Cellulose Fibre-Reinforced Biofoam for Structural Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Bio-Based Foam Preparation
2.3. Porosity Determination Using Argon Pycnometer
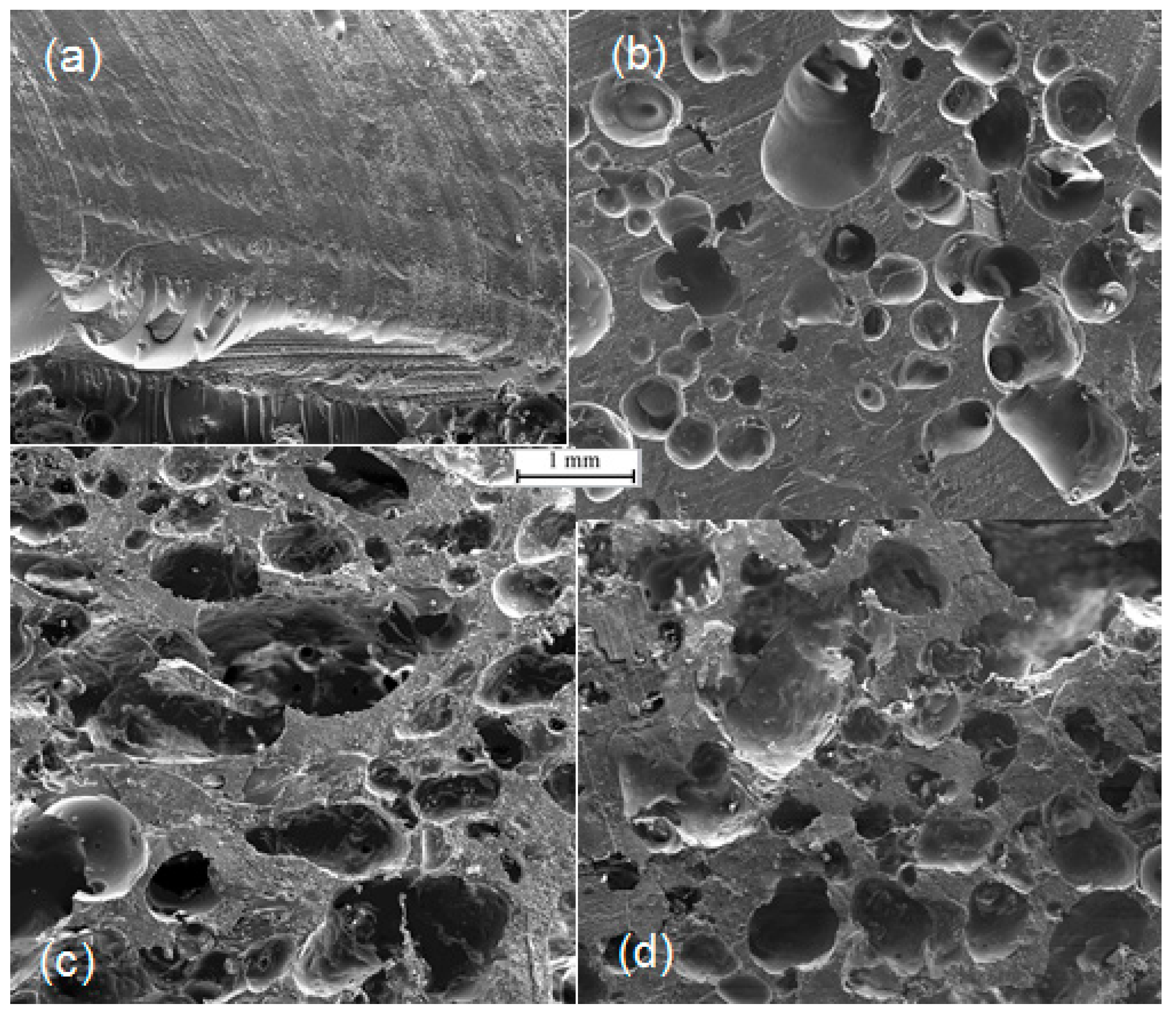
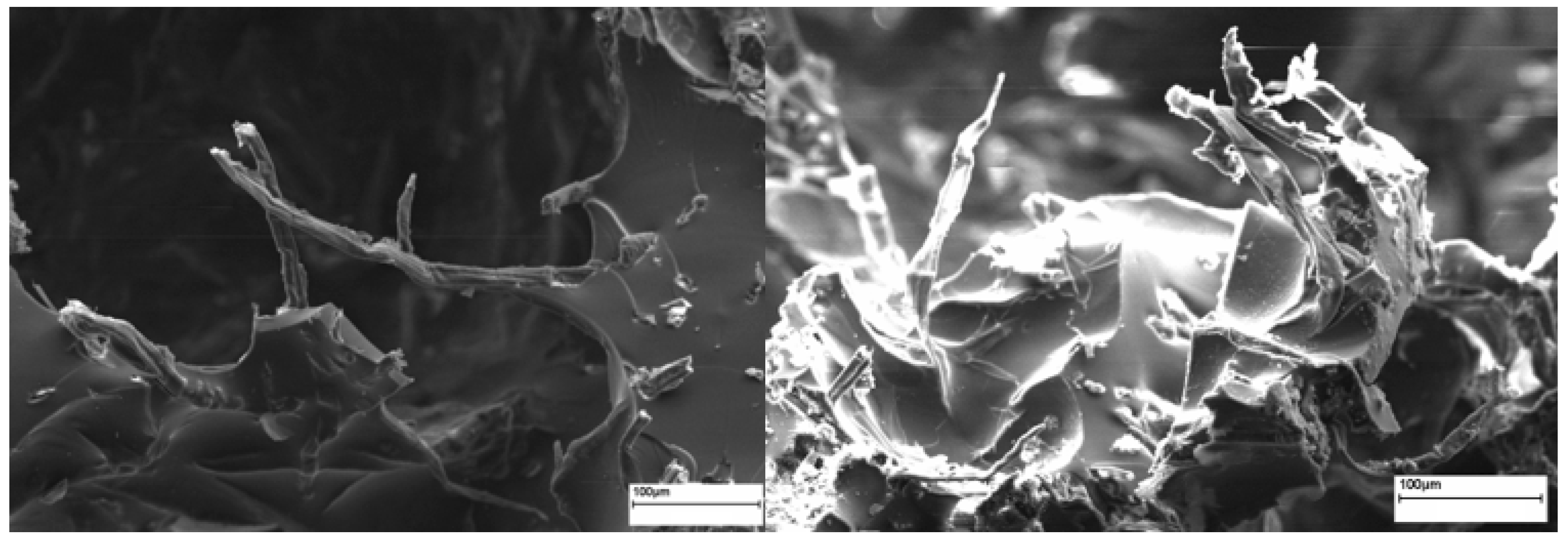
2.4. Field Emission Scanning Electron Microscope (FE-SEM)
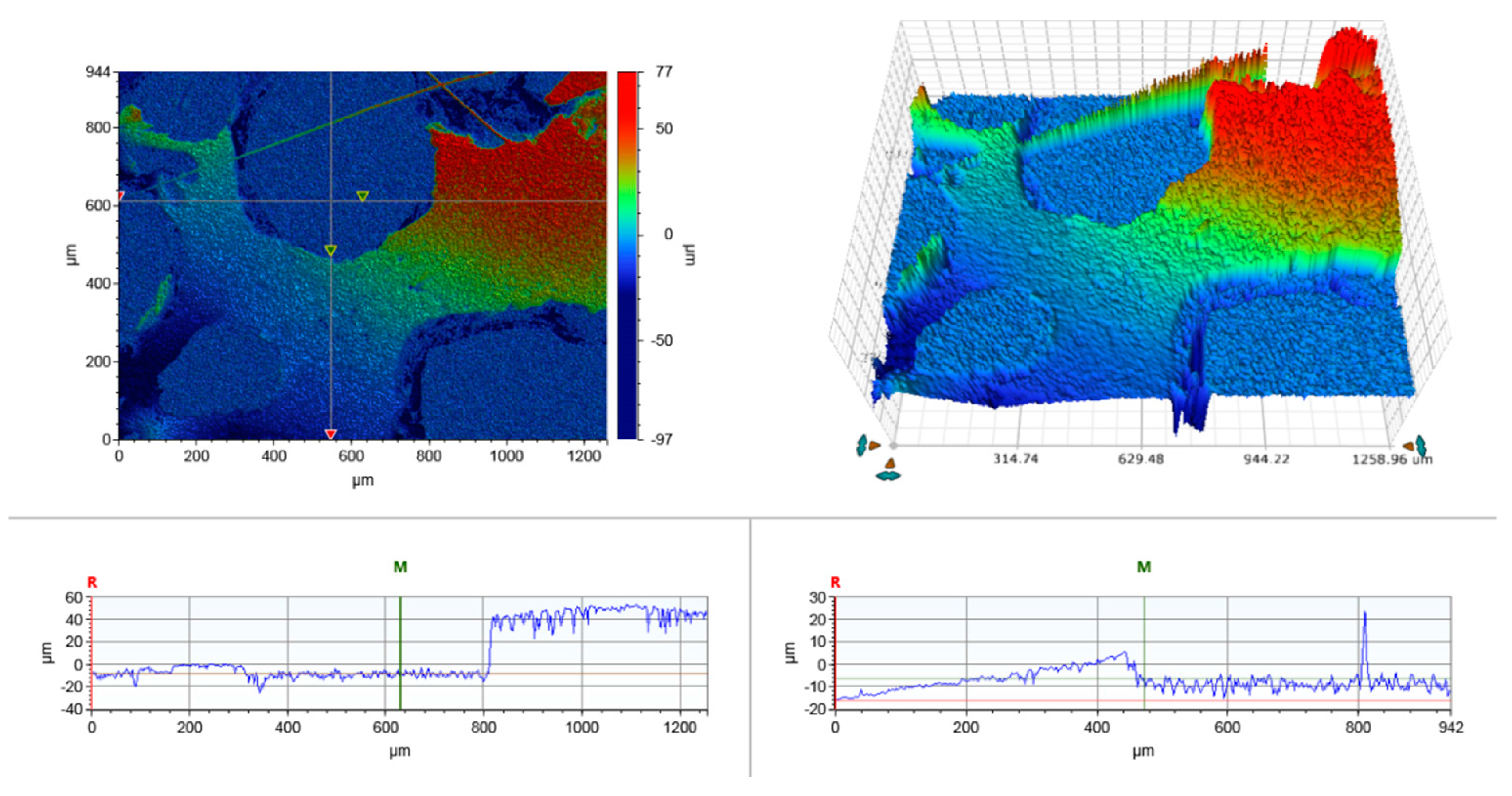
2.5. 3D Optical Microscope
2.6. Compression Test
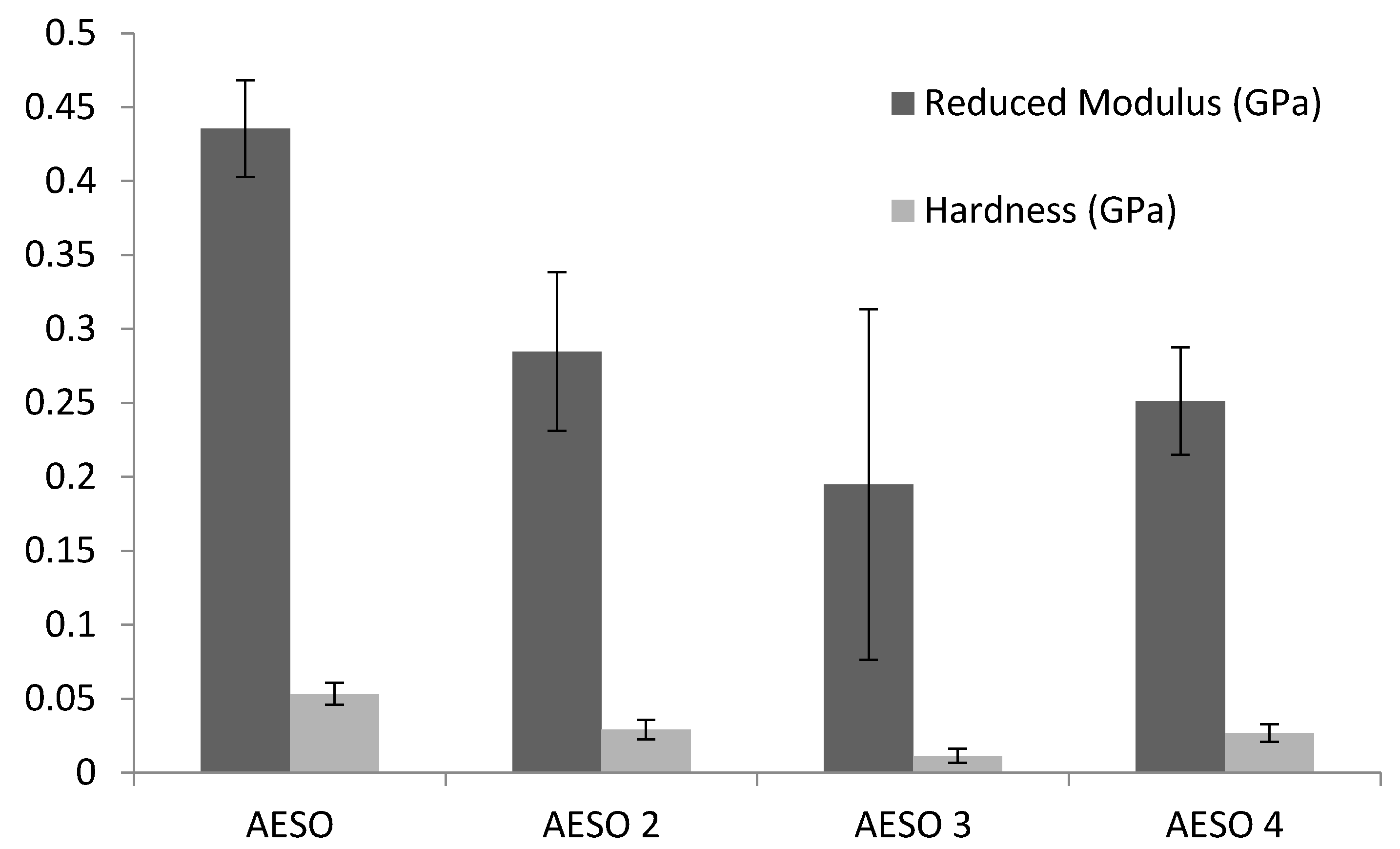
2.7. Nanoindentation Analysis
2.8. Thermal Analysis
3. Results and Discussion
3.1. Structure and Porosity of Bio-Based Foams
3.2. Mechanical Properties of Bio-Based Foams
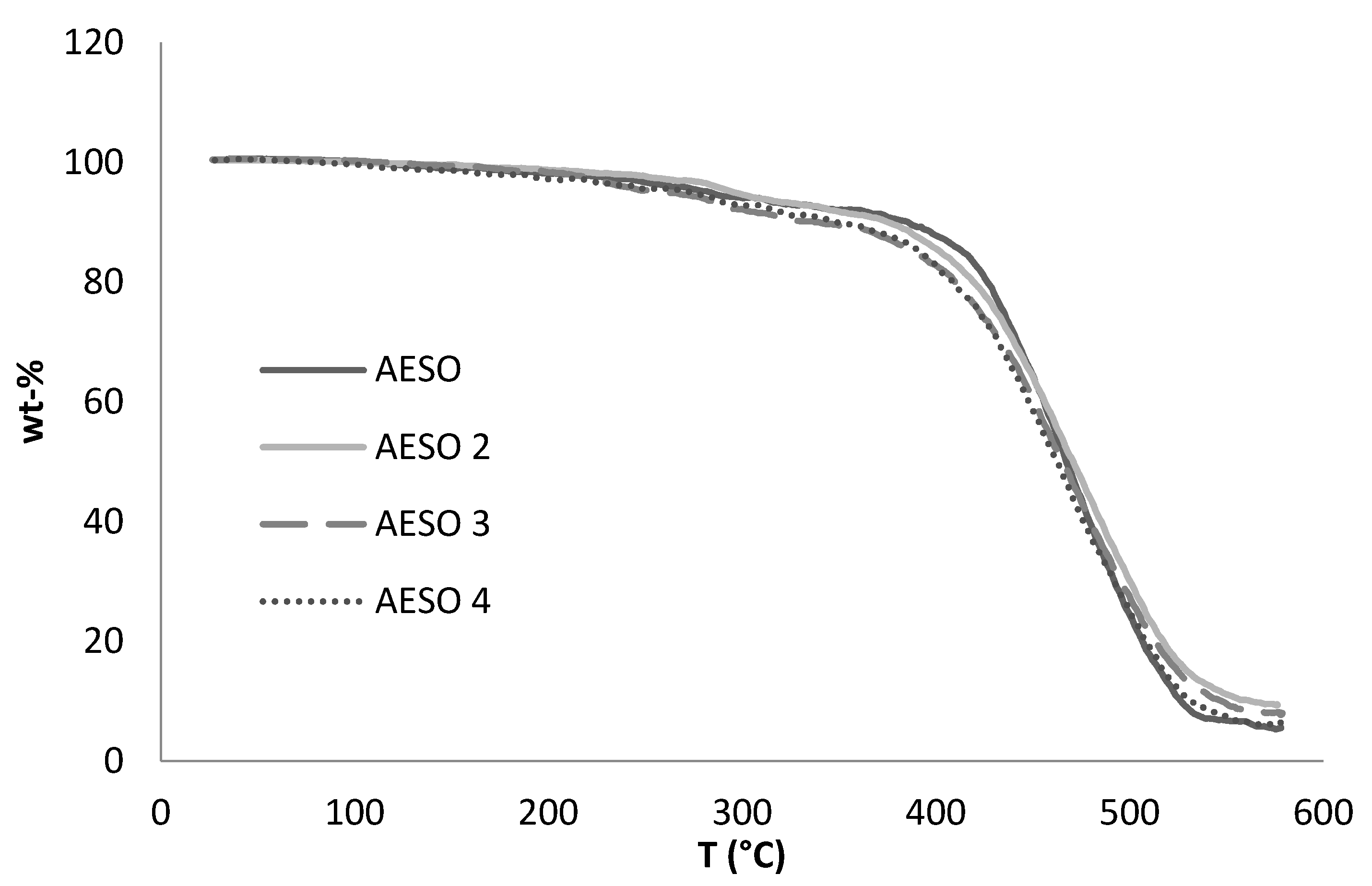
3.3. Thermal Behaviour of Bio-Based Foams
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lee, K.-Y.; Wong, L.L.C.; Blaker, J.J.; Hodgkinson, J.M.; Bismarck, A. Bio-based macroporous polymer nanocomposites made by mechanical frothing of acrylated epoxidised soybean oil. Green Chem. 2011, 13, 3117–3123. [Google Scholar] [CrossRef]
- Brencis, R.; Skujans, J.; Iljins, U.; Ziemelis, I.; Osits, N. Research on foam gypsum with hemp fibrous reinforcement. Chem. Eng. Trans. 2011, 25, 159–164. [Google Scholar]
- Siriruk, A.; Weitsman, Y.J.; Penumadu, D. Polymeric foams and sandwich composites: Material properties, environmental effects, and shear-lag modeling. Compos. Sci. Technol. 2009, 69, 814–820. [Google Scholar] [CrossRef]
- Ganjyal, G.M.; Reddy, N.; Yang, Y.Q.; Hanna, M.A. Biodegradable packaging foams of starch acetate blended with corn stalk fibers. J. Appl. Polym. Sci. 2004, 93, 2627–2633. [Google Scholar] [CrossRef]
- Lau, T.H.M.; Wong, L.L.C.; Lee, K.-Y.; Bismarck, A. Tailored for simplicity: Creating high porosity, high performance bio-based macroporous polymers from foam templates. Green Chem. 2014, 3, 1931–1940. [Google Scholar] [CrossRef]
- Schmidt, V.C.R.; Laurindo, J.B. Characterization of foams obtained from cassava starch, cellulose fibres and dolomitic limestone by a thermopressing process. Braz. Arch. Biol. Technol. 2010, 53, 185–192. [Google Scholar] [CrossRef]
- Merle, J.; Birot, M.; Deleuze, H.; Mitterer, C.; Carré, H.; Bouhtoury, F.C.E. New biobased foams from wood byproducts. Mater. Des. 2016, 91, 186–192. [Google Scholar] [CrossRef]
- Mosiewicki, M.A.; Dell’Arciprete, G.A.; Aranguren, M.I.; Marcovich, N.E. Polyurethane foams obtained from castor oil-based polyol and filled with wood flour. J. Compos. Mater. 2009, 43, 3057–3072. [Google Scholar] [CrossRef]
- Omrani, I.; Farhadian, A.; Babanejad, N.; Shendi, H.K.; Ahmadi, A.; Nabid, M.R. Synthesis of novel high primary hydroxyl functionality polyol from sunflower oil using thiol-yne reaction and their application in polyurethane coating. Eur. Polym. J. 2016, 82, 220–231. [Google Scholar] [CrossRef]
- Tan, S.; Abraham, T.; Ference, D.; Macosko, C.W. Rigid polyurethane foams from a soybean oil-based polyol. Polymer 2011, 52, 2840–2846. [Google Scholar] [CrossRef]
- Kurańska, M.; Prociak, A. The influence of rapeseed oil-based polyols on the foaming process of rigid polyurethane foams. Ind. Crops Prod. 2016, 89, 182–187. [Google Scholar] [CrossRef]
- Petrovic, Z. Polyurethanes from vegetable oils. Polym. Rev. 2008, 48, 109–155. [Google Scholar] [CrossRef]
- Altuna, F.I.; Ruseckaite, R.A.; Stefani, P.M. Biobased thermosetting epoxy foams: Mechanical and thermal characterization. ACS Sustain. Chem. Eng. 2015, 3, 1406–1411. [Google Scholar] [CrossRef]
- Bonnaillie, L.M.; Wool, R.P. Thermosetting foam with a high bio-based content from acrylated epoxidized soybean oil and carbon dioxide. J. Appl. Polym. Sci. 2007, 105, 1042–1052. [Google Scholar] [CrossRef]
- Wool, R.P.; Khot, S.N. Bio-Based resins and natural fibers. Mater. Park. ASM Int. 2001, 10, 184–193. [Google Scholar]
- Wu, S.P.; Qiu, J.F.; Rong, M.Z.; Zhang, M.Q.; Zhang, L.Y. Plant oil-based biofoam composites with balanced performance. Polym. Int. 2009, 58, 403–411. [Google Scholar] [CrossRef]
- Voutilainen, M.; Siitari-Kauppi, M.; Sardini, P.; Lindberg, A.; Timonen, J. Pore-space characterization of an altered tonalite by X-ray computed microtomography and the 14c-labeled-polymethylmethacrylate method. J. Geophys. Res. Solid Earth 2012, 117, 2156–2202. [Google Scholar] [CrossRef]
- Bonnaillie, L.M. Bio-Based Polymeric Foam from Soybean Oil and Carbon Dioxide. Ph.D. Thesis, Department of Chemical Engineering, University of Delaware, Newark, DE, USA, January 2007. [Google Scholar]
- Monnereau, C.; Vignes-Adler, M.; Kronberg, B. Influence of gravity on foams. J. Chim. Phys. 1999, 96, 958–967. [Google Scholar] [CrossRef]
- Bergeret, A.; Benezet, J.C. Natural fibre-reinforced biofoams. Int. J. Polym. Sci. 2011, 2011, 569871. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, R. Bio-Based Branched and Hyperbranched Polymers and Oligomers. U.S. Patent 20,130,345,383 A1, 26 December 2013. [Google Scholar]
- Michler, G.H.; von Schmeling, H.-H.K.-B. The physics and micro-mechanics of nano-voids and nano-particles in polymer combinations. Polymer 2013, 54, 3131–3144. [Google Scholar] [CrossRef]
- Gupta, T.K.; Singh, B.P.; Tripathi, R.K.; Dhakate, S.R.; Singh, V.N.; Panwar, O.S.; Mathur, R.B. Superior nano-mechanical properties of reduced graphene oxide reinforced polyurethane composites. RSC Adv. 2015, 5, 16921–16930. [Google Scholar] [CrossRef]
- Behera, D.; Banthia, A.K. Synthesis, characterization, and kinetics study of thermal decomposition of epoxidized soybean oil acrylate. J. Appl. Polym. Sci. 2008, 109, 2583–2590. [Google Scholar] [CrossRef]
| Samples | Porosity ɛ (%) | Grain Volume VG, (cm3) | Bulk Volume VB, (cm3) | Grain Density (g/cm3) | Bulk Density (g/cm3) |
|---|---|---|---|---|---|
| AESO | 4.5 ± 4.4 | 1.58 ± 0.05 | 1.65 ± 0.06 | 1.55 ± 0.04 | 1.48 ± 0.04 |
| AESO 2 | 57.0 ± 1.8 | 1.92 ± 0.05 | 4.45 ± 0.15 | 1.28 ± 0.02 | 0.538 ± 0.004 |
| AESO 3 | 58.3 ± 1.5 | 2.16 ± 0.05 | 5.17 ± 0.15 | 1.134 ± 0.013 | 0.474 ± 0.003 |
| AESO 4 | 54.2 ± 1.8 | 2.08 ± 0.05 | 4.55 ± 0.15 | 1.177 ± 0.014 | 0.538 ± 0.004 |
| Samples | Maximum Load (N) | Yield Strength (MPa) | Compressive Strength (MPa) |
|---|---|---|---|
| AESO | 1498.0 ± 157.6 | 28.4 ± 3.3 | 29.9 ± 3.1 |
| AESO 2 | 351.2 ± 11.5 | 3.7 ± 0.2 | 4.7 ± 0.1 |
| AESO 3 | 558.5 ± 11.0 | 4.3 ± 0.2 | 5.5 ± 0.1 |
| AESO 4 | 589.9 ± 16.7 | 4.0 ± 0.2 | 5.9 ± 0.1 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obradovic, J.; Voutilainen, M.; Virtanen, P.; Lassila, L.; Fardim, P. Cellulose Fibre-Reinforced Biofoam for Structural Applications. Materials 2017, 10, 619. https://doi.org/10.3390/ma10060619
Obradovic J, Voutilainen M, Virtanen P, Lassila L, Fardim P. Cellulose Fibre-Reinforced Biofoam for Structural Applications. Materials. 2017; 10(6):619. https://doi.org/10.3390/ma10060619
Chicago/Turabian StyleObradovic, Jasmina, Mikko Voutilainen, Pasi Virtanen, Lippo Lassila, and Pedro Fardim. 2017. "Cellulose Fibre-Reinforced Biofoam for Structural Applications" Materials 10, no. 6: 619. https://doi.org/10.3390/ma10060619
APA StyleObradovic, J., Voutilainen, M., Virtanen, P., Lassila, L., & Fardim, P. (2017). Cellulose Fibre-Reinforced Biofoam for Structural Applications. Materials, 10(6), 619. https://doi.org/10.3390/ma10060619







