Structural, Morphological, Optical and Photocatalytic Properties of Y, N-Doped and Codoped TiO2 Thin Films
Abstract
:1. Introduction
2. Results and Discussion
2.1. SEM Analysis
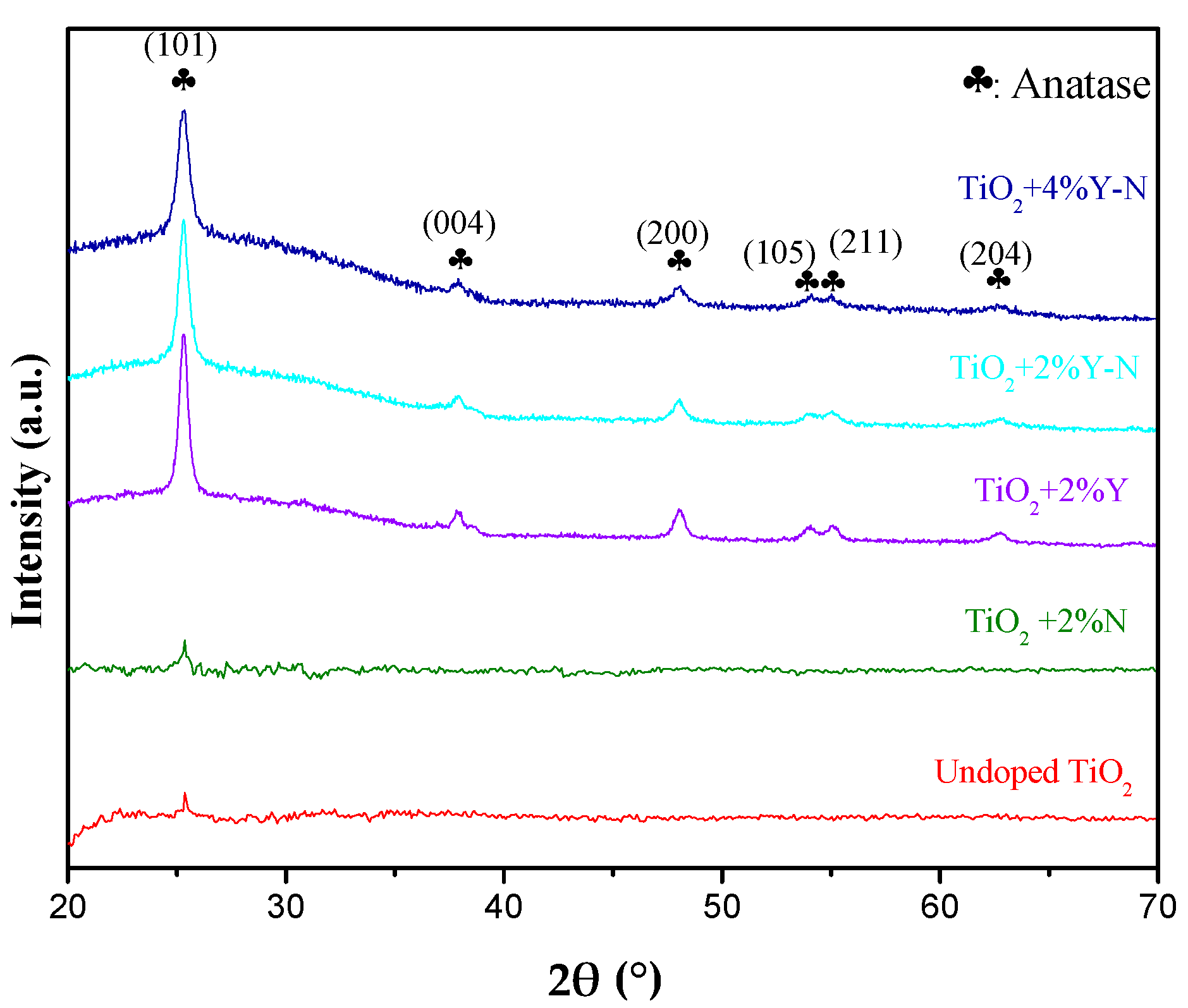
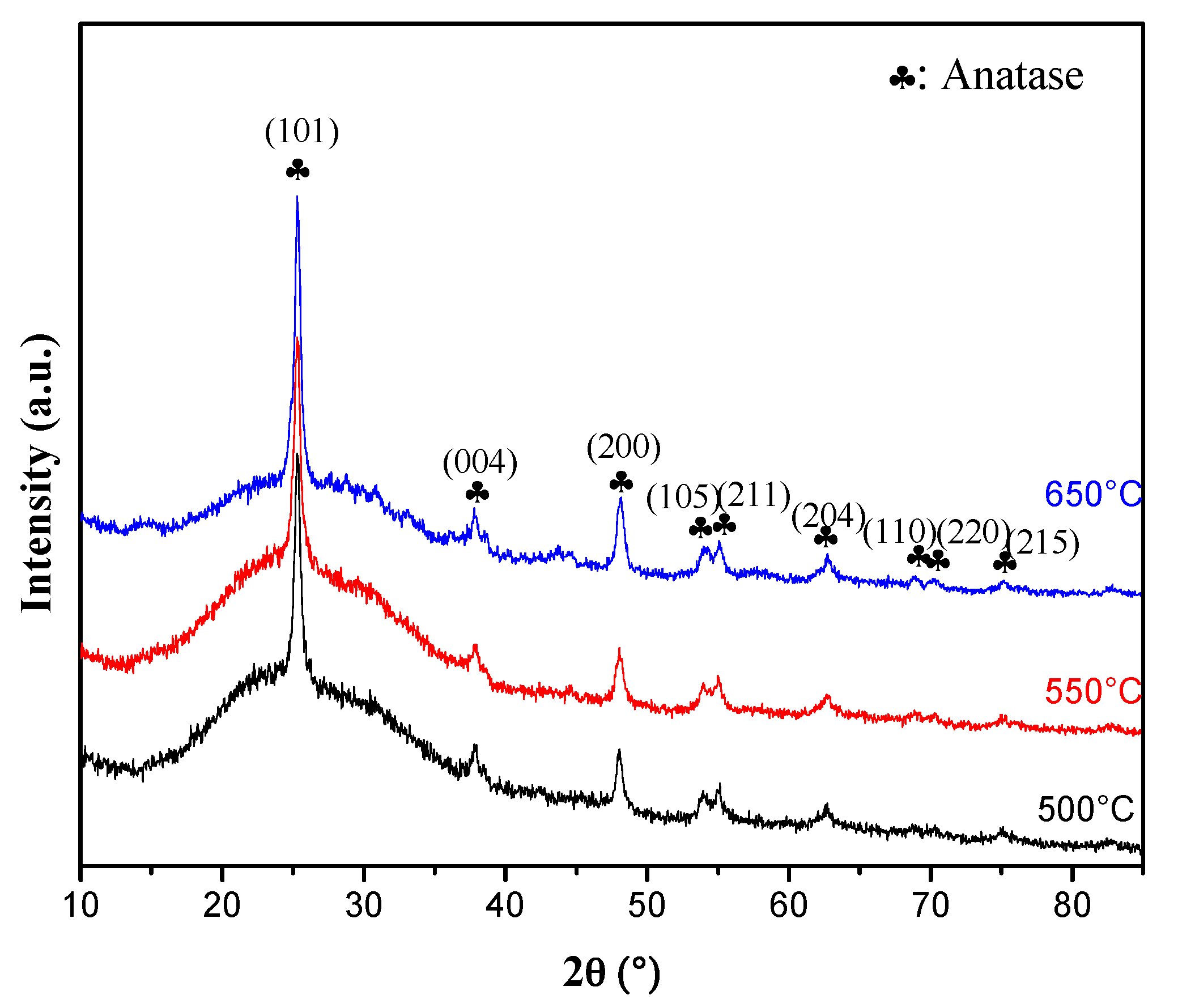
2.2. GXRD Characterization
2.3. Raman Spectroscopy
2.4. Ground State Diffuse Reflectance Spectra
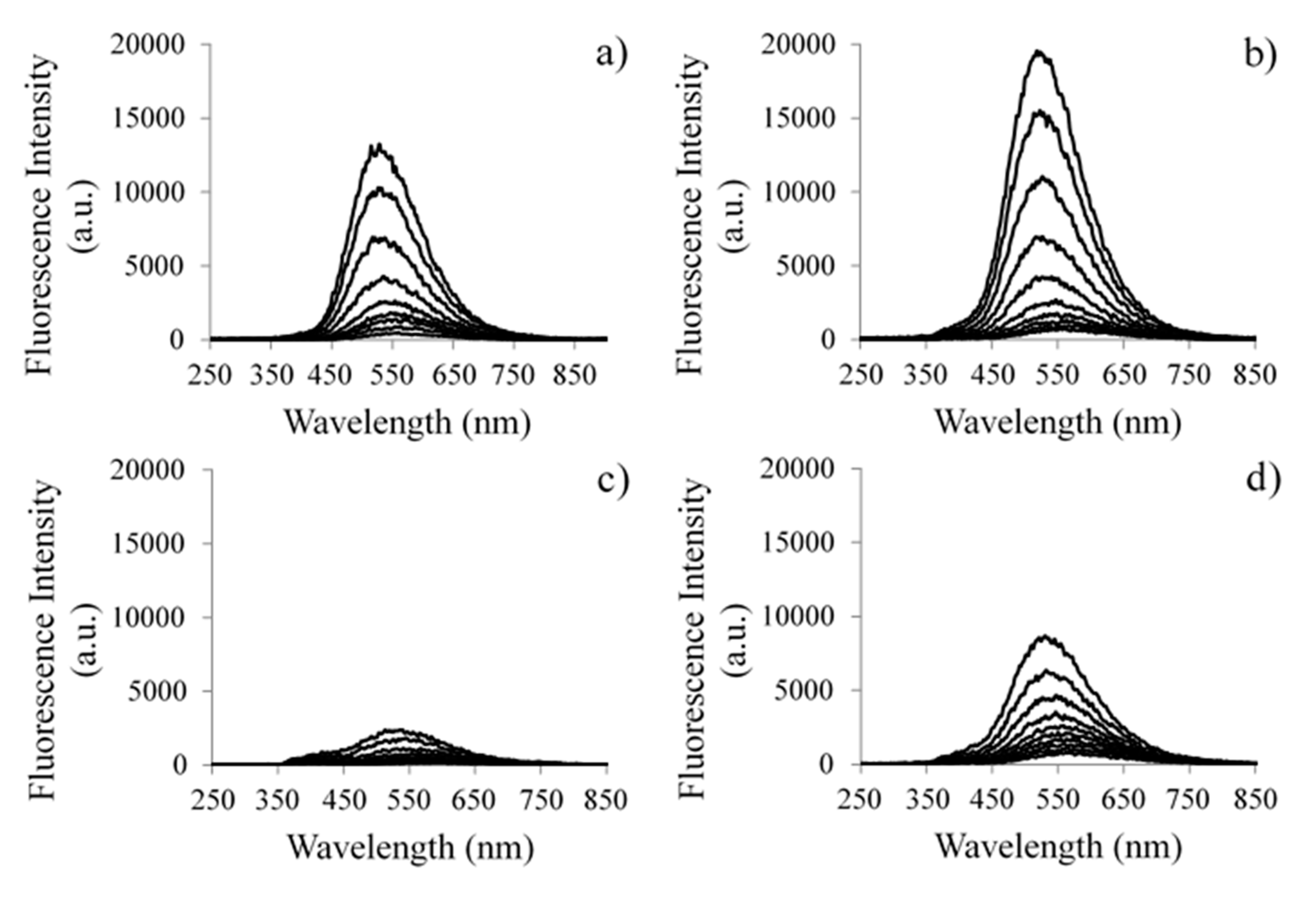
2.5. Laser Induced Luminescence Studies
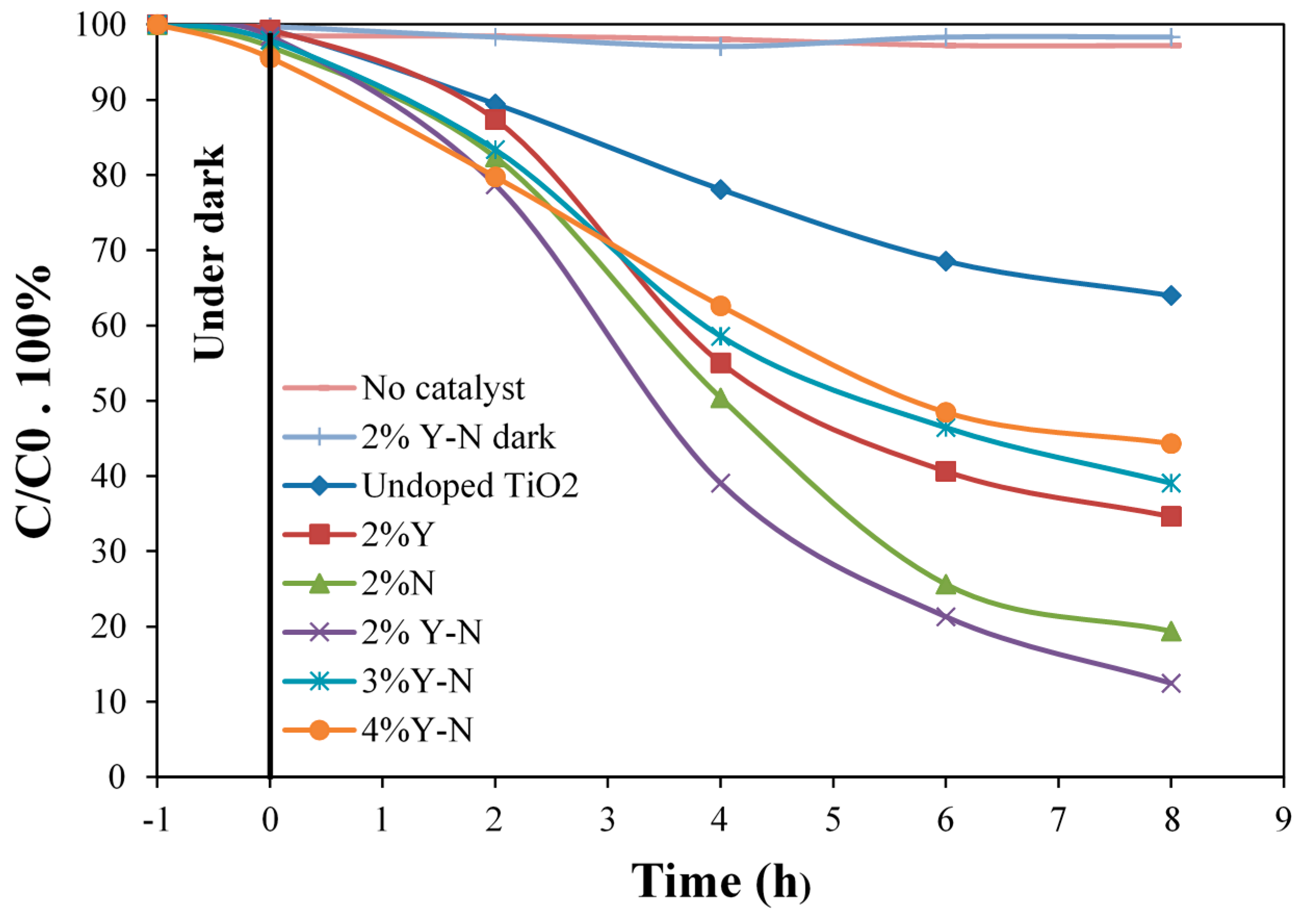
2.6. Photocatalytic Degradation
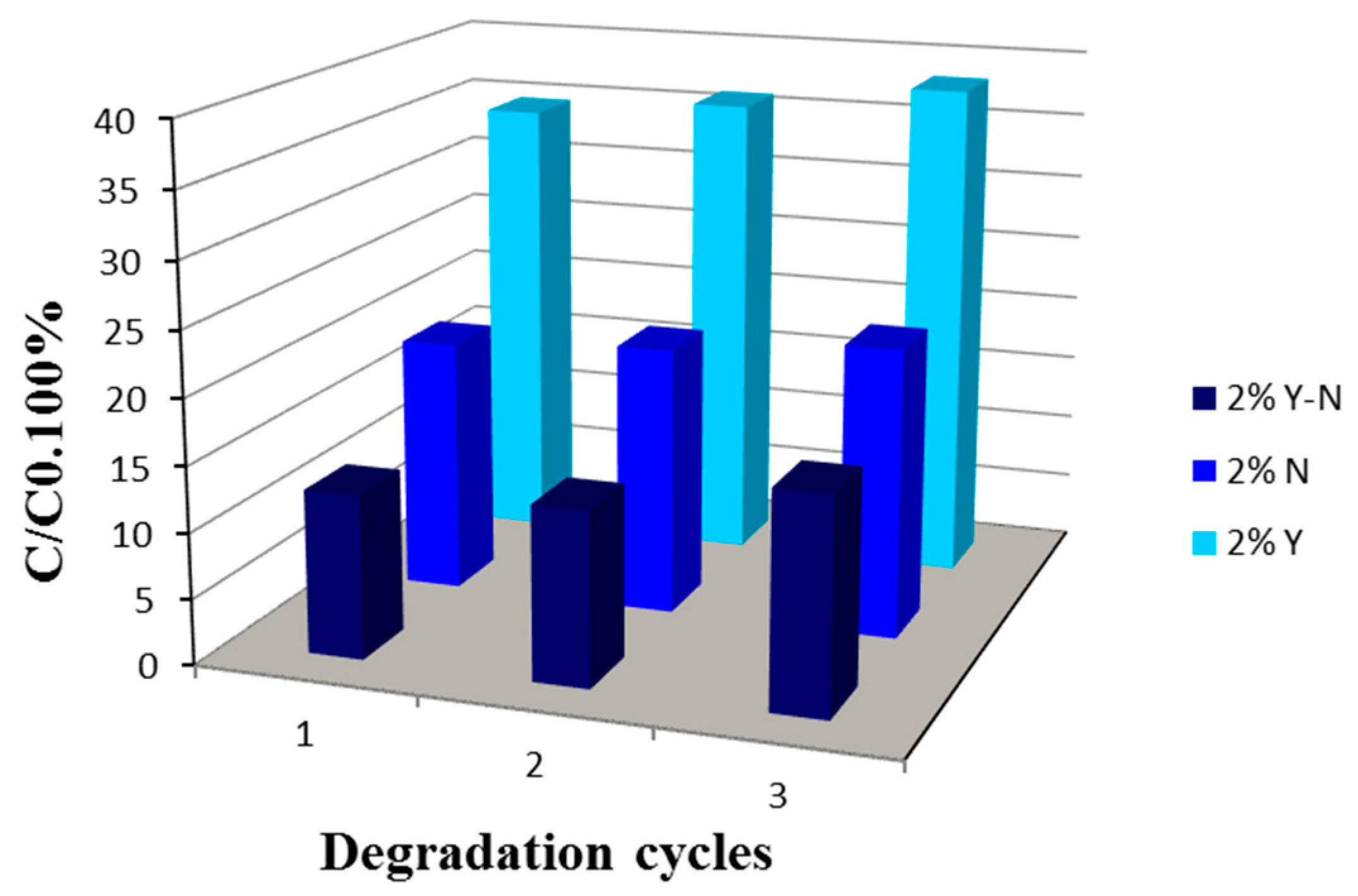
2.7. Reuse of the Photocatalyst
3. Materials and Methods
3.1. Preparation of Films and Powders
3.2. Physical and Chemical Characterization of the Catalysts
3.3. Photocatalytic Activity Measurements
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sreekanta, S.; Ahazan, R.; Lockman, Z. Photoactivity of anatase-rutile TiO2 nanotubes formed by anodization method. Thin Solid Films 2009, 518, 16–22. [Google Scholar] [CrossRef]
- Hamdi, A.; Ferraria, A.M.; Rego, A.M.B.; Ferreira, D.P.; Conceição, D.S.; Ferreira, L.F.V.; Bouattour, S. Bi–Y doped and codoped TiO2 nanoparticles: Characterization and photocatalytic activity undervisible light irradiation. J. Mol. Catal. A Chem. 2013, 380, 34–42. [Google Scholar] [CrossRef]
- Nosaka, Y.; Matsushita, M.; Nishino, J.; Nosaka, A.Y. Nitrogen-doped titanium dioxide photocatalysts for visible response prepared by using organic compounds. Sci. Technol. Adv. Mater. 2005, 6, 143–148. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-Light Photocatalysis in Nitrogen-Doped Titanium Oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, T.; Ohwaki, T.; Suzuki, K.; Moribe, S.; Kubota, S.T. Visible-light-induced photocatalytic oxidation of carboxylic acids and aldehydes over N-doped TiO2 loaded with Fe, Cu or Pt. Appl. Catal. B Environ. 2008, 83, 56–62. [Google Scholar] [CrossRef]
- Chen, X.; Luo, W. Optical spectroscopy of rare earth ion-doped TiO2nanophosphors. J. Nanosci. Nanotechnol. 2010, 10, 1482–1494. [Google Scholar] [CrossRef] [PubMed]
- Hamden, Z.; Ferreira, D.P.; Ferreira, L.V.; Bouattour, S. Li–Y doped and codopedTiO2 thin films: Enhancement of photocatalytic activity under visible light irradiation. Ceram. Int. 2014, 40, 3227–3235. [Google Scholar] [CrossRef]
- Narayan, H.; Alemu, H.; Setofolo, L.; Macheli, L. Visible Light Photocatalysis with Rare Earth Ion-Doped TiO2 Nanocomposites. ISRN Phys. Chem. 2012. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, K.; Feng, C. Photocatalytic degradation of methyl orange by polyoxometalates supported on yttrium-doped TiO2. J. Rare Earth 2011, 29, 866–877. [Google Scholar] [CrossRef]
- Bouattour, S.; Rego, A.M.B.; Ferreira, L.F.V. Photocatalytic activity of Li+–Rb+–Y3+ doped or codoped TiO2 under sunlight irradiation. Mater. Res. Bull. 2010, 45, 818–825. [Google Scholar] [CrossRef]
- Kallel, W.; Bouattour, S.; Kolsi, A.W. Structural and conductivity study of Y and Rb co-doped TiO2synthesized by the sol-gel method. J. Non-Cryst. Solids 2006, 352, 3970–3978. [Google Scholar] [CrossRef]
- Wu, Y.; Gong, Y.; Liu, J.; Zhang, Z.; Xu, Y.; Ren, H.; Li, C.; Niu, L. B and Y co-doped TiO2 photocatalyst with enhanced photodegradation efficiency. J. Alloys Compd. 2017, 695, 1462–1469. [Google Scholar] [CrossRef]
- Kuvarega, A.T.; Krause, R.W.M.; Mamba, B.B. Nitrogen/Palladium-Codoped TiO2 for Efficient Visible Light Photocatalytic Dye Degradation. J. Phys. Chem. C 2011, 115, 22110–22120. [Google Scholar] [CrossRef]
- Irie, H.; Watanabe, Y.; Hashimoto, K. Nitrogen-Concentration Dependence on Photocatalytic Activity of TiO2-xNxPowders. J. Phys. Chem. B 2003, 107, 5483–5486. [Google Scholar] [CrossRef]
- Hamden, Z.; Boufi, S.; Conceicão, D.S.; Ferraria, A.M.; Rego, A.M.B.; Ferreira, D.P.; Ferreira, L.F.V.; Bouattour, S. Li–N doped and codoped TiO2 thin films deposited by dip-coating: Characterization and photocatalytic activity under halogen lamp. Appl. Surf. Sci. 2014, 314, 910–918. [Google Scholar] [CrossRef]
- Khan, M.; Cao, W. Preparation of Y-doped TiO2 by hydrothermal method and investigation of its visible light photocatalytic activity by the degradation of methylene blue. J. Mol. Catal. A Chem. 2013, 376, 71–77. [Google Scholar] [CrossRef]
- Zhang, H.; Tan, K.; Zheng, H.; Gu, Y.; Zhang, W.F. Preparation, characterization and photocatalytic activity of TiO2 codoped with yttrium and nitrogen. Mater. Chem. Phys. 2011, 125, 156–160. [Google Scholar] [CrossRef]
- Birks, L.S.; Friedman, H. Particle Size Determination from X-Ray Line Broadening. J. Appl. Phys. 1946, 17, 687–692. [Google Scholar] [CrossRef]
- Shi, J.; Chen, J.; Feng, Z.; Chen, T.; Lian, Y.; Wang, X.; Li, C. Photoluminescence characteristics of TiO2 and their relationship to the photoassisted reaction of water/methanol mixture. J. Phys. Chem. C 2007, 111, 693–699. [Google Scholar] [CrossRef]
- Hughes, A.E.; Sexton, B.A. XPS study of an intergranular phase in yttria-zirconia. J. Mater. Sci. 1989, 24, 1057–1061. [Google Scholar] [CrossRef]
- Choudhury, B.; Borah, B.; Choudhury, A. Ce–N codoping effect on thestructural and optical properties of TiO2 nanoparticles. Mater. Sci. Eng. B 2013, 178, 239–247. [Google Scholar] [CrossRef]
- Zhu, K.; Zhang, M.; Chen, Q.; Yin, Z. Size and phonon-confinement effects on low-frequency Raman mode of anatase TiO2 nanocrystal. Phys. Lett. A 2005, 340, 220–227. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, J.; Li, H.; Wang, H.; Jia, X.; Su, P. The influence of yttrium dopant on the properties of anatase nanoparticles and the performance of dye-sensitized solar cells. Phys. Chem. Chem. Phys. 2015, 17, 14836–14842. [Google Scholar] [CrossRef] [PubMed]
- Mercado, C.; Seeley, Z.; Bandyopadhyay, A.; Bose, S.; McHale, J. Photoluminescence of dense nanocrystalline titanium dioxide thin films: Effect of doping and thickness and relation to gas sensing. ACS Appl. Mater. Interfaces 2011, 3, 2281–2288. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wang, X.; Wang, L.; Chen, Z.; Li, F.; Lu, G.; Cheng, H.M. Drastically enhanced photocatalytic activity in nitrogen doped mesoporous TiO2 with abundant surface states. J. Colloid Interface. Sci. 2009, 334, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Ohashi, N.; Hishita, S.; Kolodiazhnyi, T.; Haneda, H. Origin of visible-light-driven photocatalysis: A comparative study on N/F-doped and N–F-codoped TiO2 powders by means of experimental characterizations and theoretical calculations. J. Solid State Chem. 2005, 178, 3293–3302. [Google Scholar] [CrossRef]
- Agrios, A.G.; Gray, K.A.; Weitz, E. Narrow-band irradiation of a homologous series of chlorophenols on TiO2: Charge-transfer complex formation and reactivity. Langmuir 2004, 20, 5911–5918. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhu, L.; Huang, Y.; She, Y.; Yu, Y.; Tang, H. Drastically enhanced visible-light photocatalytic degradation of colorless aromatic pollutants over TiO2 via a charge-transfer-complex path: A correlation between chemical structure and degradation rate of the pollutants. J. Catal. 2009, 266, 199–206. [Google Scholar] [CrossRef]
- Rego, A.M.B.; Ferreira, L.F.V. Photonic and electronic spectroscopies for the characterization of organic surfaces and organic molecules adsorbed on surfaces. Exp. Methods Phys. Sci. 2001, 38, 269–354. [Google Scholar]
- Ferreira, L.F.V.; Machado, I.L.F. Surface Photochemistry: Organic Molecules within Nanocavities of Calixarenes. Curr. Drug Discov. Technol. 2007, 4, 229–245. [Google Scholar] [CrossRef]




| Sample | λabs (nm) | Eg (ev) |
|---|---|---|
| Undoped TiO2 | 396 | 3.13 |
| N doped | 392 | 3.17 |
| 2% Y doped | 400 | 3.10 |
| 2% Y-N codoped | 385 | 3.22 |
| 4% Y-N codoped | 396 | 3.13 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamden, Z.; Conceição, D.; Boufi, S.; Vieira Ferreira, L.F.; Bouattour, S. Structural, Morphological, Optical and Photocatalytic Properties of Y, N-Doped and Codoped TiO2 Thin Films. Materials 2017, 10, 600. https://doi.org/10.3390/ma10060600
Hamden Z, Conceição D, Boufi S, Vieira Ferreira LF, Bouattour S. Structural, Morphological, Optical and Photocatalytic Properties of Y, N-Doped and Codoped TiO2 Thin Films. Materials. 2017; 10(6):600. https://doi.org/10.3390/ma10060600
Chicago/Turabian StyleHamden, Zeineb, David Conceição, Sami Boufi, Luís Filipe Vieira Ferreira, and Soraa Bouattour. 2017. "Structural, Morphological, Optical and Photocatalytic Properties of Y, N-Doped and Codoped TiO2 Thin Films" Materials 10, no. 6: 600. https://doi.org/10.3390/ma10060600
APA StyleHamden, Z., Conceição, D., Boufi, S., Vieira Ferreira, L. F., & Bouattour, S. (2017). Structural, Morphological, Optical and Photocatalytic Properties of Y, N-Doped and Codoped TiO2 Thin Films. Materials, 10(6), 600. https://doi.org/10.3390/ma10060600






