Microarc Oxidation Coating Combined with Surface Pore-Sealing Treatment Enhances Corrosion Fatigue Performance of 7075-T7351 Al Alloy in Different Media
Abstract
:1. Introduction
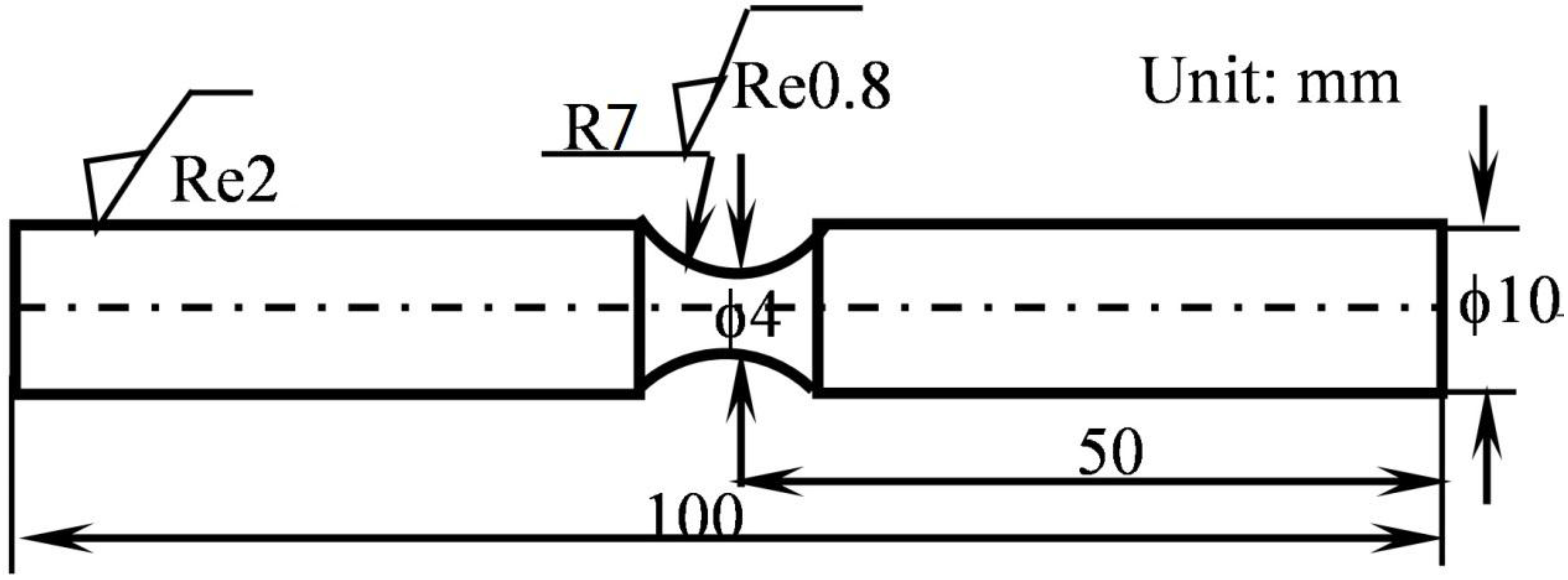
2. Materials and Experimental Methods
2.1. Coating Preparation
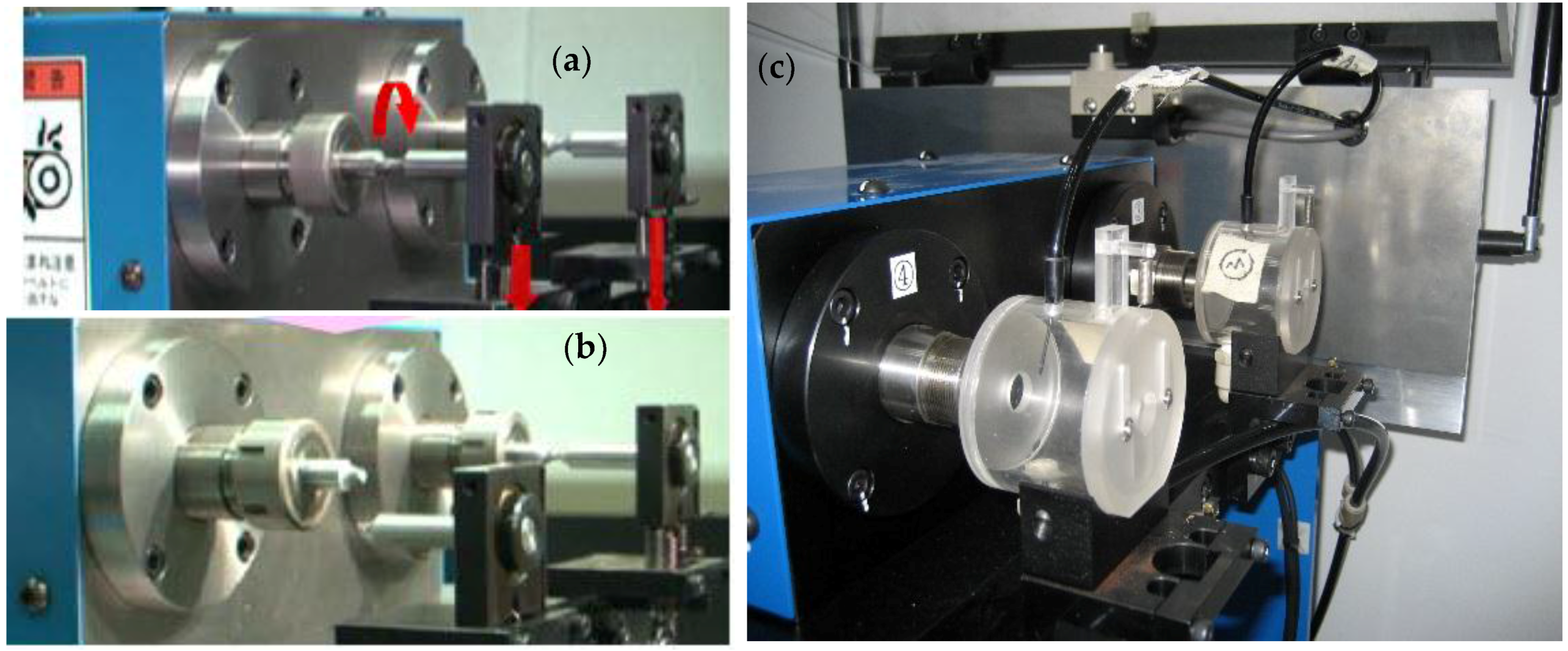
2.2. Experimental Processes
2.3. Residual Stress Measurement
3. Results
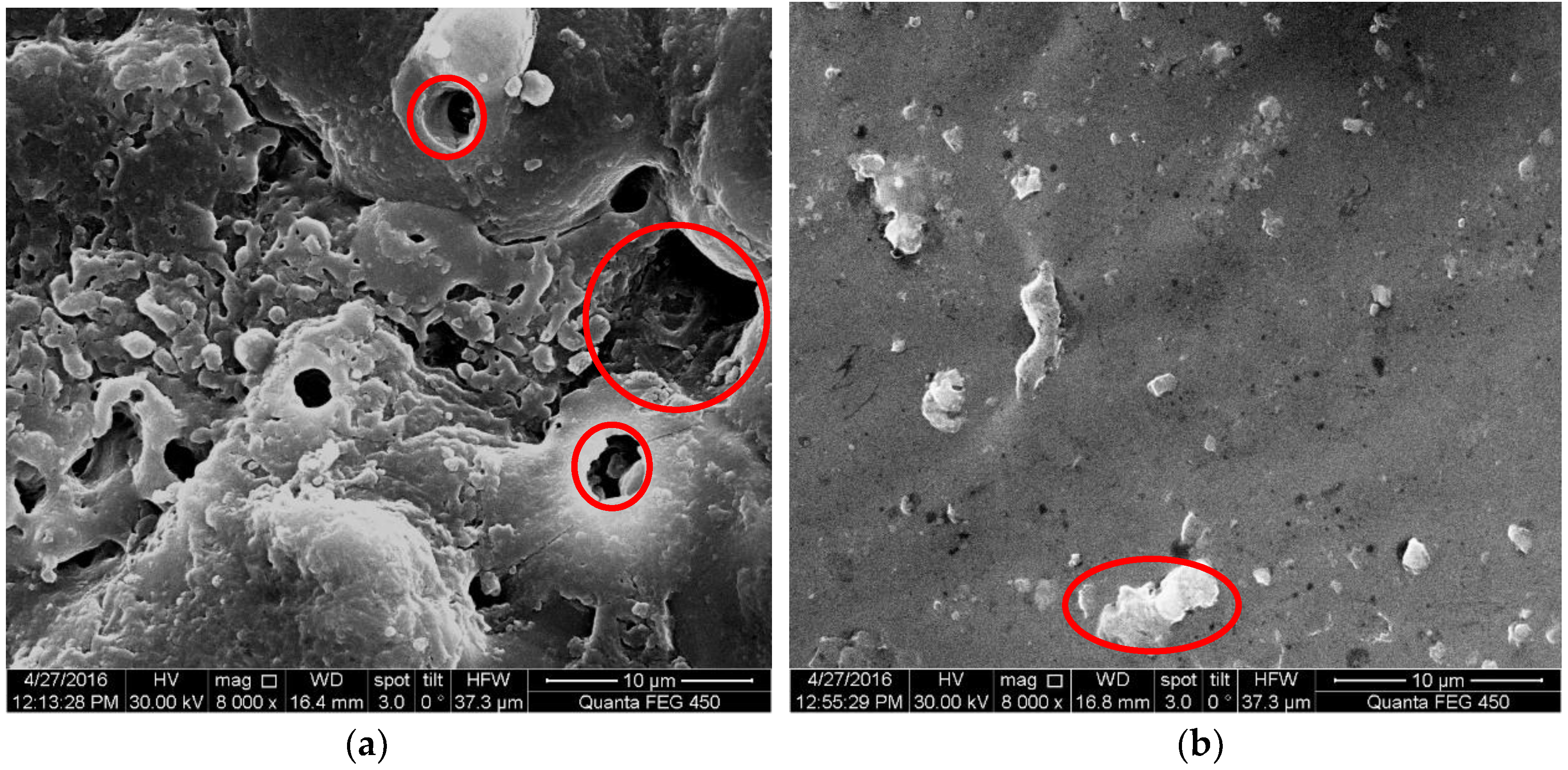
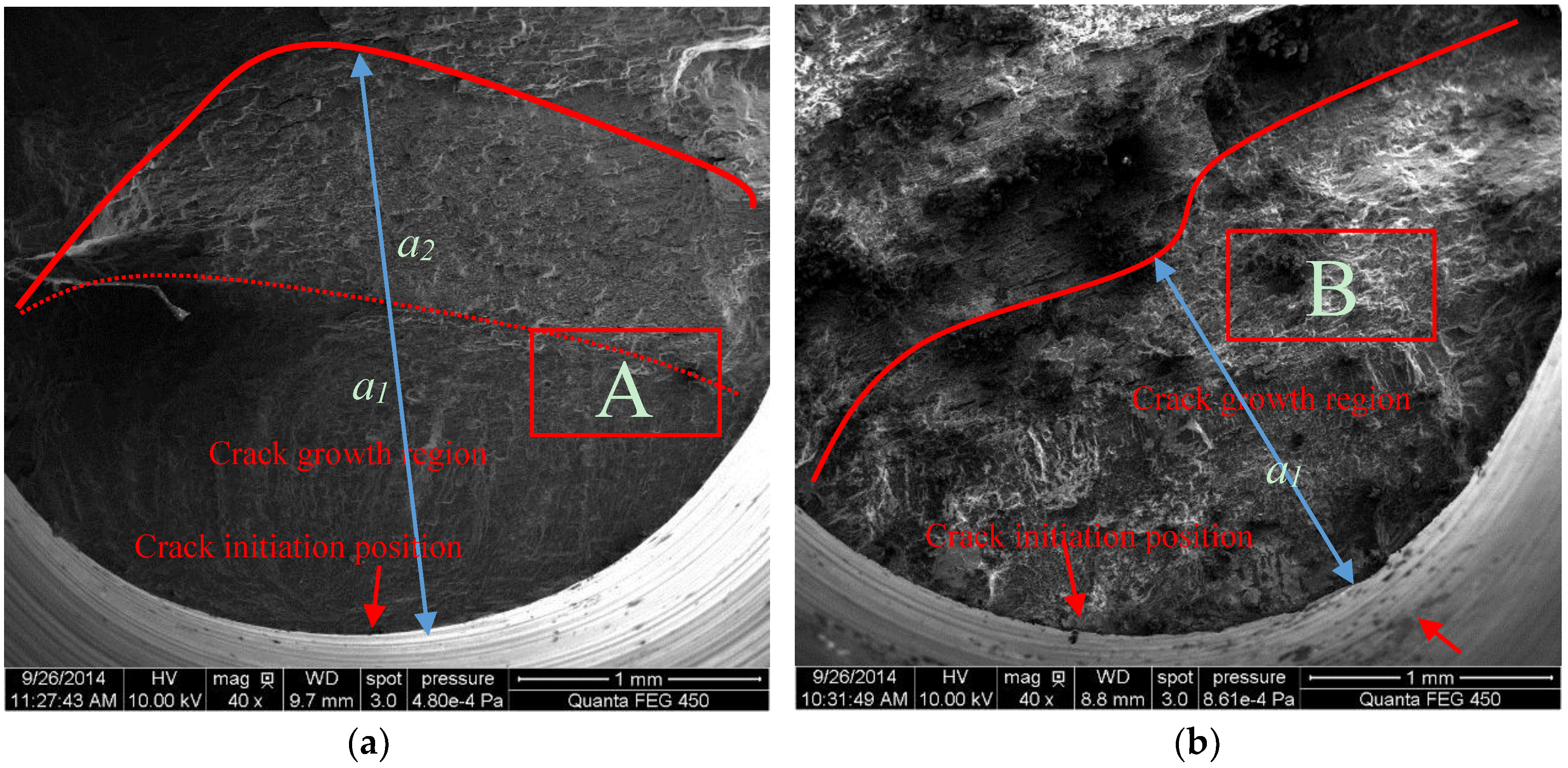
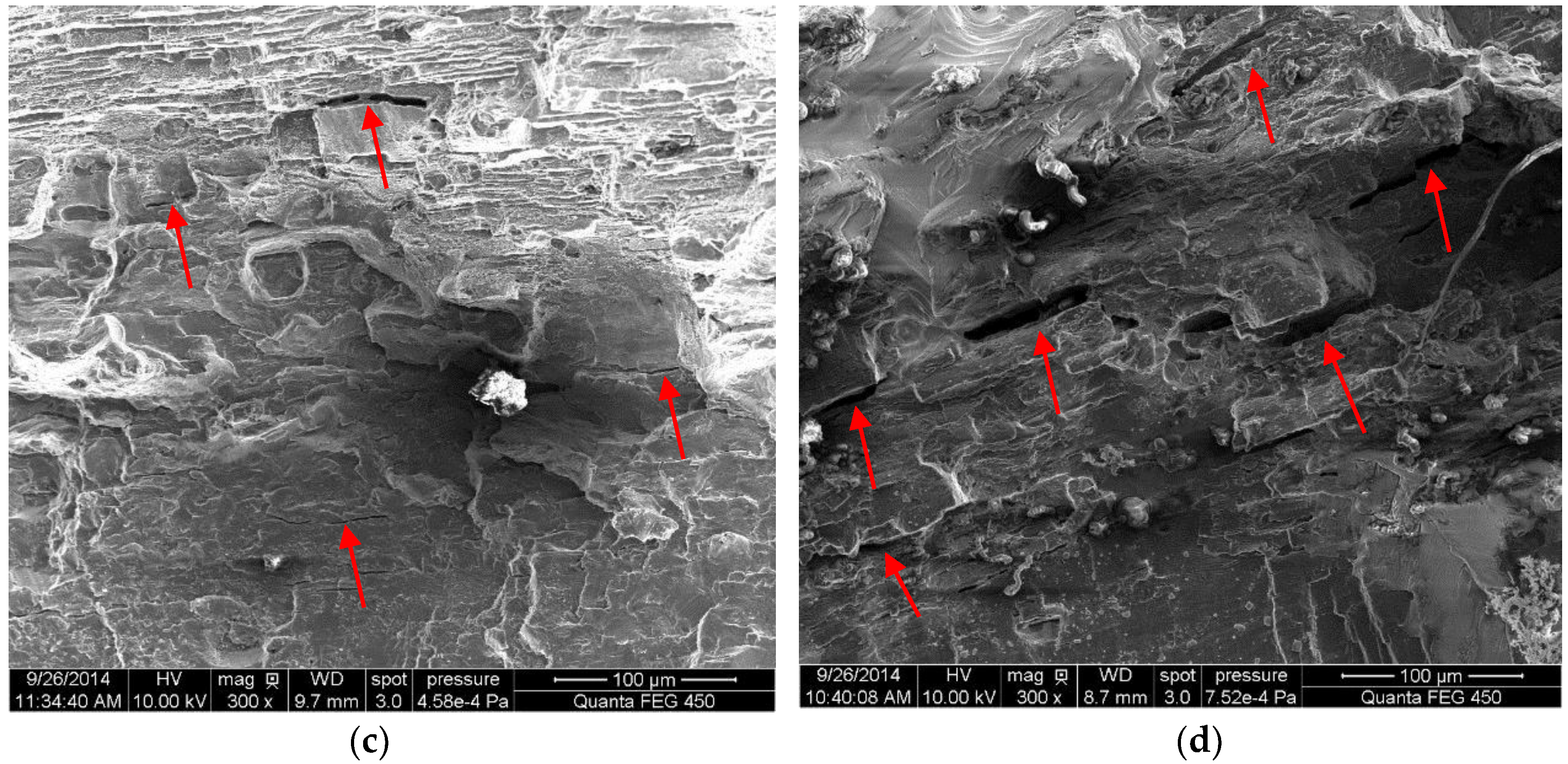
3.1. Surface Morphology of MAO Coated Samples
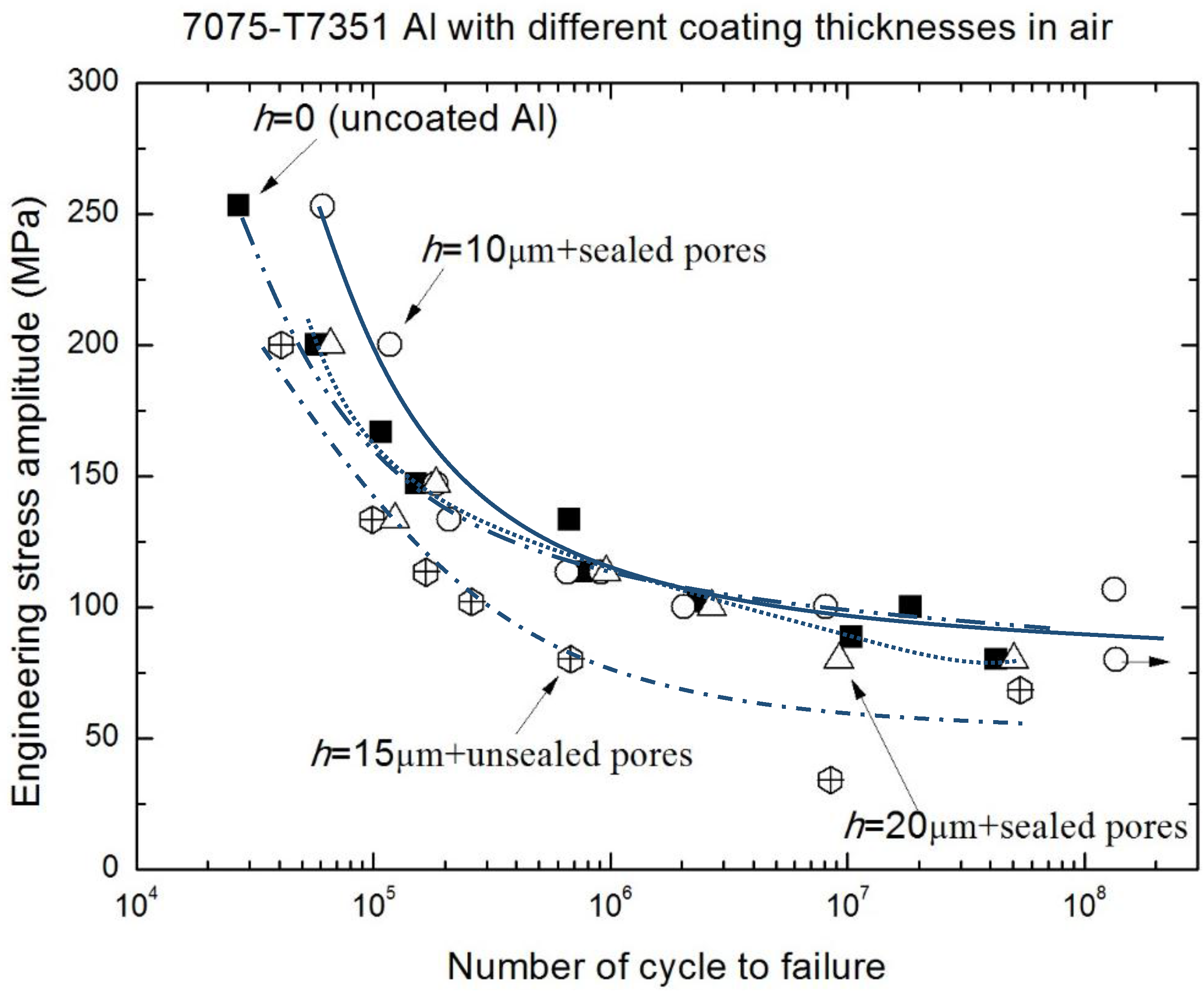
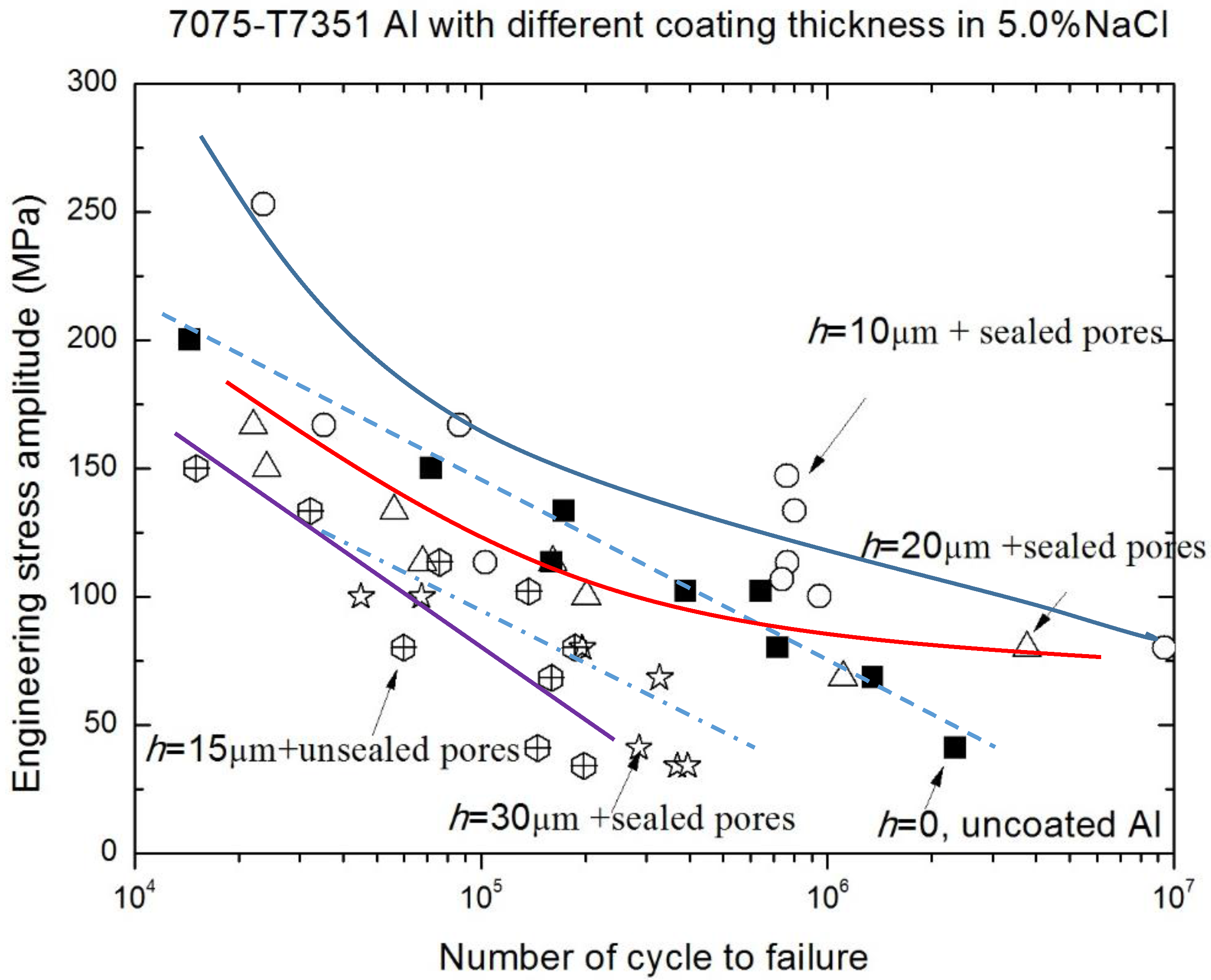
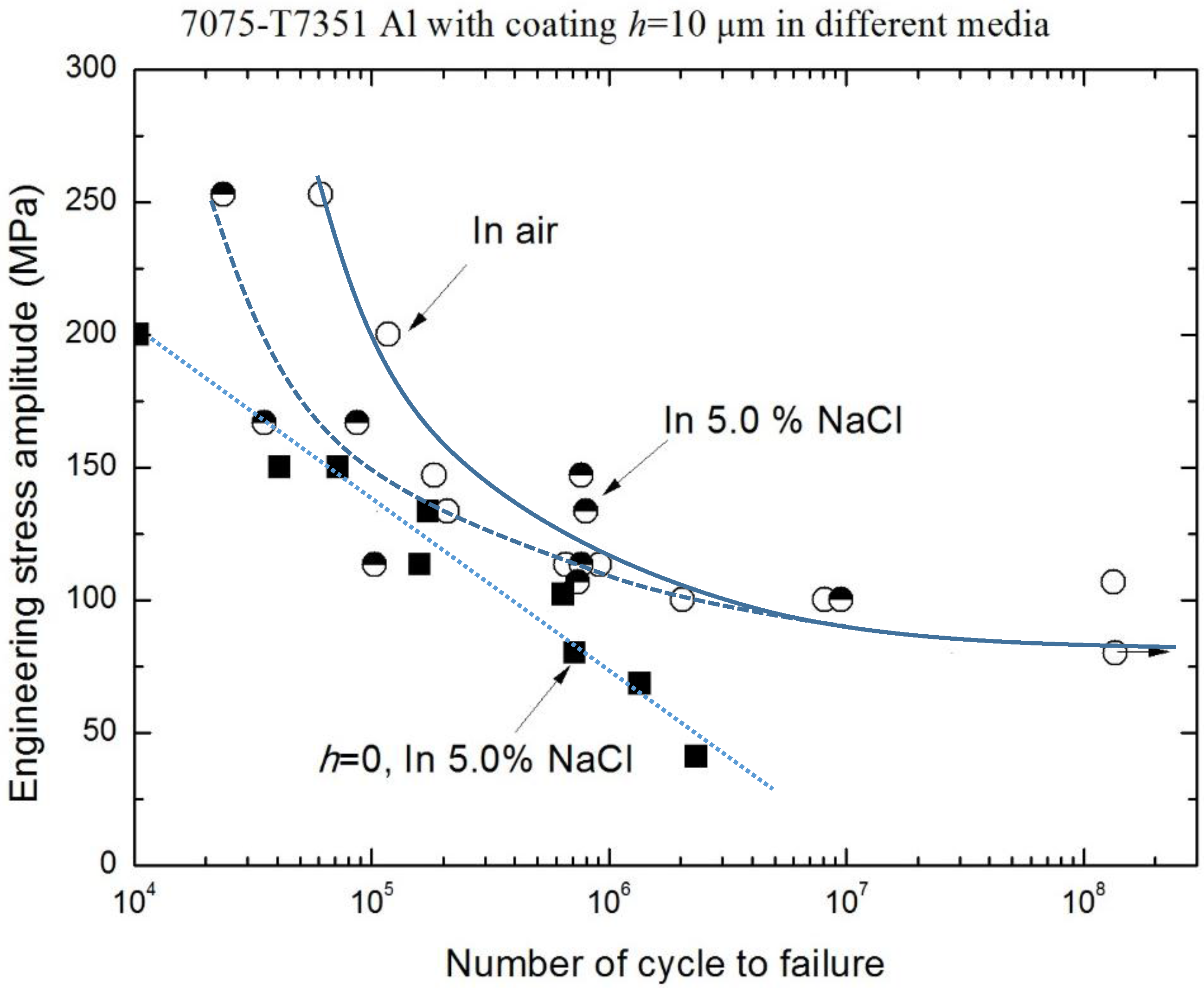
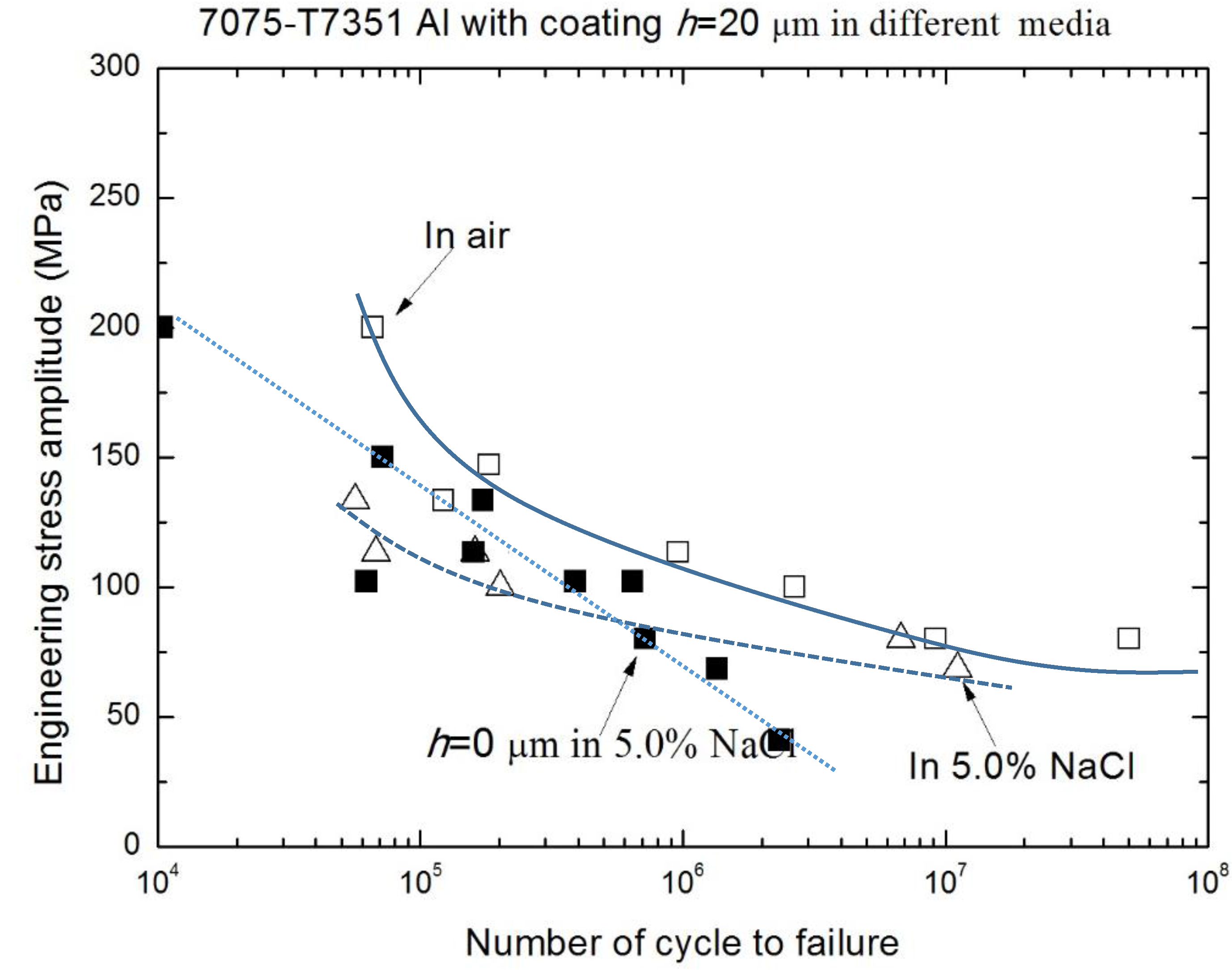
3.2. Corrosion Fatigue Performance of Coating-Substrate in the Different Conditions
4. Conclusions
- Through the pore-sealing treatment by epoxy resin, the unfavorable thermal micro-cracks and micro-pores were filled. Therefore, the pore-sealing treatment can also improve the corrosion fatigue performance of 7075-T7351Al alloy with MAO coating.
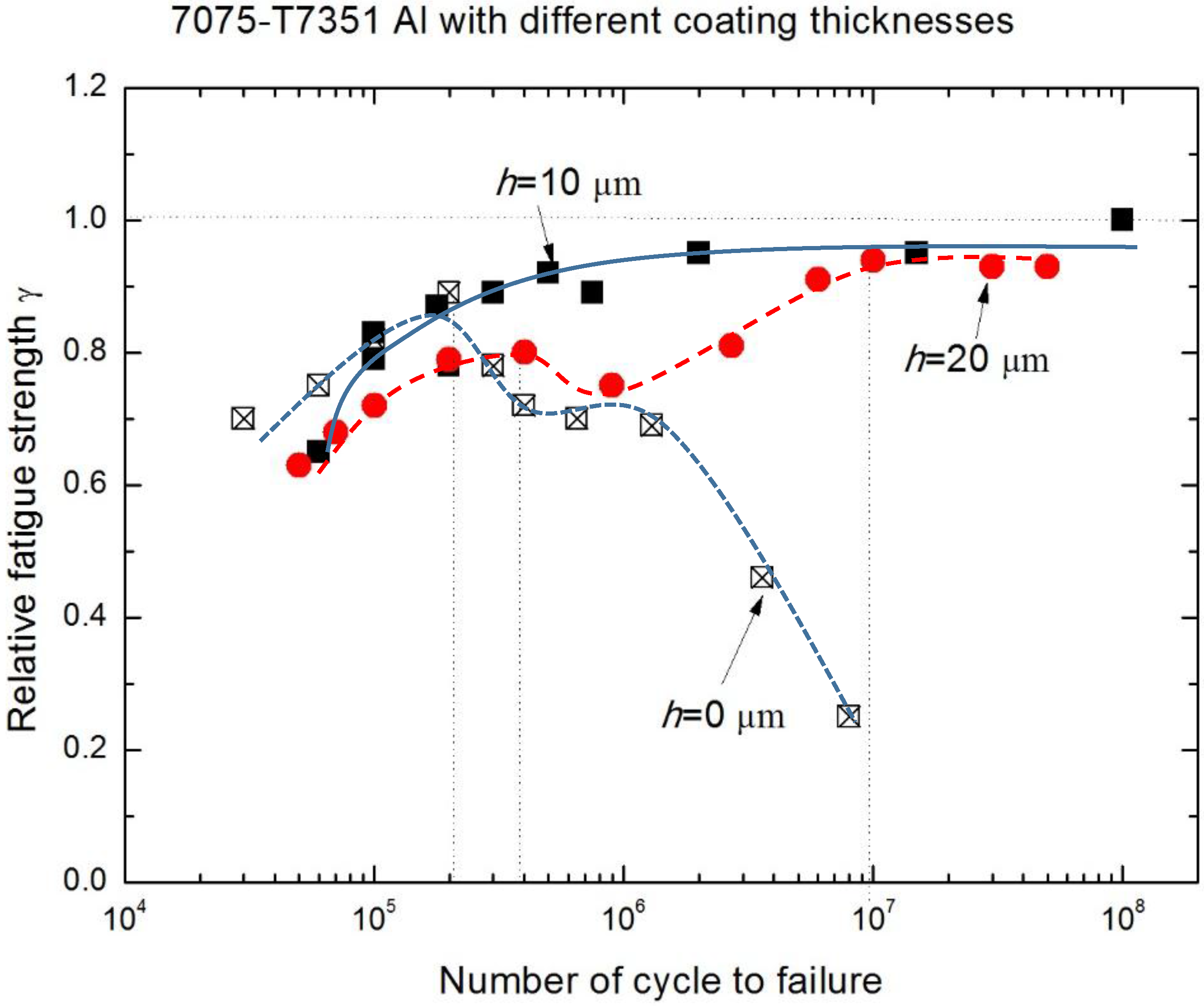
- The relative fatigue strength ratio (γ = σNaCl/σair) indicates that the major effect of MAO coating thickness on corrosion fatigue behavior occurs within the range from 1 × 105 to 1 × 107. Compared with γ of uncoated 7075-T7351 Al alloy, the MAO coating using the pore-sealing treatment can enhance the corrosion fatigue performance.
- The effect of MAO coating thickness on the corrosion fatigue performance of 7075-T7351 Al alloy suggests an optimizing value, such as h = 10 μm. For such a thickness of MAO coating on 7075-T7351 Al alloy, the fatigue limit in a 5.0 wt % NaCl aqueous solution approaches that for in air.
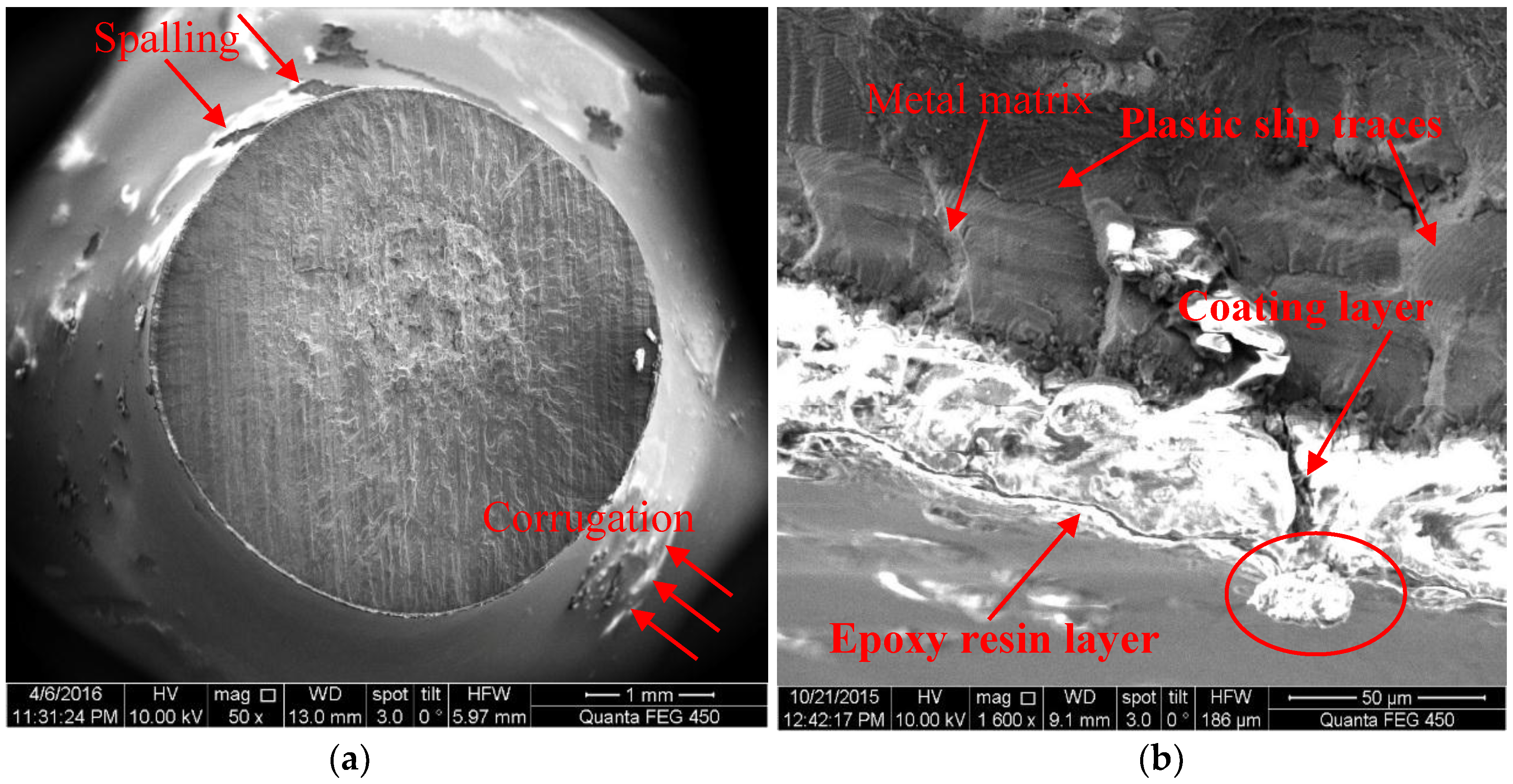
- For samples of MAO coated alloy with the pore-sealing treatment, microscopic analysis based on SEM images of surfaces and fracture cross-sections provides some evidence of corrosion fatigue cracks forming; these are breakdowns (corrugation and spalling) in high stress level, forming pits that cause a stress concentration and electrochemical attack on plastic deformation zones where the non-deformation areas act as a large cathode.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, Y.M.; Zhang, P.E.; Guo, L.X.; Ouyang, J.H.; Zhou, Y.; Jia, D.C. Effect of microarc oxidation coating on fatigue performance of Ti-Al-Zr alloy. Appl. Surf. Sci. 2009, 255, 8616–8623. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.Q.; Chen, C.F.; Gu, Y.H. Advances in microarc oxidation coated AZ31 Mg alloys for biomedical applications. Corros. Sci. 2015, 91, 7–28. [Google Scholar] [CrossRef]
- Wang, Y.M.; Wang, F.H.; Xu, M.J.; Zhao, B.; Guo, L.X.; Ouyang, J.H. Microstructure and corrosion behavior of coated AZ91 alloy by microarc oxidation for biomedical application. Appl. Surf. Sci. 2009, 255, 9124–9131. [Google Scholar] [CrossRef]
- Gupta, P.; Tenhundfeld, G.; Daigle, E.O.; Ryabkov, D. Electrolytic plasma technology: Science and engineering—An overview. Surf. Coat. Technol. 2007, 201, 8746–8760. [Google Scholar] [CrossRef]
- Wang, X.S.; Li, Y.; Meng, X.K. An estimation method on failure stress of micro thickness Cu film-substrate structure. Sci. China Ser. E 2009, 52, 2210–2215. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.S.; Meng, X.K. Buckling behavior of metal film/substrate structure under pure bending. Appl. Phys. Lett. 2008, 92, 131902. [Google Scholar] [CrossRef]
- Yerokhin, A.L.; Nie, X.; Leyland, A.; Matthews, A.; Dowey, S.J. Plasma electrolysis for surface engineering. Surf. Coat. Technol. 1999, 122, 73–93. [Google Scholar] [CrossRef]
- Yerokhin, A.L.; Shatrov, A.; Samsonov, V.; Shashkov, P.; Pikington, A.; Leyland, A.; Matthews, A. Oxide cermic coatings on aluminum alloys produced by a pulsed bipolar plasma electrolytic oxidation process. Surf. Coat. Technol. 2005, 199, 150–157. [Google Scholar] [CrossRef]
- Rajasekaran, B.; Raman, S.G.S.; Joshi, S.V.; Sundararajan, G. Effect of microarc oxidised layer thickness on plain fatigue and fretting fatigue behaviour of Al-Mg-Si alloy. Int. J. Fatigue 2008, 30, 1259–1266. [Google Scholar] [CrossRef]
- Rajasekaran, B.; Raman, S.G.S.; Krishna, L.R.; Joshi, S.V.; Sundararajan, G. Influence of microarc oxidation and hard anodizing on plain fatigue and fretting fatigue behaviour of Al-Mg-Si alloy. Surf. Coat. Technol. 2008, 202, 1462–1469. [Google Scholar] [CrossRef]
- Lonyuk, B.; Apachitei, I.; Duszcyk, J. The effect of oxide coatings on fatigue properties of 7475-T6 aluminium alloy. Surf. Coat. Technol. 2007, 201, 8688–8694. [Google Scholar] [CrossRef]
- Campanelli, L.C.; Duarte, L.T.; Silva, P.S.C.P.; Bolfarini, C. Fatigue behavior of modified surface of Ti–6Al–7Nb and CP-Ti by micro-arc oxidation. Mater. Des. 2014, 64, 393–399. [Google Scholar] [CrossRef]
- Niinomi, M. Recent metallic materials for biomedical applications. Metall. Mater. Trans. A 2002, 33, 477–486. [Google Scholar] [CrossRef]
- Wang, X.S.; Guo, X.W.; Li, X.D.; Ge, D.Y. Improvement on the fatigue performance of 2024-T4 alloy by synergistic coating technology. Materials 2014, 7, 3533–3546. [Google Scholar] [CrossRef]
- Wang, X.S.; Li, X.D.; Yang, H.H.; Kawagoishi, N.; Pan, P. Environment-induced fatigue cracking behavior of aluminum alloys and modification methods. Corros. Rev. 2015, 33, 110–137. [Google Scholar] [CrossRef]
- Wang, Y.M.; Lei, T.Q.; Jiang, B.L.; Guo, L.X. Growth, microstructure and mechanical properties of microarc oxidation coatings on titanium alloy in phosphate-containing solution. Appl. Surf. Sci. 2004, 233, 258–267. [Google Scholar] [CrossRef]
- Wang, Y.M.; Tian, H.; Shen, X.E.; Wen, L.; Ouyang, J.H.; Zhou, Y.; Jia, D.C.; Guo, L.X. An elevated temperature infrared emissivity ceramic coating formed on 2024 aluminium alloy by microarc oxidation. Ceram. Int. 2013, 39, 2869–2875. [Google Scholar] [CrossRef]
- Wang, Y.M.; Jiang, B.L.; Lei, T.Q.; Guo, L.X. Microarc oxidation coatings formed on Ti6A14V in Na2SiO3 system solution: Microstructure, mechanical and tribological properties. Surf. Coat. Technol. 2006, 201, 82–89. [Google Scholar] [CrossRef]
- Zhang, X.P.; Ouyang, J.H.; Liu, Z.G.; Wang, Y.J.; Wang, Y.M. Crack-healing behavior and strength recovery of hot-pressed TZ3Y20A-MoSi2 ceramics. Mater. Sci. Eng. A 2015, 648, 299–304. [Google Scholar] [CrossRef]
- Qian, G.A.; Zhou, C.F.; Hong, Y.S. A model to predict S-N curves for surface and subsurface crack initiation in different environmental media. Int. J. Fatigue 2015, 71, 35–44. [Google Scholar] [CrossRef]
- Yang, H.H.; Wang, Y.L.; Wang, X.S.; Pan, P.; Jia, D.W. Synergistic effect of environmental media and stress on the fatigue damage behavior of Al alloys. Fatigue Fract. Eng. Mater. Struct. 2016, 39, 1309–1316. [Google Scholar] [CrossRef]
- Moram, M.A.; Vickers, M.E. X-ray diffraction of III-nitrides. Rep. Progr. Phys. 2009, 72, 036502. [Google Scholar] [CrossRef]
- Kotsikos, G.; Sutcliffe, J.M.; Holroyd, N.J.H. Hydrogen effects in the corrosion fatigue behavior of the white zone of 7xxx series aluminum alloy welds. Corros. Sci. 2000, 42, 17–33. [Google Scholar] [CrossRef]
- Hall, M.M., Jr. Effect of cyclic crack opening displacement rate on corrosion fatigue crack velocity and fracture mode transitions for Al-Zn-Mg-Cu alloys. Corros. Sci. 2014, 81, 132–143. [Google Scholar] [CrossRef]
- Sharma, M.M.; Tomedi, J.D.; Parks, J.M. A microscopic study on the corrosion fatigue of ultra-fine grained and conventional Al-Mg alloy. Corros. Sci. 2015, 93, 180–190. [Google Scholar] [CrossRef]
- Khan, R.H.U.; Yerokhin, A.L.; Pikington, A.; Leyland, A.; Matthews, A. Residual stresses in plasma electrolytic oxidation coatings on Al alloy produced by pulsed unipolar current. Surf. Coat. Technol. 2005, 200, 1580–1594. [Google Scholar] [CrossRef]
- Rateick, R.G.; Binknowski, T.C.; Boray, B.C. Effect of hard anodize thickness on the fatigue of AA6061 and C355 aluminium. J. Mater. Sci. Lett. 1996, 15, 1321–1323. [Google Scholar] [CrossRef]
- Srivatsan, T.S.; Sudarshan, T.S. Mechanisms of fatigue crack initiation in metals: Role of aqueous environments. J. Mater. Sci. 1988, 23, 1521–1533. [Google Scholar] [CrossRef]
- Wasekar, N.P.; Jyothirmayi, A.; Sundararajan, G. Influence of prior corrosion on the high cycle fatigue behavior of microarc oxidation coated 6061-T6 aluminum alloy. Int. J. Fatigue 2011, 33, 1268–1278. [Google Scholar] [CrossRef]

| Applied Voltage (V) | Electrolyte Composition | Treatment Time (min) | Coating Thickness, h (μm) | Coating Composition |
|---|---|---|---|---|
| 600 | Na2SiO3, 8 gL−1 NaOH, 1 gL−1 (NaPO3)6, 20 gL−1 | 13 | 10 | γ-Al2O3 |
| 19 | 15 | γ-Al2O3 | ||
| 25 | 20 | γ-Al2O3 | ||
| 38 | 30 | γ-Al2O3 |
| Material | E (GPa) | μ | σ0.2 (MPa) | σb (MPa) | δ (%) |
|---|---|---|---|---|---|
| 7075-T7351 Al | 70.5 | 0.30 | 465 | 528 | 14.6 |
| γ-Al2O3 | 253 | 0.24 |
| Coating Thickness (μm) | Residual Stress (MPa) | Deviation (MPa) |
|---|---|---|
| 10 | −198.27 | ±11 |
| 20 | −263.74 | ±13 |
| 30 | −446.90 | ±28 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.-H.; Wang, X.-S.; Wang, Y.-M.; Wang, Y.-L.; Zhang, Z.-H. Microarc Oxidation Coating Combined with Surface Pore-Sealing Treatment Enhances Corrosion Fatigue Performance of 7075-T7351 Al Alloy in Different Media. Materials 2017, 10, 609. https://doi.org/10.3390/ma10060609
Yang H-H, Wang X-S, Wang Y-M, Wang Y-L, Zhang Z-H. Microarc Oxidation Coating Combined with Surface Pore-Sealing Treatment Enhances Corrosion Fatigue Performance of 7075-T7351 Al Alloy in Different Media. Materials. 2017; 10(6):609. https://doi.org/10.3390/ma10060609
Chicago/Turabian StyleYang, Hui-Hui, Xi-Shu Wang, Ya-Ming Wang, Yan-Ling Wang, and Zhi-Hao Zhang. 2017. "Microarc Oxidation Coating Combined with Surface Pore-Sealing Treatment Enhances Corrosion Fatigue Performance of 7075-T7351 Al Alloy in Different Media" Materials 10, no. 6: 609. https://doi.org/10.3390/ma10060609
APA StyleYang, H.-H., Wang, X.-S., Wang, Y.-M., Wang, Y.-L., & Zhang, Z.-H. (2017). Microarc Oxidation Coating Combined with Surface Pore-Sealing Treatment Enhances Corrosion Fatigue Performance of 7075-T7351 Al Alloy in Different Media. Materials, 10(6), 609. https://doi.org/10.3390/ma10060609





