrossRef]
- Wang, W.-N.; An, W.-J.; Ramalingam, B.; Mukherjee, S.; Niedzwiedzki, D.M.; Gangopadhyay, S.; Biswas, P. Size and structure matter: Enhanced CO2 photoreduction efficiency by size-resolved ultrafine Pt nanoparticles on TiO2 single crystals. J. Am. Chem. Soc. 2012, 134, 11276–11281. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Pan, H.; Li, W.; Zhuang, Z. Photocatalytic reduction of CO2 to methane over HNb3O8 nanobelts. Appl. Catal. A Gen. 2012, 413, 103–108. [Google Scholar] [CrossRef]
- Núñez, J.; Víctor, A.; Jana, P.; Coronado, J.M.; Serrano, D.P. Effect of copper on the performance of ZnO and ZnO 1−xNx oxides as CO2 photoreduction catalysts. Catal. Today 2013, 209, 21–27. [Google Scholar] [CrossRef]
- Mankidy, B.D.; Joseph, B.; Gupta, V.K. Photo-conversion of CO2 using titanium dioxide: Enhancements by plasmonic and co-catalytic nanoparticles. Nanotechnology 2013, 24, 405402. [Google Scholar] [CrossRef] [PubMed]
- Ong, W.-J.; Gui, M.M.; Chai, S.-P.; Mohamed, A.R. Direct growth of carbon nanotubes on Ni/TiO2 as next generation catalysts for photoreduction of CO2 to methane by water under visible light irradiation. RSC Adv. 2013, 3, 4505–4509. [Google Scholar] [CrossRef]
- Zhai, Q.; Xie, S.; Fan, W.; Zhang, Q.; Wang, Y.; Deng, W.; Wang, Y. Photocatalytic conversion of carbon dioxide with water into methane: Platinum and copper (i) oxide co-catalysts with a core–shell structure. Angew. Chem. 2013, 125, 5888–5891. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Z.; Cao, S.-W.; Xue, C. Au/Pt nanoparticle-decorated TiO2 nanofibers with plasmon-enhanced photocatalytic activities for solar-to-fuel conversion. J. Phys. Chem. C 2013, 117, 25939–25947. [Google Scholar] [CrossRef]
- Tahir, M.; Amin, N.S. Photocatalytic reduction of carbon dioxide with water vapors over montmorillonite modified TiO2 nanocomposites. Appl. Catal. B Environ. 2013, 142, 512–522. [Google Scholar] [CrossRef]
- Li, P.; Xu, H.; Liu, L.; Kako, T.; Umezawa, N.; Abe, H.; Ye, J. Constructing cubic–orthorhombic surface-phase junctions of NaNbO3 towards significant enhancement of CO2 photoreduction. J. Mater. Chem. A 2014, 2, 5606–5609. [Google Scholar] [CrossRef]
- He, Z.; Wang, D.; Fang, H.; Chen, J.; Song, S. Highly efficient and stable Ag/AgIO3 particles for photocatalytic reduction of CO2 under visible light. Nanoscale 2014, 6, 10540–10544. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Chen, G.; Zhang, C.; Zou, Z. Polymeric g-C3N4 coupled with NaNbO3 nanowires toward enhanced photocatalytic reduction of CO2 into renewable fuel. ACS Catal. 2014, 4, 3637–3643. [Google Scholar] [CrossRef]
- Cao, S.-W.; Liu, X.-F.; Yuan, Y.-P.; Zhang, Z.-Y.; Liao, Y.-S.; Fang, J.; Loo, S.C.J.; Sum, T.C.; Xue, C. Solar-to-fuels conversion over In2O3/gC3N4 hybrid photocatalysts. Appl. Catal. B Environ. 2014, 147, 940–946. [Google Scholar] [CrossRef]
- He, Y.; Zhang, L.; Fan, M.; Wang, X.; Walbridge, M.L.; Nong, Q.; Wu, Y.; Zhao, L. Z-scheme SnO2−x/gC3 N4 composite as an efficient photocatalyst for dye degradation and photocatalytic CO2 reduction. Sol. Energy Mater. Sol. Cells 2015, 137, 175–184. [Google Scholar] [CrossRef]
- Ong, W.-J.; Putri, L.K.; Tan, L.-L.; Chai, S.-P.; Yong, S.-T. Heterostructured AgX/gC3N4 (X = Cl and Br) nanocomposites via a sonication-assisted deposition-precipitation approach: Emerging role of halide ions in the synergistic photocatalytic reduction of carbon dioxide. Appl. Catal. B Environ. 2016, 180, 530–543. [Google Scholar] [CrossRef]
- Wang, D.; Yu, Y.; Zhang, Z.; Fang, H.; Chen, J.; He, Z.; Song, S. Ag/Ag2SO3 plasmonic catalysts with high activity and stability for CO2 reduction with water vapor under visible light. Environ. Sci. Pollut. Res. 2016, 23, 18369–18378. [Google Scholar] [CrossRef] [PubMed]
- Usubharatana, P.; McMartin, D.; Veawab, A.; Tontiwachwuthikul, P. Photocatalytic process for CO2 emission reduction from industrial flue gas streams. Ind. Eng. Chem. Res. 2006, 45, 2558–2568. [Google Scholar] [CrossRef]
- Das, S.; Daud, W.W. Retracted: Photocatalytic CO2 Transformation into Fuel: A Review on Advances in Photocatalyst and Photoreactor. Renew. Sustain. Energy Rev. 2014, 39, 765–805. [Google Scholar] [CrossRef]
- Fan, W.; Zhang, Q.; Wang, Y. Semiconductor-based nanocomposites for photocatalytic H2 production and CO2 conversion. Phys. Chem. Chem. Phys. 2013, 15, 2632–2649. [Google Scholar] [CrossRef] [PubMed]
- Kamat, P.V. Manipulation of charge transfer across semiconductor interface. A criterion that cannot be ignored in photocatalyst design. J. Phys. Chem. Lett. 2012, 3, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Ângelo, J.; Andrade, L.; Madeira, L.M.; Mendes, A. An overview of photocatalysis phenomena applied to NOx abatement. J. Environ. Manag. 2013, 129, 522–539. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Shen, S.; Guo, L.; Mao, S.S. Semiconductor-based photocatalytic hydrogen generation. Chem. Rev. 2010, 110, 6503–6570. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Nanayakkara, C.E.; Grassian, V.H. Titanium dioxide photocatalysis in atmospheric chemistry. Chem. Rev. 2012, 112, 5919–5948. [Google Scholar] [CrossRef] [PubMed]
- Zhdanov, V.P.; Hägglund, C.; Kasemo, B. Relaxation of plasmons in nm-sized metal particles located on or embedded in an amorphous semiconductor. Surf. Sci. 2005, 599, L372–L375. [Google Scholar] [CrossRef]
- Langhammer, C.; Yuan, Z.; Zorić, I.; Kasemo, B. Plasmonic properties of supported Pt and Pd nanostructures. Nano Lett. 2006, 6, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Torimoto, T.; Horibe, H.; Kameyama, T.; Okazaki, K.-I.; Ikeda, S.; Matsumura, M.; Ishikawa, A.; Ishihara, H. Plasmon-enhanced photocatalytic activity of cadmium sulfide nanoparticle immobilized on silica-coated gold particles. J. Phys. Chem. Lett. 2011, 2, 2057–2062. [Google Scholar] [CrossRef]
- Mubeen, S.; Hernandez-Sosa, G.; Moses, D.; Lee, J.; Moskovits, M. Plasmonic photosensitization of a wide band gap semiconductor: Converting plasmons to charge carriers. Nano Lett. 2011, 11, 5548–5552. [Google Scholar] [CrossRef] [PubMed]
- Nishijima, Y.; Ueno, K.; Yokota, Y.; Murakoshi, K.; Misawa, H. Plasmon-assisted photocurrent generation from visible to near-infrared wavelength using a Au-nanorods/TiO2 electrode. J. Phys. Chem. Lett. 2010, 1, 2031–2036. [Google Scholar] [CrossRef]
- Zhao, Z.-H.; Fan, J.-M.; Wang, Z.-Z. Photo-catalytic CO2 reduction using sol–gel derived titania-supported zinc-phthalocyanine. J. Clean. Prod. 2007, 15, 1894–1897. [Google Scholar] [CrossRef]
- Zhao, Z.; Fan, J.; Liu, S.; Wang, Z. Optimal design and preparation of titania-supported CoPc using sol–gel for the photo-reduction of CO2. Chem. Eng. J. 2009, 151, 134–140. [Google Scholar] [CrossRef]
- Zhao, Z.; Fan, J.; Xie, M.; Wang, Z. Photo-catalytic reduction of carbon dioxide with in-situ synthesized CoPc/TiO2 under visible light irradiation. J. Clean. Prod. 2009, 17, 1025–1029. [Google Scholar] [CrossRef]
- Shen, S.; Shi, J.; Guo, P.; Guo, L. Visible-light-driven photocatalytic water splitting on nanostructured semiconducting materials. Int. J. Nanotechnol. 2011, 8, 523–591. [Google Scholar] [CrossRef]
- Jia, L.; Li, J.; Fang, W. Enhanced visible-light active C and Fe co-doped LaCoO3 for reduction of carbon dioxide. Catal. Commun. 2009, 11, 87–90. [Google Scholar] [CrossRef]
- Takeda, H.; Koike, K.; Inoue, H.; Ishitani, O. Development of an efficient photocatalytic system for CO2 reduction using rhenium (i) complexes based on mechanistic studies. J. Am. Chem. Soc. 2008, 130, 2023–2031. [Google Scholar] [CrossRef] [PubMed]
- Morris, A.J.; Meyer, G.J.; Fujita, E. Molecular approaches to the photocatalytic reduction of carbon dioxide for solar fuels. Acc. Chem. Res. 2009, 42, 1983–1994. [Google Scholar] [CrossRef] [PubMed]
- Hawecker, J.; Lehn, J.-M.; Ziessel, R. Efficient photochemical reduction of CO2 to CO by visible light irradiation of systems containing re (bipy)(CO)3X or Ru(bipy)32+–CO2+ combinations as homogeneous catalysts. J. Chem. Soc. Chem. Commun. 1983, 536–538. [Google Scholar] [CrossRef]
- Takeda, H.; Koizumi, H.; Okamoto, K.; Ishitani, O. Photocatalytic CO2 reduction using a mn complex as a catalyst. Chem. Commun. 2014, 50, 1491–1493. [Google Scholar] [CrossRef] [PubMed]
- Kuramochi, Y.; Kamiya, M.; Ishida, H. Photocatalytic CO2 reduction in N,N-dimethylacetamide/water as an alternative solvent system. Inorg. Chem. 2014, 53, 3326–3332. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, Y.; Morimoto, T.; Koike, K.; Ishitani, O. Photocatalytic CO2 reduction with high turnover frequency and selectivity of formic acid formation using Ru(II) multinuclear complexes. Proc. Natl. Acad. Sci. USA 2012, 109, 15673–15678. [Google Scholar] [CrossRef] [PubMed]
- Bruckmeier, C.; Lehenmeier, M.W.; Reithmeier, R.; Rieger, B.; Herranz, J.; Kavakli, C. Binuclear rhenium(I) complexes for the photocatalytic reduction of CO2. Dalton Trans. 2012, 41, 5026–5037. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Song, J.; Geletii, Y.V.; Vickers, J.W.; Sumliner, J.M.; Musaev, D.G.; Kögerler, P.; Zhuk, P.F.; Bacsa, J.; Zhu, G. An exceptionally fast homogeneous carbon-free cobalt-based water oxidation catalyst. J. Am. Chem. Soc. 2014, 136, 9268–9271. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Annaka, M.; Sakai, K. Visible light-induced water oxidation catalyzed by molybdenum-based polyoxometalates with mono-and dicobalt(III) cores as oxygen-evolving centers. Chem. Commun. 2012, 48, 1653–1655. [Google Scholar] [CrossRef] [PubMed]
- Han, X.-B.; Zhang, Z.-M.; Zhang, T.; Li, Y.-G.; Lin, W.; You, W.; Su, Z.-M.; Wang, E.-B. Polyoxometalate-based cobalt–phosphate molecular catalysts for visible light-driven water oxidation. J. Am. Chem. Soc. 2014, 136, 5359–5366. [Google Scholar] [CrossRef] [PubMed]
- Grills, D.C.; Fujita, E. New directions for the photocatalytic reduction of CO2: Supramolecular, ScCO2 or biphasic ionic liquid-ScCO2 systems. J. Phys. Chem. Lett. 2010, 1, 2709–2718. [Google Scholar] [CrossRef]
- Hawecker, J.; Lehn, J.M.; Ziessel, R. Photochemical and electrochemical reduction of carbon dioxide to carbon monoxide mediated by (2,2′-bipyridine) tricarbonylchlororhenium(I) and related complexes as homogeneous catalysts. Helv. Chim. Acta 1986, 69, 1990–2012. [Google Scholar] [CrossRef]
- Inoue, T.; Fujishima, A.; Konishi, S.; Honda, K. Photoelectrocatalytic reduction of carbon dioxide in aqueous suspensions of semiconductor powders. Nature 1979, 277, 637–638. [Google Scholar] [CrossRef]
- Mori, K.; Yamashita, H.; Anpo, M. Photocatalytic reduction of CO2 with H2O on various titanium oxide photocatalysts. RSC Adv. 2012, 2, 3165–3172. [Google Scholar] [CrossRef]
- Handoko, A.D.; Li, K.; Tang, J. Recent progress in artificial photosynthesis: CO2 photoreduction to valuable chemicals in a heterogeneous system. Curr. Opin. Chem. Eng. 2013, 2, 200–206. [Google Scholar] [CrossRef]
- Navalón, S.; Dhakshinamoorthy, A.; Álvaro, M.; Garcia, H. Photocatalytic CO2 reduction using non-titanium metal oxides and sulfides. ChemSusChem 2013, 6, 562–577. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.; Zhou, Y.; Zou, Z. Photocatalytic conversion of CO2 into renewable hydrocarbon fuels: State-of-the-art accomplishment, challenges, and prospects. Adv. Mater. 2014, 26, 4607–4626. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, X.; Jia, Y.; Chen, X.; Han, H.; Li, C. Titanium dioxide-based nanomaterials for photocatalytic fuel generations. Chem. Rev. 2014, 114, 9987–10043. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Ciriminna, R.; Pagliaro, M.; Xu, Y.-J. Nanochemistry-derived Bi2WO6 nanostructures: Towards production of sustainable chemicals and fuels induced by visible light. Chem. Soc. Rev. 2014, 43, 5276–5287. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zhang, X.; Lee, A.F. Synthetic strategies to nanostructured photocatalysts for CO2 reduction to solar fuels and chemicals. J. Mater. Chem. A 2015, 3, 14487–14516. [Google Scholar] [CrossRef]
- Hashimoto, K.; Irie, H.; Fujishima, A. TiO2 photocatalysis: A historical overview and future prospects. Jpn. Appl. Phys. 2005, 44, 8269. [Google Scholar] [CrossRef]
- Wang, P.; Huang, B.; Dai, Y.; Whangbo, M.-H. Plasmonic photocatalysts: Harvesting visible light with noble metal nanoparticles. Phys. Chem. Chem. Phys. 2012, 14, 9813–9825. [Google Scholar] [CrossRef] [PubMed]
- Murdoch, M.; Waterhouse, G.; Nadeem, M.; Metson, J.; Keane, M.; Howe, R.; Llorca, J.; Idriss, H. The effect of gold loading and particle size on photocatalytic hydrogen production from ethanol over Au/TiO2 nanoparticles. Nat. Chem. 2011, 3, 489. [Google Scholar] [CrossRef] [PubMed]
- Linic, S.; Christopher, P.; Ingram, D.B. Plasmonic-metal nanostructures for efficient conversion of solar to chemical energy. Nat. Mater. 2011, 10, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Shao, C.; Zhang, L.; Li, X.; Liu, Y. Electrospun nanofibers of v-doped TiO2 with high photocatalytic activity. J. Coll. Interface Sci. 2010, 351, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Su, Y.; Qiao, L.; Liu, L.; Su, Q.; Zhu, C.; Liu, X. Synthesis of one-dimensional TiO2/V2O5 branched heterostructures and their visible light photocatalytic activity towards Rhodamine B. Nanotechnology 2011, 22, 225702. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Tian, B.; Zeng, C.; Li, T.; Wang, T.; Zhang, J. Ecofriendly synthesis and photocatalytic activity of uniform cubic Ag@AgCl plasmonic photocatalyst. J. Phys. Chem. C 2012, 117, 213–220. [Google Scholar] [CrossRef]
- Xie, S.; Wang, Y.; Zhang, Q.; Deng, W.; Wang, Y. MgO-and Pt-promoted TiO2 as an efficient photocatalyst for the preferential reduction of carbon dioxide in the presence of water. ACS Catal. 2014, 4, 3644–3653. [Google Scholar] [CrossRef]
- Zhang, Q.-H.; Han, W.-D.; Hong, Y.-J.; Yu, J.-G. Photocatalytic reduction of CO2 with H2O on Pt-loaded TiO2 catalyst. Catal. Today 2009, 148, 335–340. [Google Scholar] [CrossRef]
- Varghese, O.K.; Paulose, M.; LaTempa, T.J.; Grimes, C.A. High-rate solar photocatalytic conversion of CO2 and water vapor to hydrocarbon fuels. Nano Lett. 2009, 9, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Kubacka, A.; Fernández-García, M.; Colón, G. Advanced nanoarchitectures for solar photocatalytic applications. Chem. Rev. 2011, 112, 1555–1614. [Google Scholar] [CrossRef] [PubMed]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Giocondi, J.L.; Rohrer, G.S. Spatial separation of photochemical oxidation and reduction reactions on the surface of ferroelectric BaTiO3. J. Phys. Chem. B 2001, 105, 8275–8277. [Google Scholar] [CrossRef]
- Kalinin, S.V.; Bonnell, D.A.; Alvarez, T.; Lei, X.; Hu, Z.; Ferris, J.; Zhang, Q.; Dunn, S. Atomic polarization and local reactivity on ferroelectric surfaces: A new route toward complex nanostructures. Nano Lett. 2002, 2, 589–593. [Google Scholar] [CrossRef]
- Dunn, S.; Tiwari, D.; Jones, P.M.; Gallardo, D.E. Insights into the relationship between inherent materials properties of PZT and photochemistry for the development of nanostructured silver. J. Mater. Chem. 2007, 17, 4460–4463. [Google Scholar] [CrossRef]
- Dunn, S.; Tiwari, D. Influence of ferroelectricity on the photoelectric effect of LiNbO3. Appl. Phys. Lett. 2008, 93, 092905. [Google Scholar] [CrossRef]
- Yang, W.-C.; Rodriguez, B.J.; Gruverman, A.; Nemanich, R. Polarization-dependent electron affinity of LiNbO3 surfaces. Appl. Phys. Lett. 2004, 85, 2316–2318. [Google Scholar] [CrossRef]
- Ramos-Moore, E.; Baier-Saip, J.; Cabrera, A. Desorption of carbon dioxide from small potassium niobate particles induced by the particles’ ferroelectric transition. Surf. Sci. 2006, 600, 3472–3476. [Google Scholar] [CrossRef]
- Chen, X.; Yamada, H.; Horiuchi, T.; Matsushige, K.; Watanabe, S.; Kawai, M.; Weiss, P. Surface potential of ferroelectric thin films investigated by scanning probe microscopy. J. Vac. Sci. Technol. B 1999, 17, 1930–1934. [Google Scholar] [CrossRef]
- Thierfelder, C.; Sanna, S.; Schindlmayr, A.; Schmidt, W. Do we know the band gap of lithium niobate? Phys. Status Solidi (C) 2010, 7, 362–365. [Google Scholar] [CrossRef]
- Ulman, M.; Tinnemans, A.; Mackor, A.; Aurian-Blajeni, B.; Halmann, M. Photoreduction of carbon dioxide to formic acid, formaldehyde, methanol, acetaldehyde and ethanol using aqueous suspensions of strontium titanate with transition metal additives. Int. J. Sol. Energy 1982, 1, 213–222. [Google Scholar] [CrossRef]
- Stock, M.; Dunn, S. LiNbO3—A new material for artificial photosynthesis. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2011, 58. [Google Scholar] [CrossRef] [PubMed]
- Harhira, A.; Guilbert, L.; Bourson, P.; Rinnert, H. Decay time of polaron photoluminescence in congruent lithium niobate. Phys. Status Solidi 2007, 4, 926–929. [Google Scholar] [CrossRef]
- Li, D.; Zhao, M.H.; Garra, J.; Kolpak, A.; Rappe, A.; Bonnell, D.; Vohs, J. Direct in situ determination of the polarization dependence of physisorption on ferroelectric surfaces. Nat. Mater. 2008, 7, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Nath, R.K.; Zain, M.F.M.; Kadhum, A.A.H. New material LiNbO3 for photocatalytically improvement of indoor air—An overview. Adv. Nat. Appl. Sci. 2012, 6, 1030–1035. [Google Scholar]
- Nath, R.K.; Zain, M. Artificial photosynthesis in concrete surface by using LiNbO3. Adv. Environ. Biol. 2015, 9, 1–9. [Google Scholar]
- Nath, R.K.; Zain, M.F.M.; Jamil, M. An environment-friendly solution for indoor air purification by using renewable photocatalysts in concrete: A review. Renew. Sustain. Energy Rev. 2016, 62, 1184–1194. [Google Scholar] [CrossRef]
- Awazu, K.; Fujimaki, M.; Rockstuhl, C.; Tominaga, J.; Murakami, H.; Ohki, Y.; Yoshida, N.; Watanabe, T. A plasmonic photocatalyst consisting of silver nanoparticles embedded in titanium dioxide. J. Am. Chem. Soc. 2008, 130, 1676–1680. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, J.; Chen, W. Plasmonic Ag/AgBr nanohybrid: Synergistic effect of SPR with photographic sensitivity for enhanced photocatalytic activity and stability. Dalton Trans. 2012, 41, 4866–4870. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Li, H.; Zhang, L. New insight into daylight photocatalysis of AgBr@Ag: Synergistic effect between semiconductor photocatalysis and plasmonic photocatalysis. Chem. A Eur. J. 2012, 18, 6360–6369. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Liu, S.; Xu, Y.-J. Recent progress on metal core@semiconductor shell nanocomposites as a promising type of photocatalyst. Nanoscale 2012, 4, 2227–2238. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-J.; Wu, J.C.; Wu, P.C.; Tsai, D.P. Plasmonic photocatalyst for H2 evolution in photocatalytic water splitting. J. Phys. Chem. C 2010, 115, 210–216. [Google Scholar] [CrossRef]
- An, C.; Wang, R.; Wang, S.; Zhang, X. Converting AgCl nanocubes to sunlight-driven plasmonic AgCl:Ag nanophotocatalyst with high activity and durability. J. Mater. Chem. 2011, 21, 11532–11536. [Google Scholar] [CrossRef]
- Link, S.; El-Sayed, M.A. Spectral Properties and Relaxation Dynamics of Surface Plasmon Electronic Oscillations in Gold and Silver Nanodots and Nanorods. J. Phys. Chem. B 1999, 103, 8410–8426. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Pal, T. Interparticle coupling effect on the surface plasmon resonance of gold nanoparticles: From theory to applications. Chem. Rev. 2007, 107, 4797–4862. [Google Scholar] [CrossRef] [PubMed]
- Atay, T.; Song, J.-H.; Nurmikko, A.V. Strongly interacting plasmon nanoparticle pairs: From dipole− dipole interaction to conductively coupled regime. Nano Lett. 2004, 4, 1627–1631. [Google Scholar] [CrossRef]
- Kim, K.-H.; Husakou, A.; Herrmann, J. Linear and nonlinear optical characteristics of composites containing metal nanoparticles with different sizes and shapes. Opt. Express 2010, 18, 7488–7496. [Google Scholar] [CrossRef] [PubMed]
- Kolwas, K.; Derkachova, A.; Shopa, M. Size characteristics of surface plasmons and their manifestation in scattering properties of metal particles. J. Quant. Spectrosc. Radiat. Transf. 2009, 110, 1490–1501. [Google Scholar] [CrossRef]
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The Optical Properties of Metal Nanoparticles: The Influence of Size, Shape, and Dielectric Environment. J. Phys. Chem. B. 2003, 107, 668–677. [Google Scholar] [CrossRef]
- Lombardi, J.R.; Birke, R.L. A unified approach to surface-enhanced raman spectroscopy. J. Phys. Chem. C 2008, 112, 5605–5617. [Google Scholar] [CrossRef]
- Thomann, I.; Pinaud, B.A.; Chen, Z.; Clemens, B.M.; Jaramillo, T.F.; Brongersma, M.L. Plasmon enhanced solar-to-fuel energy conversion. Nano Lett. 2011, 11, 3440–3446. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.-C.; Zhang, Y.; Tao, T.-X.; Zhang, L.; Fong, H. Silver nanoparticles on amidoxime fibers for photo-catalytic degradation of organic dyes in waste water. Appl. Surf. Sci. 2010, 257, 1092–1097. [Google Scholar] [CrossRef]
- Sun, S.; Wang, W.; Zhang, L.; Shang, M.; Wang, L. Ag@C core/shell nanocomposite as a highly efficient plasmonic photocatalyst. Catal. Commun. 2009, 11, 290–293. [Google Scholar] [CrossRef]
- Christopher, P.; Xin, H.; Linic, S. Visible-light-enhanced catalytic oxidation reactions on plasmonic silver nanostructures. Nat. Chem. 2011, 3, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, J.-M. Heterogeneous photocatalysis: State of the art and present applications in honor of Pr. R.L. burwell Jr.(1912–2003), former head of ipatieff laboratories, northwestern university, evanston(Ill). Top. Catal. 2005, 34, 49–65. [Google Scholar] [CrossRef]
- Herrmann, J.-M. Heterogeneous photocatalysis: Fundamentals and applications to the removal of various types of aqueous pollutants. Catal. Today 1999, 53, 115–129. [Google Scholar] [CrossRef]
- Ingram, D.B.; Linic, S. Water splitting on composite plasmonic-metal/semiconductor photoelectrodes: Evidence for selective plasmon-induced formation of charge carriers near the semiconductor surface. J. Am. Chem. Soc. 2011, 133, 5202–5205. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Tatsuma, T. Mechanisms and applications of plasmon-induced charge separation at TiO2 films loaded with gold nanoparticles. J. Am. Chem. Soc. 2005, 127, 7632–7637. [Google Scholar] [CrossRef] [PubMed]
- Takai, A.; Kamat, P.V. Capture, store, and discharge. Shuttling photogenerated electrons across TiO2–silver interface. ACS Nano 2011, 5, 7369–7376. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Dai, G.; Huang, B. Fabrication and characterization of visible-light-driven plasmonic photocatalyst Ag/AgCl/TiO2 nanotube arrays. J. Phys. Chem. C 2009, 113, 16394–16401. [Google Scholar] [CrossRef]
- Adleman, J.R.; Boyd, D.A.; Goodwin, D.G.; Psaltis, D. Heterogenous catalysis mediated by plasmon heating. Nano Lett. 2009, 9, 4417–4423. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhu, H.Y.; Zhao, J.C.; Zheng, Z.F.; Gao, X.P. Visible-light-driven oxidation of organic contaminants in air with gold nanoparticle catalysts on oxide supports. Angew. Chem. 2008, 120, 5433–5436. [Google Scholar] [CrossRef]
- Wang, P.; Huang, B.; Lou, Z.; Zhang, X.; Qin, X.; Dai, Y.; Zheng, Z.; Wang, X. Synthesis of highly efficient Ag@AgCl plasmonic photocatalysts with various structures. Chem. A Eur. J. 2010, 16, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ding, Y. Porous AgCl/Ag nanocomposites with enhanced visible light photocatalytic properties. J. Phys. Chem. C 2010, 114, 3175–3179. [Google Scholar] [CrossRef]
- Jin, R.; Cao, Y.; Mirkin, C.A.; Kelly, K.; Schatz, G.C.; Zheng, J. Photoinduced conversion of silver nanospheres to nanoprisms. Science 2001, 294, 1901–1903. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Hung, W.H.; Pavaskar, P.; Goeppert, A.; Aykol, M.; Cronin, S.B. Photocatalytic conversion of CO2 to hydrocarbon fuels via plasmon-enhanced absorption and metallic interband transitions. ACS Catal. 2011, 1, 929–936. [Google Scholar] [CrossRef]
- Hemminger, J.; Carr, R.; Somorjai, G. The photoassisted reaction of gaseous water and carbon dioxide adsorbed on the SrTiO3 (111) crystal face to form methane. Chem. Phys. Lett. 1978, 57, 100–104. [Google Scholar] [CrossRef]
- Grätzel, M. Photoelectrochemical cells. Nature 2001, 414, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Liu, Z.; Pavaskar, P.; Hung, W.H.; Cronin, S.B. Plasmonic enhancement of photocatalytic decomposition of methyl orange under visible light. J. Catal. 2011, 277, 149–153. [Google Scholar] [CrossRef]
- Liu, Z.; Hou, W.; Pavaskar, P.; Aykol, M.; Cronin, S.B. Plasmon resonant enhancement of photocatalytic water splitting under visible illumination. Nano Lett. 2011, 11, 1111–1116. [Google Scholar] [CrossRef] [PubMed]
- Cazalilla, M.; Dolado, J.; Rubio, A.; Echenique, P. Plasmonic excitations in noble metals: The case of Ag. Phys. Rev. B 2000, 61, 8033. [Google Scholar] [CrossRef]
- Cushing, S.K.; Li, J.; Meng, F.; Senty, T.R.; Suri, S.; Zhi, M.; Li, M.; Bristow, A.D.; Wu, N. Photocatalytic activity enhanced by plasmonic resonant energy transfer from metal to semiconductor. J. Am. Chem. Soc. 2012, 134, 15033–15041. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cushing, S.K.; Bright, J.; Meng, F.; Senty, T.R.; Zheng, P.; Bristow, A.D.; Wu, N. Ag@Cu2O core-shell nanoparticles as visible-light plasmonic photocatalysts. ACS Catal. 2012, 3, 47–51. [Google Scholar] [CrossRef]


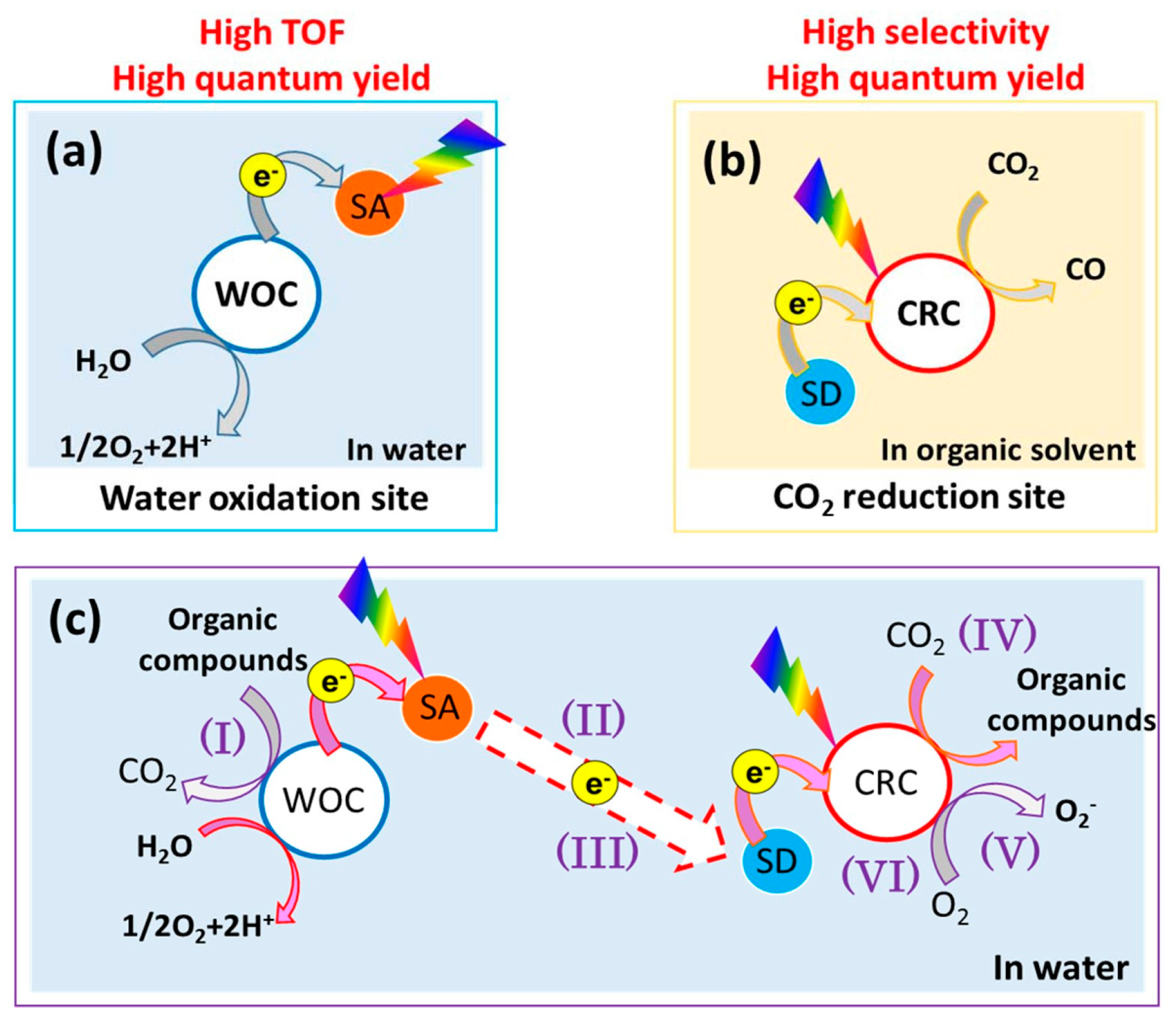



| Photocatalyst | Radiation Source | Major Products | Comments | References |
|---|---|---|---|---|
| 0.5 wt % Cu/TiO2-SiO2 | Xe lamp (2.4 mW cm−2, 250–400 nm) | CO and CH4 | The synergistic combination of Cu deposition and high surface area of SiO2 support enhanced CO2 photoreduction rates. | [36] |
| ZnGa2O4 | 300 W Xe arc lamp | CH4 | Strong gas adsorption and large specific surface area of the mesoporous ZnGa2O4 photocatalyst contribute to its high photocatalytic activity for converting CO2 into CH4. | [37] |
| (RuO + Pt)-Zn2GeO4 | 300 W Xe arc lamp | CH4 | In the presence of water, ultra-long and ultrathin geometry of the Zn2GeO4 nano-ribbon promotes CO2 photo-reduction, which was significantly enhanced by loading of Pt or RuO2. | [38] |
| Ag/ALa4Ti4O15 (A = Ca, Ba and Sr) | 400 W Hg lamp | CO, HCOOH, and H2 | On the optimized Ag/BaLa4Ti4O15 photocatalyst, CO was the reported as the main product. The molar ratio of O2 production (H2 + CO:O2 = 2:1) demonstrated that water was consumed as a reducing reagent in the photocatalytic process. | [39] |
| I-TiO2 nanoparticles | 450 W Xe lamp | CO | High photocatalytic activity was observed under visible light and the efficiency of CO2 photoreaction was much greater than undoped TiO2 due to the extension in the absorption spectra of TiO2 to the visible light region and facilitated charge separation. | [40] |
| LiNbO3 | Natural sunlight or Hg lamp (64.2 mW cm−2) | HCOOH | The MgO-doped LiNbO3 showed an energy conversion efficiency rate of 0.72% which was lower than that for the gas–solid catalytic reaction of LiNbO3 (2.2%). | [41] |
| G-Ti0.91O2 hollow spheres | 300 W Xe arc lamp | CH4, CO | The presence of G nanosheets compactly stacking with Ti0.91O2 nanosheets allows the rapid migration of photo-generated electrons from Ti0.91O2 nanosheets into G and improves the efficiency of the photocatalytic process. | [42] |
| Graphene oxides (GOs) | 300 W commercial halogen lamp | CH3OH | Among all GOs, GO-3 exhibited the highest efficiency as a photocatalyst for CO2 reduction under visible light, and the conversion rate of CO2 to CH3OH on modified GO (GO-3) was 0.172 mmol g−1 cat h−1, which is six-fold higher than that of pure TiO2. | [43] |
| W18O49 | 300 W Xe lamp | CH4 | The oxygen-vacancy-rich ultrathin W18O49 nanowires can be used to design materials with extraordinary photochemical activity because it displayed high CO2 reduction capability in presence of water. | [44] |
| Zn1.7GeN1.8O | 300 W Xe arc lamp | CH4 | Zn1.7GeN1.8O loaded with co-catalysts showed significantly higher conversion rate of CO2 into CH4. | [45] |
| Pt-, Au-, or Ag-loaded mesoporous TiO2 | 350 W Xe lamp | CH4 | The mesoporous TiO2 showed higher efficiency towards CO2 reduction when loaded with noble metal particles, and the order of enhanced photocatalytic activity was Pt > Au > Ag. The optimum loading amount of Pt was 0.2 wt %. | [16] |
| 0.5 wt % Pt loaded ZnAl2O4-modified mesoporous ZnGaNO | 300 W Xe lamp (λ = 420 nm) | CH4 | The high photocatalytic activity of this photocatalyst was attributed to the improved gas adsorption of the mesoporous structure, the chemisorption of CO2 on the photocatalyst and the narrow bandgap of ZnAl2O4-modified ZnGaNO to extend the light absorption. | [46] |
| Ga2O3 with mesopores and macropores | 300 W Xe lamp (500 mW cm−2) | CH4 | Ga2O3 with mesopores and macropores showed high photocatalytic activity due to its higher CO2 adsorption capacity (300%) and increased surface area (200%) compared to the bulk nanoparticles. | [47] |
| Pt-TiO2 thin nanostructured films | 400 W Xe lamp | CO and CH4 | The catalyst can be produced at an industrial scale for commercial application and showed high efficiency for selective CH4 formation. | [48] |
| HNb3O8 | 350 W Xe lamp | CH4 | KNb3O8 and HNb3O8 were synthesized by the conventional solid-state reaction and performed more effectively in photocatalytic CO2 reduction than commercial TiO2. | [49] |
| ZnO-based materials | 8 W fluorescent tube (average intensity 7 mW cm−2) | CO, CH4, CH3OH, H2 | N-doping did not show any important influence on the photocatalytic behavior of ZnO-based photocatalysts. The mesoporous structure of ZnO favored CO and H2 production, but catalysts with Cu showed an enhancement in the hydrocarbon production, mainly CH3OH. | [50] |
| Ag, Pt, bimetallic Ag–Pt and core–shell Ag@silica (SiO2) nanoparticles with TiO2 | 100 W Hg lamp (330 nm) | CH4 | The use of a reactor with three optical windows, a combination of both bimetallic co-catalysts, and Ag@SiO2 nanoparticles increased the product formation significantly compared to bare TiO2. | [51] |
| Carbon nanotubes Ni/TiO2 Nano-composites | 75 W visible daylight lamp (λ > 400 nm) | CH4 | Compared to Ni/TiO2 and pure anatase TiO2, Ni/TiO2 incorporated with carbon nanotubes demonstrated maximum CH4 product yield of 0.145 mmol h−1 g−1 catalysts after 4.5 h of irradiation under visible light. | [52] |
| Pt/Cu/TiO2 | 200 W Xe lamp | CH4, CO, H2 | The addition of co-catalyst Pt decreases the selectivity for CO2 photo-reduction; however, loading Cu onto TiO2 increases the selectivity from 60 to 80%. | [53] |
| Au/Pt/TiO2 | 500 W Xe lamp | CH4, CO | Plasmonic photocatalyst Au/Pt/TiO2 provided a more effective way to harvest solar energy by consuming a high-energy photon in the solar spectrum (UV region) and using it for charge carrier generation. Moreover, it also utilized visible light to enhance the photocatalytic activity. | [54] |
| 20 wt % montmorillonite modified TiO2 | 500 W Hg lamp (365 nm) | CH4 | Loading of montmorillonite on TiO2 enhanced the surface area and reduced particle size, thus improving charge separation, resulting in maximum yield for CH4 (441.5 mmol·g·cat−1 h−1). | [55] |
| 0.5 wt % Pt/NaNbO3 | 300 W Xe lamp (λ > 300 nm) | CH4, CO, H2 | The cubic-orthorhombic surface-junctions of mixed-phase NaNbO3 enhanced the charge separation, thereby improving its photoactivity. | [56] |
| Ag supported on AgIO3 (Ag/AgIO3 particles) | 500 W Xe arc lamp | CH4 and CO | In the conversion of CO2 to CH4 and CO using water vapor, Ag/AgIO3 particles showed high and stable activity because of the surface plasmon resonance effect of Ag particles. | [57] |
| g-C3N4/NaNbO3 nanowires | 300 W Xe arc lamp | CH4 | An intimate interface formation was suggested between the C3N4 and NaNbO3 nanowires in g-C3N4/NaNbO3 heterojunction photocatalyst, resulting in almost eight-fold higher CO2 reduction than individual C3N4 under visible light irradiation. | [58] |
| In2O3/g-C3N4 | 500 W Xe lamp | CH4 | The addition of In2O3 nanocrystals onto g-C3N4 surface improved the photocatalytic CO2 reduction process significantly due to the interfacial transfer of photo-generated electrons and holes between g-C3N4 and In2O3. | [59] |
| SnO2−x/g-C3N4 composite | 500 W Xe lamp | CO, CH3OH, and CH4 | Enhancement in the surface area of g-C3N4 was observed by introducing SnO2−x. Improve photocatalytic performance was attributed to the increased light absorption and accelerated the separation of electron–hole pairs. | [60] |
| AgX/g-C3N4 (X = Cl and Br) nanocomposites | 15 W energy-saving daylight bulb. | CH4 | Under ambient condition and low-power energy-saving lamps, the optimal 30 AgBr/pCN (protonated graphitic carbon nitride photocatalyst) sample showed highest photocatalytic activity with significant enhancement in CH4 formation compared to individual AgBr and pCN photocatalyst. | [61] |
| Ag supported on Ag2SO3 (Ag/Ag2SO3) | 500 W Xe lamp | CH4 and CO | Plasmonic photocatalyst Ag/Ag2SO3 was stable towards CO2 photoreduction after 10 repetitive catalytic cycles with high efficiency under visible light irradiation. | [62] |
| Reactions | E0/eV |
|---|---|
| CO2 + e− → CO2 | ≥−1.9 |
| CO2 + 2e− + 2H+ → HCOOH | −0.61 |
| CO2 + 2e−+ 2H+ → CO + H2O | −0.53 |
| CO2 + 4e− + 4H+ → HCHO + H2O | −0.48 |
| CO2 + 6e− + 6H+ → CH3OH + H2O | −0.38 |
| CO2 + 8e− + 8H+→ CH4 + 2H2O | −0.24 |
| Reaction Mode | Photocatalyst | Formation Rate (μmol·g−1h−1) | R (Electron) (μmol·g−1h−1) | Selectivity for CO2 Reduction (%) | ||
|---|---|---|---|---|---|---|
| CO | CH4 | H2 | ||||
| Solid–gas | TiO2 | 1.2 | 0.38 | 2.1 | 10 | 56 |
| solid–liquid | TiO2 | 0.80 | 0.11 | 5.3 | 13 | 19 |
| solid–gas | Pt-TiO2 | 1.1 | 5.2 | 33 | 110 | 40 |
| solid–liquid | Pt-TiO2 | 0.76 | 1.4 | 55 | 123 | 11 |
| Photo-Catalyst | Band Gap/eV | Co-Catalyst (wt %) | Loading Method | Activity/μmol·h−1 | |||
|---|---|---|---|---|---|---|---|
| H2 | O2 | CO | HCOOH | ||||
| BaLa4Ti4O15 | 3.9 | none | - | 5.3 | 2.4 | 0 | 0 |
| BaLa4Ti4O15 | 3.9 | NiOx b (0.5) | impregnation | 58 | 29 | 0.02 | 0 |
| BaLa4Ti4O15 | 3.9 | Ru (0.5) | photodeposition | 84 | 41 | 0 | 0 |
| BaLa4Ti4O15 | 3.9 | Cu (0.5) | photodeposition | 96 | 45 | 0.6 | 0 |
| BaLa4Ti4O15 | 3.9 | Au (0.5) | photodeposition | 110 | 51 | 0 | 0 |
| BaLa4Ti4O15 | 3.9 | Ag (1.0) | photodeposition | 10 c | 7.0 c | 4.3 c | 0.3 c |
| CaLa4Ti4O15 | 3.9 | none | - | 1.3 | 0.6 | 0.07 | 0 |
| CaLa4Ti4O15 | 3.9 | Ag (1.0) | photodeposition | 5.6 | 2.1 | 2.3 | 1.3 |
| SrLa4Ti4O15 | 3.8 | none | - | 0.8 | 0.5 | 0.06 | 0 |
| SrLa4Ti4O15 | 3.8 | Ag (1.0) | photodeposition | 2.7 | 1.8 | 1.8 | 0.5 |
| Photocatalyst | Loading Amount/wt % | Loading Method | Activity/μmol·h−1 | |||
|---|---|---|---|---|---|---|
| H2 | O2 | CO | HCOOH | |||
| BaLa4Ti4O15 | 1.0 | Impregnation b | 8.2 | 5.7 | 5.2 | 0.2 |
| BaLa4Ti4O15 | 1.0 | Impregnation b + H2 red c | 5.6 | 8.7 | 8.9 | 0.3 |
| BaLa4Ti4O15 | 0.5 | Liquid-phase reduction | 4.5 | 6.8 | 11 | 0.03 |
| BaLa4Ti4O15 | 1.0 | Liquid-phase reduction | 5.6 | 12 | 19 | 0.4 |
| BaLa4Ti4O15 | 2.0 | Liquid-phase reduction | 10 | 16 | 22 | 0.7 |
| BaLa4Ti4O15 | 3.0 | Liquid-phase reduction | 9.7 | 14 | 19 | 0.1 |
| BaLa4Ti4O15 | 5.0 | Liquid-phase reduction | 4.8 | 6.6 | 12 | 0.02 |
| BaLa4Ti4O15 | 1.0 | Liquid-phase reduction | 20 d | 11 d | 0 d | 0 d |
| SrLa4Ti4O15 | 1.0 | Liquid-phase reduction | 4.8 | 5.8 | 7.1 | 0.8 |
| CaLa4Ti4O15 | 1.0 | Liquid-phase reduction | 3.2 | 6.6 | 9.3 | 0.4 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nahar, S.; Zain, M.F.M.; Kadhum, A.A.H.; Hasan, H.A.; Hasan, M.R. Advances in Photocatalytic CO2 Reduction with Water: A Review. Materials 2017, 10, 629. https://doi.org/10.3390/ma10060629
Nahar S, Zain MFM, Kadhum AAH, Hasan HA, Hasan MR. Advances in Photocatalytic CO2 Reduction with Water: A Review. Materials. 2017; 10(6):629. https://doi.org/10.3390/ma10060629
Chicago/Turabian StyleNahar, Samsun, M. F. M. Zain, Abdul Amir H. Kadhum, Hassimi Abu Hasan, and Md. Riad Hasan. 2017. "Advances in Photocatalytic CO2 Reduction with Water: A Review" Materials 10, no. 6: 629. https://doi.org/10.3390/ma10060629
APA StyleNahar, S., Zain, M. F. M., Kadhum, A. A. H., Hasan, H. A., & Hasan, M. R. (2017). Advances in Photocatalytic CO2 Reduction with Water: A Review. Materials, 10(6), 629. https://doi.org/10.3390/ma10060629







