Fabrication Method Study of ZnO Nanocoated Cellulose Film and Its Piezoelectric Property
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
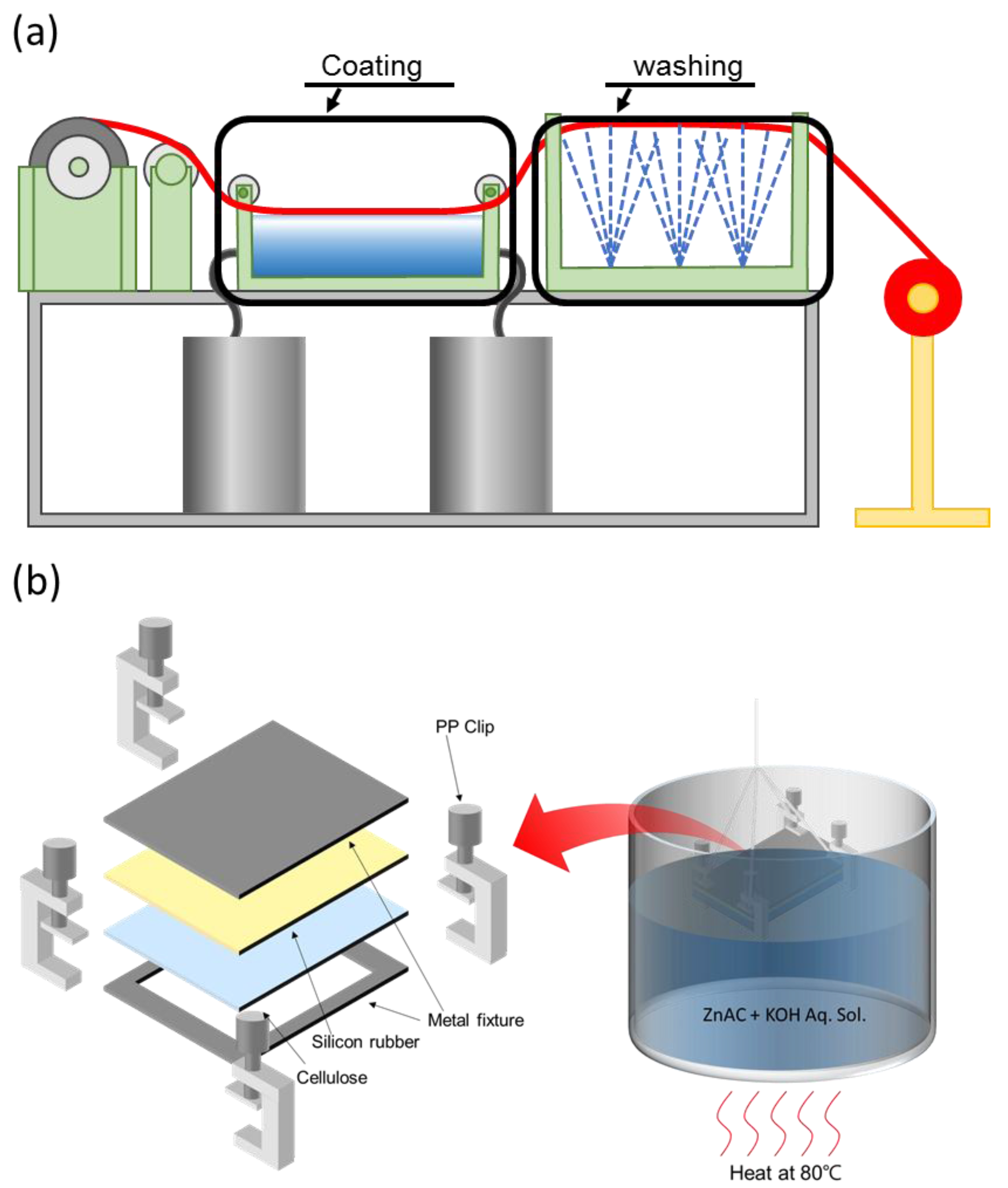
2.2. Fabrication Process
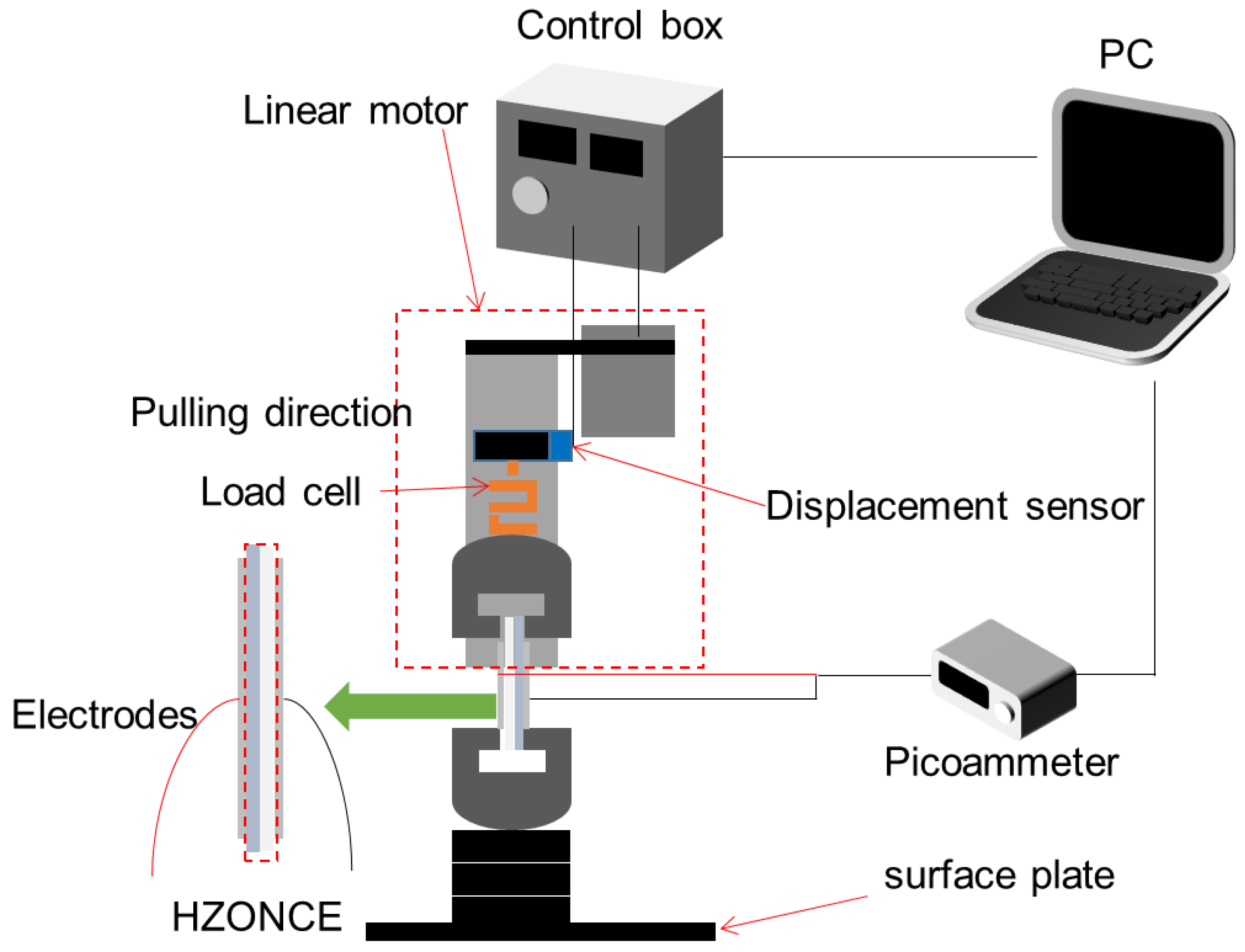
2.3. Characterization
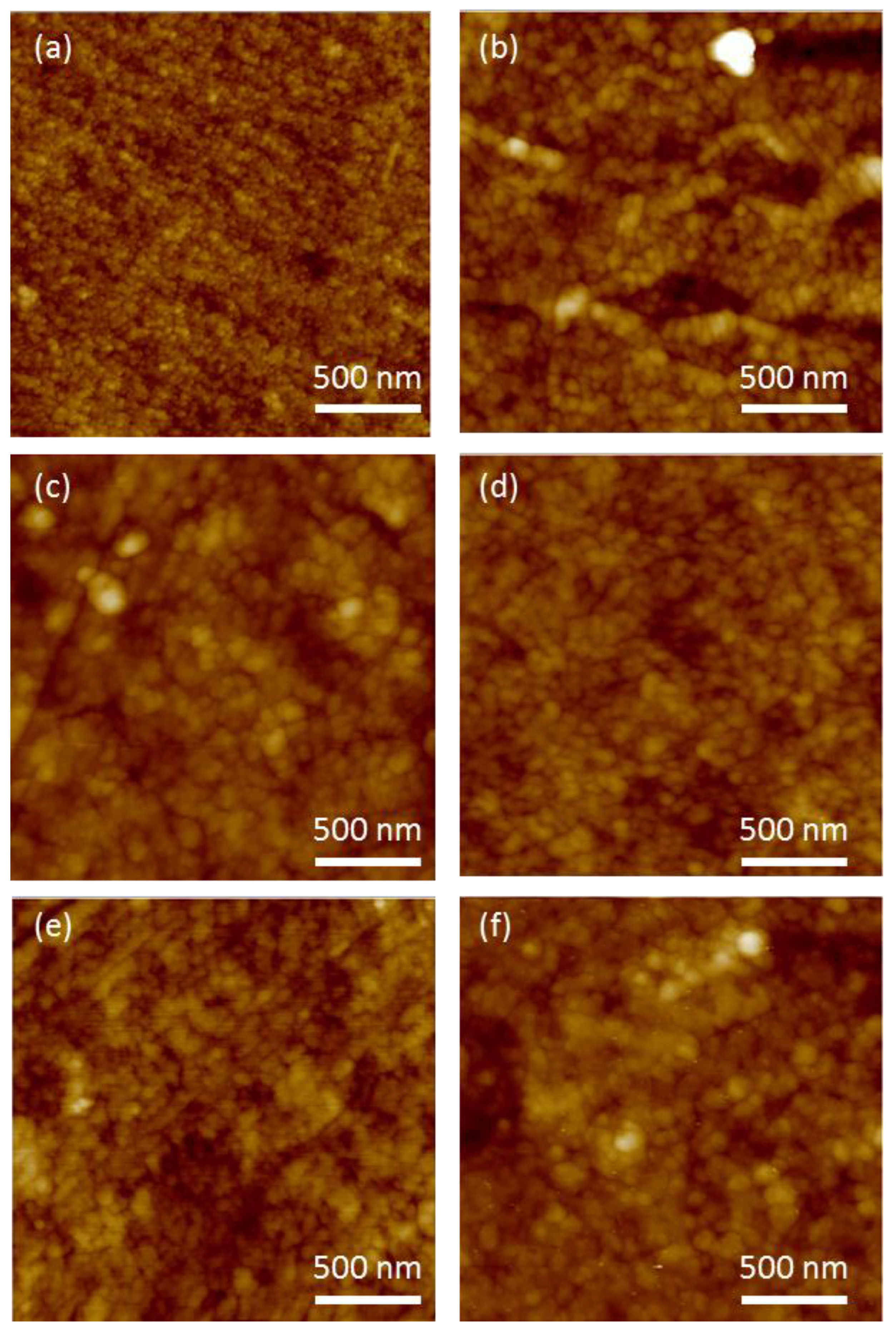
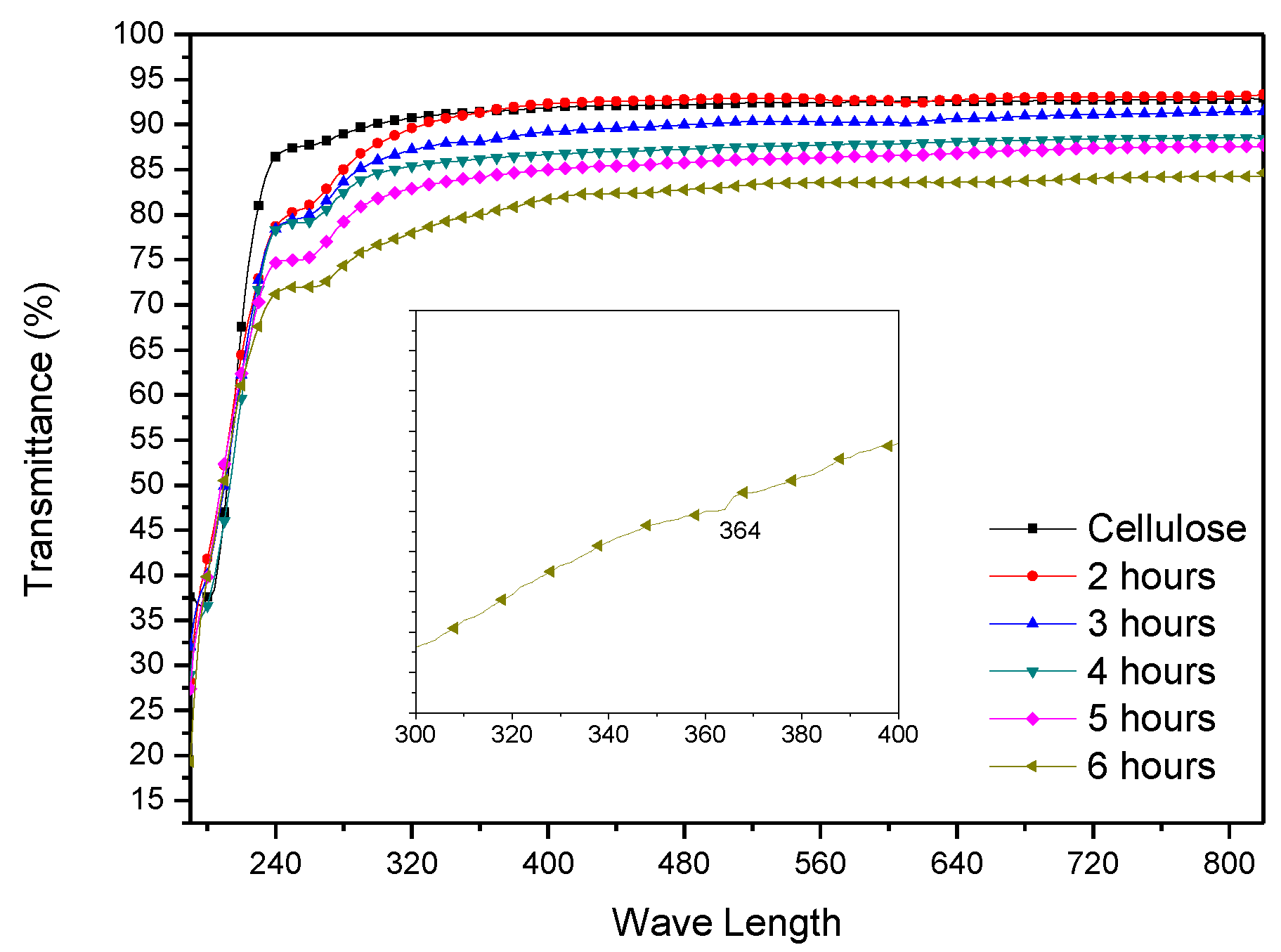
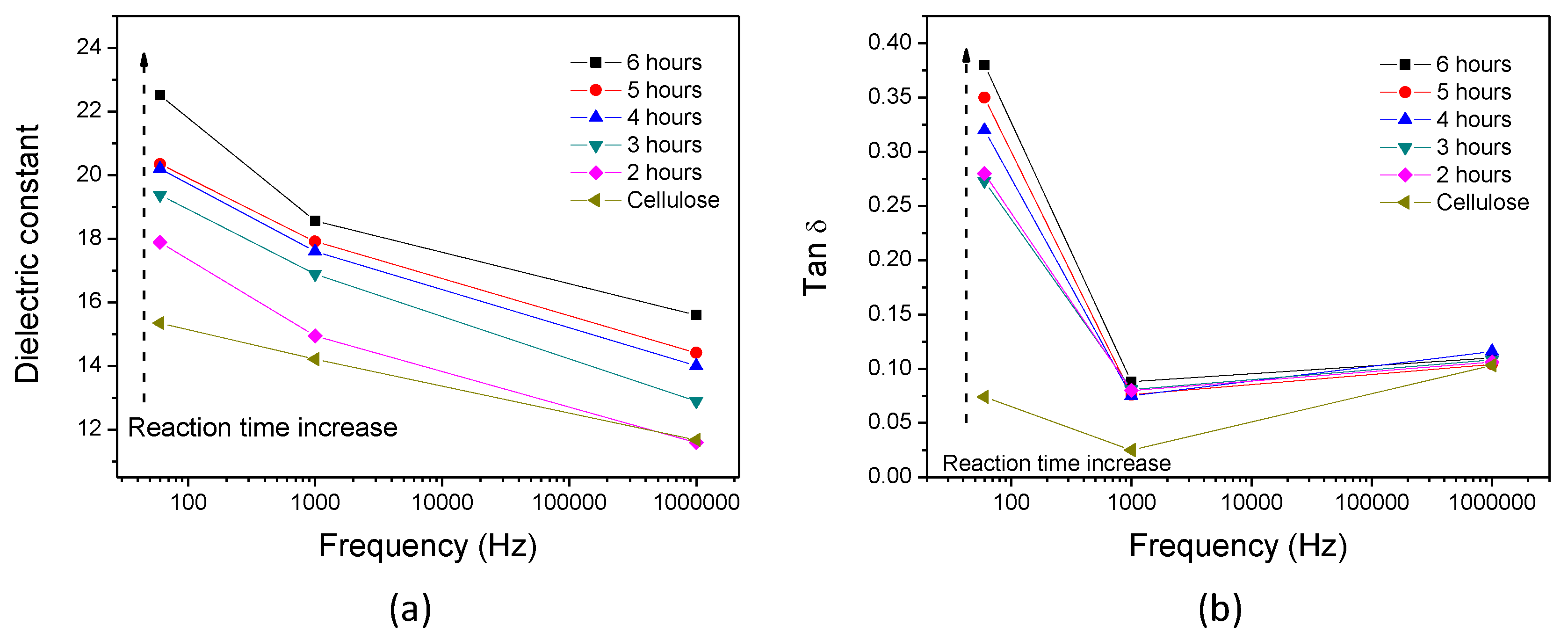
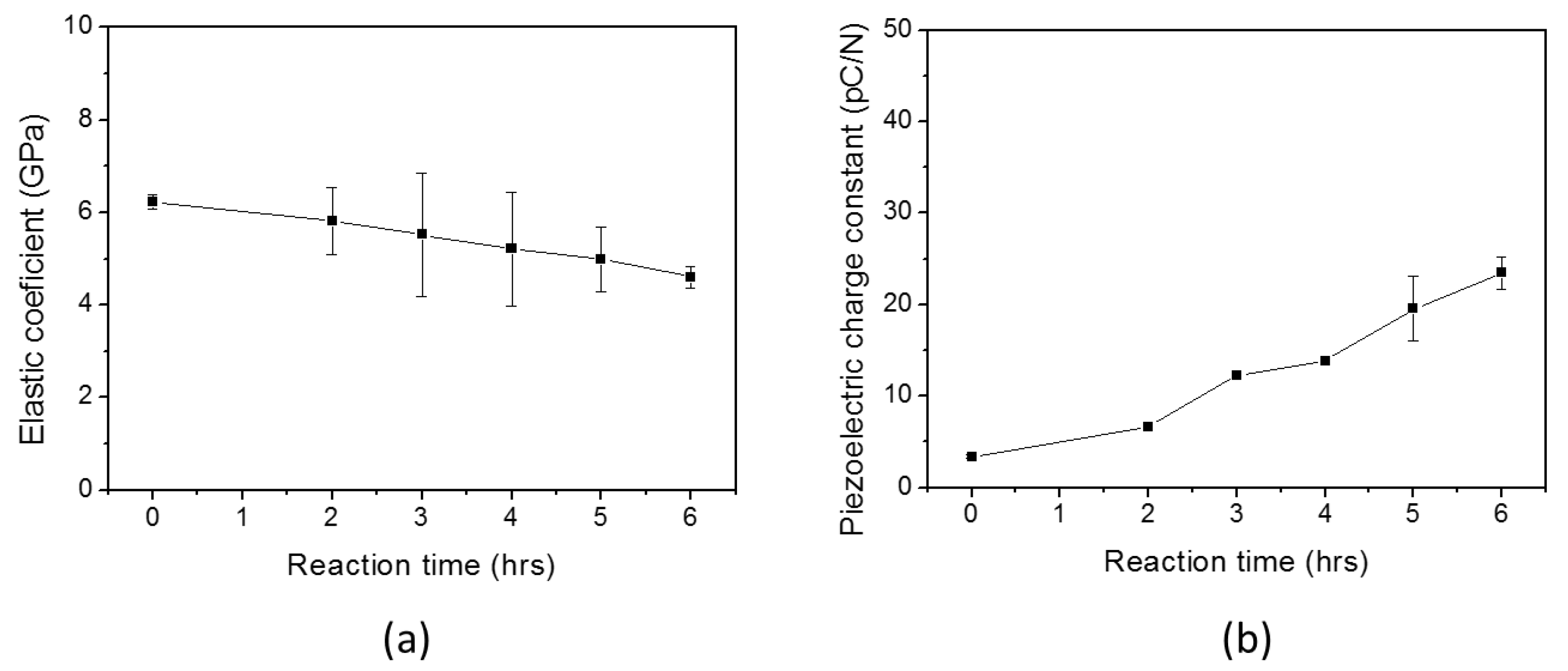
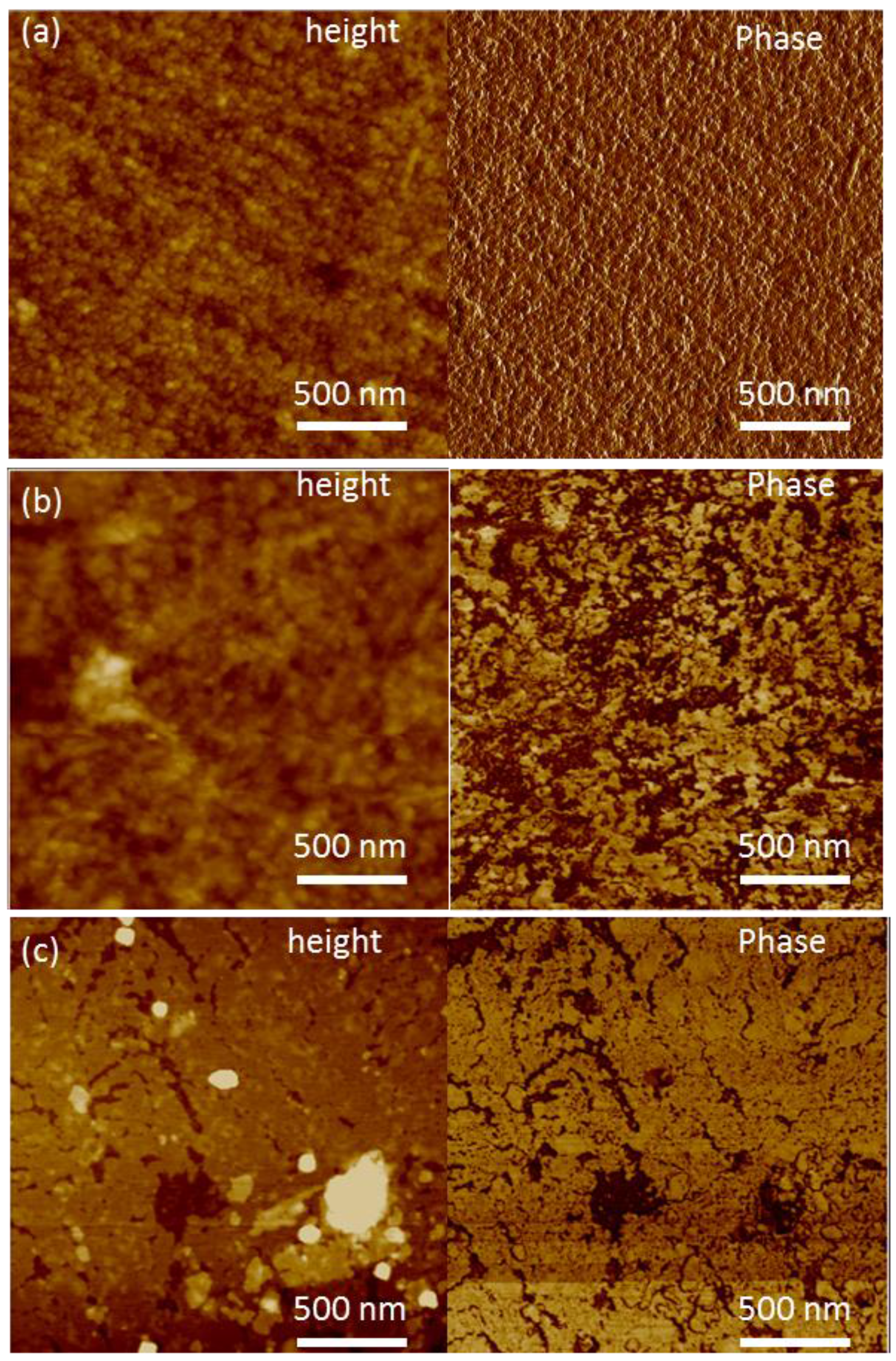
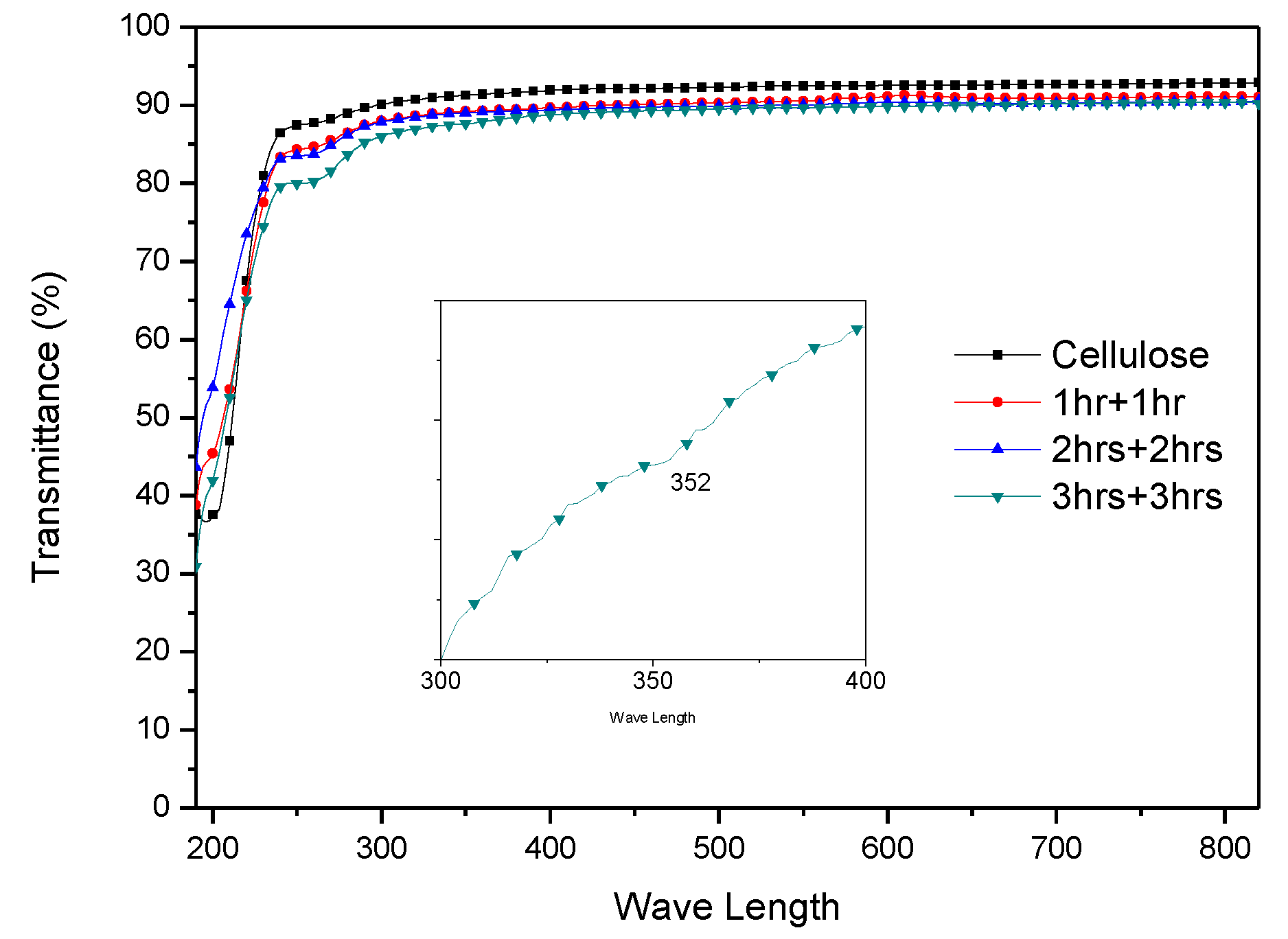
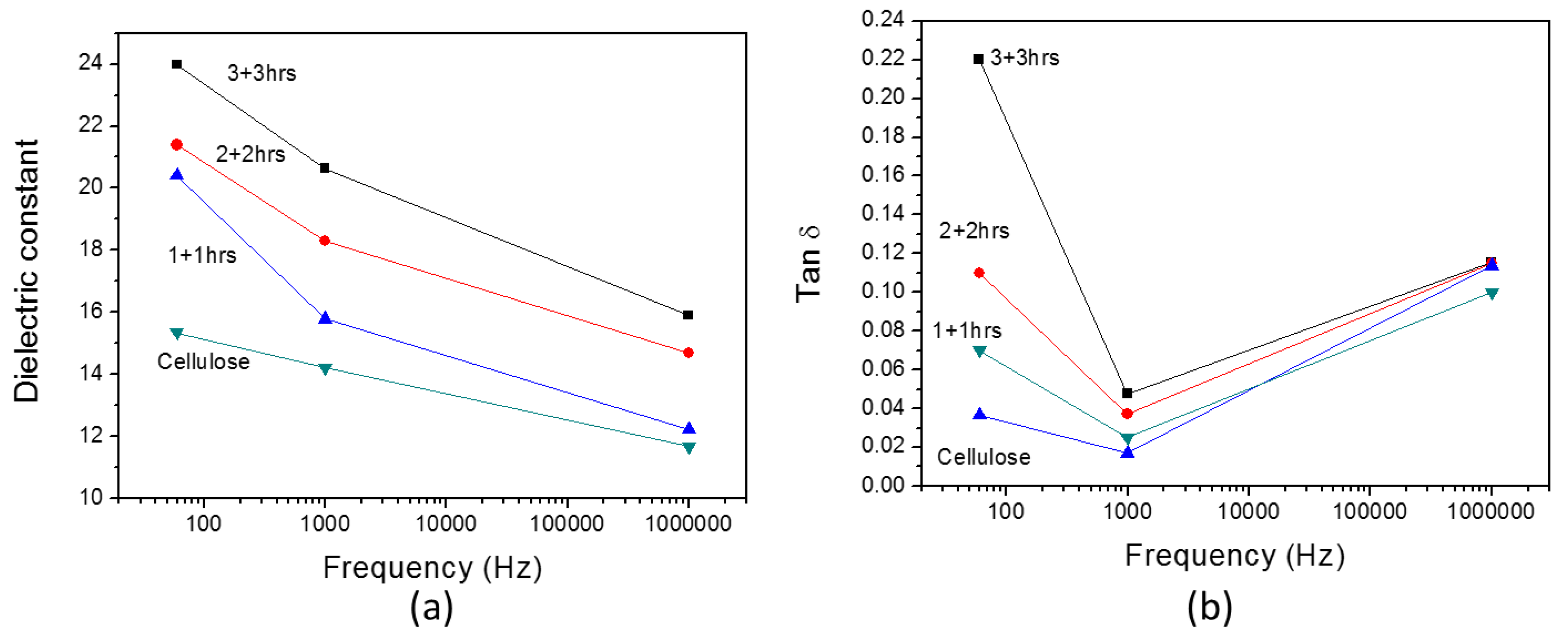
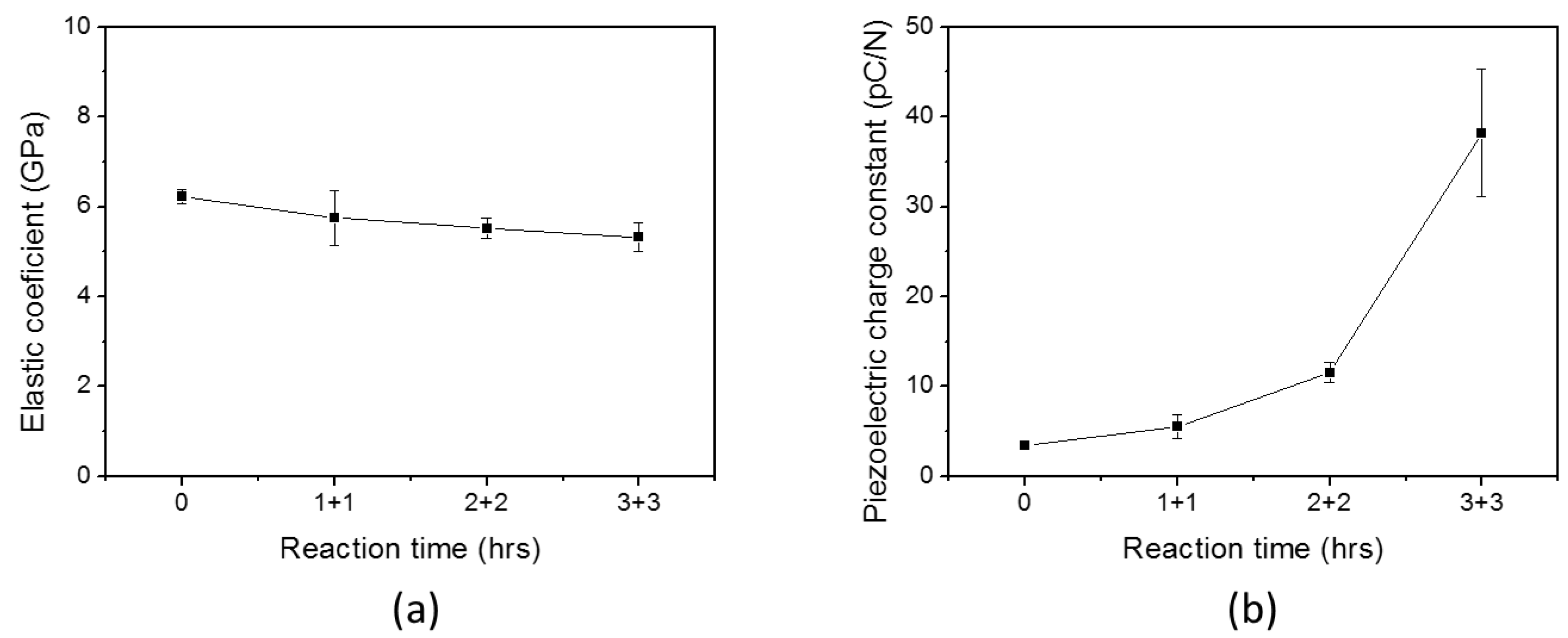
3. Results
3.1. Initial HZONCE
3.2. Improved HZONCE
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pandey, J.K.; Ahn, S.H.; Lee, C.S.; Mohanty, A.K. Recent advances in the application of natural fiber based composites. Macromol. Mater. Eng. 2010, 295, 975–989. [Google Scholar] [CrossRef]
- Eichhorn, S.J.; Dufresne, A.; Aranguren, M.; Marcovich, N.E.; Capadona, J.R.; Rowan, S.J.; Weder, C.; Thielemans, W.; Roman, M.; Renneckar, S.; et al. Review: Current international research into cellulose nanofibres and nanocomposites. J. Mater. Sci. 2010, 45. [Google Scholar] [CrossRef]
- Bazhenov, V.A. Piezoelectric Properties of Woods, 1st ed.; Consultants Bureau: New York, NY, USA, 1961; pp. 1–180. [Google Scholar]
- Kim, J.; Yun, S.; Ounaies, Z. Discovery of cellulose as a smart material. Macromolecules 2006, 39, 4202–4206. [Google Scholar] [CrossRef]
- Jeon, J.H.; Oh, I.K.; Kee, C.D.; Kim, S.J. Bacterial cellulose actuator with electrically driven bending deformation in hydrated condition. Sens. Actuators B Chem. 2010, 146, 307–313. [Google Scholar] [CrossRef]
- Kim, J.; Lee, H.; Kim, H.S. Beam vibration control using cellulose-based Electro-Active Paper sensor. Int. J. Precision Eng. Manuf. 2010, 11, 823–827. [Google Scholar] [CrossRef]
- Kim, J.; Yun, G.Y.; Kim, J.H.; Lee, J.; Kim, J.H. Piezoelectric electro-active paper (EAPap) speaker. J. Mech. Sci. Tech. 2011, 25, 2763–2768. [Google Scholar] [CrossRef]
- Mahadeva, S.K.; Kim, J. Conductometric glucose biosensor made with cellulose and tin oxide hybrid nanocomposite. Sens. Act. B Chem. 2011, 157, 177–182. [Google Scholar] [CrossRef]
- Yun, S.; Jang, S.D.; Yun, G.Y.; Kim, J.H.; Kim, J. Paper transistor made with covalently bonded multiwalled carbon nanotube and cellulose. Appl. Phys. Lett. 2009, 95, 104102. [Google Scholar] [CrossRef]
- Mun, S.; Chen, Y.; Kim, J. Cellulose–titanium dioxide–multiwalled carbon nanotube hybrid nanocomposite and its ammonia gas sensing properties at room temperature. Sens. Act. B Chem. 2012, 171, 1186–1191. [Google Scholar] [CrossRef]
- Maniruzzaman, M.; Jang, S.D.; Kim, J. Titanium dioxide–cellulose hybrid nanocomposite and its glucose biosensor application. Mate. Sci. Eng. B 2012, 177, 844–848. [Google Scholar] [CrossRef]
- Meilert, K.T.; Laub, D.; Kiwi, J. Photocatalytic self-cleaning of modified cotton textiles by TiO2 clusters attached by chemical spacers. J. Mol. Catal. A Chem. 2005, 237, 101–108. [Google Scholar] [CrossRef]
- Zhu, G.; Yang, R.; Wang, S.; Wang, Z.L. Flexible high-output nanogenerator based on lateral ZnO nanowire array. Nano Lett. 2010, 10, 3151–3155. [Google Scholar] [CrossRef]
- Tan, S.T.; Chen, B.J.; Sun, X.W.; Fan, W.J.; Kwok, H.S.; Zhang, X.H.; Chua, S.J. Blueshift of optical band gap in ZnO thin films grown by metal-organic chemical-vapor deposition. J. Appl. Phys. 2005, 98, 013505. [Google Scholar] [CrossRef]
- Hoffman, R.L.; Benjamin, J.N.; Wager, J.F. ZnO-based transparent thin-film transistors. Appl. Phys. Lett. 2003, 82, 733–735. [Google Scholar] [CrossRef]
- Gonçalves, G.; Marques, P.A.; Neto, C.P.; Trindade, T.; Peres, M.; Monteiro, T. Growth, structural, and optical characterization of ZnO-coated cellulosic fibers. Cryst. Growth Des. 2008, 9, 386–390. [Google Scholar] [CrossRef]
- Gullapalli, H.V.; Vemuru, S.M.; Kumar, A.; Botello-Mendez, A.; Vajtai, R.; Terrones, M.; Nagarajaiah, S.; Ajayan, P.M. Flexible piezoelectric ZnO–paper nanocomposite strain sensor. Small 2010, 6, 1641–1646. [Google Scholar] [CrossRef]
- Mun, S.; Ko, H.U.; Zhai, L.; Min, S.K.; Kim, G.W.; Kim, J. Enhanced electromechanical behavior of cellulose film by ZnO nanocoating and its vibration energy harvesting. Acta Mater. 2016, 114, 1–6. [Google Scholar] [CrossRef]
- KO, H.U.; John, A.; Mun, S.; Im, J.; Kim, J. Preparation and characterization of cellulose-ZnO nanolayer film by blending method. Macromol. Res. 2015, 23, 814–818. [Google Scholar] [CrossRef]
- Mun, S.; Maniruzzaman, M.; Ko, H.U.; Kafy, A.; Kim, J. Preparation and characterization of cellulose-ZnO hybrid film by blending method and its glucose biosensor application. Mater. Tech. Adv. Biometer. 2015, 2, B150–B154. [Google Scholar] [CrossRef]
- Mun, S.; Kim, J.; Lee, K.S. Evaluation of cellulose electro-active paper made by tape casting and zone stretching methods. Int. J. Prec. Eng. Manuf. 2010, 11, 987–990. [Google Scholar]
- John, A.; Ko, H.U.; Kim, D.G.; Kim, J. Preparation of cellulose-ZnO by a wet chemical method and their characterization. Cellulose 2011, 18, 675–680. [Google Scholar]
- Kang, B.W.; Mun, S.; Ko, H.U.; Zhai, L.; Kim, J.H.; Kim, J. Electromechanical behavior of green cellulose-ZnO hybrid nanocomposite. J. Biobased Mater. 2014, 8, 137–142. [Google Scholar] [CrossRef]
- Kim, H.S.; Li, Y.; Kim, J. Electro-mechanical behavior and direct piezoelectricity of cellulose electro-active paper. Sens. Act. A Phys. 2008, 147, 304–309. [Google Scholar] [CrossRef]
- Bouropoulos, N.; Psarras, G.C.; Moustakas, N.; Chrissanthopoulos, A.; Baskoutas, S. Optical and dielectric properties of ZnO-PVA nanocomposites. Physica. Status. solidi. (a) 2008, 205, 2033–2037. [Google Scholar] [CrossRef]
- Zubko, P.; Catalan, G.; Tagantsev, A.K. Flexoelectric effect in Solids. Annu. Rev. Mater. Res. 2013, 43, 387–421. [Google Scholar] [CrossRef]
- Alamusi; Xue, J.M.; Wu, L.K.; Hu, N.; Qiu, J.; Chang, C.; Atobe, S.; Fukunaga, H.; Watanabe, T.; Liu, Y.L.; et al. Evaluation of piezoelectric property of reduced graphene oxide (rGO)–poly(vinylidene fluoride) nanocomposites. Nanoscale 2012, 4, 7250. [Google Scholar]
- Dai, S.; Gharbi, M.; Sharma, P.; Park, H.S. Surface piezoelectricity: size effects in nanostructures and the emergence of piezoelectricity in non-piezoelectric materials. J. Appl. Phys. 2011, 110, 104305. [Google Scholar] [CrossRef]
- Viswanatha, R.; Sapra, S.; Satyam, P.V.; Dev, B.N.; Srama, D.D. Understanding the quantum size effects in ZnO nanocrystals. J. Mater. Chem. 2004, 14, 661–668. [Google Scholar] [CrossRef]
- Li, W.J.; Shi, E.W.; Zheng, Y.Q.; Yin, Z.W. Hydrothermal preparation of nanometer ZnO powders. J. Mater. Sci. Lett. 2001, 20, 1381–1383. [Google Scholar] [CrossRef]
- Mendes, S.F.; Costa, C.M.; Caparros, C.; Sencadas, V.; Lanceros-Me´ndez, S. Effect of filler size and concentration on the structure and properties of poly (vinylidene fluoride)/BaTiO3 nanocomposites. J. Meter. Sci. 2012, 47, 1378–1388. [Google Scholar] [CrossRef]

| Properties | ZONCE [18] | Initial HZONCE | Improved HZONCE |
|---|---|---|---|
| Young’s modulus (GPa) | 3.5 | 4.6 | 5.3 |
| Dielectric constant (at 1 kHz) | 21.3 | 18.6 | 24.0 |
| Piezoelectric charge constant (pC/N) | 93.5 | 23.4 | 37.4 |
| Optical transparency (at 480 nm) | 86.0 | 82.8 | 90.0 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, H.-U.; Kim, H.C.; Kim, J.W.; Zhai, L.; Kim, J. Fabrication Method Study of ZnO Nanocoated Cellulose Film and Its Piezoelectric Property. Materials 2017, 10, 611. https://doi.org/10.3390/ma10060611
Ko H-U, Kim HC, Kim JW, Zhai L, Kim J. Fabrication Method Study of ZnO Nanocoated Cellulose Film and Its Piezoelectric Property. Materials. 2017; 10(6):611. https://doi.org/10.3390/ma10060611
Chicago/Turabian StyleKo, Hyun-U, Hyun Chan Kim, Jung Woong Kim, Lindong Zhai, and Jaehwan Kim. 2017. "Fabrication Method Study of ZnO Nanocoated Cellulose Film and Its Piezoelectric Property" Materials 10, no. 6: 611. https://doi.org/10.3390/ma10060611
APA StyleKo, H.-U., Kim, H. C., Kim, J. W., Zhai, L., & Kim, J. (2017). Fabrication Method Study of ZnO Nanocoated Cellulose Film and Its Piezoelectric Property. Materials, 10(6), 611. https://doi.org/10.3390/ma10060611








