Collagen-Fibrinogen Lyophilised Scaffolds for Soft Tissue Regeneration
Abstract
:1. Introduction
2. Results
2.1. Production of Collagen–Fibrinogen Scaffolds and Films
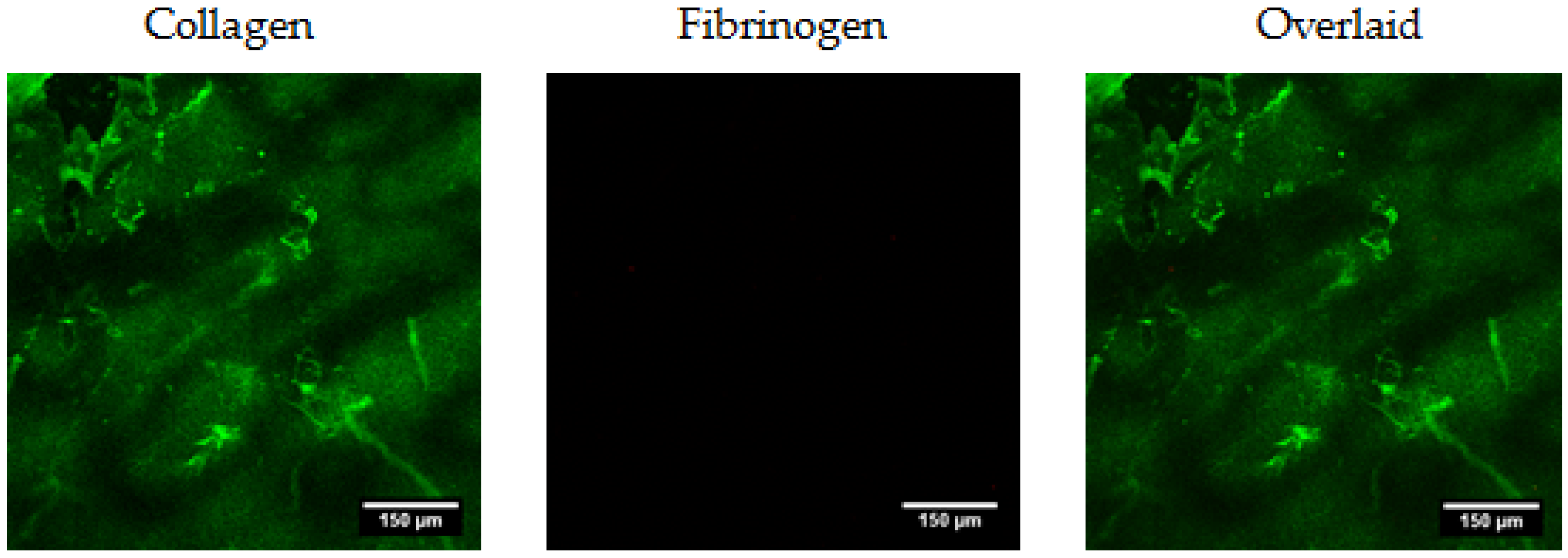
2.1.1. Structural Analysis with Micro-CT and Scanning Electron Microscopy (SEM)
2.2. Films for Cellular Analysis
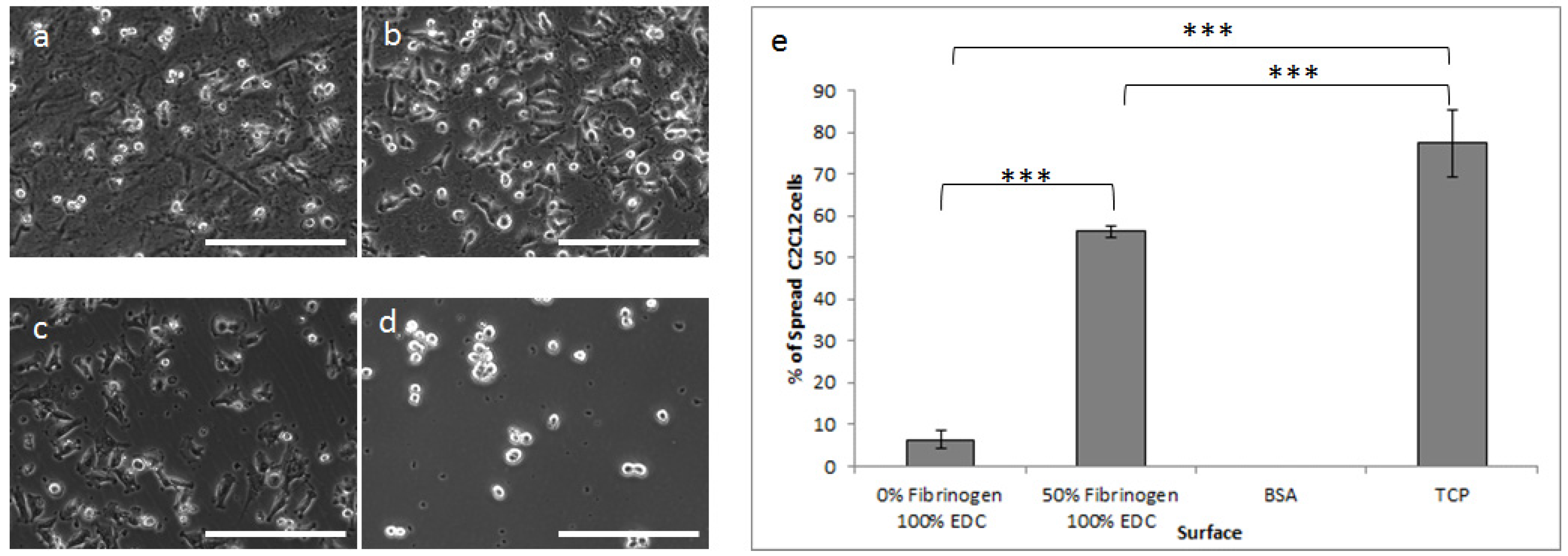
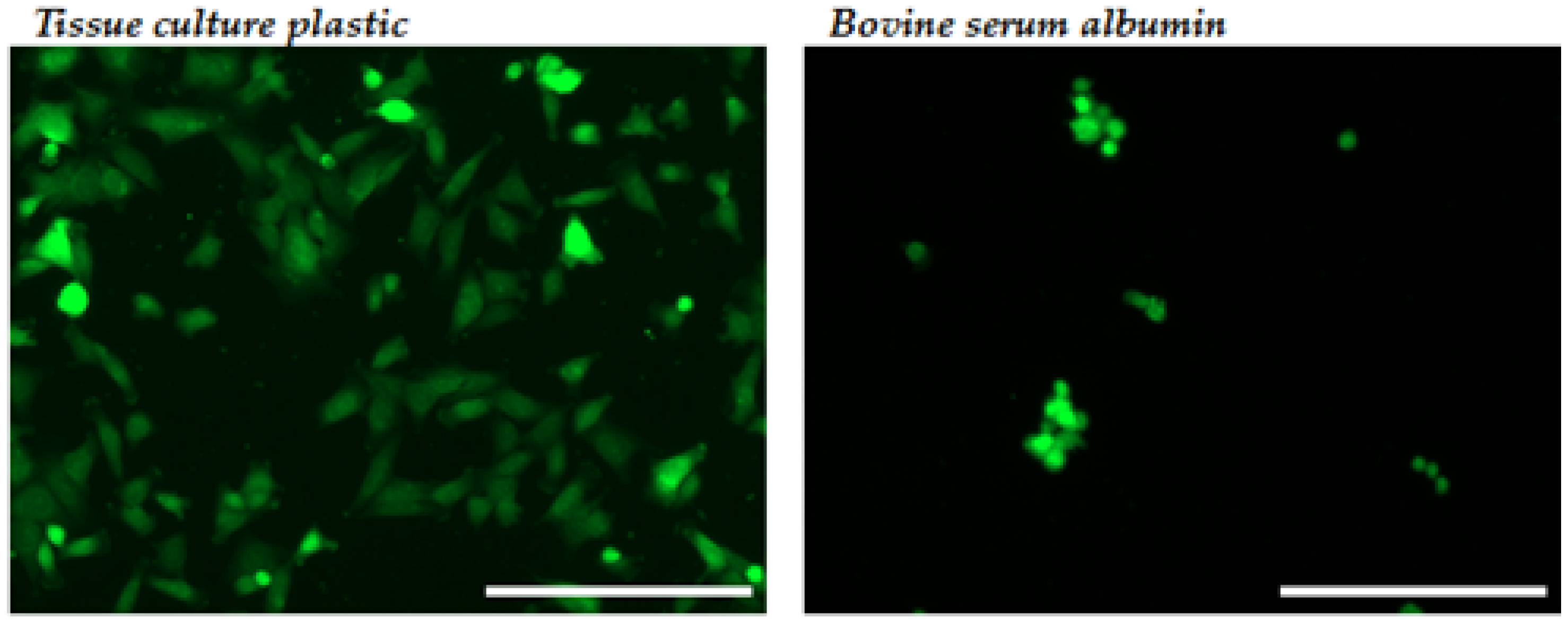
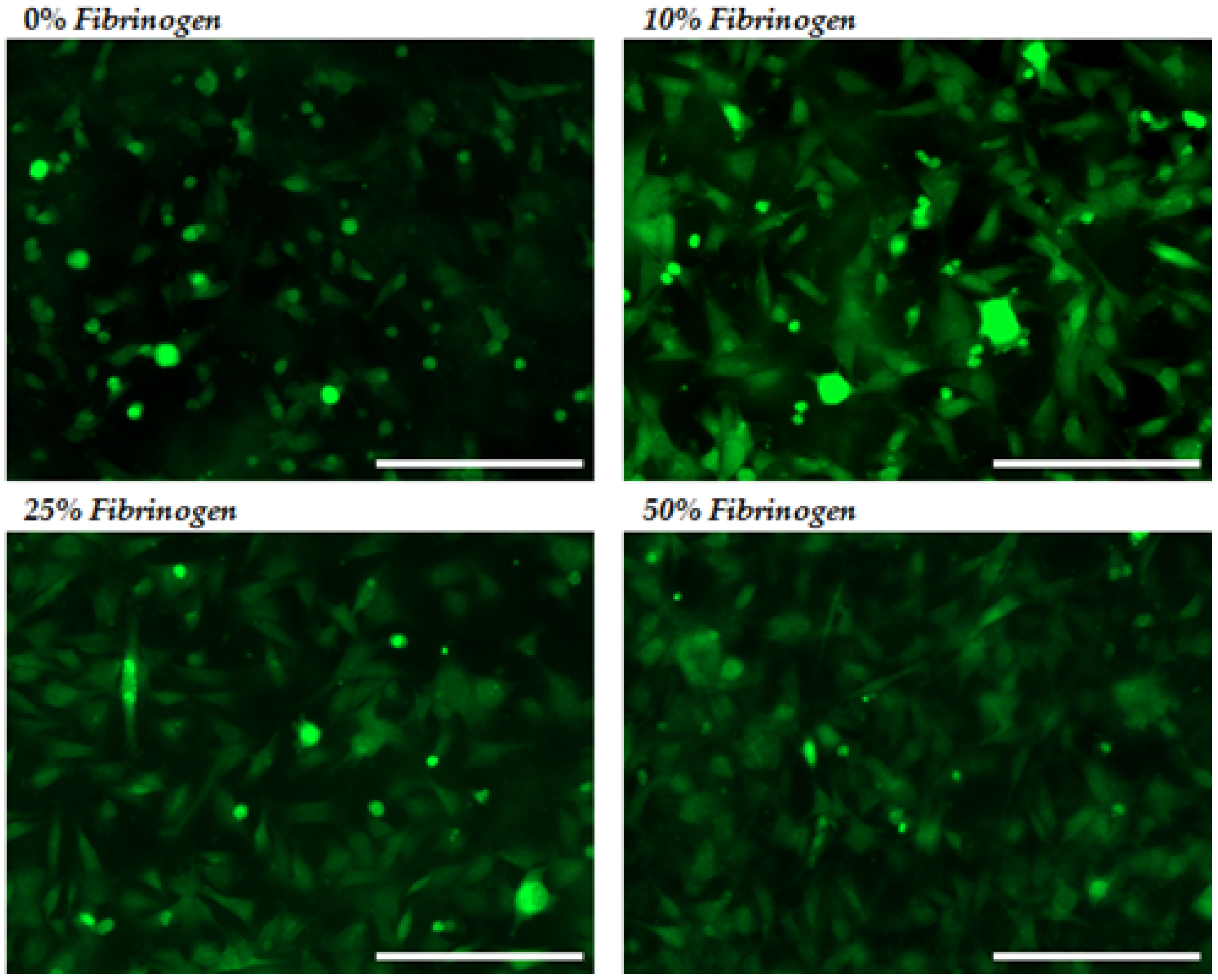
2.2.1. Cellular Adhesion and Spreading
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Collagen–Fibrinogen Scaffolds
4.3. Cross-Linking
4.4. Analysis of Distribution of Collagen and Fibrinogen
4.5. Characterisation of Scaffold Pore Structure
4.6. Collagen-Fibrinogen Films
4.7. Cell Culture
4.7.1. Cell Adhesion Analysis
4.7.2. Cell Spreading Analysis
4.8. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cen, L.; Liu, W.E.; Cui, L.E.; Zhang, W.; Cao, Y. Collagen tissue engineering: Development of novel biomaterials and applications. Pediatr. Res. 2008, 63, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Faraj, K.A.; Van Kuppevelt, T.H; Daamen, W.F. Construction of collagen scaffolds that mimic the three-dimensional architecture of specific tissues. Tissue Eng. 2007, 13, 2387–2394. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.G.; Kawazoe, N.; Tateishi, T.; Chen, G. Preparation of novel collagen sponges using an ice particulate template. J. Bioact. Compat. Polym. 2010, 25, 360–373. [Google Scholar] [CrossRef]
- Lu, H.; Ko, Y.G.; Kawazoe, N.; Chen, G. Cartilage tissue engineering using funnel-like collagen sponges prepared with embossing ice particulate templates. Biomaterials 2010, 31, 5825–5835. [Google Scholar] [CrossRef] [PubMed]
- Parenteau-Bareil, R.; Gauvin, R; Berthod, F. Collagen-based biomaterials for tissue engineering applications. Materials 2010, 3, 1863–1887. [Google Scholar] [CrossRef]
- Ellis, D.L.; Yannas, I.V. Recent advances in tissue synthesis in vivo by use of collagen-glycosaminoglycan copolymers. Biomaterials 1996, 17, 291–299. [Google Scholar] [CrossRef]
- Hanthamrongwit, M.; Reid, W.H.; Grant, M.H. Chondroitin-6-sulphate incorporated into collagen gels for the growth of human keratinocytes: the effect of cross-linking agents and diamines. Biomaterials 1996, 17, 775–780. [Google Scholar] [CrossRef]
- Matsuda, K.; Suzuki, S.; Isshiki, N.; Yoshioka, K.; Okada, T.; Ikada, Y. Influence of glycosaminoglycans on the collagen sponge component of a bilayer artificial skin. Biomaterials 1990, 11, 351–355. [Google Scholar] [CrossRef]
- Park, S.N.; Lee, H.J.; Lee, K.H.; Suh, H. Biological characterization of EDC-crosslinked collagen–hyaluronic acid matrix in dermal tissue restoration. Biomaterials 2003, 24, 1631–1641. [Google Scholar] [CrossRef]
- Powell, H.M.; Boyce, S.T. EDC cross-linking improves skin substitute strength and stability. Biomaterials 2006, 27, 5821–5827. [Google Scholar] [CrossRef] [PubMed]
- Tierney, C.M.; Haugh, M.G.; Liedl, J.; Mulcahy, F.; Hayes, B.; O’Brien, F.J. The effects of collagen concentration and crosslink density on the biological, structural and mechanical properties of collagen-GAG scaffolds for bone tissue engineering. J. Mech. Behav. Biomed. Mater. 2009, 2, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, J.H.; Ghose, S.; Kew, S.J.; Moavenian, A.; Best, S.M.; Cameron, R.E. Effect of fiber crosslinking on collagen-fiber reinforced collagen–chondroitin-6-sulfate materials for regenerating load-bearing soft tissues. J. Biomed. Mater. Res. A 2013, 101, 176–184. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, F.J.; Harley, B.A.; Yannas, I.V.; Gibson, L. Influence of freezing rate on pore structure in freeze-dried collagen-GAG scaffolds. Biomaterials 2004, 25, 1077–1086. [Google Scholar] [CrossRef]
- Davidenko, N.; Campbell, J.J.; Thian, E.S.; Watson, C.J.; Cameron, R.E. Collagen–hyaluronic acid scaffolds for adipose tissue engineering. Acta Biomater. 2010, 6, 3957–3968. [Google Scholar] [CrossRef] [PubMed]
- Yannas, I.V.; Burke, J.F.; Huang, C.; Gordon, P.L. Suppression of In vivo Degradability and of Immunogenicity of Collagen by Reaction with Glycosaminoglycans. Polym. Prepr. Am. Chem. Soc. Div. Polym. Chem. 1975, 16, 209–214. [Google Scholar]
- Daamen, W.F.; Van Moerkerk, H.T.B.; Hafmans, T.; Buttafoco, L.; Poot, A.A.; Veerkamp, J.H.; Van Kuppevelt, T.H. Preparation and evaluation of molecularly-defined collagen–elastin–glycosaminoglycan scaffolds for tissue engineering. Biomaterials 2003, 24, 4001–4009. [Google Scholar] [CrossRef]
- Buttafoco, L.; Kolkman, N.G.; Engbers-Buijtenhuijs, P.; Poot, A.A.; Dijkstra, P.J.; Vermes, I.; Feijen, J. Electrospinning of collagen and elastin for tissue engineering applications. Biomaterials 2006, 27, 724–734. [Google Scholar] [CrossRef] [PubMed]
- Herrick, S.; Blanc-Brude, O; Gray, A.; Laurent, G. Fibrinogen. Int. J. Biochem. Cell Biol. 1999, 31, 741–746. [Google Scholar] [CrossRef]
- Spotnitz, W.D. Commercial fibrin sealants in surgical care. Am. J. Surg. 2001, 182, S8–S14. [Google Scholar] [CrossRef]
- Jackson, M.R. Fibrin sealants in surgical practice: An overview. Am. J. Surg. 2001, 182, S1–S7. [Google Scholar] [CrossRef]
- Wnek, G.E.; Carr, M.E.; Simpson, D.G.; Bowlin, G.L. Electrospinning of nanofiber fibrinogen structures. Nano. Lett. 2003, 3, 213–216. [Google Scholar] [CrossRef]
- Sell, S.A.; McClure, M.J.; Garg, K.; Wolfe, P.S.; Bowlin, G.L. Electrospinning of collagen/ biopolymers for regenerative medicine and cardiovascular tissue engineering. Adv. Drug Deliv. Rev. 2009, 61, 1007–1019. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.K.; Petersen, M.A.; Murray, S.G.; Baeten, K.M.; Meyer-Franke, A.; Chan, J.P.; Vagena, E.; Bedard, C.; Machado, M.R.; Coronado, P.E.R.; et al. Blood coagulation protein fibrinogen promotes autoimmunity and demyelination via chemokine release and antigen presentation. Nat. Commun. 2015, 6, 8164. [Google Scholar] [CrossRef] [PubMed]
- McManus, M.C.; Boland, E.D.; Simpson, D.G.; Barnes, C.P.; Bowlin, G.L. Electrospun fibrinogen: Feasibility as a tissue engineering scaffold in a rat cell culture model. J. Biomed. Mater. Res. A 2007, 81, 299–309. [Google Scholar] [CrossRef] [PubMed]
- McManus, M.; Boland, E.; Sell, S.; Bowen, W.; Koo, H.; Simpson, D.; Bowlin, G. Electrospun nanofibre fibrinogen for urinary tract tissue reconstruction. Biomed. Mater. 2007, 2, 257. [Google Scholar] [CrossRef] [PubMed]
- Underwood, S.; Afoke, A.; Brown, R.A.; MacLeod, A.J.; Dunnill, P. The physical properties of a fibrillar fibronectin-fibrinogen material with potential use in tissue engineering. Bioproc. Biosyst. Eng. 1999, 20, 239–248. [Google Scholar] [CrossRef]
- Underwood, S.; Afoke, A.; Brown, R.A.; MacLeod, A.J.; Shamlou, P.A.; Dunnill, P. Wet extrusion of fibronectin–fibrinogen cables for application in tissue engineering. Biotechnol. Bioeng. 2001, 73, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Linnes, M.P.; Ratner, B.D.; Giachelli, C.M. A fibrinogen-based precision microporous scaffold for tissue engineering. Biomaterials 2007, 28, 5298–5306. [Google Scholar] [CrossRef] [PubMed]
- Mol, A.; van Lieshout, M.I.; Dam-de Veen, C.G.; Neuenschwander, S.; Hoerstrup, S.P.; Baaijens, F.P.; Bouten, C.V. Fibrin as a cell carrier in cardiovascular tissue engineering applications. Biomaterials 2005, 26, 3113–3121. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.A.; Lanigan, J.M.; DellaPelle, P.; Manseau, E.; Dvorak, H.F.; Colvin, R.B. Fibronectin and fibrin provide a provisional matrix for epidermal cell migration during wound reepithelialization. J. Investig. Dermatol. 1992, 79, 264–269. [Google Scholar] [CrossRef]
- McManus, M.C.; Boland, E.D.; Koo, H.P.; Barnes, C.P.; Pawlowski, K.J.; Wnek, G.E.; Simpson, D.G.; Bowlin, G.L. Mechanical properties of electrospun fibrinogen structures. Acta Biomater. 2006, 2, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Zünd, G.; Benedikt, P.; Jockenhoevel, S.; Hoerstrup, S.P.; Sakyama, S.; Hubbell, J.A.; Turina, M. Fibrin gel as a three dimensional matrix in cardiovascular tissue engineering. Eur. J. Cardiothorac. Surg. 2000, 17, 587–591. [Google Scholar] [CrossRef]
- Dainiak, M.B.; Allan, I.U.; Savina, I.N.; Cornelio, L.; James, E.S.; James, S.L.; Mikhalovsky, S.V.; Jungvid, H.; Galaev, I.Y. Gelatin–fibrinogen cryogel dermal matrices for wound repair: Preparation, optimisation and in vitro study. Biomaterials 2010, 31, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, R.; De Cicco, G.; Vizzardi, E.; Gelsomino, S. Human fibrinogen/thrombin-coated collagen patch to control intraoperative severe pulmonary hemorrhage and air leakage after correction of a ruptured thoracic aortic aneurysm. Ann. Thorac. Surg. 2011, 91, 917–919. [Google Scholar] [CrossRef] [PubMed]
- Cummings, C.L.; Gawlitta, D.; Nerem, R.M.; Stegemann, J.P. Properties of engineered vascular constructs made from collagen, fibrin, and collagen–fibrin mixtures. Biomaterials 2004, 25, 3699–3706. [Google Scholar] [CrossRef] [PubMed]
- Rowe, S.L.; Stegemann, J.P. Interpenetrating collagen-fibrin composite matrices with varying protein contents and ratios. Biomacromolecules 2006, 7, 2942–2948. [Google Scholar] [CrossRef] [PubMed]
- Park, S.N.; Park, J.C.; Kim, H.O.; Song, M.J.; Suh, H. Characterization of porous collagen/hyaluronic acid scaffold modified by 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide cross-linking. Biomaterials 2002, 23, 1205–1212. [Google Scholar] [CrossRef]
- Cornwell, K.G.; Lei, P.; Andreadis, S.T.; Pins, G.D. Crosslinking of discrete self-assembled collagen threads: Effects on mechanical strength and cell–matrix interactions. J. Biomed. Mater. Res. A 2007, 80, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Osborne, C.S.; Reid, W.H.; Grant, M.H. Investigation into cell growth on collagen/chondroitin-6-sulphate gels: The effect of crosslinking agents and diamines. J. Mater. Sci. Mater. Med. 1997, 8, 179–184. [Google Scholar] [CrossRef]
- Ahmad, Z.; Shepherd, J.H.; Shepherd, D.V.; Ghose, S.; Kew, S.J.; Cameron, R.E.; Best, S.M.; Brooks, R.A.; Wardale, J.; Rushton, N. Effect of 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide and N-hydroxysuccinimide concentrations on the mechanical and biological characteristics of cross-linked collagen fibres for tendon repair. Regen. Biomater. 2015, 2, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Damink, L.O.; Dijkstra, P.J.; Van Luyn, M.J.A.; Van Wachem, P.B.; Nieuwenhuis, P.; Feijen, J. In vitro degradation of dermal sheep collagen cross-linked using a water-soluble carbodiimide. Biomaterials 1996, 17, 679–684. [Google Scholar] [CrossRef]
- Ashworth, J.C.; Mehr, M.; Buxton, P.G.; Best, S.M.; Cameron, R.E. Cell invasion in collagen scaffold architectures characterized by percolation theory. Adv. Healthc. Mater. 2015, 4, 1317–1321. [Google Scholar] [CrossRef] [PubMed]
- Pawelec, K.M.; Shepherd, J.; Jugdaohsingh, R.; Best, S.M.; Cameron, R.E.; Brooks, R.A. Collagen scaffolds as a tool for understanding the biological effect of silicates. Mater. Lett. 2015, 157, 176–179. [Google Scholar] [CrossRef]
- Grover, C.N.; Cameron, R.E.; Best, S.M. Investigating the morphological, mechanical and degradation properties of scaffolds comprising collagen, gelatin and elastin for use in soft tissue engineering. J. Mech. Behav. Biomed. Mater. 2012, 10, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Harley, B.A.; Lynn, A.K.; Wissner-Gross, Z.; Bonfield, W.; Yannas, I.V.; Gibson, L.J. Design of a multiphase osteochondral scaffold. II. Fabrication of a mineralized collagen–glycosaminoglycan scaffold. J. Biomed. Mater. Res. A 2010, 92, 1066–1077. [Google Scholar] [CrossRef] [PubMed]
- Davidenko, N.; Schuster, C.F.; Bax, D.V.; Raynal, N.; Farndale, R.W.; Best, S.M.; Cameron, R.E. Control of crosslinking for tailoring collagen-based scaffolds stability and mechanics. Acta Biomater. 2015, 25, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Davidenko, N.; Schuster, C.F.; Bax, D.V.; Farndale, R.W.; Hamaia, S.; Best, S.M.; Cameron, R.E. Evaluation of cell binding to collagen and gelatin: A study of the effect of 2D and 3D architecture and surface chemistry. J. Mater. Sci. Mater. Med. 2016, 27, 148. [Google Scholar] [CrossRef] [PubMed]
- Ber, S.; Köse, G.T.; Hasırcı, V. Bone tissue engineering on patterned collagen films: An in vitro study. Biomaterials 2005, 26, 1977–1986. [Google Scholar] [CrossRef] [PubMed]
- Grover, C.N.; Gwynne, J.H.; Pugh, N.; Hamaia, S.; Farndale, R.W.; Best, S.M.; Cameron, R.E. Crosslinking and composition influence the surface properties, mechanical stiffness and cell reactivity of collagen-based films. Acta Biomater. 2012, 8, 3080–3090. [Google Scholar] [CrossRef] [PubMed]
- Bax, D.V.; Davidenko, N.; Gullberg, D.; Hamaia, S.W.; Farndale, R.W.; Best, S.M.; Cameron, R.E. Fundamental insight into the effect of carbodiimide crosslinking on cellular recognition of collagen-based scaffolds. Acta Biomater. 2016, 49, 218–234. [Google Scholar] [CrossRef] [PubMed]
- Giancotti, F.G.; Ruoslahti, E. Integrin signaling. Science 1999, 285, 1028–1033. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.O. Integrins: Versatility, modulation, and signaling in cell adhesion. Cell 1992, 69, 11–25. [Google Scholar] [CrossRef]
- Ma, L.; Gao, C.; Mao, Z.; Zhou, J.; Shen, J.; Hu, X.; Han, C. Collagen/chitosan porous scaffolds with improved biostability for skin tissue engineering. Biomaterials 2003, 24, 4833–4841. [Google Scholar] [CrossRef]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric scaffolds in tissue engineering application: A review. Int. J. Polym. Sci. 2011. [Google Scholar] [CrossRef]
- Murphy, C.M.; Haugh, M.G.; O'Brien, F.J. The effect of mean pore size on cell attachment, proliferation and migration in collagen–glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 2010, 31, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- Garcı́a, A.J.; Vega, M.D.; Boettiger, D. Modulation of cell proliferation and differentiation through substrate-dependent changes in fibronectin conformation. Mol. Biol. Cell 1999, 10, 785–798. [Google Scholar] [CrossRef] [PubMed]
- Damink, L.O.; Dijkstra, P.J.; Van Luyn, M.J.A.; Van Wachem, P.B.; Nieuwenhuis, P.; Feijen, J. Cross-linking of dermal sheep collagen using a water-soluble carbodiimide. Biomaterials 1996, 17, 765–773. [Google Scholar] [CrossRef]
- Doolittle, R.F.; Watt, K.W.; Cottrell, B.A.; Strong, D.D.; Riley, M. The amino acid sequence of the alpha-chain of human fibrinogen. Nature 1979, 280, 464. [Google Scholar] [CrossRef] [PubMed]
- Watt, K.W.; Takagi, T.; Doolittle, R.F. Amino acid sequence of the beta chain of human fibrinogen: Homology with the gamma chain. Proc. Natl. Acad. Sci. USA 1978, 75, 1731–1735. [Google Scholar] [CrossRef] [PubMed]
- Otsu, N. A threshold selection method from gray-level histograms. Automatica 1975, 11, 23–27. [Google Scholar] [CrossRef]
- Lorensen, W.E.; Cline, H.E.; Lorensen, W.E.; Cline, H.E. Marching cubes: A high resolution 3D surface construction algorithm. ACM Siggraph Comput. Graph. 1987, 21, 163–169. [Google Scholar] [CrossRef]
- Sotta, P.; Long, D. The crossover from 2D to 3D percolation: Theory and numerical simulations. EPJE 2003, 11, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.-H.; Carman, C.V.; Springer, T.A. Structural basis of integrin regulation and signaling. Annu. Rev. Immunol. 2007, 25, 619–647. [Google Scholar] [CrossRef] [PubMed]
- Humphries, M.J. Cell-substrate adhesion assays. In Current Protocols in Cell Biology; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2011. [Google Scholar] [CrossRef]
- Shepherd, J.; Bax, D.; Best, S.; Cameron, R. Collagen-Fibrinogen Lyophilised Scaffolds for Soft Tissue Regeneration; Cambridge University’s Apollo Depository: Cambridge, UK, 2017. [Google Scholar]

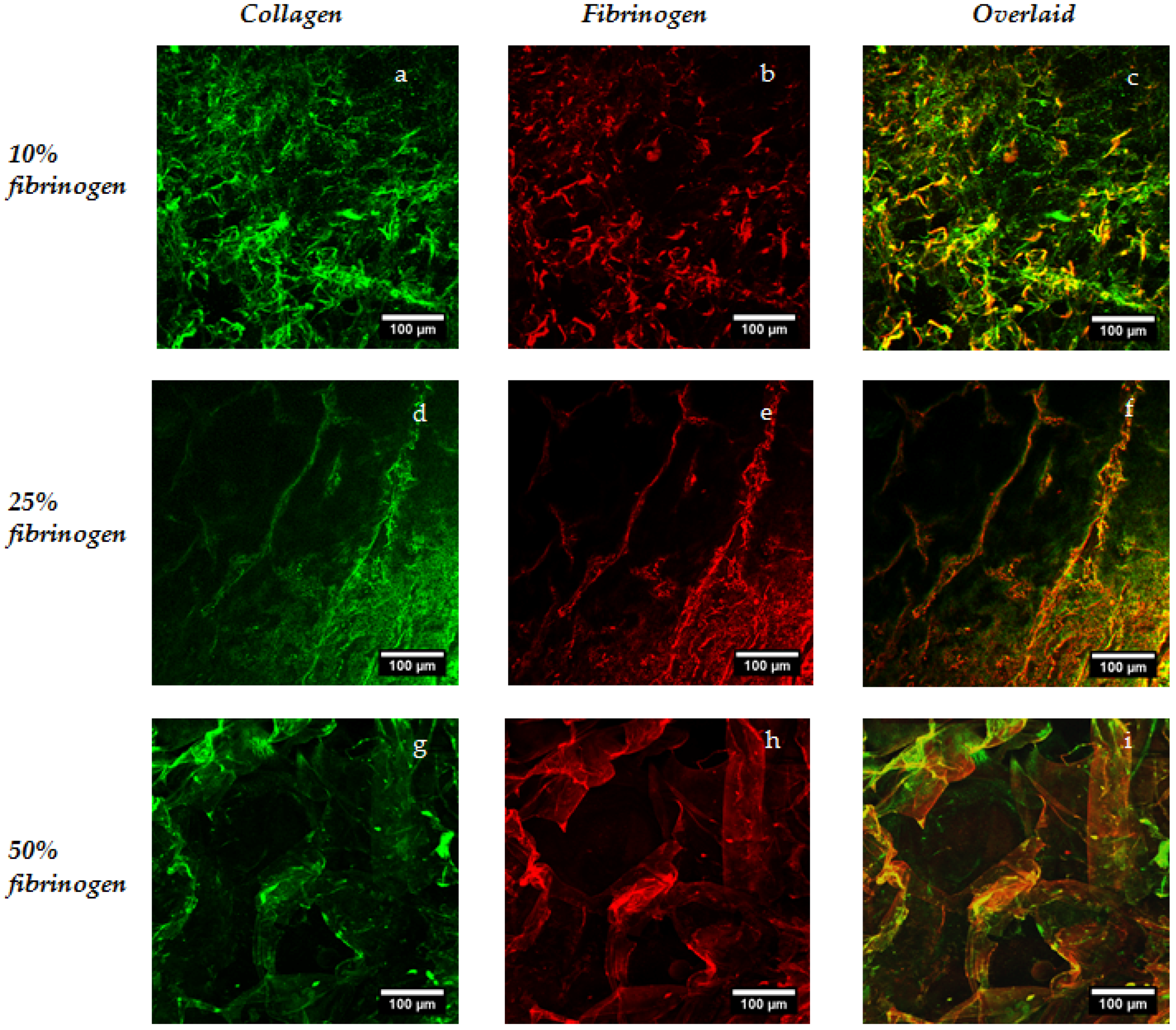
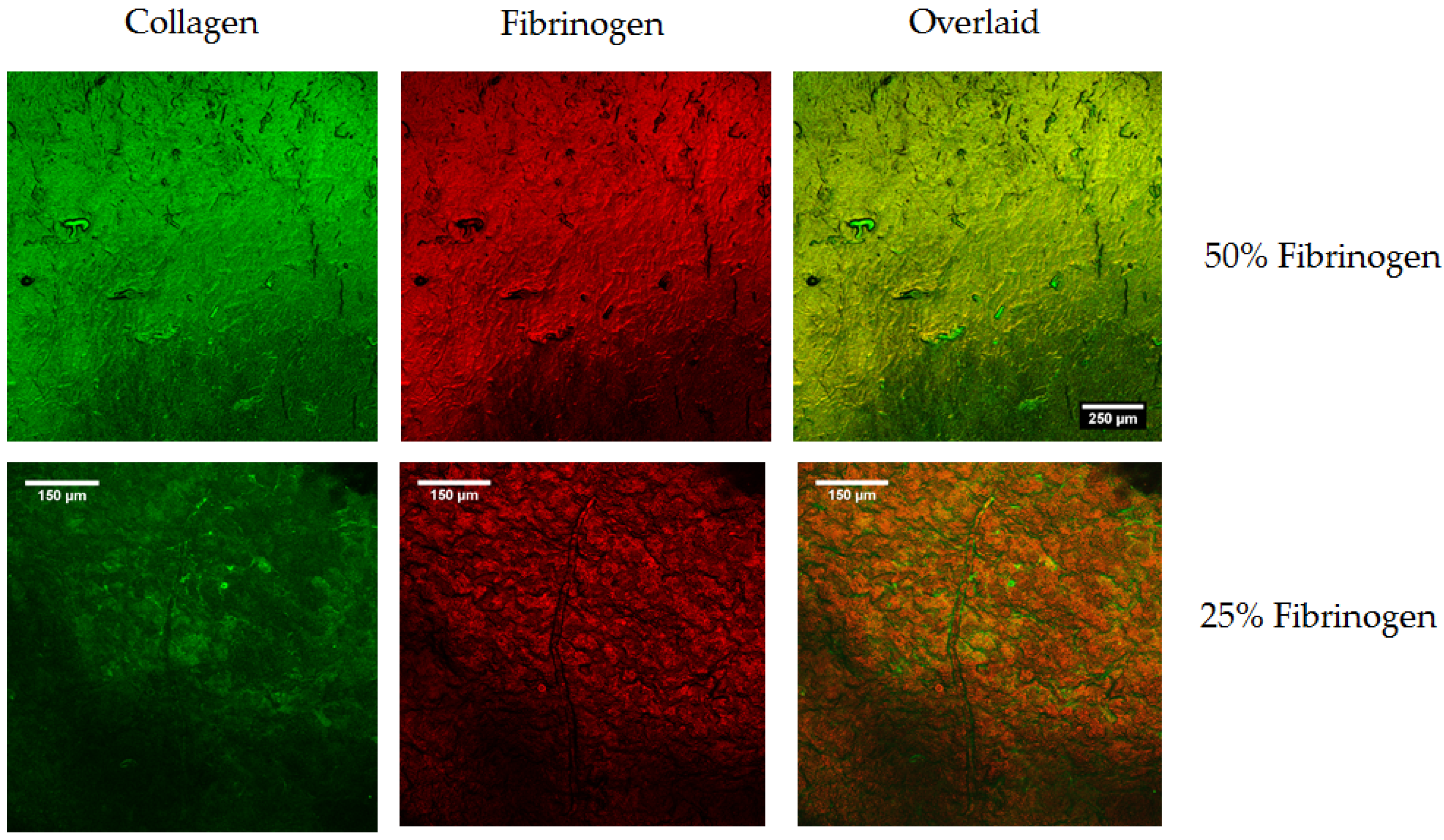
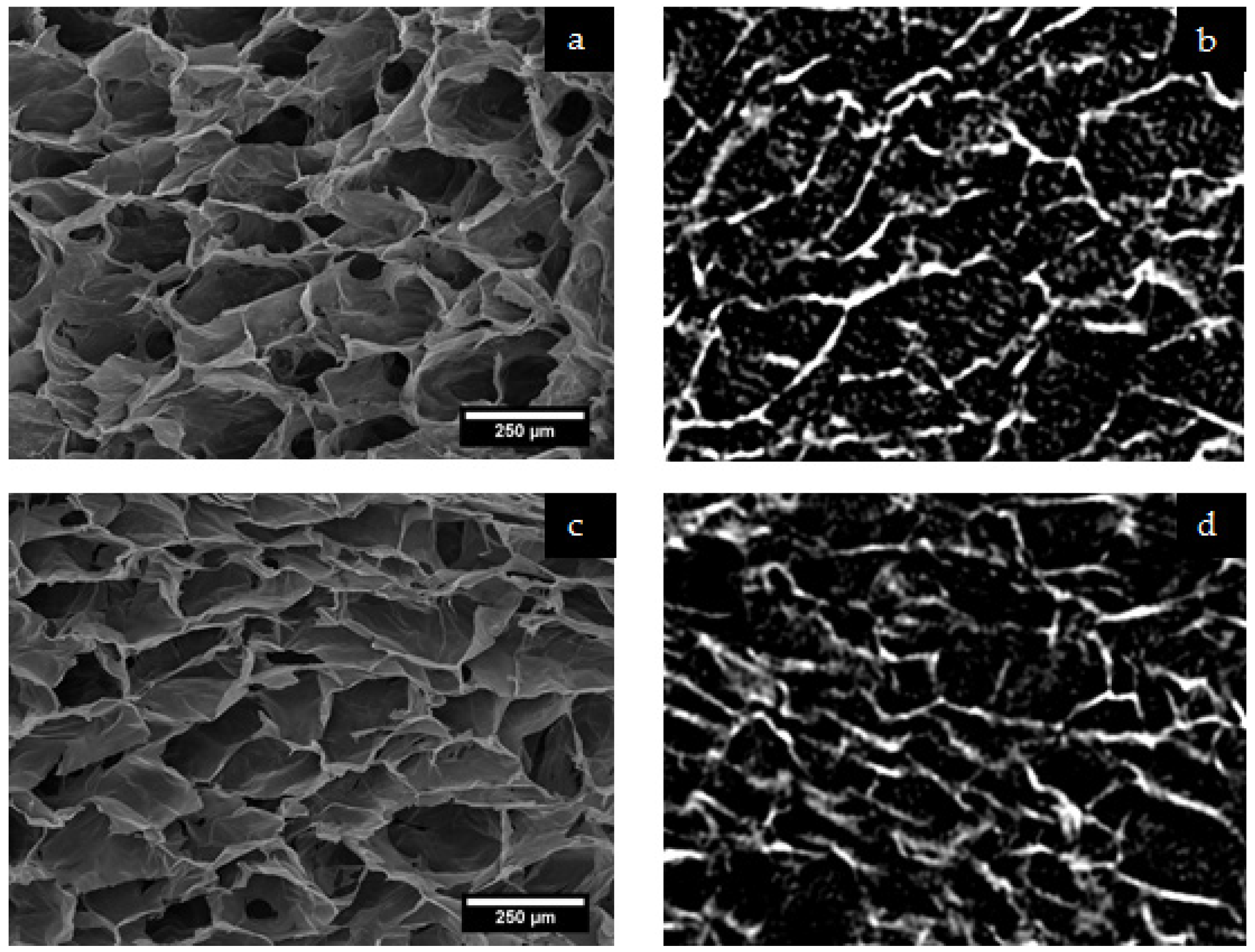
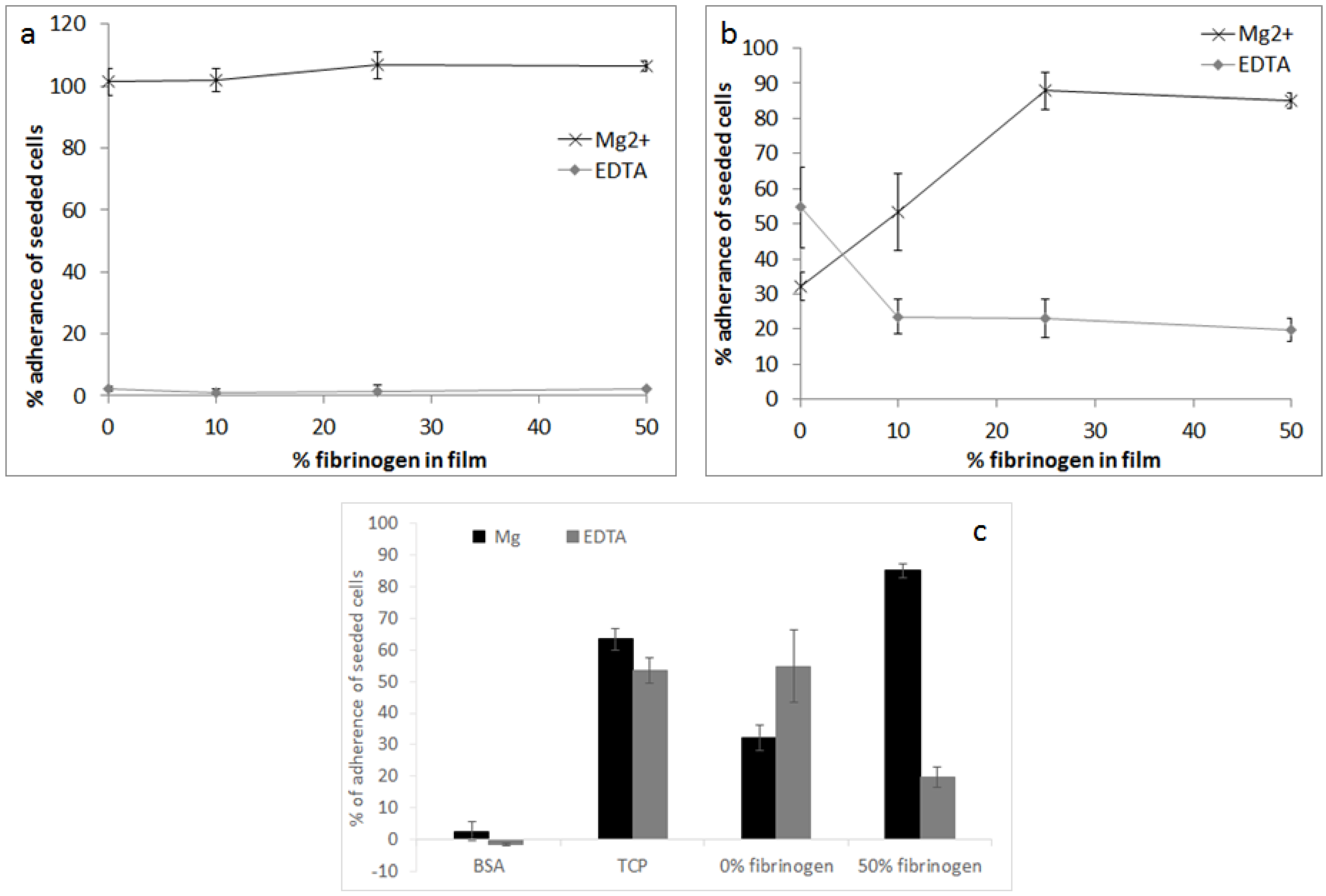
| Fibrinogen Content | Cross-Linking Concentration | ||||
|---|---|---|---|---|---|
| 1% | 10% | 30% | 50% | 100% | |
| 0% | μCT, sIF | μCT, sIF, CA | μCT, sIF, fIF | μCT, sIF | μCT, sIF, CA, CS |
| 10% | μCT, sIF | μCT, sIF, CA | μCT, sIF, fIF | μCT, sIF | μCT, sIF, CA, CS |
| 25% | μCT, sIF | μCT, sIF, CA | μCT, sIF, fIF | μCT, sIF | μCT, sIF, CA, CS |
| 50% | μCT, sIF | μCT, sIF, CA | μCT, sIF, fIF | μCT, sIF | μCT, sIF, CA, CS |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shepherd, J.; Bax, D.; Best, S.; Cameron, R. Collagen-Fibrinogen Lyophilised Scaffolds for Soft Tissue Regeneration. Materials 2017, 10, 568. https://doi.org/10.3390/ma10060568
Shepherd J, Bax D, Best S, Cameron R. Collagen-Fibrinogen Lyophilised Scaffolds for Soft Tissue Regeneration. Materials. 2017; 10(6):568. https://doi.org/10.3390/ma10060568
Chicago/Turabian StyleShepherd, Jennifer, Daniel Bax, Serena Best, and Ruth Cameron. 2017. "Collagen-Fibrinogen Lyophilised Scaffolds for Soft Tissue Regeneration" Materials 10, no. 6: 568. https://doi.org/10.3390/ma10060568
APA StyleShepherd, J., Bax, D., Best, S., & Cameron, R. (2017). Collagen-Fibrinogen Lyophilised Scaffolds for Soft Tissue Regeneration. Materials, 10(6), 568. https://doi.org/10.3390/ma10060568





