Current Insights into the Modulation of Oral Bacterial Degradation of Dental Polymeric Restorative Materials
Abstract
:1. Introduction
2. Concept for Restorative Polymeric Direct Materials
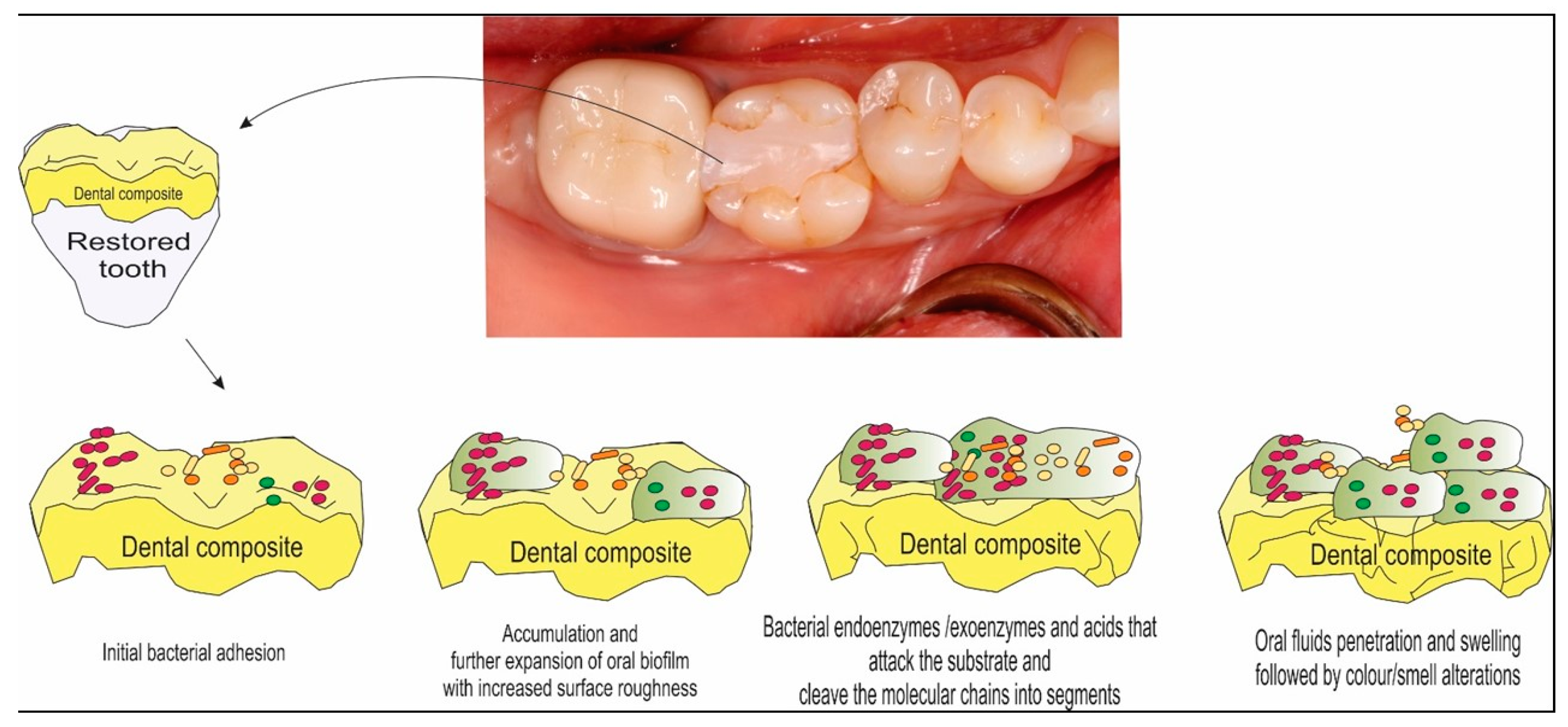
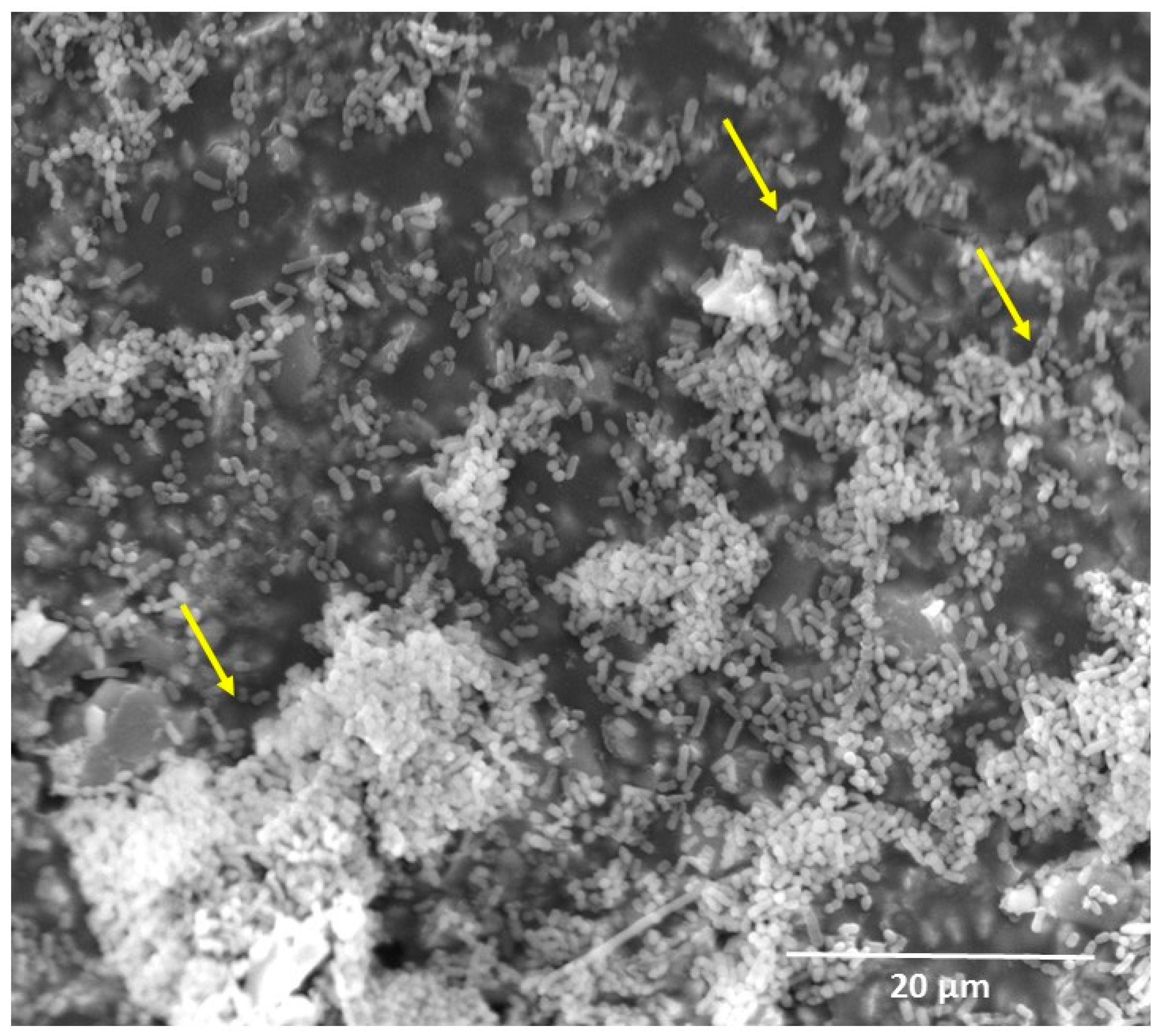
3. Oral Biofilm Degradation of Polymeric Restorative Dental Materials
4. Emerging Approaches to Reduce Oral Biofilm Degradation
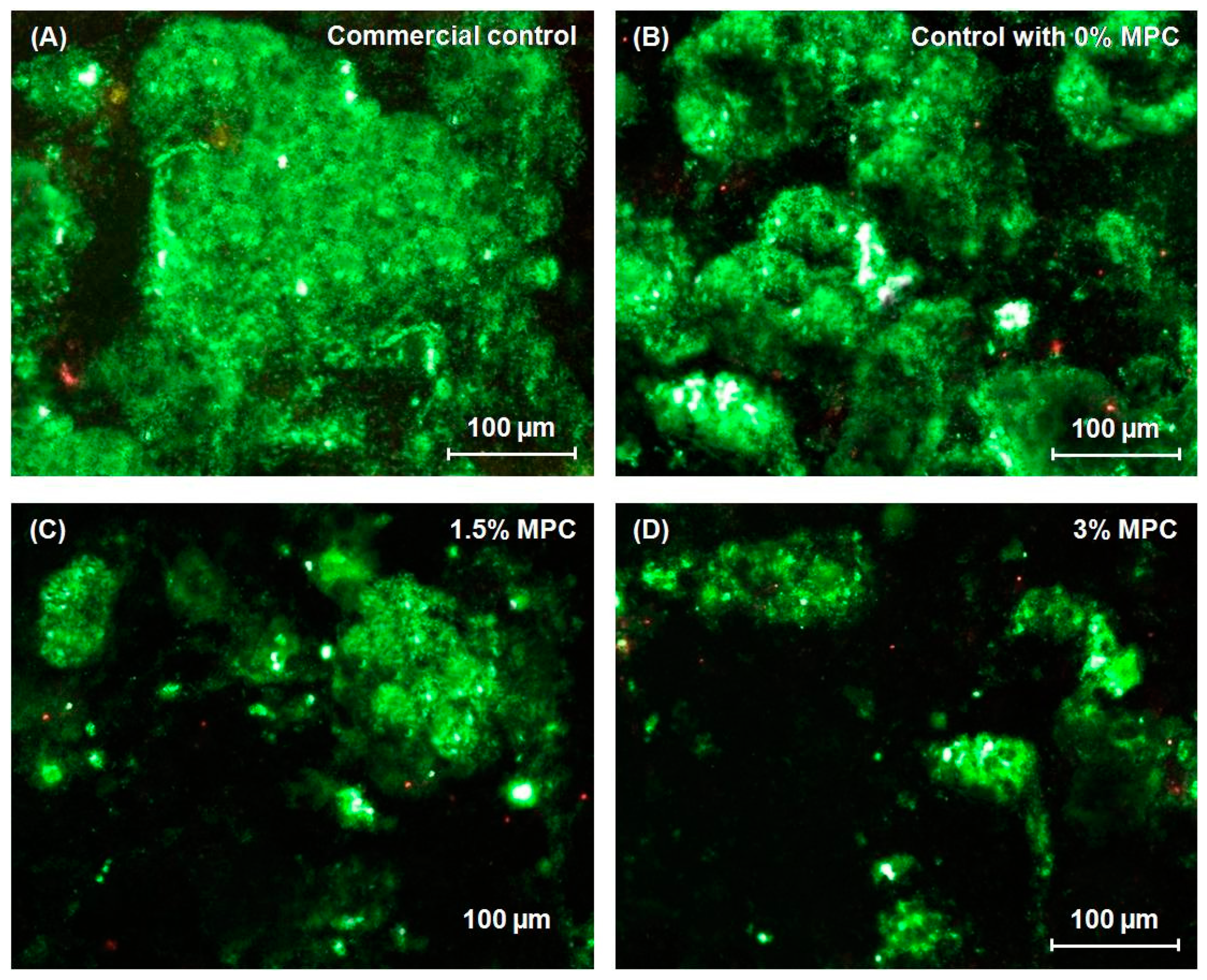
4.1. Resin Composite Containing Releasing Antibacterial Agents
4.2. Resin Composite Containing Non-Releasing Antibacterial Agents
4.3. Resin Composite Containing Protein-Repellent Monomers
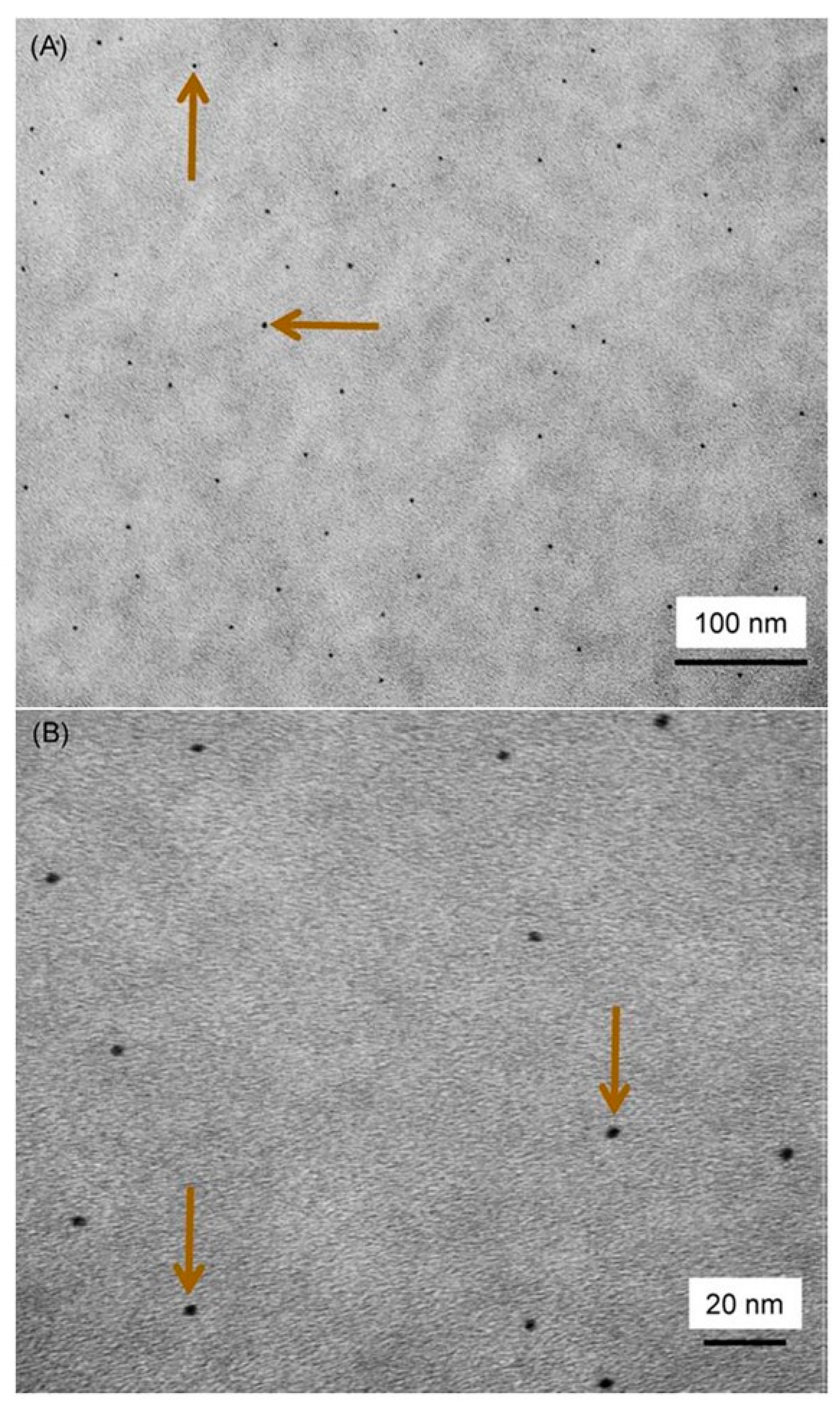
4.4. Other Approaches to Mitigation of Oral Biofilm Degradation
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Disclaimer
References
- Ferracane, J.L. Resin composite—State of the art. Dent. Mater. 2011, 27, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Drummond, J.L. Degradation, fatigue, and failure of resin dental composite materials. J. Dent. Res. 2008, 87, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Bayne, S.C. Correlation of clinical performance with ‘in vitro tests’ of restorative dental materials that use polymer-based matrices. Dent. Mater. 2012, 28, 52–71. [Google Scholar] [CrossRef] [PubMed]
- Beyth, N.; Bahir, R.; Matalon, S.; Domb, A.J.; Weiss, E.I. Streptococcus mutans biofilm changes surface-topography of resin composites. Dent. Mater. 2008, 24, 732–736. [Google Scholar]
- Beyth, N.; Domb, A.J.; Weiss, E.I. An in vitro quantitative antibacterial analysis of amalgam and composite resins. J. Dent. 2007, 35, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Kassebaum, N.J.; Bernabé, E.; Dahiya, M.; Bhandari, B.; Murray, C.J.; Marcenes, W. Global burden of untreated caries: A systematic review and meta regression. J. Dent. Res. 2015, 94, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Cramer, N.B.; Stansbury, J.W.; Bowman, C.N. Recent advances and developments in composite dental restorative materials. J. Dent. Res. 2011, 90, 402–416. [Google Scholar] [CrossRef] [PubMed]
- Busscher, H.J.; Rinastiti, M.; Siswomihardjo, W.; Mei, H.C.V.D. Biofilm formation on dental restorative and implant materials. J. Dent. Res. 2010, 89, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Bourbia, M.; Ma, D.; Cvitkovitch, D.G.; Santerre, J.P.; Finer, Y. Cariogenic bacteria degrade dental resin composites and adhesives. J. Dent. Res. 2013, 92, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Delaviz, Y.; Finer, Y.; Santerre, J.P. Biodegradation of resin composites and adhesives by oral bacteria and saliva: A rationale for new material designs that consider the clinical environment and treatment challenges. Dent. Mater. 2014, 30, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Gajewski, V.E.; Pfeifer, C.S.; Fróes-Salgado, N.R.; Boaro, L.C.; Braga, R.R. Monomers used in resin composites: Degree of conversion, mechanical properties and water sorption/solubility. Braz. Dent. J. 2012, 23, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, F.; Kawano, Y.C.; Stansbury, J.W.; Braga, R.R. Influence of BisGMA, TEGDMA, and BisEMA contents on viscosity, conversion, and flexural strength of experimental resins and composites. Eur. J. Oral Sci. 2009, 117, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Nagem, F.H.; Nagem, H.D.; Francisconi, P.A.; Franco, E.B.; Mondelli, R.F.; Coutinho, K.Q. Volumetric polymerization shrinkage of contemporary composite resins. J. Appl. Oral. Sci. 2007, 15, 448–452. [Google Scholar] [CrossRef]
- Spencer, P.; Ye, Q.; Park, J.; Topp, E.M.; Misra, A.; Marangos, O.; Wang, Y.; Bohaty, B.S.; Singh, V.; Sene, F.; et al. Adhesive/dentin interface: the weak link in the composite restoration. Ann. Biomed. Eng. 2010, 38, 1989–2003. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Bonet, A.; Kaufman, G.; Yang, Y.; Wong, C.; Jackson, A.; Huyang, G.; Bowen, R.; Sun, J. Preparation of Dental Resins Resistant to Enzymatic and Hydrolytic Degradation in Oral Environments. Biomacromolecules 2015, 16, 3381–3388. [Google Scholar] [CrossRef] [PubMed]
- Daghighi, S.; Sjollema, J.; Hc, V.D.M.; Busscher, H.J.; Rochford, E.T. Infection resistance of degradable versus non-degradable biomaterials: An assessment of the potential mechanisms. Biomaterials 2013, 34, 8013–8017. [Google Scholar] [CrossRef] [PubMed]
- Mayanagi, G.; Igarashi, K.; Washio, J.; Takahashi, N. PH Response and Tooth Surface Solubility at the Tooth/Bacteria Interface. Caries Res. 2017, 51, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Spencer, P.; Ye, Q.; Misra, A.; Goncalves, S.E.; Laurence, J.S. Proteins, pathogens, and failure at the composite-tooth interface. J Dent Res. 2014, 93, 1243–1249. [Google Scholar] [CrossRef] [PubMed]
- Grumezescu, A.M.; Chifiriuc, C.M. Prevention of microbial biofilms—The contribution of micro and nanostructured materials. Curr. Med. Chem. 2014, 21, 3311. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.D. Microbiological deterioration and degradation of synthetic polymeric materials: recent research advances. Int. Biodeter. Biodegr. 2003, 52, 69–91. [Google Scholar] [CrossRef]
- Khvostenko, D.; Salehi, S.; Naleway, S.E.; Hilton, T.J.; Ferracane, J.L.; Mitchell, J.C.; Kruzic, J.J. Cyclic mechanical loading promotes bacterial penetration along composite restoration marginal gaps. Dent. Mater. 2015, 31, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Loguercio, A.D.; Reis, A.; Bortoli, G.; Patzlaft, R.; Kenshima, S.; Kenshima, S. Influence of adhesive systems on interfacial dentin gap formation in vitro. Oper. Dent. 2006, 31, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Awliya, W.Y.; El-Sahn, A.M. Leakage pathway of Class V cavities restored with different flowable resin composite restorations. Oper. Dent. 2008, 33, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Fucio, S.B.P.; Carvalho, F.G.; Sobrinho, L.C.; Sinhoreti, M.A.C.; Puppin-Rontani, R.M. The influence of 30-day-old Streptococcus mutans biofilm on the surface of esthetic restorative materials-an in vitro study. J. Dent. 2008, 36, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Leprince, J.G.; Palin, W.M.; Hadis, M.A.; Devaux, J.; Leloup, G. Progress in dimethacrylate-based dental composite technology and curing efficiency. Dent. Mater. 2012, 29, 139–156. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, L.; Filho, J.D.; Guimarães, J.G.; Poskus, L.T.; Silva, E.M. Solubility, salivary sorption and degree of conversion of dimethacrylate-based polymeric matrixes. J. Biomed. Mater. Res. B App. Biomater. 2008, 85, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Khalichi, P.; Cvitkovitch, D.G.; Santerre, J.P. Effect of composite resin biodegradation products on oral streptococcal growth. Biomaterials. 2004, 25, 5467–5472. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Zhang, K.; Weir, M.D.; Melo, M.A.; Zhou, X.; Xu, H.H. Nanotechnology strategies for antibacterial and remineralizing composites and adhesives to tackle dental caries. Nanomedicine 2015, 10, 627–641. [Google Scholar] [CrossRef] [PubMed]
- Melo, M.A.S.; Guedes, S.F.F.; Xu, H.H.K.; Rodrigues, L.K.A. Nanotechnology-based restorative materials for dental caries management. Trends Biotechnol. 2013, 31, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.F.; Wu, R.; Fan, Y.; Liao, S.; Wang, Y.; Wen, Z.T.; Xu, X. Antibacterial dental composites with chlorhexidine and mesoporous silica. J. Dent. Res. 2014, 93, 1283–1289. [Google Scholar] [CrossRef] [PubMed]
- Chatzistavrou, X.; Fenno, J.C.; Faulk, D.; Badylak, S.; Kasuga, T.; Boccaccini, A.R.; Papagerakis, P. Fabrication and characterization of bioactive and antibacterial composites for dental applications. Acta. Biomater. 2014, 10, 3723–3732. [Google Scholar] [CrossRef] [PubMed]
- Fraise, A.P.; Maillard, J.Y.; Sattar, S. Types of Antimicrobial Agents. In Russell, Hugo and Ayliffe’s Principles and Practice of Disinfection, Preservation and Sterilization, 5th ed.; Wiley-Blackwell Publishing Ltd: Hoboken, NJ, USA, 2013; pp. 88–97. [Google Scholar]
- Teughels, W.; Van Assche, N.; Sliepen, I.; Quirynen, M. Effect of material characteristics and/or surface topography on biofilm development. Clin. Oral Implant. Res. 2006, 17, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Hori, K.; Matsumoto, S. Bacterial adhesion: From mechanism to control. Biochem. Eng. J. 2010, 48, 424–434. [Google Scholar] [CrossRef]
- Zhang, K.; Melo, M.A.S.; Cheng, L.; Weir, M.D.; Bai, Y.; Xu, H.H. Effect of quaternary ammonium and silver nanoparticle-containing adhesives on dentin bond strength and dental plaque microcosm biofilms. Dent. Mater. 2012, 28, 842–852. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Zhang, K.; Melo, M.A.S.; Weir, M.D.; Zhou, X.; Xu, H.H. Anti-biofilm Dentin Primer with Quaternary Ammonium and Silver Nanoparticles. J. Dent. Res. 2012, 91, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Beyth, N.; Yudovin-Farber, I.; Bahir, R.; Domb, A.J.; Weiss, E.I. Antibacterial activity of dental composites containing quaternary ammonium polyethylenimine nanoparticles against Streptococcus mutans. Biomaterials 2006, 27, 3995–4002. [Google Scholar] [CrossRef] [PubMed]
- Imazato, S. Antibacterial properties of resin composites and dentin bonding systems. Dent. Mater. 2003, 19, 449–457. [Google Scholar] [CrossRef]
- Xu, X.; Wang, Y.; Liao, S.; Wen, Z.T.; Fan, Y. Synthesis and characterization of antibacterial dental monomers and composites. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 1151–1162. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Weir, M.D.; Xu, H.H.; Antonucci, J.M.; Kraigsley, A.M.; Lin, N.J.; Lin-Gibson, S.; Zhou, X.D. Antibacterial amorphous calcium phosphate nanocomposites with a quaternary ammonium dimethacrylate and silver nanoparticles. Dent. Mater. 2012, 28, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Weir, M.D.; Xu, H.H. Effects of quaternary ammonium chain length on antibacterial bonding agents. J. Dent. Res. 2013, 92, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Simoncic, B.; Tomcis, B. Structures of novel antimicrobial agents for textiles—A review. Textile. Res. J. 2010, 80, 1721–1737. [Google Scholar] [CrossRef]
- Zhang, K.; Cheng, L.; Weir, M.D.; Bai, Y.X.; Xu, H.H. Effects of quaternary ammonium chain length on the antibacterial and remineralizing effects of a calcium phosphate nanocomposite. Int. J. Oral. Sci. 2016, 8, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Wang, C.; Zhou, J.; Jiang, L.; Xue, J.; Li, W. Influence of Surface Properties on Adhesion Forces and Attachment of Streptococcus mutans to Zirconia In Vitro. Biomed Res Int. 2016. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, K.; Ueda, T.; Nakabayashi, N. Preparation of Phospholipid Polylners and Their Properties as Polymer Hydrogel Membranes. Polym. J. 1990, 22, 355–360. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, Z.; Pan, F.; Ma, Y.; Armes, S.P.; Lewis, A.L.; Lu, J.R. Solution pH-regulated interfacial adsorption of diblock phosphorylcholine copolymers. Langmuir 2005, 21, 9597–9603. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Zhang, Z.; Chen, S.F.; Bryers, J.D.; Jiang, S.Y. Inhibition of bacterial adhesion and biofilm formation on zwitterionic surfaces. Biomaterials 2007, 28, 4192–4199. [Google Scholar] [CrossRef] [PubMed]
- Mashaghi, S.; Jadidi, T.; Koenderink, G.; Mashaghi, A. Lipid Nanotechnology. Int. J. Mol. Sci. 2013, 14, 4242–4282. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.L.; Tolhurst, L.A.; Stratford, P.W. Analysis of a phosphorylcholine-based polymer coating on a coronary stent pre- and post-implantation. Biomaterials. 2002, 23, 1697–1706. [Google Scholar] [CrossRef]
- Takahashi, N.; Iwasa, F.; Inoue, Y.; Morisaki, H.; Ishihara, K.; Baba, K. Evaluation of the durability and antiadhesive action of 2-methacryloyloxyethyl phosphorylcholine grafting on an acrylic resin denture base material. J. Prosthet. Dent. 2014, 112, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Chen, C.; Melo, M.A.; Bai, Y.; Cheng, L.; Xu, H.H. A novel protein-repellent dental composite containing 2-methacryloyloxyethyl phosphorylcholine. Int. J. Oral. Sci. 2015, 7, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Weir, M.D.; Fouad, A.F.; Xu, H.H. Effect of salivary pellicle on antibacterial activity of novel antibacterial dental adhesives using a dental plaque microcosm biofilm model. Dent. Mater. 2014, 30, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Weir, M.D.; Romberg, E.; Bai, Y.; Xu, H.H. Development of novel dental adhesive with double benefits of protein-repellent and antibacterial capabilities. Dent. Mater. 2015, 31, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Ma, J.; Melo, M.A.; Weir, M.D.; Bai, Y.; Xu, H.H. Protein-repellent and antibacterial dental composite to inhibit biofilms and caries. J. Dent. 2015, 43, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Mandracci, P.; Mussano, F.; Ceruti, P.; Pirri, C.F.; Carossa, S. Reduction of bacterial adhesion on dental composite resins by silicon-oxygen thin film coatings. Biomed. Mater. 2015, 10, 15–17. [Google Scholar] [CrossRef] [PubMed]
- Goldman, G.; Starosvetsky, J.; Armon, R. Inhibition of biofilm formation on UF membrane by use of specific bacteriophages. J. Membrane. Sci. 2009, 342, 145–152. [Google Scholar] [CrossRef]
- Rodrigues, L.; Van Der Mei, H.; Banat, I.M.; Teixeira, J.; Oliveira, R. Inhibition of microbial adhesion to silicone rubber treated with biosurfactant from Streptococcus thermophilus A. FEMS Immunol. Med. Microbiol. 2006, 46, 107–112. [Google Scholar]
- Thorat, S.B.; Diaspro, A.; Salerno, M. In vitro investigation of coupling-agent-free dental restorative composite based on nano-porous alumina fillers. J. Dent. 2014, 42, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Thorat, S.B.; Diaspro, A.; Scarpellini, A.; Povia, M.; Salerno, M. Comparative study of loading of anodic porous alumina with silver nanoparticles using different methods. Materials 2013, 6, 206–216. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, N.; Ma, Y.; Weir, M.D.; Xu, H.H.K.; Bai, Y.; Melo, M.A.S. Current Insights into the Modulation of Oral Bacterial Degradation of Dental Polymeric Restorative Materials. Materials 2017, 10, 507. https://doi.org/10.3390/ma10050507
Zhang N, Ma Y, Weir MD, Xu HHK, Bai Y, Melo MAS. Current Insights into the Modulation of Oral Bacterial Degradation of Dental Polymeric Restorative Materials. Materials. 2017; 10(5):507. https://doi.org/10.3390/ma10050507
Chicago/Turabian StyleZhang, Ning, Yansong Ma, Michael D. Weir, Hockin H. K. Xu, Yuxing Bai, and Mary Anne S. Melo. 2017. "Current Insights into the Modulation of Oral Bacterial Degradation of Dental Polymeric Restorative Materials" Materials 10, no. 5: 507. https://doi.org/10.3390/ma10050507
APA StyleZhang, N., Ma, Y., Weir, M. D., Xu, H. H. K., Bai, Y., & Melo, M. A. S. (2017). Current Insights into the Modulation of Oral Bacterial Degradation of Dental Polymeric Restorative Materials. Materials, 10(5), 507. https://doi.org/10.3390/ma10050507







